Abstract
Angiotensin-converting enzyme inhibition potentiates basal and bradykinin-stimulated tissue-type plasminogen activator (t-PA) release to a greater extent in women than in men. This study tested the hypothesis that 17β-estradiol enhances the effect of angiotensin-converting enzyme inhibition on t-PA release in young postmenopausal women. We conducted a double-blind, prospective, crossover study in 14 young postmenopausal women (mean age 48.2±2.3 years) who were randomized to receive 17β-estradiol (1 mg/d) or matching placebo for 4 weeks. At the end of each treatment period, we measured the effect of intraarterial infusion of bradykinin, methacholine, and nitroprusside on forearm blood flow and net t-PA release, before and during intraarterial enalaprilat (0.33 μg/min/100 mL forearm volume). 17β-estradiol significantly reduced baseline venous plasminogen activator inhibitor-1 antigen (4.4±1.4 versus 10.4±2.5 ng/mL, P=0.001) and t-PA antigen (5.5±0.6 versus 7.5±1.3 ng/mL, P=0.022) compared with placebo. 17β-estradiol increased basal forearm vascular release of active t-PA compared with placebo (1.2±0.3 IU/mL/min versus 0.4±0.1 IU/mL/min respectively, P=0.032), without increasing t-PA antigen release (P=0.761). Enalaprilat significantly increased basal net t-PA antigen release (from −0.8±1.0 to 3.2±1.2 ng/min/100 mL, P=0.012), but not the release of active t-PA, during either placebo or 17β-estradiol. Enalaprilat potentiated bradykinin-stimulated vasodilation and t-PA antigen and activity release similarly during placebo and 17β-estradiol treatment. 17β-estradiol treatment does not alter the effect of angiotensin-converting enzyme inhibition on basal t-PA antigen or on bradykinin-stimulated t-PA antigen or activity release. 17β-estradiol increases basal release of active t-PA in young postmenopausal women, consistent with enhanced vascular fibrinolytic function.
Keywords: estradiol, angiotensin-converting enzyme inhibition, postmenopausal women, plasminogen activator, bradykinin
Estrogen deficiency of menopause causes vascular and metabolic changes that increase the risk for cardiovascular disease.1,2 In contrast to observational studies that suggest that hormone replacement therapy (HRT) reduces the risk of coronary heart disease (CHD),3–5 recent randomized controlled trials indicate that treatment with conjugated estrogen plus progestin increases the risk of pulmonary emboli, CHD, and stroke, whereas treatment with conjugated estrogen alone increases risk of stroke without affecting embolic events or risk of CHD.6–8 This discrepancy in results has been attributed to the type of HRT used, the timing of intervention of HRT after menopause,9,10 or the route of administration.11 For example, the Heart and Estrogen/progestin Replacement Study (HERS)8 and the Women's Health Initiative (WHI)6,7 study assessed the effect of treatment with conjugated equine estrogens, a mixture of estrogens extracted from horse urine that also contains progestins and androgens, whereas estradiol is the major endogenous estrogen.12
Differences in the outcomes of epidemiological studies and clinical trials are best interpreted in the context of an understanding of the biological effects of estrogen. On the one hand, oral HRT increases circulating fibrin split products (FSP), concentrations of inflammatory cytokines, and C-reactive protein.13–15 On the other, estradiol exerts many salutary effects on the vasculature, including improving endothelial-dependent vasodilation,16 decreasing fibrinogen and plasminogen activator inhibitor (PAI)-1 (PAI-1),17–19 increasing the secretion of nitric oxide from intact platelets,20 and attenuating platelet-derived growth factor (PDGF) receptor-activated smooth muscle cell migration and proliferation.21
HRT decreases circulating t-PA antigen in parallel with PAI-1 antigen, to which it is complexed. Circulating t-PA antigen does not reflect the capacity of the vasculature to release t-PA, however, and the effect of HRT or estrogen on endothelial fibrinolytic function has not been studied extensively. Hoetzer et al16 observed that acute intraarterial infusion of 17β-estradiol enhanced bradykinin-stimulated t-PA antigen release. The investigators also reported that bradykinin-stimulated t-PA release was increased in women chronically taking estrogen, but treatment was not randomized.
We have previously demonstrated that angiotensin-converting enzyme (ACE) inhibition increases basal vascular t-PA antigen release in women but not in men.22 In addition, ACE inhibition potentiates bradykinin-stimulated t-PA release to a greater extent in premenopausal women than in postmenopausal women and in either group of women compared with age-matched men,23 suggesting that estrogen may modulate the effect of ACE inhibition on bradykinin-stimulated t-PA release. This study tested the hypothesis that 17β-estradiol enhances t-PA release in the presence of ACE inhibition in young postmenopausal women.
Methods
Subjects
Fourteen healthy postmenopausal women participated in the study. Women were defined as postmenopausal if it had been at least 1 year since their last menstruation and if their untreated estradiol concentration was less than 30 pg/mL or their follicle-stimulating hormone (FSH) concentration exceeded 50 mIU/mL. After written informed consent was obtained, all subjects underwent a complete history and physical examination, and an ECG and routine laboratory were obtained. Subjects with renal, pulmonary, endocrine, hematologic, or cardiovascular disease (including hypertension defined as an untreated seated systolic/diastolic blood pressure greater than 140/90) were excluded. All subjects were within 30% of their ideal body weight. Subjects with fasting cholesterol greater than 5.7 mmol/L (220 mg/dL) and smokers were excluded.
Four subjects (or 30% of subjects) were taking HRT, which was washed out for 1 month before the start of the study. Two subjects were taking conjugated estrogens (Premarin), 1 subject was taking transdermal estradiol (Climara), and 1 subject was taking estradiol vaginal tablet (Vagifem). These subjects were taking HRT for relief of postmenopausal symptoms including hot flashes and vaginal dryness.
Experimental Protocol
The study protocol was approved by the Vanderbilt University Institutional Review Board and conducted according to the Declaration of Helsinki. Subjects were randomized to receive either 17β-estradiol (1 mg/d, Watson Pharmaceuticals) or placebo for 4 weeks followed by the first study day (please see Figure S1 at http://hyper.ahajournals.org). After the first study day, subjects were crossed-over (placebo or 17β-estradiol) for 4 weeks followed by study day 2. Four subjects completed only 1 study day (2 during 17β-estradiol and 2 during placebo treatment arm). One subject developed a gastrointestinal illness before her second study day. One subject developed bradycardia during arterial line placement, while another developed tachycardia. In the 4th subject we were not able to obtain arterial access on 1 of the study days.
Studies were performed in the morning in a temperature-controlled room. FBF was measured by silastic-in-mercury strain-gauge plesthysmography (please see the online data supplement).24 After measurement of baseline forearm blood flow (FBF) and blood sampling, graded doses of sodium nitroprusside (SNP, Gensia Siccor Pharmaceuticals), methacholine (MCH, Pharmaceutical Compounding Center), and bradykinin (Clinalfa AG) were infused in random order. SNP (endothelium-independent control) was infused at 1.6, 3.2, and 6.4 μg/min; MCH (endothelium-dependent, bradykinin-receptor independent control) at 3.2, 6.4, and 12.8 μg/min, and bradykinin at 100, 200, and 400 ng/min. Each dose was infused for 5 minutes and FBF was measured during the last 2 minutes of infusion.
Thirty minutes after administration of these three drugs, baseline measurements were repeated and subjects then received a continuous intraarterial infusion of enalaprilat (Ben Venue Laboratories, Inc) at 0.33 μg/min/100 mL forearm volume. While continuing the infusion of enalaprilat, baseline measurements and infusions of MCH and bradykinin were repeated. Bradykinin doses were reduced to 25, 50, and 100 ng/min during enalaprilat because 2 subjects in a prior study developed transient arm swelling after infusion of higher doses of bradykinin in the presence of enalaprilat.22
Blood Sampling and Biochemical Assays
After measurement of FBF, simultaneous arterial and venous samples were obtained from the infused arm before and after each dose of MCH and bradykinin. Blood samples were collected on ice and centrifuged immediately, and plasma was stored at −70°C until the time of assay. Please see the data supplement for details regarding analyses.
Statistical Analysis
Data are presented as mean±SEM. Because 4 subjects only completed 1 study day we analyzed the data using 2 different methods. First we analyzed paired data measured in the 10 subjects who completed both days. In addition, we analyzed the unpaired data of all 14 subjects who had completed any study day. Because both analyses yielded the same result, we present here the findings of the paired analysis. The effect of agonists on hemodynamic and fibrinolytic variables was determined using a general linear model-repeated measures ANOVA in which the within-subject variables were vasodilator dose and/or drug arm (17β-estradiol or placebo). The effect of enalaprilat on the t-PA response to 100 ng/min bradykinin was determined using a paired t test. To determine whether hormonal status (17β-estradiol or placebo) affected sensitivity to exogenous bradykinin, we constructed individual dose response curves in which bradykinin dose was log transformed. The slopes of these individual curves were then compared between treatments. A 2-tailed probability value less than 0.05 was considered statistically significant. Statistical analysis were performed with the statistical package SPSS for Windows (Version 15.0, SPSS) and GraphPad Prism for Windows (Version 4.0, GraphPad).
Results
Effect of 17β-Estradiol and Enalaprilat on Baseline Characteristics
Subject characteristics at the time of screening are presented in Table S1. There were no significant changes in systolic blood pressure (P=0.678), diastolic blood pressure (P=0.481), or mean arterial pressure (P=0.502) in subjects taking HRT after washout. Also, treatment with 17β-estradiol in subjects taking HRT before the study had no effect on systolic blood pressure, diastolic blood pressure, or mean arterial pressure. The time between the last normal menstruation and study date was 5.0±1.2 years for women not taking any HRT. During the study, estradiol concentrations were 2-fold higher during estradiol compared with during placebo (53.7±7.5 versus 24.8±2.5 pg/mL, P=0.017). Systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate were similar during treatment with placebo and 17β-estradiol (Table). Baseline FBF and FVR were also similar in the 2 treatment arms. Venous PAI-1 antigen and venous t-PA antigen were significantly decreased during 17β-estradiol compared with during placebo. However, baseline venous t-PA activity was not significantly different between the 2 treatment arms. Intraarterial administration of enalaprilat did not affect mean arterial pressure, FBF, or FVR during either the 17β-estradiol or placebo arm (all probability values >0.367).
Table.
Effect of Treatment on Baseline Characteristics
| Parameter | Placebo | 17β-Estradiol | P Value |
|---|---|---|---|
| Systolic blood pressure, mm Hg | 112.0±3.1 | 110.3±2.7 | 0.584 |
| Diastolic blood pressure, mm Hg | 69.4±1.6 | 68.3±2.4 | 0.747 |
| Mean arterial pressure, mm Hg | 83.6±1.7 | 82.3±2.3 | 0.650 |
| Heart rate, bpm | 67.9±2.4 | 68.9±2.7 | 0.492 |
| Baseline FBF, mL/min per 100 mL | 3.6±0.6 | 4.0±0.7 | 0.174 |
| Baseline FVR, mlL/min/mm Hg per 100 mL | 30.3±4.9 | 28.4±3.3 | 0.108 |
| Baseline venous PAI-1 antigen, ng/mL | 10.4±2.5 | 4.4±1.4 | 0.001 |
| Baseline venous t-PA antigen, ng/mL | 7.5±1.3 | 5.5±0.6 | 0.022 |
| Baseline venous t-PA activity, IU/mL | 0.6±0.1 | 0.7±0.1 | 0.415 |
FBF indicates forearm blood flow; FVR, forearm vascular resistance; PAI-1, plasminogen activator inhibitor-1; t-PA, tissue-type plasminogen activator.
Effect of 17β-Estradiol and Enalaprilat on Basal Vascular Net t-PA Release
Basal net t-PA antigen release (Figure 1A) was similar during treatment with 17β-estradiol or placebo (P=0.887). Enalaprilat significantly increased net t-PA antigen release (P=0.012 for effect of enalaprilat, P=0.761 for placebo versus 17β-estradiol). Because 17β-estradiol treatment significantly decreased PAI-1 antigen, we also assessed the effect of 17β-estradiol and enalaprilat on basal vascular release of active t-PA (Figure 1B). Basal net release of active t-PA was significantly higher during 17β-estradiol treatment than during placebo treatment (1.2±0.3 IU/mL/min versus 0.4±0.1 IU/mL/min respectively, P=0.032). In contrast to the effect of enalaprilat on net t-PA antigen release, enalaprilat did not enhance the net release of active t-PA (P=0.884).
Figure 1.
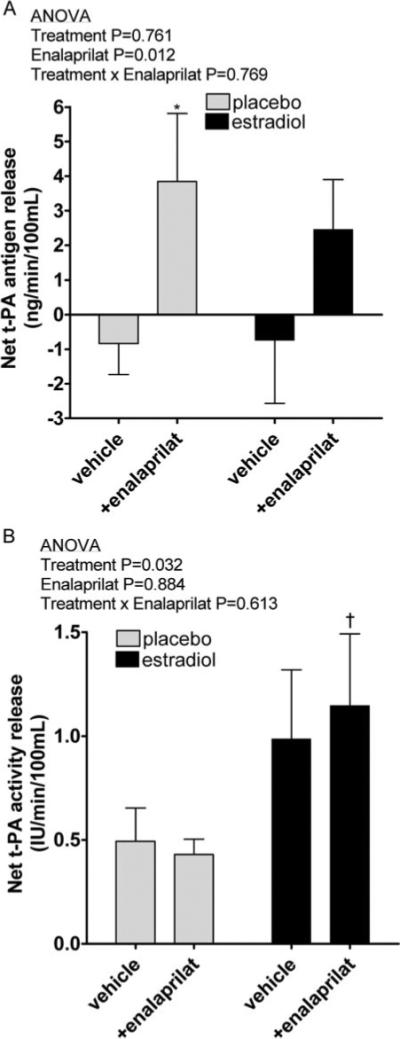
Basal net t-PA antigen release (A) and activity release (B) during placebo and 17β-estradiol (1 mg/d) treatment, in the presence and absence of intraarterial enalaprilat. *P=0.039 vs vehicle, †P=0.033 vs placebo, in posthoc analysis.
Effect of 17β-Estradiol and Enalaprilat on FBF Response to Exogenous Agonist
Figure 2 illustrates the effect of bradykinin on FBF in the presence and absence of enalaprilat during placebo and 17β-estradiol treatment. Bradykinin caused a dose-dependant increase in FBF during placebo (P=0.004) as well as during 17β-estradiol (P=0.002) treatment, and there was no significant difference in the vasodilator response during the 2 treatment arms (P=0.155, Figure 2A). Enalaprilat significantly enhanced bradykinin-stimulated FBF during placebo treatment (from 9.6±2.1 to 19.3±3.0 mL/min/100 mL at 100 ng/min bradykinin, P<0.001) as well as during 17β-estradiol treatment (from 11.2±1.6 to 23.8±4.2 mL/min/100 mL at 100 ng/min bradykinin, P=0.001). The magnitude of the effect of enalaprilat was similar during the 2 treatment arms (P=0.134, Figure 2B). The slope of the relationship between bradykinin dose and FBF during enalaprilat was not significantly different between placebo and 17β-estradiol treatment (Figure 2C and 2D, P=0.274), indicating that 17β-estradiol treatment did not enhance the sensitivity of the vasodilator response to exogenous bradykinin.
Figure 2.
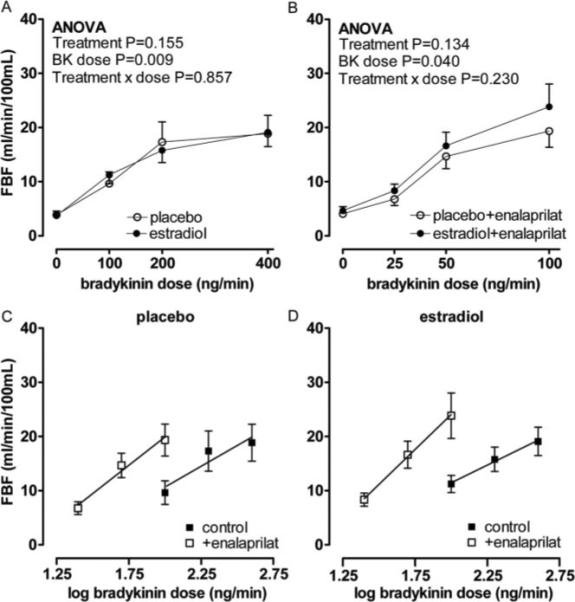
Forearm blood flow (FBF) response in postmenopausal women to bradykinin (A), bradykinin plus enalaprilat (B) during placebo (○) and 17β-estradiol (1 mg/d) treatment (●). Probability values are for repeated measures ANOVA with pairwise comparisons between placebo and 17β-estradiol. Forearm blood flow versus log bradykinin dose in postmenopausal women in the absence (■) and presence of enalaprilat (□) during placebo (C) and 17β-estradiol treatment (D).
MCH significantly increased FBF during both placebo (P=0.005) and 17β-estradiol (P=0.001) treatment, and there was no significant difference between the 2 treatment arms (P=0.243, data not shown). As expected, enalaprilat did not enhance MCH-stimulated FBF during either placebo (P=0.786) or 17β-estradiol (P=0.118) treatment. Similarly, SNP (endothelium-independent control) significantly increased FBF during placebo (P=0.004) and 17β-estradiol (P=0.002) treatment and the response to SNP was similar during the 2 arms (P=0.810, data not shown).
Effect of 17β-Estradiol and Enalaprilat on t-PA Response to Exogenous Bradykinin
Bradykinin caused a significant dose-dependent increase in t-PA antigen release during both placebo (P=0.007) and 17β-estradiol (P=0.007) treatment (please see Figure S2 at http://hyper.ahajournals.org). Enalaprilat significantly enhanced bradykinin-stimulated t-PA antigen release during placebo treatment (from 11.9±4.7 to 217.9±52.5 ng/min/100 mL at 100 ng/min bradykinin, P=0.002) as well as during 17β-estradiol treatment (from 16.9±8.1 to 209.0±59.1 ng/min/100 mL at 100 ng/min bradykinin, P=0.007). There was no effect of placebo versus 17β-estradiol treatment on bradykinin-stimulated t-PA antigen release in the absence (P=0.929, Figure S2A) or presence (P=0.401, Figure S2 B) of enalaprilat. The slope of the relationship between brady-kinin dose and t-PA antigen release during enalaprilat was not significantly different (Figure S2C and S2D, P=0.437) between the 2 treatment arms.
Because 17β-estradiol treatment decreased PAI-1 antigen, we also assessed the effect of exogenous bradykinin on the release of active t-PA. Enalaprilat significantly enhanced the release of active t-PA during both placebo (from 7.5±1.9 to 58.3±14.2 IU/min/100 mL at 100 ng/min bradykinin, P=0.002) and 17β-estradiol treatment (from 6.6±3.7 to 66.3±18.0 IU/min/100 mL at 100 ng/min bradykinin, P=0.007). As with bradykinin-stimulated t-PA antigen release, there was no effect of 17β-estradiol treatment on bradykinin-stimulated release of active t-PA in the absence or presence of enalaprilat (P=0.744 and P=0.217 respectively, Figure 3A and 3B). The slope of the relationship between bradykinin dose and active t-PA release during enalaprilat was not significantly different (Figure 3C and 3D, P=0.812) between the 2 treatment arms.
Figure 3.
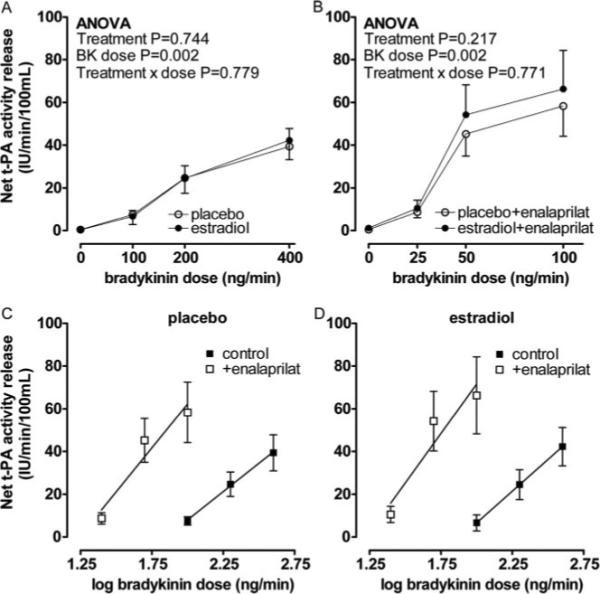
Net release of t-PA activity in response to bradykinin (A), bradykinin plus enalaprilat (B) during placebo (○) and 17β-estradiol treatment (●). Probability values are for repeated measures ANOVA with pairwise comparisons between placebo and 17β-estradiol. Release of t-PA activity versus log bradykinin dose in postmenopausal women in the absence (■) and presence of enalaprilat (□) during placebo (C) and 17β-estradiol treatment (D).
Discussion
This study examined the effect of 17β-estradiol treatment on vascular fibrinolytic function in the absence and presence of ACE inhibition in young, otherwise healthy postmenopausal women. As previously described,17,25 17β-estradiol decreased circulating PAI-1 antigen concentrations. ACE inhibition increased basal release of t-PA antigen, but not activity, and enhanced bradykinin-stimulated vasodilation and t-PA antigen and activity release. 17β-estradiol did not alter these effects of ACE inhibition but enhanced the basal forearm vascular release of active t-PA.
The endogenous fibrinolytic system plays a critical role in maintaining vascular patency. For example, endothelial fibrinolytic capacity, as measured by stimulated t-PA release from the forearm vasculature, predicts the future risk of adverse cardiovascular events in patients with CHD, with individuals with the lowest t-PA release experiencing the highest rate of adverse events.26 In contrast, increased venous t-PA antigen predicts an increased risk of CHD.27 These apparently contradictory findings may be explained by the different physiological determinants of these 2 measures of t-PA. A single measurement of venous t-PA antigen captures both free active t-PA and t-PA bound to PAI-1; because greater than 70% of t-PA circulates bound to PAI-1, increased t-PA antigen usually manifests an increase in PAI-1 rather than an increase in active t-PA.28,29 In contrast, measurement of net release of t-PA takes the arteriovenous gradient and blood flow into account and reflects the capacity of the vascular endothelium to release stored t-PA.30 Thus, in the current study 17β-estradiol decreased venous t-PA antigen in parallel with PAI-1 antigen without affecting peripheral t-PA activity. Moreover, 17β-estradiol increased basal forearm vascular release of active t-PA, consistent with a beneficial effect on endothelial fibrinolytic function.
Numerous studies have reported a favorable effect of estrogens on PAI-1 activity and antigen with a concomitant decrease in venous t-PA antigen.17,18,25,31,32 The route of administration also determines the effect of HRT on the fibrinolytic system. In this regard, transdermal HRT avoids the first-pass effect of estrogen in the liver and decreases PAI-1 and t-PA antigen to a lesser extent than oral HRT.18 Estrogen has been reported to have no effect or to increase31 peripheral t-PA activity. The present study is unique in measuring the effect of 17β-estradiol on basal vascular release of active t-PA and confirms that 17β-estradiol has a profibrinolytic effect. Although it is important to note that this contrasts with the overall finding of an increased risk of thrombotic events in the WHI study,6,7 our subjects were treated with 17β-estradiol alone rather than with conjugated equine estrogens, did not receive concomitant progesterone and were younger than those enrolled in the WHI study and many prior studies.12 Thus, these data are consistent with the recent observation that the effects of 17β-estradiol on vascular endothelial function may depend on age.33,34
Stored t-PA is released from the endothelium both constitutively and in response to activation of G protein– coupled receptors such as the bradykinin B2 receptor.29 We have reported previously that ACE inhibition enhances basal t-PA antigen release through bradykinin B2 receptor–dependent pathway22 and that this effect is seen in premenopausal and postmenopausal women but not in men.23 However, ACE inhibition potentiates bradykinin-stimulated t-PA release to a greater extent in premenopausal women compared with postmenopausal women, suggesting that hormonal status may influence stimulated t-PA release during ACE inhibition.23 In the present study we again found that ACE inhibition increased basal net t-PA antigen release in postmenopausal women. Interestingly, there was no effect of ACE inhibition on basal release of active t-PA, suggesting that t-PA was rapidly inactivated by PAI-1 or other inhibitors.
Contrary to our hypothesis, however, 1-month treatment with oral 17β-estradiol did not affect sensitivity to exogenous bradykinin in the presence or absence of ACE inhibition. These data conflict with the findings of a prior study indicating that acute intraarterial administration of 17β-estradiol increased bradykinin-stimulated t-PA antigen release in postmenopausal women, although forearm venous concentrations of 17β-estradiol achieved in that study (169.0±13.6 pg/mL) were higher than those achieved with usual doses of oral 17β-estradiol.16 Our data also conflict with observational data from the same study suggesting that bradykinin-stimulated t-PA release was higher in women who took unopposed estrogen therapy. As addressed by the investigators, this prior study was not randomized; in addition, subjects were older than in the current study, and subjects were exposed to estrogen for at least 1 year. On the other hand, data from the present study are compatible with the work of Jern and coworkers who report no effect of acute 17β-estradiol infusion on methacholine-stimulated forearm blood flow or net t-PA release in postmenopausal diabetic women.35 Taken together with our prior observation that basal and bradykinin-stimulated t-PA release are increased in ACE inhibitor-treated postmenopausal women compared with age-matched men, the data support an estrogen-independent effect of gender on vascular t-PA release during ACE inhibition. In this regard, decreased bradykinin-stimulated t-PA release in men compared with women during ACE inhibition could reflect a reported negative effect of testosterone on bradykinin-stimulated calcium influx,36 a prospect that merits further investigation.
Perspectives
CHD is the leading cause of death in women. ACE inhibitors decrease mortality attributable to CHD in both men and women.37 Previous studies indicate that ACE inhibition increases basal and bradykinin-stimulated t-PA antigen release in pre- and postmenopausal women compared with age-matched men, but enhances bradykinin-stimulated t-PA release to a greater extent in premenopausal women compared with postmenopausal women.23 The present study confirms that ACE inhibition enhances basal t-PA antigen release in postmenopausal women but suggests that released antigen is rapidly inactivated. The study indicates that 1-month therapy with oral 17β-estradiol does not alter the effects of ACE inhibition on bradykinin-stimulated t-PA release. However, 17β-estradiol does increase the basal release of active t-PA in young postmenopausal women, confirming that decreased PAI-1 antigen concentrations results in enhanced vascular fibrinolytic function. Ongoing clinical trials such as the Kronos Early Estrogen Protection Study (KEEPS, ClinicalTrials.gov Identifier: NCT00154180) and the Early versus Late Intervention Trial with estradiol (ELITE, ClinicalTrials.gov Identifier: NCT0011451) will address whether early versus late estradiol treatment affects thrombotic risk in healthy postmenopausal women.
Supplementary Material
Acknowledgments
We thank Delia Woods, RN, for her nursing assistance and Rhoda Jones, BS, for her technical assistance.
Sources of Funding
This research was funded by NIH grants HL085740, HL060906, HL065193, RR00095, and T32 GM007569.
Footnotes
Disclosures
None.
References
- 1.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 2.Klouche M. Estrogens in human vascular diseases. Ann N Y Acad Sci. 2006;1089:431–443. doi: 10.1196/annals.1386.032. [DOI] [PubMed] [Google Scholar]
- 3.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 4.Henderson BE, Paganini-Hill A, Ross RK. Estrogen replacement therapy and protection from acute myocardial infarction. Am J Obstet Gynecol. 1988;159:312–317. doi: 10.1016/s0002-9378(88)80074-7. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan JM, Vander Zwaag R, Lemp GF, Hughes JP, Maddock V, Kroetz FW, Ramanathan KB, Mirvis DM. Postmenopausal estrogen use and coronary atherosclerosis. Ann Intern Med. 1988;108:358–363. doi: 10.7326/0003-4819-108-3-358. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.The Women's Health Initiative Steering C Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Jayachandran M, Miller VM. Mechanisms of estrogenic vascular protection. Am J Physiol Heart Circ Physiol. 2006;290:H507–H508. doi: 10.1152/ajpheart.01086.2005. [DOI] [PubMed] [Google Scholar]
- 10.Rosano GM, Vitale C, Fini M. Hormone replacement therapy and cardioprotection: what is good and what is bad for the cardiovascular system? Ann N Y Acad Sci. 2006;1092:341–348. doi: 10.1196/annals.1365.031. [DOI] [PubMed] [Google Scholar]
- 11.Ho JY-P, Chen M-J, Sheu WH-H, Yi Y-C, Tsai AC-W, Guu H-F, Ho ES-C. Differential effects of oral conjugated equine estrogen and transdermal estrogen on atherosclerotic vascular disease risk markers and endothelial function in healthy postmenopausal women. Hum Reprod. 2006;21:2715–2720. doi: 10.1093/humrep/del245. [DOI] [PubMed] [Google Scholar]
- 12.Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, Virkamaki A, Hovatta O, Hamsten A, Taskinen MR, Yki-Jarvinen H. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost. 2001;85:619–625. [PubMed] [Google Scholar]
- 14.Decensi A, Omodei U, Robertson C, Bonanni B, Guerrieri-Gonzaga A, Ramazzotto F, Johansson H, Mora S, Sandri MT, Cazzaniga M, Franchi M, Pecorelli S. Effect of transdermal estradiol and oral conjugated estrogen on C-reactive protein in retinoid-placebo trial in healthy women. Circulation. 2002;106:1224–1228. doi: 10.1161/01.cir.0000028463.74880.ea. [DOI] [PubMed] [Google Scholar]
- 15.Wakatsuki A, Ikenoue N, Shinohara K, Watanabe K, Fukaya T. Effect of lower dosage of oral conjugated equine estrogen on inflammatory markers and endothelial function in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. 2004;24:571–576. doi: 10.1161/01.ATV.0000115383.49802.0c. [DOI] [PubMed] [Google Scholar]
- 16.Hoetzer GL, Stauffer BL, Irmiger HM, Ng M, Smith DT, DeSouza CA. Acute and chronic effects of oestrogen on endothelial tissue-type plasminogen activator release in postmenopausal women. J Physiol. 2003;551:721–728. doi: 10.1113/jphysiol.2003.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh KK, Mincemoyer R, Bui MN, Csako G, Pucino F, Guetta V, Waclawiw M, Cannon RO., 3rd Effects of hormone-replacement therapy on fibrinolysis in postmenopausal women. N Engl J Med. 1997;336:683–690. doi: 10.1056/NEJM199703063361002. [DOI] [PubMed] [Google Scholar]
- 18.Brosnan JF, Sheppard BL, Norris LA. Haemostatic activation in postmenopausal women taking low-dose hormone therapy: less effect with transdermal administration? Thromb Haemost. 2007;97:558–565. [PubMed] [Google Scholar]
- 19.Hahn L, Mattsson LA, Andersson B, Tengborn L. The effects of oestrogen replacement therapy on haemostatic variables in postmenopausal women with non-insulin-dependent diabetes mellitus. Blood Coagul Fibrinolysis. 1999;10:81–86. doi: 10.1097/00001721-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Jayachandran M, Mukherjee R, Steinkamp T, LaBreche P, Bracamonte MP, Okano H, Owen WG, Miller VM. Differential effects of 17{beta}-estradiol, conjugated equine estrogen, and raloxifene on mRNA expression, aggregation, and secretion in platelets. Am J Physiol Heart Circ Physiol. 2005;288:H2355–H2362. doi: 10.1152/ajpheart.01108.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kappert K, Caglayan E, Huntgeburth M, Baumer AT, Sparwel J, Uebel M, Rosenkranz S. 17{beta}-Estradiol attenuates PDGF signaling in vascular smooth muscle cells at the postreceptor level. Am J Physiol Heart Circ Physiol. 2006;290:H538–H546. doi: 10.1152/ajpheart.00240.2005. [DOI] [PubMed] [Google Scholar]
- 22.Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. 2003;107:579–585. doi: 10.1161/01.cir.0000046268.59922.a4. [DOI] [PubMed] [Google Scholar]
- 23.Pretorius M, Luther JM, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases basal vascular tissue plasminogen activator release in women but not in men. Arterioscler Thromb Vasc Biol. 2005;25:2435–2440. doi: 10.1161/01.ATV.0000186185.13977.94. [DOI] [PubMed] [Google Scholar]
- 24.Hokanson DE, Sumner DS, Strandness DE., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22:25–29. doi: 10.1109/tbme.1975.324535. [DOI] [PubMed] [Google Scholar]
- 25.Brown NJ, Abbas A, Byrne D, Schoenhard JA, Vaughan DE. Comparative effects of estrogen and angiotensin-converting enzyme inhibition on plasminogen activator inhibitor-1 in healthy postmenopausal women. Circulation. 2002;105:304–309. doi: 10.1161/hc0302.102570. [DOI] [PubMed] [Google Scholar]
- 26.Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2007;27:1651–1656. doi: 10.1161/ATVBAHA.107.143248. [DOI] [PubMed] [Google Scholar]
- 27.Lowe GDO, Danesh J, Lewington S, Walker M, Lennon L, Thomson A, Rumley A, Whincup PH. Tissue plasminogen activator antigen and coronary heart disease: Prospective study and meta-analysis. Eur Heart J. 2004;25:252–259. doi: 10.1016/j.ehj.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator Inhibitor type 1 (PAI-1) complex relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96:761–768. doi: 10.1161/01.cir.96.3.761. [DOI] [PubMed] [Google Scholar]
- 29.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–2479. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 30.Jern C, Selin L, Jern S. Application of the perfused-forearm model to study release mechanisms of tissue-type plasminogen activator in man. Fibrinolysis. 1994;8:13–15. [Google Scholar]
- 31.Brussaard HE, Leuven JA, Krans HM, Kluft C. The effect of 17 beta-oestradiol on variables of coagulation and fibrinolysis in postmenopausal women with type 2 diabetes mellitus. Vascul Pharmacol. 2002;39:141–147. doi: 10.1016/s1537-1891(02)00303-8. [DOI] [PubMed] [Google Scholar]
- 32.Post MS, van der Mooren MJ, van Baal WM, Blankenstein MA, Merkus HM, Kroeks MV, Franke HR, Kenemans P, Stehouwer CD. Effects of low-dose oral and transdermal estrogen replacement therapy on hemostatic factors in healthy postmenopausal women: a randomized placebo-controlled study. Am J Obstet Gynecol. 2003;189:1221–1227. doi: 10.1067/s0002-9378(03)00599-4. [DOI] [PubMed] [Google Scholar]
- 33.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17{beta}-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 34.Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol. 2007;27:1669–1672. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- 35.Manhem K, Dotevall A, Wilhelmsen L, Jern S. Preserved tissue-type plasminogen activator release and endothelium-dependent vasodilation in postmenopausal women with NIDDM. J Diabetes Complications. 2000;14:127–134. doi: 10.1016/s1056-8727(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 36.Rubio-Gayosso I, Garcia-Ramirez O, Gutierrez-Serdan R, Guevara-Balcazar G, Munoz-Garcia O, Morato-Cartajena T, Zamora-Garza M, Ceballos-Reyes G. Testosterone inhibits bradykinin-induced intracellular calcium kinetics in rat aortic endothelial cells in culture. Steroids. 2002;67:393–397. doi: 10.1016/s0039-128x(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 37.Lonn E, Roccaforte R, Yi Q, Dagenais G, Sleight P, Bosch J, Suhan P, Micks M, Probstfield J, Bernstein V, Yusuf S. Effect of long-term therapy with ramipril in high-risk women. J Am Coll Cardiol. 2002;40:693–702. doi: 10.1016/s0735-1097(02)02035-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


