Abstract
Objectives
This study was conducted to compare viral dynamics in blood and semen between subjects with antibody negative, acute HIV-1 infection and other subjects with later stages of infection.
Design
A prospective cohort study was embedded within a cross-sectional study of HIV screening in a Lilongwe, Malawi STD clinic.
Methods
Blood samples from HIV antibody negative or indeterminate volunteers were used to detect HIV RNA in plasma using a pooling strategy. Blood and seminal plasma HIV-1 RNA concentrations were measured over 16 weeks.
Results
Sixteen men with acute HIV infection and 25 men with chronic HIV infection were studied. Blood viral load in subjects with acute HIV infection was highest about 17 days after infection (mean ± SE, 6.9 ± 0.5 log10 copies/ml), while semen viral load peaked about 30 days after infection (4.5 ± 0.4 log10 copies/ml). Semen viral load declined by 1.7 log10 to a nadir by week 10 of HIV infection. Semen and blood viral loads were more stable in chronically infected subjects over 16 weeks. Higher semen levels of HIV RNA were noted in subjects with low CD4 cell counts.
Conclusions
These results provide a biological explanation for reported increases in HIV transmission during the very early (acute) and late stages of infection. Recognizing temporal differences in HIV shedding in the genital tract is important in the development of effective HIV prevention strategies.
Keywords: semen, HIV transmission, acute HIV infection, peak viral load
Introduction
It has been estimated that 80% of HIV infections are acquired through sexual transmission [1]. A better understanding of sexual transmission of HIV is essential to the interpretation of the HIV pandemic, and to develop prevention strategies. HIV transmission depends on the infectiousness of the host and susceptibility of the sexual partner, and neither parameter is uniform over time [2]. Infectiousness can be directly correlated with the concentration of HIV in blood [3,4], which (for sexual transmission) must serve as a surrogate for shedding of HIV into genital tract secretions [2]. Recent epidemiologic data suggest that infectiousness is amplified during the earliest days of infection (acute HIV) and in the latest stage of the disease (AIDS), when the viral burden is also increased [5].
We and others have defined acute HIV infection (AHI) as the time before and during HIV seroconversion [6]. The study of patients with AHI is critical to understanding the biology of HIV transmission, but such patients have been extremely difficult to find. Heretofore, there have been no longitudinal studies of the dynamics of HIV shedding in the genital tract in patients with AHI. We have developed a cross-sectional surveillance strategy that allows detection of subjects with AHI before antibody formation. Using this strategy we have detected a considerable number of subjects with acute HIV infection in North Carolina [7], Malawi [8,9], and South Africa [10] and Brazil [11].
The current study was designed to recruit subjects with acute HIV infection among men in Malawi to characterize the biology of HIV acquisition. In this paper, we focus on the longitudinal dynamics of HIV shedding in the genital tract, and examine differences between HIV shedding in AHI and later stages of HIV disease. The results allow prediction of the magnitude of peak HIV in the genital tract and the time(s) of maximal risk for HIV transmission.
Methods
Study design
We conducted a prospective cohort study of viral dynamics among participants with acute and chronic HIV infections. Patients presenting for care at the Kamuzu Central Hospital (KCH) sexually transmitted infection (STI) clinic in Lilongwe, Malawi during February 2003 to October 2004 were considered eligible if they were at least 18 years of age, able to speak English or Chichewa, able and willing to return in 1 week and to attend required follow-up visits. All eligible patients initially classified as possibly having AHI were invited to enroll in the prospective cohort for verification. As only five women with AHI were recruited [9] this report focuses on the men enrolled.
Study procedures
All study procedures were approved by the IRB at the University of North Carolina and the Malawi Health Sciences Research Committee. All study participants gave written informed consent before entering the screening and recruitment protocol. Screening assessments included physical exams and a questionnaire regarding signs and symptoms of AHI and STI, demographics and basic risk behaviors. STI were treated based on Malawi STI Syndromic Guidelines. Participants were screened for HIV infection via a dual, parallel HIV antibody testing protocol using approved HIV rapid tests, Determine (Abbott Laboratories, Abbott Park, Illinois, USA) and Unigold (Trinity Biotech, IDA Business Park Bray, Wicklow, Ireland), according to Malawi country guidelines. Patients with one or more negative rapid test result underwent further testing in real time, by HIV Western blot (Biorad, Hercules, California, USA); plasma HIV p24 antigen (Perkin Elmer, Waltham, Massachusetts, USA) and HIV RNA RT–PCR (Roche HIV-1 Amplicor Monitor, version 1.5, Roche Diagnostics Corporation, Indianapolis, USA). RNA testing followed a 50 : 5 : 1 pooling algorithm as described previously [12]. The comparison between these laboratory assays is the subject of a separate report [9].
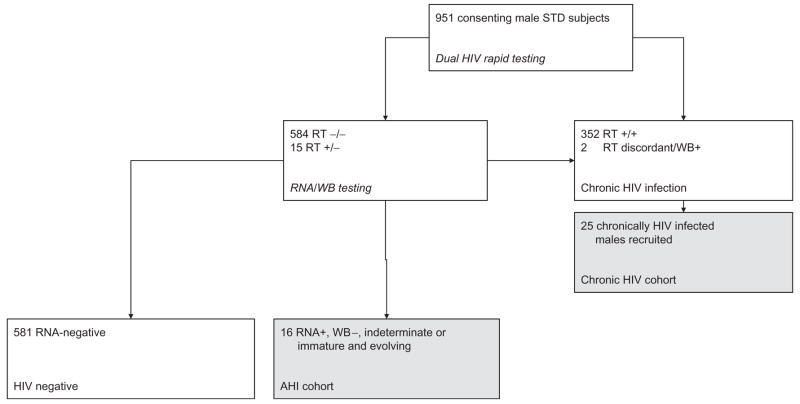
Prospective cohort participants were recruited based on HIV results available at the 1-week follow-up visit (Fig. 1). All patients for whom baseline HIV rapid tests were negative or discordant, and baseline p24 antigen or RNA assay was positive (at any level) were suspected of having AHI and were recruited. At each follow-up visit (weeks 1, 2, 4, 8, 12 and 16), patients with suspected AHI had repeat HIV rapid testing and HIV western blot to detect antibody seroconversion; subjects had directed clinical assessments, and donated blood and genital secretions for measurement of HIV-1 RNA concentrations. Whenever a patient with suspected AHI was recruited to the study, the next (male) patient screened and found to be HIVantibody positive was recruited into the chronic HIV (CHI) cohort. Enrolled CHI participants were followed in a similar protocol, but without repeat antibody testing, and with CD4 cell counts recorded at weeks 1 and 16. Semen was obtained by masturbation. Blood plasma HIV RNA levels were measured with Roche Monitor, version 1.5; seminal plasma HIV RNA levels were measured using a NucliSens assay (HIV-1 QT, BioMeriéux, Durham, North Carolina, USA). The limits of sensitivity of this assay are approximately 200 copies/ml plasma.
Fig. 1. Flowchart of the study population.
Cohorts contributing data to this report are represented by the shaded boxes. RT, Rapid testing; WB, insert full term here.
Clinical staging
Patients were classified as having AHI if they had a positive HIV RNA result at baseline and either two negative rapid tests or one positive rapid test with either: (i) weakly positive Western blot with seroconversion documented by an evolving Western blot pattern at follow-up, or (ii) negative or indeterminate Western blot. CHI infection was defined by two positive rapid HIV antibody tests and/or a characteristic converted Western blot pattern at baseline.
For AHI subjects, we estimated infection date as occurring 14 days prior to onset of characteristic symptoms [8,13] including fever, headache, or body ache. The mean interval from infection to seroconversion, calculated for symptomatic subjects, was used to estimate infection date for asymptomatic acute subjects, deducting time from the observed date of seroconversion.
Statistical analysis strategy and methods
Cohort comparisons in Table 1 made use of a two-tailed Fisher exact test procedure. AHI cohort viral load measurements were indexed by elapsed time since estimated infection date (day 0). CHI cohort measurements were indexed by elapsed time enrolled in the study. Average CD4 cell count, used as an explanatory variable in the CHI cohort, was treated as a continuous covariate or was dichotomized at its median value, 351 mean cells/μl. The primary analyses focused on longitudinal dynamics of mean HIV-1 RNA log10 copies/ml as a function of elapsed time and patient characteristics [14]. These analyses required statistical methods and non-linear models appropriate for modest sample sizes and incomplete, mis-timed, bivariate, log-normal, longitudinal measurements that are subject to left-censoring by the lower limit of quantitation of the assay methods used. The models fitted assumed that the population mean viral load is a smooth function of time which has an initial value of − 4 log10 copies/ml on day 0 [14,15]. In the first stage of the analysis, the mixed-effects model proposed by Edwards et al. [15] was fitted using all available blood (BVL) and semen (SVL) viral load data. The algorithm for fitting the model involved correctly specifying a log-normal likelihood function for the data to account for left-censoring. Imputation methods were not used in the fitting of this non-linear model. In addition, this model was generalized to handle the two-dimensional measure (BVL, SVL) in order to facilitate inferences about the longitudinal bivariate relationship between blood and semen viral load. The computations for these analyses relied on customized programming code executed iteratively using SAS procedure NLMIXED. In a second stage of analysis, numerous non-linear and linear mixed-effects models were fitted in an exploratory manner. These included, for example, non-linear models designed to cope with uncertainty about the date (day 0) the patient became infected. Models of this kind, and some other kinds of models, were not supported by the available data. None were found to be superior to the initial model fitted. In the third stage of the analysis, the initial bestfitting model was used to address the primary aims of the study: the fitted model yielded hypothesis tests, confidence regions, and estimates of means, variances, correlations, viral-load-versus-time curves, patient-specific trends and population percentiles. In this report, statistical estimates are presented with their standard errors (e.g., mean ± SE) unless stated otherwise. Approximate 95% confidence intervals may be inferred (e.g., mean ± SE). All statistical computations were performed using SAS System software (version 9.1.3, SAS Institute, Cary, North Carolina, USA).
Table 1.
Baseline characteristics of male subjects with acute and chronic HIV infection.
| Acute HIV infection |
Chronic HIV infection |
||
|---|---|---|---|
| All subjects (n =16) | Cohort subjects (n =25) | All subjects (n =354) | |
| STI syndromes [n (%)] | |||
| Urethral discharge | 7 (44) | 13 (52) | 161 (45) |
| Scrotal Swelling | 0 (0) | 1 (4) | 7 (2) |
| Balanitis | 2 (13) | 0 (0) | 31 (9) |
| Bubo | 1 (6) | 2 (8) | 33 (9) |
| Genital ulcer disease | 13 (81)* | 14 (56) | 160 (45) |
| Warts | 2 (13) | 3 (12) | 34 (10) |
| Asymptomatic partner | 0 (0) | 0 (0) | 29 (8) |
| Acute retroviral symptoms*,** [n (%)] | |||
| Any acute retroviral symptom | 13 (81) | 19 (76) | 299 (85) |
| Fever | 9 (56)*,** | 3 (12) | 103 (29) |
| Any adenopathy | 8 (50) | 7 (28) | 131 (37) |
| Headache | 7 (44) | 4 (16) | 79 (22) |
| Body ache | 7 (44)* | 4 (16) | 65 (18) |
| Stomach pain | 7 (44)* | 4 (16) | 69 (20) |
| Inguinal adenopathy | 7 (44) | 7 (28) | 127 (36) |
| Pharyngitis | 1 (6) | 0 (0) | 8 (2) |
| Rash | 1 (6) | 5 (20) | 61 (17) |
| Mean (SD) log10 blood viral load (baseline) | 6.03 (1.52)*,** | 5.02 (0.64) | 4.92 (0.67) |
| Mean (SD) log10 blood viral load (week 1) | 5.98 (0.89)** | 5.00 (0.66) | – |
| Mean (SD) log10 semen viral load (week 1) | 4.36 (0.85) | 3.93 (0.88) | – |
The results represent self-reported history of symptoms in previous month, except for adenopathy, which was assessed during the physical examination. Other symptoms assessed were diarrhea, night sweats, appetite loss, cough, fatigue, joint pain, nausea, weight loss, oral patches, and oral ulcers; the frequency of these symptoms did not differ significantly between acutely infected patients and each group of chronically infected patients (chronically infected cohort subjects; all chronically infected male subjects). Acute retroviral symptoms are described in the references [8,20].
Difference statistically significant (P <0.05) between acute and all chronically infected male subjects.
Difference statistically significant (P <0.05) between acute and chronically infected cohort subjects.
Results
A total of 951 men attending the STI clinic at Kamuzu Central Hospital, Lilongwe, Malawi were enrolled and received HIV testing through a screening protocol. The data for 497 women evaluated are presented in a separate report [9]. Of the participants, 370 (38.9%) were HIV infected: acute HIV infection was confirmed in 16 (1.7%) whereas 354 (37.2%) had CHI. All 16 acutely infected men and 25 of the chronically infected men were included in the prospective cohort study (Fig. 1).
Baseline characteristics for AHI and CHI cohorts are compared in Table 1. Most (81%) of the subjects with AHI reported recent acute retroviral symptoms at study entry including fever (56%), headache (44%), body ache (44%), or abdominal pain (44%). Genital ulcer disease (81%) and inguinal adenopathy (44%) were more common among acutely infected men than among the men with CHI. The proportion of subjects with urethritis, which can influence seminal shedding of HIV-1, was similar in the AHI (44%) and CHI (52%) cohorts.
At study entry, the median duration of infection for acutely infected men (estimated from clinical data) was 28 days (range, 17–44 days). At the initial screening visit, blood viral load was higher in the AHI cohort (mean ± SE, 6.0 ± 0.4 log10 copies/ml) than for the CHI cohort (5.0 ± 0.1 log10 copies/ml; P = 0.01). At week 1 (the initial visit for semen donation) mean semen viral load was higher in the AHI cohort (4.4 ± 0.3 log10 copies/ml) than in the CHI cohort (3.9 ± 0.2 log10 copies/ ml). The latter difference was not statistically significant (P =0.31).
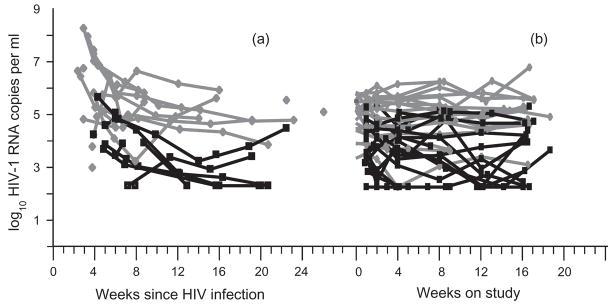
As indicated in Fig. 2, semen viral loads were persistently lower than concurrent levels of HIV in blood in subjects with both AHI and CHI. The correlation between blood viral load and semen viral load were smaller during AHI (r, 0.3; SE, 0.2; P =0.17) than during CHI (r, 0.4; SE, 0.1). These estimates were obtained by fitting blood and semen viral load data to a longitudinal joint statistical model which appropriately accounted for left-censored values.
Fig. 2. HIV-1 viremia and HIV-1 shedding over time in acutely (a) or chronically (a) HIV infected men.
HIV-1 RNA measurements in blood plasma (grey) and seminal plasma (black) are shown for 16 weeks. The acute infection data (a) are shown with the x-axis representing the time from estimated infection date, which is different for each subject. Chronic infection data (b) are shown with the x-axis representing the time from study entry and therefore appear to be more synchronized. Sixteen acutely infected subjects contributed 60 blood and 34 semen time-points; 25 chronically infected subjects contributed 123 blood and 89 semen time-points. HIV-1 shedding was detectable in 26/34 (76%) acute semen samples and in 70 (79%) of 89 chronically infected semen samples.
Viral dynamics: acutely infected subject data
For purposes of investigating the dynamic changes occurring in blood and semen for acutely infected patients, HIV-1 RNA concentrations were indexed by the estimated time since infection (time 0) and are plotted against elapsed time in Fig. 2. Blood and semen viral load longitudinal measurements closest to time 0 tended to rapidly decrease (Fig. 2a), suggesting that our sampling may have begun near or after the times of peak shedding for the patients studied.
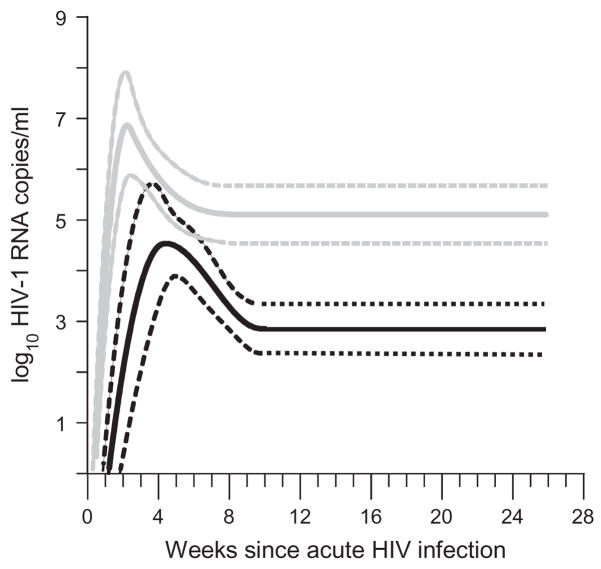
Population mean curves for blood and semen viral load were estimated by fitting compartment-specific longitudinal statistical models (Fig. 3). The peaks of the fitted curves occurred within the first month of infection and were followed immediately by rapid decline. The estimated time of peak viral load was later for semen (30 ± 4 days) than for blood data (17 ± 1 days). In each compartment, mean viral load decreased rapidly to a nadir within 10 weeks from infection (by day 68 for semen viral load, by day 61 for blood viral load). The difference between the peak (4.5 ± 0.4 log10 copies/ml) and nadir (2.9 ± 0.2 log10 copies/ml) mean semen viral loads was similar to the difference in blood (peak, 6.9 ± 0.5 and nadir, 5.1 ± 0.3) and both differences were statistically significant (P <0.001).
Fig. 3. Viral dynamics in acute HIV infection.
To describe the magnitude and timing of trends in the observed data, expected concentration versus time curves for mean HIV-1 RNA level were generated using a statistical model. Shown here are mean estimates for blood (grey) and semen (black) data along with their 95% confidence intervals (dotted lines) for acute infection subjects only. The final, unified model used in all primary analyses was a linear mixed-effects model including all data, featuring two-knot polynomial regression splines for acute subject blood and semen HIV-1 RNA level that were quadratic for day 0 to day k1, cubic for days k1 to k2, and constant for days k2 to day 200 [17]. For [k1,k2] was taken to be [18, 60.66] for blood and [30, 68.39] for semen. From an assumed mean HIV-1 RNA level of − 4 logs on day 0 [14,15], the model featured a peak in viral load during the first 3–4 weeks followed by decay of the viral loads to nadirs and chronic levels by 8–10 weeks after initial infection. To account for a possible relationship between shedding and CD4 cell count, chronic infection blood and semen data were stratified by the median CD4 cell count for chronically infected cohort subjects (351 cells/μl). Reported model estimates were robust to substantial changes in model assumptions (including assumed day 0 viral load and to the placement of regression knots).
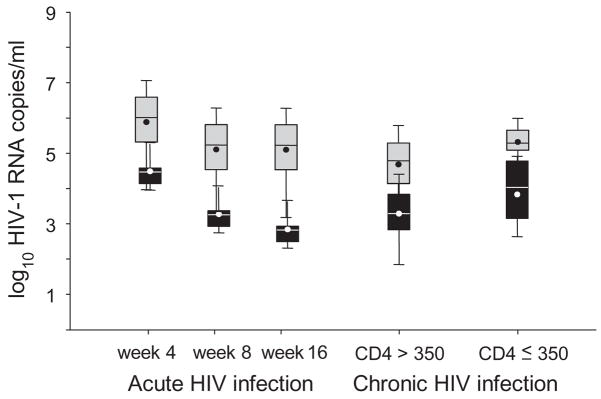
The distributions of the predicted patient-specific mean blood and semen viral loads (predicted by an eBLUP method) for each patient at selected time points are summarized by box-and-whisker plots in Fig. 4. In blood, the median and interquartile range (IQR) were 6.8 (6.2–7.5) log10 copies/ml at day 17 peak (data not shown), 5.9 (5.3–6.6) at week 4, and 5.2 (4.5–5.8) at week 8 when the nadir occurred. The pattern in semen was similar with peak shedding occurring at about day 30 and nadir occurring at the end of week 10. Median and IQR were 4.4 (4.3–4.6) log10 copies/ml at week 4, 3.2 (3.1–3.4) at week 8, and 2.8 (2.7–2.9) at week 16.
Fig. 4. HIV viremia and shedding, measured over the natural course of HIV infection.
Box-and-whisker plots represent HIV-1 RNA levels in blood (grey) and semen (black), estimated for individual study subjects by disease stage. Numerical values at each time-point were estimated for each individual subject by empirical best linear unbiased prediction (eBLUP) making use of the final, unified best fit model including all observed data. Boxes and whiskers denote the 25th, 75thquartiles and total range of values. Internal circles and horizontal lines represent mean and median, respectively. Data for acutely infected subjects are shown at weeks 4, 8 and 16. The data for chronically HIV infected subjects represent the mean values of specimens collected over 16 weeks and are shown separately for high (>350 cells/μl) and low (≤ 350 cells/μl) CD4 cell count subjects.
Viral dynamics: chronically HIV infected subject data
We found a strong relationship between having a low CD4 cell count and high semen viral load (P =0.0162), consistent with reports relating HIV transmission and advanced disease [5]. Each 1 log10 decrease in CD4 cell count predicted a semen viral load increase of 2.1 ± 0.8 log10 copies/ml. CD4 cell count was also predictive of blood viral load (P =0.0009): each 1 log10 decrease in CD4 cell count predicted a blood viral load increase of 1.6 ± 0.5 log10 copies/ml.
In contrast to the dynamics seen in the AHI cohort, both viremia and shedding measurements were more stable for subjects in the CHI cohort (Fig. 2b). In the CHI cohort, within-patient serial correlation of viral load measurements was high (r, 0.83 ± 0.05) for blood and was moderate for semen (r, 0.60 ± 0.10). Furthermore, temporal declines in semen mean viral load were modest in magnitude (estimate ± SE; 1.1 ± 0.6 log10/month if CD4 cell count was ≥ 351/μl, and 0.6 ± 0.5 log10/ month if CD4 cell count was <351/μl) were not statistically significant (P >0.06). A time-invariant (stable) model appeared to provide the best fit for the chronic infection viral load data.
The potential for temporal declines in viral load associated with treatment of urethritis (urethral discharge, UD) or genital ulcer disease (GUD) were explored in the CHI cohort. In patients with either early or late chronic infection, no temporal declines were detected for the subgroups of patients with GUD. In contrast, patients treated for UD exhibited a decline in mean semen viral load of −0.30 log10/month (SE, 0.15) if CD4 cell count was > 351/μl, and − 0.24 log10/month (SE, 0.10) if CD4 cell count was <351/μl. Under the assumption that the effect of UD is the same in both early and late chronic infection, a test of the null hypothesis ’the effects of UD on rate of decline is zero’ yielded P =0.0555.
The proportion of subjects with urethritis was similar for subjects in AHI (7/16) and CHI (13/25) cohorts. However, the AHI sample size was not large enough to support direct estimation of the magnitude of temporal declines that might be associated with resolution of STI.
The mean semen viral load for men with CD4 cell count ≥ 350/μl (3.2 ± 0.3) was lower than for men with CD4 cell count <350/μl (3.8 ± 0.3), although this difference was not statistically significant (P >0.15). The distributions of the mean blood and semen viral loads predicted (by an eBLUP method) for each patient at selected time points are summarized by the box-and-whisker plots in Fig. 4.
Viral dynamics: comparison of acutely and chronically HIV infected subjects
In blood, the peak mean viral load in the AHI cohort was greater (difference ± SE, 2.2 ± 0.5; P =0.0002) than the stable mean of men with ‘early stage’ CHI (average CD4 cell count ≥ 351/μl) The peak for AHI was also greater compared to men with ‘later stage’ CHI (1.5 ± 0.5; P =0.0066). In semen, the AHI peak mean viral load was greater than the mean for ‘early stage’ CHI men (difference ± SE, 1.4 ± 0.5; P =0.0168). However, in comparison to men with ‘later stage’ CHI, the peak AHI mean was not detectably different from the chronic mean level (0.7 ± 0.5; P =0.1962).
Discussion
The probability of the sexual transmission of HIV has been the subject of a very large number of studies [16]. However, the results provided by these epidemiologic and cohort studies make it very difficult to understand the massive HIV pandemic [1] because sexual transmission has appeared to be so inefficient, requiring hundreds if not thousands of episodes of sexual intercourse. Recently, Wawer et al. were able to monitor HIV transmission in a closed population in which couples could be identified retrospectively [5]. Their results demonstrate ‘amplified transmission’ of HIV [17] by subjects who had newly acquired HIV (including AHI), as well as by subjects with AIDS (shortly before death). Forty-three per cent of all susceptible partners with sexual contact to an individual infected in the prior 10 months became HIV infected [5].
The sexual transmission of HIV must depend on the concentration of HIV in genital secretions [3,4]. The current study was designed to compare shedding of HIV in genital secretions in subjects with acute and chronic infection. Heretofore it has been virtually impossible to recruit subjects with acute (pre-seroconversion) HIV infection. We were able to recruit such subjects in an STI clinic in Malawi because of the strong association between STI and HIV acquisition [2,18,19] and the use of novel strategies to detect HIV in antibody-negative subjects [7–13,20]. Contrary to the belief that AHI is rare in places such as Malawi and South Africa (where HIV is highly prevalent and in a later epidemic phase [1]), we have demonstrated that AHI is all too common in these sites, consistently representing more than 2% of all HIV cases detected [8–11]. We have previously emphasized the importance of detection of subjects with acute HIV infection for purposes of secondary prevention of HIV and earlier therapeutic intervention [6].
In the current study, we found that HIV in the seminal plasma reached its peak 4 weeks after infection, and that this burst of HIV shedding was almost completely contained by week 10, most likely reflecting the host immune response. Far greater change in semen viral load was observed in subjects with acute HIV infection compared to those with chronic infection, suggesting that observed dynamics did not simply result from treatment and resolution of STI. However, the decline in semen HIV observed in chronically infected subjects after treatment of urethritis is consistent with our previous report [19]. The relatively modest declines in HIV shedding seen likely reflect the fact that measurement of HIV in semen began only 1 week after antibiotic treatment, so that the maximal effect of antibiotic therapy could not be observed [19].
The patterns of HIV dynamics measured in blood and semen in this study were consistent with earlier cross-sectional observations [21,22], and with previous data observed in acutely infected rhesus macaques [23]. The concentration of HIV in blood is generally higher than that in semen (and often by a substantial amount [19,22]) emphasizing the compartmentalization of HIV infection [24]. The concentration of HIV in semen of men with AHI in Malawi generally exceeded that reported for subjects with acute HIV studied in the United States [8,25,26]; concentrations of HIV in blood and semen of men with chronic HIV infection were also greater than in comparable US subjects [26]. In addition, many chronically HIV infected men in Malawi with later stages of disease had levels of HIV shedding that were similar to those seen at the AHI peak.
These results lend themselves to further consideration of statistical and mathematical models of the spread of HIV. Early models estimated that AHI and very late chronic HIV infection [27] could play a key role in the spread of HIV, and the high semen viral burdens we observed and other empirical observations [5] support this idea. Recently, Fraser et al. [28] have argued that patients with early chronic infection (and many years of infectious potential) may contribute greatly to pandemic spread based on the blood viral loads characteristic of this stage. Our results suggest that both models are likely correct, and help to explain the continued spread of HIV in different kinds of populations.
This study has clear limitations. First, we did not measure HIV transmission directly, but rather HIV concentrations in semen. Second, only 16 men with AHI were studied, and both the subjects with AHI and CHI represent a convenience sample from a single clinic in Malawi; accordingly, the results may not be generalizable. Third, while appropriate and robust statistical methods were used to model and interpret these data, future larger studies should confirm the results; larger sample sizes may be required to fully understand the putative role of particular STIs on viral dynamics.
In summary, the high concentrations of HIV in semen in men at the extremes of disease demonstrate a substantial increased risk for sexual transmission of the virus. The data emphasize the importance of detection of all people with HIV, including those in the first few days of infection. The need for development of prevention intervention(s) focused on patients in the very earliest stages of HIV infection and their exposed and at-risk sexual partners is urgent.
Acknowledgments
The authors thank the patients and staff of Kamuzu Central Hospital, and all the personnel of the UNC Project in Lilongwe, Malawi led by Dr. Mina Hosseinipour. We also thank the staff of the UNC CFAR Core Virology Laboratory (Melissa Kerkau, Amy James, and Priya Joshi) for technical expertise in performing viral load assays.
These data were presented at the Twelfth Conference on Retroviruses and Opportunistic Infections. Boston, MA, February 2005 [abstract 20].
Sponsorship: This work was funded in part by the the UNC STD Cooperative Research Center (U19-AI31496), UNC Center for AIDS Research (NICHD/ NIAID 9-P30-AI50410), the UNC Fogarty Center (D43-TW01039), NIDDK (R01-49381), NIAID (AI-07001, K23-AI01781) and NIMH (R01-MH068686), the UNC HIV Prevention Treatment Network (U01-AI48005)and the Center for HIV Vaccine Immunology.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) UNAIDS 2006 report on the global AIDS epidemic. http://www.u-naids.org.
- 2.Galvin SR, Cohen MS. Sexual transmission of HIV. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 6.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute HIV infections during HIV testing in North Carolina. New Engl J Med. 2005;152:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 8.Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FE, Kazembe PN, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:517–524. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 9.Fiscus SA, Pilcher CD, Miller WC, Powers KA, Hoffman IF, Price M, et al. Rapid real time detection of patients with acute HIV infection in Africa. J Infect Dis. 195:416–424. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 10.Stevens W, Akkers E, Myers M, Pilcher C, Venter F, et al. High Prevalence of undetected acute HIV infection in a South African primary care clinic. Third IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro. August 2005; [abstract MoOa0108] [Google Scholar]
- 11.DeSouza R, Pilcher C, Fisus SA, Cachafenro A, Scherer L, Sperhacker RD, Silva M, et al. Rapid and efficient acute HIV detection by 4th generation Ag/Ab ELISA. XVI International Conference on AIDS; Toronto. August 2006; [abstract THLB0201] [Google Scholar]
- 12.Pilcher CD, McPherson JT, Leone PA, Smurzynski M, Owen-O’Dowd J, Peace-Brewer AL, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288:216–221. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 13.Quinn TC, Brookmeyer R, Kline, et al. Feasibility of pooling sera for HIV-1 RNA to diagnose acute primary HIV-1 infection and estimate HIV incidence. AIDS. 2000;14:2751–2757. doi: 10.1097/00002030-200012010-00015. [DOI] [PubMed] [Google Scholar]
- 14.Stafford MA, Corel L, Gao Y, et al. Modeling plasma virus concentration during primary HIV infection. J Theor Biol. 2000;203:285–301. doi: 10.1006/jtbi.2000.1076. [DOI] [PubMed] [Google Scholar]
- 15.Edwards LJ, Stewart PW, MacDougall JE, Helmsa R. A method for fitting regression splines with varying polynomial order in the linear mixed model. Stat Med. 2006;15:513–527. doi: 10.1002/sim.2232. [DOI] [PubMed] [Google Scholar]
- 16.Royce AR, Seña A, Cates W, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MS, Pilcher C. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391– 1393. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 18.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. SexTrans Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 20.Bollinger RC, Brookmeyer RS, Mehendale SM, Paranjape RS, Shepherd ME, Gadkari DA, et al. Risk factors and clinical presentation of acute primary HIV infection in India. JAMA. 1997;278:2085–2089. [PubMed] [Google Scholar]
- 21.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: Acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 22.Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 23.Pullium JK, Adams DR, Jackson E, Kim CN, Smith DK, Janssen R, et al. Pig-tailed macaques infected with human immunodeficiency virus (HIV) type 2GB122 or simian/HIV89. 6p express virus in semen during primary infection: new model for genital tract shedding and transmission. J Infect Dis. 2001;183:1023–1030. doi: 10.1086/319293. [DOI] [PubMed] [Google Scholar]
- 24.Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Stekler J, Syckr BJ, Holte S, Mgenza J, Stevens C, Dragavon J, et al. Semen HIV dynamics and effect of antiretrovirals following primary HIV infection. Thirteenth Conference on Retro-viruses and Opportunistic Infections; Denver, CO. February 2006; [abstract 396] [Google Scholar]
- 26.Dyer JR, Vernazza PL, Gilliam B, et al. High levels of human immunodeficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J Infect Dis. 1998;177:1742– 1746. doi: 10.1086/517436. [DOI] [PubMed] [Google Scholar]
- 27.Koopman JS, Jacquez JA, Welch GW, et al. The role of early HIV infection in the spread of HIV through populations. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 28.Fraser C, Hollingsworth T, Chapman R, Anderson R. Quantitating the impact of primary HIV on HIV transmission and control. Thirteenth Conference on Retroviruses and Opportunistic Infections; Denver, CO. February 2006; [abstract 62] [Google Scholar]