Abstract
The International Agency for Research on Cancer has declared smoking to be a risk factor for hepatocellular carcinoma (HCC). However, passive exposure to cigarette smoke and use of noncigarette tobacco products on the risk of HCC has not been examined. Therefore, we evaluated the independent effects of different types of smoking exposure along with multiple risk factors for HCC and determined whether the magnitude of smoking was modified by other risk factors in men and women. We conducted a case-control study at The University of Texas M. D. Anderson Cancer Center where 319 HCC patients and 1,061 healthy control subjects were personally interviewed for several HCC risk factors. Multivariate logistic regression analysis was performed to estimate the adjusted odds ratio (AOR) and 95% confidence interval (CI) for each potential risk factor. Use of smokeless tobacco (chewing tobacco and snuff), cigars, pipes and passive smoking exposure were not related to HCC among noncigarette smokers. However, regular cigarette smoking was associated with HCC in men: AOR, 1.9 (95% CI, 1.1–3.1). Heavy alcohol consumption was associated with HCC in women: AOR, 7.7 (95% CI, 2.3–25.1). Cigarette smoking interacted synergistically with chronic infection of hepatitis C virus in men: AOR, 136.3 (95% CI, 43.2–429.6) and with heavy alcohol consumption in women: AOR, 13.7 (95% CI, 3.2–57.9). We conclude that sex differences were observed in HCC relationship with cigarette smoking and alcohol consumption. Controlling for smoking exposure might be a prudent approach to the prevention of HCC, especially in patients with chronic viral hepatitis infections.
Keywords: passive smoking, HCC, cigarette smoking, gender, pipes, cigars, tobacco
The incidence of hepatocellular carcinoma (HCC) has increased significantly in the United States over the past 2 decades.1 Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most established risk factors.2 However, in the United States, approximately 60% of HCC cases are not attributable to hepatitis viral infection.3 Additional risk factors—such as heavy alcohol consumption, diabetes and cigarette smoking—have been linked to the etiology of HCC.3–18
Smoking prevalence in the United States has declined since 1997; however, despite the overwhelming evidence of smoking’s harmful effects, almost 22% of U.S. adults still smoked cigarettes in 2000.19 This prevalence may have disproportionately widespread implications in the etiology of different malignancies. The association between smoking and HCC was demonstrated by the results of a meta-analysis of 14 studies, which revealed that cigarette smoking significantly elevated the overall risk of developing HCC (p = 0.001).20 Moreover, a dose-response effect was reported in 11 studies, with an overall summary odds ratio (OR) of 2.6 (95% confidence interval [CI], 2.1–3.3; p = 0.001) for heavy smokers. In support of this association, the International Agency for Research on Cancer of the World Health Organization recently declared smoking to be a risk factor for liver cancer.21
As cigarette smoking has declined in popularity, the use of cigars, pipes and nonsmoked tobacco has been increasing in the United States.22 Men who exclusively smoke cigars and/or pipes have a higher risk of cancer of the larynx, lung and oral cavity.23–26 During smoking of cigarettes, cigars, pipes and other tobacco products, not only mainstream smoke is inhaled by the smoker, but a side stream of smoke is also released into the air; a smoker inhales only 15% of a cigarette’s smoke—the rest goes into the surrounding air.27 Once released, this side-stream smoke is mixed with exhaled mainstream smoke; together, they make up secondhand tobacco smoke, also referred to as “involuntary,” “environmental” or “passive” smoke, to which both smokers and non-smokers are exposed. The association between passive smoking and lung cancer has been confirmed by several meta-analyses.28–30 However, the association between passive smoking and breast cancer, childhood cancers and renal cell carcinoma is inconclusive.21 Despite evidence sufficient to judge the positive association between active smoking and liver cancer, smoking-HCC relationship in men and women separately has not been widely addressed by epidemiological studies. In addition, no previous studies have examined the impact of passive smoking on HCC. To further assess the associations between the risk of HCC and passive smoking and the use of noncigarette tobacco products, we embarked on this large-scale case-control study, taking into consideration the confounding effect of cigarette smoking and other significant risk factors for HCC.
Material and methods
Study design and population
This was a hospital-based case-control study in which case patients and control subjects were prospectively ascertained. The study was approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center. Written informed consent for an interview and for a biological sample was obtained from each participant. A total of 1,380 subjects (319 case patients with HCC and 1,061 healthy control subjects) were enrolled.
Case patients were recruited from the population of patients with newly diagnosed HCC who were evaluated and treated at the institution’s gastrointestinal medical and surgical oncology outpatient clinics. Inclusion criteria were as follows: a pathologically confirmed diagnosis of HCC, U.S. residency and the ability to communicate in English. Patients with concurrent or past history of other types of cancers were excluded.
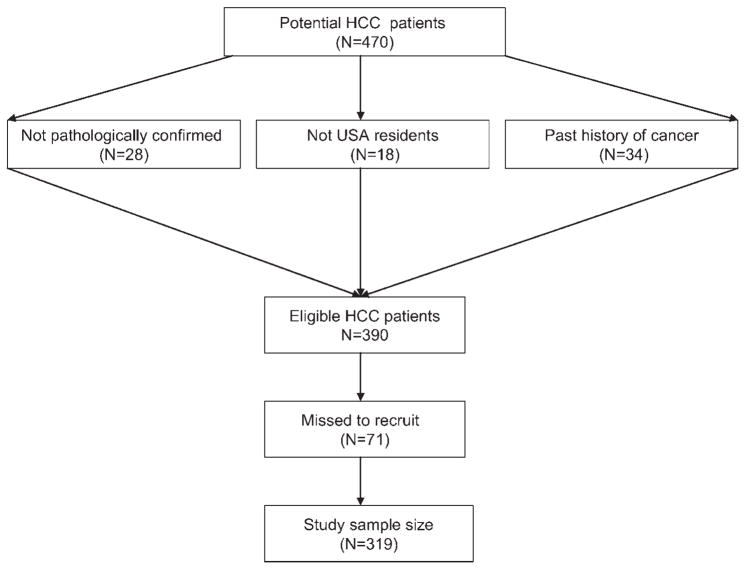
From January 2000 through December 2006, 470 patients with suspected HCC were approached, 390 eligible patients were identified and 319 patients were enrolled (Fig. 1). We failed to recruit 71 eligible patients (71/390 [18.2%]) for the following reasons: patient refusal (30%), physician refusal (n = 5%), severity of illness or sadness of the patient or family (19%), language barrier (19%), inadequate time to complete interview (8%) and change in the patient’s schedule (19%). The clinical characteristics, demographic features and medical histories of these unrecruited patients were retrieved from their medical records and recorded in a database. Statistical analysis indicated that the unrecruited patients did not differ from enrolled patients in terms of stage of disease, age, sex, educational level, state of residency, race/ethnicity, smoking and alcohol exposure or hepatitis virus reactivity.
Figure 1.
Flow diagram showing ascertainment of US eligible cases who were patients with pathologically diagnosed hepatocellular carcinoma at The University of Texas M. D. Anderson Cancer Center (2000–2006).
Control subjects were healthy nonblood relatives, particularly spouses, of patients with cancers other than liver, gastrointestinal, lung or head and neck (smoking-related cancers) who were undergoing treatment at our institution. Eligibility criteria for control subjects were the same as those for patients, except that control subjects could not have ever had cancer. A short, structured questionnaire was used to screen potential control subjects on the basis of eligibility criteria. Control subjects and patients were recruited simultaneously.
Of potential control subjects, 81.9% agreed to participate. There were no significant differences in age, sex, race/ethnicity, educational level or accompanied patient’s type of cancer between those who consented and those who refused to participate.
Case patients and control subjects were personally interviewed by well-trained interviewers; no proxy interviews were conducted. The interviewers used a structured and validated questionnaire to collect information about demographic characteristics and potential risk factors for HCC such as personal smoking history, alcohol use, medical history, occupational history and family history of cancer.
Cigarette smokers were defined as subjects who had smoked ≥100 cigarettes during their lifetimes. Former smokers were defined as subjects who had quit smoking at least 1 year before study enrollment. Former and current smokers were asked to indicate the average number of cigarettes they smoked per day, the age at which they began smoking and the duration of smoking. Former smokers were questioned about the age at which they stopped smoking. Pack-years were estimated by multiplying the number of years of smoking and the number of packs of cigarettes smoked per day (1 pack-year = 1 pack of cigarettes/day for a year). Heavy smokers were defined as those who had >20 pack-years of smoking.
Subjects were also asked about their use of pipes, cigars and smokeless tobacco products (chewing tobacco and snuff). Frequency of daily intake (times per day) and duration of exposure to each type (years of use) were documented for each subject. The total intake-years of exposure were calculated by multiplying daily intake by duration of exposure for each tobacco type for all users. Participants were then classified according to total intake-years of exposure; the median value of intake-years of exposure for control subjects was used as a cutoff point to discriminate between heavy users (greater than the median value) and mild/moderate users (less than or equal to the median value).
Case patients and control subjects were asked about their history of passive smoking during childhood (birth to 18 years), adulthood at home (age >18 years) and adulthood at work. For exposed individuals, the starting age or year of exposure and ending age or year of exposure were recorded for each period. The cumulative duration of passive smoking was estimated by summing the duration of exposure for the 3 periods after controlling for possible overlap between exposure during adulthood at home and adulthood at work. Exposed individuals were then classified according to cumulative life-years of passive smoking. Information on passive smoking was incomplete for 3 control subjects; statistical analysis was performed on data from 319 case patients and 1,058 control subjects.
Alcohol drinkers were defined as subjects who had consumed at least 4 alcoholic drinks of beer, wine or hard liquor each month for 6 months during their lifetimes. The age at which drinking started and (if applicable) ended were recorded for each type of alcoholic beverage for all participants. Drinkers were further classified according to the total lifetime volume of ethanol consumed in milliliters, which was computed according to the frequency of drinking, type of serving (glass, bottle or can), number and size of each serving and duration of consumption, summed over the whole period of alcohol use. Consumed serving units were defined as 12.0 oz for beer, 4.0 oz for wine and 1.5 oz for hard liquor (each equivalent to approximately 12.0 mL of ethanol).31 Heavy alcohol consumption was defined as consumption of more than 60 mL ethanol/day during the subject’s period of alcohol drinking.32,33
Ten milliliters of blood was drawn from a peripheral vein for each participant. The samples were tested for the presence of anti-HCV antibodies (anti-HCV) using a third-generation enzyme-linked immunosorbent assay (Abbott Laboratories, North Chicago, IL). The samples were also tested using the enzyme-linked immunosorbent assay for the presence of HBV surface antigen (HBsAg) and HBV core antibody (anti-HBc). Plasma samples were available for only 889 control subjects who were tested for virus markers with all HCC patients.
Statistical methods
Microsoft Access software was used for data entry and data management, and Stata software (Stata, College Station, TX) was used for statistical analysis. Chi-square tests were used to assess the significance of differences in the distributions of categorical variables between case patients and control subjects. We performed univariate single-factor unconditional logistic regression analyses to assess the marginal effects of each HCC risk factor using maximum-likelihood estimation. We also performed multivariate unconditional logistic regression analyses using all variables that were significant at p < 0.05 in the single-factor analyses. For each factor, we calculated the adjusted OR (AOR) and 95% CI using maximum-likelihood estimation. All AORs were adjusted for age, sex, race/ethnicity, education, marital status, place of residency and other significant HCC risk factors by use of the likelihood-ratio test. The final model was chosen on the basis of biological plausibility and lowest -2 Log Likelihood function. Using the additive scale, we investigated possible interactions between significant risk factors. To assess deviations from the additive model (which assumes that there is no interaction between variables), we calculated the synergism index [S = (OR11 − 1/([OR01 + OR10) − 2, where OR11 is the OR of the joint effect of 2 risk factors and OR10 and OR01 is the OR of each risk factor in the absence of the other], and its 95% CI. A value of S equal to unity was interpreted to be indicative of additivity, whereas a value greater than unity was indicative of superadditivity and synergism.34
Results
Participants characteristics
Table I shows the distribution of case patients and control subjects according to demographic characteristics. Most study subjects were male and non-Hispanic white. Case patients were slightly older than control subjects, with a mean difference ± standard deviation of 2.2 ± 0.7 years (case patients, 62 ± 0.7; control subjects, 60 ± 0.3 years). Case patients also had a lower education level. In the multivariate logistic regression analysis, we adjusted for all significant demographic variables. Stratified analysis by gender was performed to examine HCC risk factors in men and women separately.
TABLE I.
CHARACTERISTICS OF STUDY PARTICIPANTS
| Demographic variable | Cases |
Controls |
p-Value | ||
|---|---|---|---|---|---|
| N = 319 | % (95% CI) | N = 1,061 | % (95% CI) | ||
| Sex | 0.02 | ||||
| Male | 225 | 70.5 (65.2–75.5) | 670 | 63.1 (60.2–66.1) | |
| Female | 94 | 29.5 (24.5–34.8) | 391 | 36.9 (34.0–39.8) | |
| Age (years) | 0.004 | ||||
| ≤40 | 15 | 4.7 (2.7–7.6) | 46 | 4.3 (3.2–5.7) | |
| 41–50 | 39 | 12.2 (8.8–16.3) | 176 | 16.6 (14.4–19.0) | |
| 51–59 | 89 | 27.9 (23.0–33.2) | 313 | 29.5 (26.8–32.3) | |
| 60–69 | 91 | 28.5 (23.6–33.8) | 342 | 32.2 (29.4–35.1) | |
| ≥70 | 85 | 26.7 (21.9–31.9) | 184 | 17.4 (15.1–19.8) | |
| Ethnicity | 0.001 | ||||
| White | 215 | 67.4 (62.0–72.5) | 939 | 88.5 (86.4–90.4) | |
| African Americans | 31 | 9.7 (6.7–13.5) | 40 | 3.8 (2.7–5.1) | |
| Hispanics | 49 | 15.4 (11.6–19.8) | 74 | 7.0 (5.5–8.7) | |
| Asians | 24 | 7.5 (4.9–11.0) | 8 | 0.7 (0.3–1.5) | |
| Educational level | 0.001 | ||||
| ≤High school degree | 154 | 48.3 (42.7–53.9) | 316 | 29.8 (27.0–32.6) | |
| Some college years | 68 | 21.3 (17.0–26.2) | 274 | 25.8 (23.2–28.6) | |
| College degree | 97 | 30.4 (25.4–35.8) | 471 | 44.4 (41.4–47.4) | |
| Marital status | 0.001 | ||||
| Married/lived like married | 243 | 76.2 (71.1–80.7) | 952 | 89.7 (87.7–91.5) | |
| Single | 19 | 6.0 (3.6–9.1) | 17 | 1.6 (0.9–2.6) | |
| Widow | 23 | 7.2 (4.6–10.6) | 27 | 2.6 (1.7–3.7) | |
| Divorced/separated | 34 | 10.6 (7.5–14.6) | 65 | 6.1 (4.8–7.7) | |
| Residency by USA state | 0.5 | ||||
| Texas and neighboring states1 | 234 | 73.3 (68.1–78.1) | 760 | 71.6 (68.8–74.3) | |
| Other sates | 85 | 26.7 (21.9–31.9) | 301 | 28.4 (25.7–31.2) | |
Neighboring states included NM, LA, AR and OK.
Risk factors for HCC
Nonsmoking risk factors
Large, statistically significant differences in the prevalence of anti-HCV, HBsAg, anti-HBc positivity were observed between case patients and control subjects (p < 0.0001). Multivariate AORs for the association of HCC with anti-HCV and HCC with HBsAg were statistically significant in both men and women. However, the multivariate AOR for anti-HBc and HCC was not statistically significant in women (Table II).
TABLE II.
RISK FACTORS OF HCC: AOR (95% CI)1 USING MULTIVARIABLE LOGISTIC REGRESSION
| Smoking variable | Total population |
Men |
Women |
|||
|---|---|---|---|---|---|---|
| Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | |
| HCV2 | 319/1,061 | 225/670 | 94/391 | |||
| Anti-HCV− | 219/880 | 1 | 144/533 | 1 | 75/347 | 1 |
| Anti-HCV+ | 100/9 | 41.1 (18.9–89.7) | 81/7 | 46.3 (18.4–116.3) | 19/2 | 41.5 (8.8–195.3) |
| HBV2 | ||||||
| HBsAg−/Anti-HBc− | 248/859 | 1 | 171/523 | 1 | 77/336 | 1 |
| HBsAg+/Anti-HBc+ | 25/1 | 6.7 (2.2–20.3) | 18/1 | 4.1 (1.1–16.3) | 7/0 | |
| HBsAg−/Anti-HBc+ | 46/29 | 2.7 (1.4–5.2) | 36/16 | 3.7 (1.6–8.6) | 10/13 | 1.5 (0.5–5.1) |
| Diabetes mellitus | ||||||
| No | 211/952 | 1 | 140/586 | 1 | 71/366 | 1 |
| Yes | 108/109 | 4.5 (3–6.6) | 85/84 | 4.9 (3.1–7.9) | 23/25 | 3.8 (1.8–8.2) |
| ≤1 year diabetes | 15/29 | 3.1 (1.5–6.6) | 12/20 | 4.6 (1.9–10.9) | 3/9 | 1.5 (0.3–7.2) |
| >1 year diabetes | 93/80 | 4.7 (3.1–7.3) | 73/64 | 4.8 (2.9–8.1) | 20/16 | 5 (2.2–11.6) |
| Alcohol drinking | ||||||
| No | 114/463 | 1 | 58/205 | 1 | 56/258 | 1 |
| Yes | 205/598 | 1.8 (1.1–3.2) | 167/465 | 0.9 (0.5–1.4) | 38/133 | 1.9 (1.1–3.4) |
| Ethanol consumption/day3 | ||||||
| <60 mL/day | 134/530 | 1.1 (0.7–1.5) | 106/405 | 0.7 (0.4–1.2) | 28/125 | 1.5 (0.8–2.9) |
| ≥60 mL/day | 69/65 | 2.7 (1.5–4.7) | 59/57 | 1.8 (1.1–3.4) | 10/8 | 7.7 (2.3–25.1) |
| Family history of cancer | ||||||
| Never | 103/333 | 1 | 77/232 | 1 | 26/101 | 1 |
| Ever | 216/728 | 1.5 (1.1–2.1) | 148/438 | 1.6 (1.1–2.5) | 68/290 | 1.1 (0.6–2.1) |
Odds ratios were adjusted for age, sex, race, education, marital status, state of residency, HCV, HBV, diabetes, heavy alcohol consumption, cigarette smoking and family history of cancer.
Hepatitis virus markers were missing in 172 controls (130 men and 42 women).
Total ethanol intake could not be determined in 2 HCC men and 3 control men.
The mean lifetime intake of ethanol was 730,853.8 mL in case patients and 291,350.8 mL in control subjects (p = 0.0001); a similar difference between case patients and control subjects was observed in men (p < 0.01) and women (p = 0.01). A significant effect of heavy alcohol consumption on elevated risk of HCC was observed in both men and women. However, the magnitude of the measurement for association was larger in women (AOR = 7.7) than in men (AOR = 1.8), Table II. As compared to nondrinking men, AORs (95% CIs) were 5.2 (1.6–17.4), 0.7 (0.4–1.3) and 2.1 (1.1–4.1) for heavy drinking women, nondrinking women and heavy drinking men, respectively. The estimated S index for interaction between heavy drinking and female sex = 5.3 (95% CI, 3.6–6.8). Mild or moderate drinking (<60 mL/day) had no significant effect on HCC development in men or women.
Table II shows that patients with diabetes mellitus had a risk of HCC 4.5 times greater than that of patients without diabetes. However, 13.9% of case patients (n = 15) and 26.6% (n = 29) of control subjects had diabetes first diagnosed at the time of cancer diagnosis or recruitment in the study. When time of diabetes diagnosis was considered, the estimated multivariate AORs for patients with a history of diabetes for >1 year were 4.7 (95% CI, 3.1–7.3), 4.8 (95% CI, 2.9–8.1) and 5 (95% CI, 2.2–11.6) in all subjects, in men and in women, respectively.
Table II also shows that a family history of cancer was associated with a significantly elevated risk of HCC. The effect of a family history of cancer was significant in men (AOR = 1.6; 95% CI, 1.1–2.5) than in women (AOR = 1.1; 95% CI, 0.6–2.1) after controlling for other significant risk factors.
Cigarette smoking
Table III shows that cigarette smoking was a significant risk factor for HCC. This relationship was observed among regular smokers (but not among irregular smokers) and among men (but not women). Control subjects started smoking at an earlier age than case patients (mean ± standard error [SE] age, 19 ± 0.5 years for case patients and 18 ± 0.2 years for control subjects; p = 0.03). However, the overall duration of smoking was longer in case patients than control subjects (mean ± SE duration, 30.9 ± 1.8 years for case patients and 27.1 ± 1.1 years for control subjects; p = 0.06). The median and range of cigarettes smoked per day were identical for patients and control subjects (median, 20; range, 1–80 cigarettes/day; p = 0.5). Compared to nonsmokers, smokers had no significant trend in risk for HCC by number of smoked cigarettes/day. However, when considering both parameters (years of smoking and number of cigarettes smoked each day), the median of pack-years was higher for patients (25.3; range, 0.1–130.0) than for controls (20; range, 0.1–145.0), p = 0.06. Men who had quit smoking within 10 years of diagnosis or recruitment into the study continued to be at a significantly increased risk for HCC development.
TABLE III.
SMOKING AND RISK FOR HCC: AOR (95% CI)1 USING MULTIVARIABLE LOGISTIC REGRESSION
| Smoking variable | Total population1 |
Men2 |
Women2 |
|||
|---|---|---|---|---|---|---|
| Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | |
| Cigarette smoking | 319/1,061 | 225/670 | 94/391 | |||
| Never | 103/540 | 1 | 52/289 | 1 | 51/251 | 1 |
| Ever | 216/521 | 1.5 (1.02–2.1) | 173/381 | 1.9 (1.2–3.1) | 43/140 | 1 (0.5–1.8) |
| Regular smoking | 206/488 | 1.5 (1.1–2.2) | 163/363 | 1.9 (1.1–3.1) | 43/125 | 1.1 (0.6–1.9) |
| Nonregular smoking | 10/33 | 1 (0.4–2.6) | 10/18 | 1.9 (0.6–5.1) | 0/15 | |
| Duration of smoking | ||||||
| ≤20 years | 67/252 | 1.1 (0.7–1.8) | 57/184 | 1.6 (0.9–2.9) | 10/68 | 0.4 (0.2–1.2) |
| >20 years | 149/269 | 1.8 (1.2–2.7) | 116/197 | 2.1 (1.2–3.6) | 33/72 | 1.5 (0.7–2.9) |
| Smoking cigarettes/day | ||||||
| ≤20 cigarettes/day | 155/366 | 1.5 (1.1–2.2) | 115/250 | 1.9 (1.2–3.3) | 40/116 | 1 (0.5–1.9) |
| >20 cigarettes per day | 61/155 | 1.4 (0.8–2.3) | 58/131 | 1.7 (1–3.2) | 3/24 | 0.8 (0.2–3.1) |
| Smoked-pack-year | ||||||
| ≤20 pack-year | 87/263 | 1.3 (0.8–1.9) | 68/178 | 1.9 (1.1–3.3) | 19/85 | 0.6 (0.3–1.3) |
| >20 pack-year | 129/258 | 1.7 (1.1–2.6) | 105/203 | 1.9 (1.1–3.2) | 24/55 | 1.6 (0.8–3.4) |
| Former smokers | ||||||
| Quit smoking | 154/400 | 1.4 (0.9–2.1) | 125/306 | 1.8 (1.1–2.9) | 29/94 | 0.9 (0.5–1.8) |
| ≤10 years quit smoking | 53/86 | 1.7 (0.96–3.1) | 40/59 | 2.4 (1.2–4.9) | 13/27 | 1.1 (0.4–2.9) |
| >10 years quit smoking | 101/314 | 1.3 (0.8–1.9) | 85/247 | 1.7 (0.9–2.8) | 16/67 | 0.8 (0.4–1.8) |
Odds ratios of smoking parameters were adjusted for age, sex, race, education, marital status, state of residency, HCV, HBV, diabetes, heavy alcohol consumption and family history of cancer in all subjects.
Odds ratios of smoking parameters, in men and women separately, were adjusted for age, race, education, marital status, state of residency, HCV, HBV, diabetes, heavy alcohol consumption and family history of cancer.
Noncigarette tobacco products
Table IV shows the distribution of exposure to chewing tobacco, snuff, pipes and cigars among case patients and control subjects according to cigarette smoking status. The proportions of subjects using chewing tobacco or snuff were higher among control subjects than case patients. However, pipes and cigars were more frequently used by case patients than by control subjects. As shown in Table IV, non-cigarette tobacco products use was not significantly associated with an elevated risk of HCC development. However, heavy use of pipes and cigars conferred approximately 2.6- and 1.7-fold increases, respectively, in risk of HCC after controlling for demographic characteristics and other significant risk factors for HCC; but the association was observed among cigarette smokers and was not statistically significant (p = 0.07 and 0.09, respectively).
TABLE IV.
USE OF NONCIGARETTE TOBACCO PRODUCTS AND RISK FOR HCC: AOR (95% CI)1 USING MULTIVARIABLE LOGISTIC REGRESSION
| Smoking variable | Total population |
Cigarette smokers |
Noncigarette smokers |
|||
|---|---|---|---|---|---|---|
| Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | |
| Chewing tobacco | 319/1,061 | 216/521 | 103/540 | |||
| Never use | 305/994 | 1 | 203/480 | 1 | 102/514 | 1 |
| Ever use | 14/67 | 0.7 (0.3–1.4) | 13/41 | 0.9 (0.4–2.1) | 1/26 | 0.2 (0.02–1.9) |
| Low or moderate intake2 | 9/39 | 0.9 (0.4–2.2) | 8/30 | 1.2 (0.5–2.9) | 1/9 | 0.3 (0.02–3.9) |
| High intake3 | 5/28 | 0.2 (0.04–1.2) | 5/11 | 0.3 (0.1–2.1) | 0/17 | |
| Snuff | ||||||
| Never use | 310/1,009 | 1 | 208/492 | 1 | 102/517 | 1 |
| Ever use | 9/52 | 0.5 (0.2–1.2) | 8/29 | 0.5 (0.2–1.5) | 1/23 | 0.2 (0.02–2.3) |
| Low or moderate intake2 | 8/24 | 0.8 (0.3–2.3) | 7/16 | 0.9 (0.3–2.8) | 1/8 | 0.4 (0.03–5.1) |
| High intake3 | 1/28 | 0.1 (0.01–1) | 1/13 | 0.1 (0.03–1) | 0/15 | |
| Pipe | ||||||
| Never use | 292/996 | 1 | 195/474 | 1 | 97/522 | 1 |
| Ever use | 27/65 | 1.4 (0.8–2.6) | 21/47 | 1.5 (0.9–3) | 6/18 | 1.5 (0.4–5.3) |
| Low or moderate intake2 | 17/50 | 1.2 (0.6–2.5) | 13/37 | 1.2 (0.5–2.7) | 4/13 | 1.7 (0.4–7.1) |
| High intake3 | 10/15 | 2 (0.7–5.6) | 8/10 | 2.6 (0.9–8.2) | 2/5 | 1.2 (0.1–11.2) |
| Cigars | ||||||
| Never use | 285/976 | 1 | 189/462 | 1 | 96/514 | 1 |
| Ever use | 34/85 | 1.5 (0.9–2.5) | 27/59 | 1.6 (0.9–2.8) | 7/26 | 1.4 (0.4–4.7) |
| Low or moderate intake2 | 28/74 | 1.5 (0.9–2.8) | 23/53 | 1.3 (0.8–2.1) | 5/21 | 1.6 (0.4–6.1) |
| High intake3 | 6/11 | 1.2 (0.3–4.3) | 4/6 | 1.7 (0.9–2.9) | 2/5 | 0.9 (0.1–10.1) |
Adjusted odds ratios for age, sex, race, education, marital status, state of residency, HCV, HBV, diabetes, heavy alcohol consumption, cigarette smoking and family history of cancer.
Low or moderate intake: ≤20 intake-years of exposure.
High intake: >20 intake-years of exposure.
Passive smoking
Because every smoker is involuntarily exposed to his or her own exhaled and side-stream smoke in the place where he or she smoked, active smokers (cigarettes, pipe, cigars and tobacco products) were reclassified as involuntarily exposed to others’ mainstream or own exhaled smoke (mainstream) and side-stream smoke. This categorization may eliminate the random misclassification of passive smoke exposure. Total of 88 HCC patients and 471 control subjects were defined as non-smokers who never used cigarettes, cigars, pipes, snuff and tobacco, among whom, 36 HCC patients and 120 control subjects had no passive smoking exposure. Using these individuals as the reference group, we estimated the risk of HCC associated with passive smoking exposure among nonsmokers in the whole population and in men and women separately (Table V).
TABLE V.
PASSIVE SMOKING EXPOSURE AND RISK FOR HCC AMONG NONSMOKERS: AOR (95% CI)1 USING MULTIVARIABLE LOGISTIC REGRESSION
| None-smoking variable | Total population |
Men |
Women |
|||
|---|---|---|---|---|---|---|
| Cases/controls1 | AOR (95% CI) | Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | |
| Passive smoking exposure | 88/471 | 41/224 | 47/247 | |||
| Never | 36/120 | 1 | 19/60 | 1 | 17/60 | 1 |
| Childhood only | 12/100 | 0.4 (0.2–1.1) | 7/55 | 0.1 (0.02–0.5) | 5/45 | 0.6 (0.2–1.9) |
| Adulthood only | 15/78 | 0.6 (0.3–1.4) | 6/40 | 0.2 (0.1–1.1) | 9/38 | 0.9 (0.3–2.5) |
| Childhood and adulthood | 25/173 | 0.6 (0.3–1.1) | 9/69 | 0.3 (0.1–1.2) | 16/104 | 0.7 (0.3–1.5) |
| Childhood exposure | 37/273 | 16/124 | 21/149 | |||
| Occasional | 1/52 | 0.2 (0.02–1.2) | 0/27 | 1/25 | 0.3 (0.03–2.5) | |
| Regular | 36/221 | 0.6 (0.3–1.1) | 16/97 | 0.3 (0.1–1.1) | 20/124 | 0.7 (0.3–1.7) |
| ≤10 years | 3/40 | 0.4 (0.1–1.7) | 1/19 | 0.4 (0.03–4.9) | 2/21 | 0.4 (0.1–2.4) |
| >10 years | 34/233 | 0.6 (0.3–1.2) | 15/105 | 0.2 (0.1–0.8) | 19/128 | 0.8 (0.3–1.7) |
| Adulthood exposure2 | 40/251 | 15/109 | 25/142 | |||
| Occasional | 3/87 | 0.1 (0.02–05) | 2/52 | 0.1 (0.02–0.6) | 1/35 | 0.1 (0.01–1.5) |
| Regular | 37/164 | 0.9 (0.4–1.7) | 13/57 | 0.6 (0.2–2.5) | 24/107 | 1 (0.4–2.2) |
| 20 years | 27/136 | 0.8 (0.4–1.6) | 13/58 | 0.5 (0.1–2.1) | 14/78 | 1 (0.4–2.4) |
| >20 years | 13/115 | 0.4 (0.2–1.3) | 2/51 | 0.1 (0.01–1.1) | 11/64 | 0.7 (0.3–1.8) |
| Lifetime exposure | 52/351 | 22/164 | 30/187 | |||
| Occasional | 4/83 | 0.2 (0.1–0.9) | 2/47 | 0.1 (0.01–0.6) | 2/36 | 0.3 (0.1–1.6) |
| Regular | 48/268 | 0.6 (0.3–1.1) | 20/117 | 0.2 (0.1–0.8) | 28/151 | 0.8 (0.4–1.7) |
| ≤20 years | 23/183 | 0.4 (0.2–0.9) | 13/93 | 0.1 (0.02–0.6) | 10/90 | 0.6 (0.2–1.5) |
| >20 years | 29/168 | 0.7 (0.3–1.3) | 9/71 | 0.3 (0.1–1.1) | 20/97 | 0.8 (0.4–1.9) |
Adjusted odds ratios for age, sex, race, education, marital status, state of residency, HCV, HBV, diabetes, heavy alcohol consumption and family history of cancer.
Adult exposure estimated from summation of exposure years during adulthood at home and at work exclusive from childhood period.
A significant difference in passive smoke exposure was observed between nonsmokers cases (n = 52, 59.1%) and controls (n = 351, 74.5%); p = 0.002. Such difference is related to the significantly higher exposure of control subjects, compared to case patients, to passive smoke during childhood (37 cases [42%] and 273 controls [58%], p = 0.0003). For adulthood exposure, however, there were no significant differences between case patients (45.5%) and control subjects (53.3%), p = 0.3.
Among nonsmokers, the exposure time of passive smoking was similar for exposed case patients and control subjects (mean ± SE, 24.2 ± 2.5 years for case patients; 26.5 ± 0.7 years for control subjects) (p = 0.5). Moreover, Table V shows no significant relationship between the development of HCC and years of exposure during childhood, adulthood (at home and at work) or total lifetime. Furthermore, subjects who were exposed to passive smoking had no increased risk of HCC that could be correlated with the age at first exposure or age at cancer diagnosis. The estimated AORs were 0.5 (95% CI, 0.1–1.9) and 0.6 (95% CI, 0.3–1.1) for patients who were ≤50 and >50 years old, respectively, at HCC diagnosis.
Risk modification of cigarette smoking by other risk factors
Table VI shows the independent effects of HCV infection, HBV infection, diabetes mellitus and heavy alcohol consumption, along with the effect of each risk factor combined with cigarette smoking, on HCC risk in women and men. After adjustment for the effect of demographic characteristics and other significant risk factors for HCC, there was synergy between cigarette smoking and HCV infection in men and between cigarette smoking and heavy alcohol consumption and between cigarette smoking and HBV infection in women. These interactions fit the assumption of additive scales. When the AOR was used as an estimate of the relative risk of disease development, the relative excess risk for patients with a history of cigarette smoking along with HCV positivity in men or along with heavy alcohol consumption in women exceeded the sum of the relative excess risks for each risk factor alone [e.g., 136.3 − 1.0 > (1.8 − 1.0) + (28.9 − 1.0)]. The estimated synergism index (S) was 4.7 (95% CI, 2.8–6.6) for smoking and HCV in men and 5.8 (95% CI, 2.4–9.2) for smoking and heavy alcohol consumption in women. However, for HBV and smoking in women, the S index was not significant (2.6; 95% CI, 0.8–4.4). No risk modification between smoking and other risk factors was observed in men or women.
TABLE VI.
RISK MODIFICATION OF CIGARETTE SMOKING BY OTHER RISK FACTORS. AOR (95% CI) USING MULTIVARIABLE LOGISTIC REGRESSION
| Men |
Women |
||||
|---|---|---|---|---|---|
| Variables | Cases/controls | AOR (95% CI) | Cases/controls | AOR (95% CI) | |
| HCV | Smoking | 225/540 | Model (1)1 | 94/349 | Model (1)1 |
| No | No | 40/229 | 1 | 45/222 | 1 |
| Yes | No | 12/3 | 28.9 (7.3–114.2) | 6/2 | 13.4 (2.3–78.8) |
| No | Yes | 104/304 | 1.8 (1.1–2.9) | 30/125 | 1.2 (0.6–2) |
| Yes | Yes | 69/4 | 136.3 (43.2–429.6) | 13/0 | |
| HBV2 | Smoking | 225/540 | Model (2)3 | 94/349 | Model (2)3 |
| No | No | 39/225 | 1 | 42/217 | 1 |
| Yes | No | 13/7 | 9.7 (3.3–28.7) | 9/7 | 3.1 (1.1–10.1) |
| No | Yes | 132/298 | 2 (1.2–3.3) | 35/119 | 1.1 (0.6–1.9) |
| Yes | Yes | 41/10 | 9.6 (3.9–2.4) | 8/6 | 6.7 (1.9–24.1) |
| Heavy drinking | Smoking | 117/262 | Model (3)4 | 66/266 | Model (3)4 |
| No | No | 28/121 | 1 | 38/184 | 1 |
| Yes | No | 9/13 | 3.9 (1.1–14.9) | 1/3 | 3.1 (0.3–35.8) |
| No | Yes | 30/84 | 1.5 (1.1–3.4) | 18/74 | 1.1 (0.5–2.3) |
| Yes | Yes | 50/44 | 2.8 (1.2–6.8) | 9/5 | 13.7 (3.2–57.9) |
| Diabetes mellitus | Smoking | 225/670 | Model (4)5 | 94/391 | Model (4)5 |
| No | No | 30/260 | 1 | 37/233 | 1 |
| Yes | No | 22/29 | 8.4 (3.6–19.8) | 14/18 | 4 (1.6–9.8) |
| No | Yes | 110/326 | 2.4 (1.3–4.2) | 34/133 | 1.2 (0.7–2.3) |
| Yes | Yes | 63/55 | 9.1 (4.6–17.9) | 9/7 | 4.6 (1.3–15.9) |
Adjusted OR for age, race, education, marital status, state of residency, family history of cancer, HBV, diabetes and alcohol.
HBV includes HBsAg+ or Anti-HBc+.
Adjusted OR for age, race, education, marital status, state of residency, family history of cancer, HCV, diabetes and alcohol.
Adjusted OR for age, race, education, marital status, state of residency, family history of cancer, HCV, HBV and diabetes.
Adjusted OR for age, race, education, marital status, state of residency, family history of cancer, HCV, HBV and alcohol.
Discussion
The results of this large case-control study confirm the significance of previously reported risk factors for HCC development, including heavy alcohol consumption, diabetes mellitus, family history of cancer and chronic HCV and HBV infection. Also consistent with previous studies, smoking-related HCC was significant among men.4,10,35,36 Moreover, it appeared that the magnitude of AOR for HCC risk was larger with smoking duration than with the intensity of smoking; this may, in part, reflect the accuracy with which these 2 parameters were measured. While the duration of smoking can be reasonably accurately measured in epidemiological studies, the intensity of smoking is subject to misclassification bias. Intensity is influenced not only by the number of cigarettes per day but also by the depth of inhalation and number of puffs taken per cigarette. It is possible that smokers compensate for a reduction in the number of cigarettes smoked per day by smoking each cigarette more intensively.21 The same finding was observed for the association between cigarette smoking and lung cancer.21,37 In addition, we found that the excess risk persisted among men who had quit smoking 10 or fewer years before the study, independent of the presence of other significant risk factors. This finding supports the results of early Japanese reports14,36 and a U.S. military veterans study38,39 in which a positive association was found between former smoking and liver cancer.
The exact mechanism of tobacco hepatocarcinogenesis is unknown; however, of approximately 4,000 components identified in tobacco smoke, at least 55 are known carcinogens. The major chemical carcinogens include polycyclic aromatic hydrocarbons, such as benzo[a]pyrene; aromatic amines, such as 4-aminobi-phenyl; and nitrosamines, such as 4-(methylnitrosamine)-1-(3-pyr-idyl)-1-butanone. A case control study demonstrated that 4-amino-biphenyl DNA adducts contained in tobacco smoke is a liver carcinogen.40 In addition, tobacco smoke contains volatile compounds (e.g., benzene), radioactive elements (e.g., polonium-210) and free radicals that may also play a role in hepatocarcinogenicity.41,42
Unlike cigarette smoking, the magnitude of AOR for the association between heavy alcohol consumption and HCC was larger in women than in men, which may be partially attributable to the synergism between female sex and heavy alcohol consumption. A recent review by Mancinelli et al.43 suggested that women may experience a more rapid progression of alcohol damage than men. The lower body mass index and body fluid content in women than men may contribute to lowered ethanol diffusion and high blood concentration in women.44 Moreover, the activity of gastric alcohol dehydogenase, which is responsible for the first-pass metabolism of ethanol in the stomach, is significantly lower in women than in men, which implies that large amounts of alcohol will be metabolized by hepatic alcohol dehydogenase.45,46 It is also possible that genetic variations in carcinogen metabolism, inflammatory response, DNA repair and cell-cycle regulation play a role in determining individual susceptibility to tobacco and alcohol carcinogenesis, which may partially explain variations in HCC risk by sex.
The most notable findings of the current study were the synergy between cigarette smoking and HCV in men and cigarette smoking and heavy alcohol consumption in women on the etiology of HCC. An increased risk of HCC associated with cigarette smoking was previously reported for chronic carriers of HBV10 and HCV5,12 and for alcohol drinkers.11,16 The mechanisms by which cigarette smoking enhances the HCC risk associated with HCV infection or alcohol consumption are unknown. However, it is possible that, in some people with these risk factors, smoking-induced oxidative stress increases the liver’s susceptibility to chronic inflammation, DNA damage and HCC development. A direct effect of smoking that promotes the rapid progression of HCV- or alcohol-induced cirrhosis cannot be excluded.
Despite the significant association between cigarette smoking and the risk of HCC, we found no significant association between the risk of HCC and passive smoking. Few studies have examined the relationship between passive smoking and cancer. Except for lung cancer, the evidence for an association between passive smoking and several types of cancer is inconclusive. The lack of risk association between passive smoking and HCC in women was consistent with the results of Nishino et al.,47 who reported no significant increase in the relative risk of liver cancer among women whose husbands smoked (relative risk, 1.2; 95% CI, 0.5–3.2). To our knowledge, no previous studies have explored this risk in men. Our observed inverse association between HCC development and adulthood exposure to passive smoking in men has been previously reported for breast cancer development.47 A possible explanation for this finding could be attributed to chance or to residual confounding of unmeasured protective factors, such as dietary intake of fruits and vegetables, vitamins, physical activity and normal body weight among nonsmokers exposed to others’ smoke. In addition, potential selection bias related to the use of hospital visitors as control subjects is also possible. Because the control subjects were companions of cancer patients, there is a possibility that the prevalence of passive smoking among control subjects was higher than general population. However, it is unlikely that any association between passive smoking and HCC risk was masked by selection bias in our study, for the following reasons. We found a positive association between active smoking and HCC. In addition, our control recruitment excluded subjects who were accompanying patients with cancers that are strongly associated with cigarette smoking (e.g., lung and head and neck cancers). As a result, the prevalence of passive smoking exposure among noncigarette smoking control subjects was comparable to that reported for U.S. adults by other population-based studies.29,48 Moreover, comparing the current controls with other cancers (smoking-unrelated), revealed no significant effect of smoking on these cancers. Until other epidemiological studies confirm our finding, the lack of risk-relationship between HCC and passive smoking should be interpreted with caution.
The use of chewing tobacco and snuff was also not related to HCC development in general or in nonsmokers. However, a potential risk of HCC for heavy users of pipes and cigars may exist among cigarette smokers. To our knowledge, our study is the first to explore the association between the use of noncigarette products or passive smoking and the risk of HCC after taking into consideration the confounding effect of known HCC risk factors. We also assessed both the intensity and duration of exposure during different life periods. The observed moderate association of heavy pipe and cigar use with HCC is not surprising, given the previously reported high mortality rate from liver cancer among cigar users39,49; however, the previous reports did not adjust for major HCC risk factors, particularly viral hepatitis. With respect to chewing tobacco, we found no previous epidemiological study that investigated any relationship between chewing tobacco and HCC. However, an association between HCC and chewing betel quid,50,51 a common habit in Taiwan52 has been reported.
It is generally accepted that accurately assessing the relationship between noncigarette tobacco use and cancer risk is difficult. Obstacles include the lack of standard measurements for cigar size and tobacco type, variations in the behavior of people using these types of products (inhalation versus chewing), the low prevalence of noncigarette tobacco exposure in the general population (compared to the marked prevalence of cigarette smoking) and the potential confounding effect of high socioeconomic status among cigar and pipe users. All of these factors may bias measurements of the cumulative intake of noncigarette tobacco products. In our study, however, we were able to assess several smoking types with proper adjustment for confounding factors. Both HCC patients and control subjects were personally interviewed using a structured and validated questionnaire for several sources of smoke exposure.
The study was specifically designed to minimize ascertainment or selection biases related to misdiagnosis of the case patients. All of our case patients had pathologically confirmed HCC, and both case patients and control subjects were prospectively enrolled simultaneously at M. D. Anderson Cancer Center. Moreover, we believe that our control selection was appropriate and representative of our study base. Control subjects were cancer-free at the time of enrollment and less likely than case patients to have cancer, chronic illness or previous exposure to tobacco and alcohol use. Moreover, all spouses of other cancers in the control group reported that they would have chosen to be referred to the same hospital if they had been diagnosed with cancer during the same time period, probably because spouses tend to share the same family physician, have the same health insurance coverage, and live in the same geographic location. Moreover, altruism was the main reason given by controls for accompanying cancer patients during hospital visit. It is unlikely that such reason is related to the exposures under investigation by the current study. All of these results indicated that the patients and control subjects had the same catchments, which further supports the idea that the study control subjects were representative of the hospital (M. D. Anderson Cancer Center) population from which the case patients were selected.53–55
In conclusion, in this American hospital-based case-control study, after adjusting for other known risk factors for HCC, we did not find significant associations between passive smoking or use of noncigarette tobacco products and risk of HCC among non-smokers. However, sex differences were observed in HCC relationship with cigarette smoking and alcohol consumption. We did find a significant association between HCC and cigarette smoking in men and heavy alcohol consumption in women. A synergistic interaction between cigarette smoking and HCV and cigarette smoking and alcohol consumption was observed for men and women, respectively. The observed sex difference in HCC development could be attributed to genetic variation between men and women with different susceptibility to HCC development upon their environmental exposure to smoking and alcohol. However, these findings need to be confirmed in different populations. In the meantime, control of all sources of smoking exposure would appear to be a prudent approach for the prevention of HCC, especially in patients with chronic hepatitis infections.
Acknowledgments
National Institutes of Health; Grant numbers: RO3 ES11481, CA106458-01; Grant sponsor: Texas Tobacco Settlement.
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans: Hepatitis viruses. Lyon, France: IARC; 1994. pp. 182–221. [Google Scholar]
- 3.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 4.Zhu K, Moriarty C, Caplan LS, Levine RS. Cigarette smoking and primary liver cancer: a population-based case-control study in US men. Cancer Causes Control. 2007;18:315–21. doi: 10.1007/s10552-006-0105-8. [DOI] [PubMed] [Google Scholar]
- 5.Franceschi S, Montella M, Polesel J, La VC, Crispo A, Dal ML, Casarin P, Izzo F, Tommasi LG, Chemin I, Trepo C, Crovatto M, et al. Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:683–9. doi: 10.1158/1055-9965.EPI-05-0702. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–24. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U. S Cancer. 2004;101:1009–17. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 8.Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004;96:1851–6. doi: 10.1093/jnci/djh334. [DOI] [PubMed] [Google Scholar]
- 9.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Wang LY, You SL, Lu SN, Ho HC, Wu MH, Sun CA, Yang HI, Chien-Jen C. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control. 2003;14:241–50. doi: 10.1023/a:1023636619477. [DOI] [PubMed] [Google Scholar]
- 11.Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- 12.Mori M, Hara M, Wada I, Hara T, Yamamoto K, Honda M, Naramoto J. Prospective study of hepatitis B and C viral infections, cigarette smoking, alcohol consumption, and other factors associated with hepatocellular carcinoma risk in Japan. Am J Epidemiol. 2000;151:131–9. doi: 10.1093/oxfordjournals.aje.a010180. [DOI] [PubMed] [Google Scholar]
- 13.Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study Int J Cancer. 1999;81:695–9. doi: 10.1002/(sici)1097-0215(19990531)81:5<695::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Hirohata T, Fukuda K, Shibata A, Tsukuma H, Hiyama T. Risk factors for hepatocellular carcinoma among Japanese women. Cancer Causes Control. 1995;6:91–8. doi: 10.1007/BF00052768. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Hirohata T, Takeshita S, Hirohata I, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, Ishibashi H. Hepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Int J Cancer. 1992;51:509–14. doi: 10.1002/ijc.2910510402. [DOI] [PubMed] [Google Scholar]
- 16.Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF, Chang WY, Sheen MC, Lin TM. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- 17.Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst. 1991;83:1820–6. doi: 10.1093/jnci/83.24.1820. [DOI] [PubMed] [Google Scholar]
- 18.Tsukuma H, Hiyama T, Oshima A, Sobue T, Fujimoto I, Kasugai H, Kojima J, Sasaki Y, Imaoka S, Horiuchi N. A case-control study of hepatocellular carcinoma in Osaka, Japan. Int J Cancer. 1990;45:231–6. doi: 10.1002/ijc.2910450205. [DOI] [PubMed] [Google Scholar]
- 19.Levy DT, Nikolayev L, Mumford E. Recent trends in smoking and the role of public policies: results from the SimSmoke tobacco control policy simulation model. Addiction. 2005;100:1526–36. doi: 10.1111/j.1360-0443.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 20.Austin H. The role of tobacco use and alcohol consumption in the etiology of hepatocellular carcinoma. In: Tabor E, DiBisceglie A, Purcell R, editors. Etiology, pathology and treatment of hepatocellular carcinoma in North America. Vol. 13. The Woodlands, Texas: Portfolio Publishing Company; 2007. pp. 57–70. [Google Scholar]
- 21.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans: tobacco smoke and involuntary smoking. Vol. 83. Lyon, France: IARC; 2004. pp. 161–176. [PMC free article] [PubMed] [Google Scholar]
- 22.Baker F, Ainsworth SR, Dye JT, Crammer C, Thun MJ, Hoffmann D, Repace JL, Henningfield JE, Slade J, Pinney J, Shanks T, Burns DM, et al. Health risks associated with cigar smoking. JAMA. 2000;284:735–40. doi: 10.1001/jama.284.6.735. [DOI] [PubMed] [Google Scholar]
- 23.Lubin JH, Richter BS, Blot WJ. Lung cancer risk with cigar and pipe use. J Natl Cancer Inst. 1984;73:377–81. doi: 10.1093/jnci/73.2.377. [DOI] [PubMed] [Google Scholar]
- 24.Boffetta P, Pershagen G, Jockel KH, Forastiere F, Gaborieau V, Heinrich J, Jahn I, Kreuzer M, Merletti F, Nyberg F, Rosch F, Simonato L. Cigar and pipe smoking and lung cancer risk: a multicenter study from Europe. J Natl Cancer Inst. 1999;91:697–701. doi: 10.1093/jnci/91.8.697. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro JA, Jacobs EJ, Thun MJ. Cigar smoking in men and risk of death from tobacco-related cancers. J Natl Cancer Inst. 2000;92:333–7. doi: 10.1093/jnci/92.4.333. [DOI] [PubMed] [Google Scholar]
- 26.Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L, Munoz N, Franceschi S. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fielding JE, Phenow KJ. Health effects of involuntary smoking. N Engl J Med. 1988;319:1452–60. doi: 10.1056/NEJM198812013192205. [DOI] [PubMed] [Google Scholar]
- 28.Hackshaw AK. Lung cancer and passive smoking. Stat Methods Med Res. 1998;7:119–36. doi: 10.1177/096228029800700203. [DOI] [PubMed] [Google Scholar]
- 29.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–8. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong L, Goldberg MS, Parent ME, Hanley JA. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer. 2000;27:3–18. doi: 10.1016/s0169-5002(99)00093-8. [DOI] [PubMed] [Google Scholar]
- 31.International Agency for Research on Cancer (IARC) Monographs on the evaluation of carcinogenic risks to humans: alcohol Drinking. Vol. 44. Lyon, France: IARC; 1988. [Google Scholar]
- 32.Diaz LE, Montero A, Gonzalez-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56(Suppl 3):S50–S53. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- 33.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C, Porru S, Nardi G. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 34.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–11. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 35.Yun YH, Jung KW, Bae JM, Lee JS, Shin SA, Min PS, Yoo T, Yul HB. Cigarette smoking and cancer incidence risk in adult men: National Health Insurance Corporation Study. Cancer Detect Prev. 2005;29:15–24. doi: 10.1016/j.cdp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Goodman MT, Moriwaki H, Vaeth M, Akiba S, Hayabuchi H, Mabuchi K. Prospective cohort study of risk factors for primary liver cancer in Hiroshima and Nagasaki, Japan. Epidemiology. 1995;6:36–41. doi: 10.1097/00001648-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2:1525–36. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Smoking and cancer mortality among U.S. veterans: a 26-year follow-up. Int J Cancer. 1995;60:190–3. [PubMed] [Google Scholar]
- 39.Hsing AW, McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Cigarette smoking and liver cancer among US veterans. Cancer Causes Control. 1990;1:217–21. doi: 10.1007/BF00117473. [DOI] [PubMed] [Google Scholar]
- 40.Wang LY, Chen CJ, Zhang YJ, Tsai WY, Lee PH, Feitelson MA, Lee CS, Santella RM. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am J Epidemiol. 1998;147:315–23. doi: 10.1093/oxfordjournals.aje.a009452. [DOI] [PubMed] [Google Scholar]
- 41.Vineis P, Pirastu R. Aromatic amines and cancer. Cancer Causes Control. 1997;8:346–55. doi: 10.1023/a:1018453104303. [DOI] [PubMed] [Google Scholar]
- 42.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 43.Mancinelli R, Binetti R, Ceccanti M. Woman, alcohol and environment: emerging risks for health. Neurosci Biobehav Rev. 2007;31:246–53. doi: 10.1016/j.neubiorev.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Ely M, Hardy R, Longford NT, Wadsworth ME. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol Alcohol. 1999;34:894–902. doi: 10.1093/alcalc/34.6.894. [DOI] [PubMed] [Google Scholar]
- 45.Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–7. [PubMed] [Google Scholar]
- 46.Frezza M, di PC, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–9. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 47.Nishino Y, Tsubono Y, Tsuji I, Komatsu S, Kanemura S, Nakatsuka H, Fukao A, Satoh H, Hisamichi S. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. doi: 10.1023/a:1012273806199. [DOI] [PubMed] [Google Scholar]
- 48.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233–40. [PubMed] [Google Scholar]
- 49.Carstensen JM, Pershagen G, Eklund G. Mortality in relation to cigarette and pipe smoking: 16 years’ observation of 25,000 Swedish men. J Epidemiol Community Health. 1987;41:166–72. doi: 10.1136/jech.41.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun CA, Wu DM, Lin CC, Lu SN, You SL, Wang LY, Wu MH, Chen CJ. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol. 2003;157:674–82. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 51.Tsai JF, Chuang LY, Jeng JE, Ho MS, Hsieh MY, Lin ZY, Wang LY. Betel quid chewing as a risk factor for hepatocellular carcinoma: a case-control study. Br J Cancer. 2001;84:709–13. doi: 10.1054/bjoc.1999.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko YC, Chiang TA, Chang SJ, Hsieh SF. Prevalence of betel quid chewing habit in Taiwan and related sociodemographic factors. J Oral Pathol Med. 1992;21:261–4. doi: 10.1111/j.1600-0714.1992.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 53.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135:1019–28. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 54.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol. 1992;135:1029–41. doi: 10.1093/oxfordjournals.aje.a116397. [DOI] [PubMed] [Google Scholar]
- 55.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992;135:1042–50. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]