Abstract
Background
We have recently shown that the cardioprotection afforded by cardioplegia is affected by age and gender and is less effective in the aged female rabbit heart compared with the aged male rabbit heart. We hypothesized that these differences were due to age and gender-specific modulation of mitochondrial oxygen consumption and mitochondrial free matrix calcium ([Ca2+]Mito) content occurring during early reperfusion.
Methods
To test this hypothesis, 104 male and female rabbit hearts, mature (15 to 20 weeks) and aged (>32 months), were subjected to Langendorff perfusion. Control hearts were perfused for 75 minutes. Global ischemia hearts were underwent 30 minutes of equilibrium, 30 minutes of global ischemia, and 15 minutes of reperfusion. Cardioplegia (potassium/magnesium) ± diazoxide was infused 5 minutes before global ischemia. Mitochondria were isolated from left ventricular tissue and used for the measurement of oxygen consumption and [Ca2+]Mito.
Results
Mitochondrial oxygen consumption was significantly increased in the mature and aged female hearts in all treatment groups (p < 0.001 versus male). Cardioplegia ± diazoxide modulated mitochondrial oxygen consumption, but these effects were significantly decreased in the aged heart and in the female heart (p < 0.001 each versus male). Cardioplegia (potassium/magnesium) significantly decreased [Ca2+]Mito (p < 0.001 versus global ischemia) in aged but not mature hearts. The addition of diazoxide to potassium/magnesium significantly decreased [Ca2+]Mito in mature and aged males (p < 0.001 versus potassium/magnesium) but not in females.
Conclusions
These results demonstrate that mitochondrial oxygen consumption and [Ca2+]Mito are modulated by age and gender and play an important role in the differences observed between mature and aged male and female response to global ischemia and the cardioprotection afforded by cardioplegia ± diazoxide.
We have previously shown that magnesium (Mg) supplemented potassium (K) cardioplegia (K/Mg) is superior to high potassium cardioplegia and that the cardioprotection afforded by K/Mg cardioplegia can be enhanced with the addition of diazoxide (DZX), a mitochondrial adenosine 5′-triphosphate (ATP)-sensitive K+ (mitoKATP) channel opener [1]. We have recently reported the effects of ischemia and reperfusion and the cardioprotection afforded by cardioplegia ± diazoxide in a rabbit model using male and female, sexually mature and aged (retired breeder, not senescent) hearts [2]. Our data demonstrated that aging significantly decreased postischemic functional recovery and significantly increased infarct size.
We also demonstrated that the effects of global ischemia were partially ameliorated by cardioplegia ± diazoxide, but this cardioprotection was significantly decreased in the aged compared with the mature heart and significantly decreased in the aged female compared with the aged male rabbit heart [2]. These animal studies are consistent with human studies indicating that women have a significantly greater risk potential compared with men and worse outcomes after cardiac surgery [3, 4].
The Society of Thoracic Surgeons National Cardiac Surgery Database provides evidence that in patients undergoing coronary artery bypass grafting (CABG), women have a significantly higher operative mortality than men, and long-term survival is generally less favorable in women than in men [5]. Multivariate analysis also shows that women have higher mortality rates than equally matched men in low-risk and medium-risk groups [5]. It is only among very high-risk patients that sex is not found to be an independent predictor of adverse outcomes [5].
Our model was chosen because previous studies in animal models have shown that age-related ischemic intolerance develops well before senescence, being primarily evident by middle age [2, 6, 7]. It has been demonstrated in animal models that there is an apparent nadir in ischemic tolerance in middle-aged to aged hearts, with a subsequent modest improvement with senescence [6, 7]. This pattern is consistent with empiric clinical observations and with prior studies showing an improvement in the senescent compared with the aged heart that may be related to selection of a subpopulation resistant to myocardial injury [6, 7]. Support for this hypothesis comes from the study of Vaccarino and colleagues [8], who have reported that in CABG patients aged between 50 and 60 years, women experienced an 86% higher risk of in-hospital death than men; however, sex differences in in-hospital mortality were less marked in the older-age subgroup.
The mechanism(s) modulating age-associated and gender-associated differences in cardioprotection remain to be fully elucidated; however, we and others have suggested that alterations in mitochondrial function and mitochondrial free matrix calcium accumulation ([Ca2+]Mito) occurring after global ischemia and apparent during early reperfusion may play a significant role [9-15]. Results from our recent studies led us to hypothesize that age and gender modulated mitochondrial function and [Ca2+]Mito and that these changes are associated with the age-specific and gender-specific differential cardioprotection afforded by cardioplegia ± diazoxide. To test this hypothesis, age-matched mature (sexually mature) and aged (retired breeders, not senescent) male and female hearts were subjected to either no ischemia (control) or ischemia–reperfusion with and without cardioplegia ± diazoxide. Mitochondria were isolated from postischemic hearts to determine the effects of ischemia on mitochondrial function and calcium accumulation.
Material and Methods
Animals
Male (n = 56) and female mature (n = 52) (sexually mature, 15 to 20 weeks; 3 to 4 kg) and male (n = 52) and female (n = 52) aged (retired breeders, not senescent, >32 months; 5 to 6 kg) New Zealand White rabbits were obtained from Millbrook Farm, Amherst, MA. The classification of mature and aged is derived from normative rabbit data meeting accepted criteria for aging [16]. All experiments were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Committee and the Harvard Medical Area Standing Committee on Animals and conformed to the Guidelines Regulating the Care and Use of Laboratory Animals (NIH publication no. 5377−3, 1996).
Langendorff Perfusion
All rabbits were anesthetized with pentobarbital sodium (Nembutal, 100 mg/kg; Abbott Laboratories, North Chicago, IL), and heparin (200 U/kg) was given intravenously through the marginal ear vein [1, 2, 10]. Langendorff perfusion was performed as previously described [1, 2, 10]. Hearts were paced via the right atrium so that heart rate was maintained at 180 ± 3 beats/min throughout the experiment using a Medtronic Model 5330 stimulator (Minneapolis, MN). Hemodynamic variables were acquired using the PO-NE-MAH digital data acquisition system (Gould, Valley View, OH), with an Acquire Plus processor board (Gould, Valley View, OH), and left ventricular pressure analysis software [1, 2].
Experimental Protocol
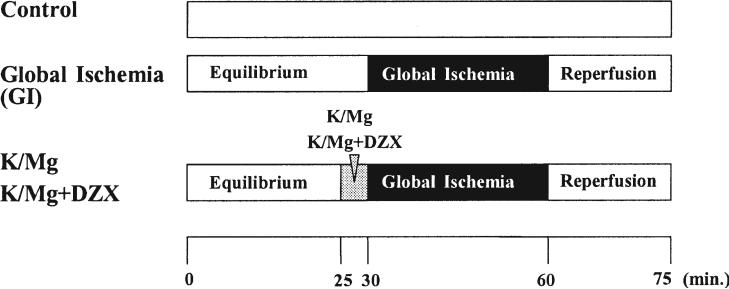
The experimental protocol is shown in Figure 1. To determine the effects of ischemia–reperfusion on mitochondrial function and mitochondrial free calcium accumulation during early reperfusion, hearts were perfused for 30 minutes to establish equilibrium hemodynamics. Control hearts were perfused without global ischemia at 37°C for 75 minutes. Global ischemia (GI) hearts underwent 30 minutes of perfusion for equilibrium, 30 minutes of global ischemia, and 15 minutes of reperfusion. The K/Mg hearts and K/Mg + DZX hearts were perfused for 30 minutes of equilibrium, received either K/Mg or K/Mg + DZX at the start of global ischemia, and then underwent 15 minutes of reperfusion. The reperfusion time of 15 minutes was used because previous investigations have shown that a 15-minute reperfusion allows for verification of hemodynamic recovery of samples and for comparison with previous results using 120 minutes of reperfusion as an end point [2].
Fig 1.
Experimental protocol. Hearts were perfused for 30 minutes to establish equilibrium hemodynamics. Control hearts (top bar) were perfused without global ischemia at 37°C for 75 minutes. Global ischemia (GI) hearts (middle bar) underwent 30 minutes of perfusion for equilibrium (left clear area), 30 minutes of GI (black area), and 15 minutes of reperfusion (right clear area). Potassium/magnesium (K/Mg) hearts and K/Mg + diazoxide (DZX) hearts (bottom bar) underwent 30 minutes of perfusion for equilibrium (right clear area), then received either K/Mg or K/Mg + DZX (gray area) at the start of global ischemia (black area) and 15 minutes of reperfusion (right clear area).
After the completion of reperfusion, mature male hearts (n = 8 each group), mature female hearts (n = 7 each group), aged male hearts (n = 7 each group), and aged female hearts (n = 7 each group) were used for the immediate isolation of mitochondria.
A separate group of hearts consisting of mature male hearts (n = 6 each group), mature female hearts (n = 6 each group), aged male hearts (n = 6 each group), and aged female hearts (n = 6 each group) were immediately freeze-clamped at the end of reperfusion and stored in liquid nitrogen. They were then used for mitochondrial free calcium determination and tissue ATP content.
Mitochondrial Oxygen Consumption
Mitochondria were isolated from postischemic left ventricular tissue and suspended in 3 to 4 mL of respiration medium as previously described [10]. Mitochondrial oxygen consumption was determined using a Clark-type electrode (Yellow Springs Instruments Co, Yellow Springs, OH) as previously described [10]. In brief, oxygen consumption in isolated mitochondria was determined in the presence of the substrate malate/glutamate (complex I) or succinate (complex II). The addition of adenosine diphosphate (ADP) causes a sudden burst of oxygen uptake as the ADP is converted into ATP. Before the assay, the instrument was calibrated to 100% saturation in air-saturated respiration media at 30°C.
Oxygen consumption at complex I, the first of the complexes of the electron transport chain, was determined using glutamate and malate (5 mM of each) added to the respiration media. Complex II mitochondrial oxygen consumption, the second of the complexes of the electron transport chain, was determined using succinate (8 mM) and rotenone (4 μM), an inhibitor of complex I, added to the respiration media in the place of glutamate and malate. State 3, (active) mitochondrial oxygen consumption defined as ADP-stimulated respiration, was initiated by the addition of 1 mmol/L ADP to the respiration media and allowed to proceed for 2 minutes, as we have previously described [10]. Oxygen consumption was calculated based on an initial air-saturated [O2] = 195 nM/mL [10].
Mitochondrial Free Matrix Calcium
[Ca2+]Mito was determined using an in-house spectrofluorescence system as previously described [9]. Two observations per heart were determined for [Ca2+]Mito, and the mean was used as n = 1 observation. Calibration was performed as previously described [10].
Tissue Adenosine 5′-Triphosphate Content
Tissue ATP content was determined in freeze-clamped tissue samples (approximately 200 mg) according to the method as described by Lowry and Passonneao [17] using a LS 55 luminescence spectrometer (Perkin Elmer, Wellesley, MA). Tissue ATP content was expressed as ATP content per gram of dry weight, based on an ATP standard curve (ATP standard, FL-AAS, Sigma-Aldrich, St. Louis, MO). Two observations per heart were determined for ATP content and the mean used as n = 1 observation. Wet weight/dry weight ratios were determined as previously described [18].
Statistical Analysis
Statistical analysis was performed with SAS 8.02 software (SAS Institute, Cary, NC). The mean ± standard error of the mean is shown for all results. Standard t tests were used for comparing GI results with the control group. The same approach was used for comparisons between male and female hearts within an age and treatment group. Post hoc pair-wise testing was performed using a Bonferroni correction. Values for p for the between-group comparisons were reported adjusted for the six comparisons within each analysis of variance. A two by two analysis of variance with factors for age, gender, and age by gender interaction was performed. Results presented always include the age by gender interaction. Statistical significance was set at p < 0.05.
Results
Effects of Global Ischemia and Cardioplegia in the Mature and Aged Heart
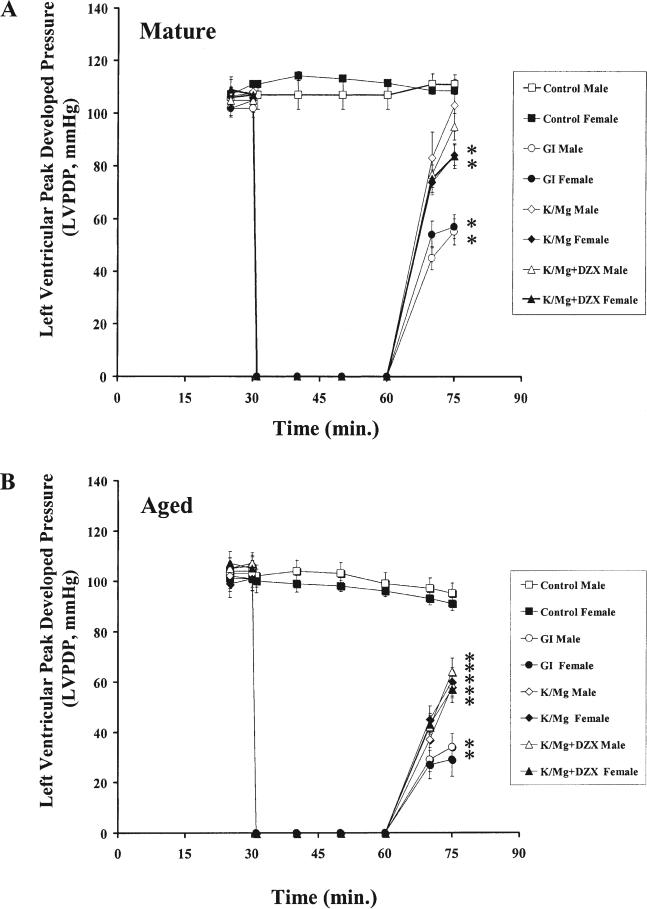
postischemic functional recovery. No significant difference in left ventricular peak developed pressure (LVPDP) was observed within or between mature and aged, male and female hearts at the end of equilibrium (p = 1.000 for each, Fig 2). There was no significant difference LVPDP in control hearts within or between mature and aged, male and female hearts at the end of 15 minutes of reperfusion (p = 1.000 for each). LVPDP in GI hearts was significantly decreased compared with control at the end of 15 minutes of reperfusion in the mature and aged, male and female (p < 0.001 for each, Fig 2).
Fig 2.
Left ventricular peak developed pressure (LVPDP) during 30 minutes of equilibrium, 30 minutes of global ischemia, and 15 minutes of reperfusion for control (clear square, filled square), global ischemia (GI; open circle, male; filled circle, female), potassium/magnesium (K/Mg; clear diamond, male; solid diamond, female), and K/Mg + diazoxide (DZX; clear triangle, male; filled triangle, female) hearts in (A) mature male (n = 8) and mature female (n = 7) and (B): aged male (n = 7) and aged female (n = 7). All results are shown as the mean ± standard error of the mean. *Significant differences at p < 0.001 versus control.
In mature male hearts, no significant difference was observed in LVPDP compared with control at the end of 15 minutes reperfusion in K/Mg or K/Mg + DZX (p = 1.000 for each, Fig 2A), However, LVPDP was significantly decreased compared with control in K/Mg hearts in the mature female (p = 0.008) and in the aged male and female (p < 0.001 for each; Fig 2). LVPDP was also significantly decreased compared with control in K/Mg + DZX hearts in the mature female (p = 0.006) and in the aged male and female (p < 0.001 for each; Fig 2). These results are not significantly different (p = 1.000 for each) from that observed previously [2].
tissue adt content after 15 minutes of reperfusion. No significant difference in tissue ATP content was observed between mature and aged male and female control hearts (p = 1.000, Table 1). Tissue ATP content after 15 minutes of reperfusion was significantly decreased compared with control in GI, K/Mg, and K/Mg + DZX in both mature and aged male and female hearts (p < 0.001 for each). K/Mg and K/Mg + DZX significantly increased tissue ATP content compared with GI in both mature and aged male and female hearts (p < 0.001 for each). Tissue ATP content was significantly decreased in aged males and females compared with mature male and female hearts in GI, K/Mg, and K/Mg + DZX hearts (Table 1). Of significance, tissue ATP content was significantly decreased in aged female K/Mg + DZX hearts compared with aged male K/Mg + DZX hearts (Table 1).
Table 1.
Tissue ATP Content in Mature and Aged Male and Female Hearts at 15 Minutes of Reperfusion
| ATP μM/g Dry Weight |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
| Control | 23.0 (1.4) | 22.6 (1.2) | 22.4 (1.5) | 22.8 (1.5) | 0.8794 | 0.9977 | 0.7902 | 0.8390 | 0.8612 |
| GI | 9.0 (1.2) | 9.6 (0.8) | 7.5 (1.0) | 6.8 (1.0) | 0.0397 | 0.9582 | 0.5652 | 0.7079 | 0.6650 |
| K/Mg | 11.7 (0.9) | 11.4 (1.2) | 8.6 (0.7) | 7.8 (0.4) | 0.0004 | 0.4984 | 0.8387 | 0.7880 | 0.3645 |
| K/Mg + DZX | 12.2 (1.0) | 13.5 (0.8) | 9.4 (0.7) | 7.6 (0.4) | <0.0001 | 0.7354 | 0.0497 | 0.3240 | 0.0452 |
Mature and aged, male and female tissue ATP concentration content (μM/g dry weight) for Control (after 75 minutes of perfusion) and in GI, K/Mg and K/Mg + DZX after 30 minutes of equilibrium, 30 minutes of ischemia. 15 minutes of reperfusion. All results are shown as mean (SEM) for n = 6 animals for each group. P values for specific comparisons between male and female animals, within an age group were calculated using a t test. Significant differences at p < 0.05 are shown in bold face.
ATP = adenosine triphosphate; DZX = diazoxide; GI = global ischemia; K/Mg = potassium/magnesium.
mitochondrial free matrix calcium. No significant difference in [Ca2+]Mito was observed between mature and aged male and female control hearts (p = 1.000, Table 2). [Ca2+]Mito was significantly increased in GI in both mature and aged male and female hearts (p < 0.001 for each, Table 2). No significant difference between mature male and female and aged male and female GI hearts was observed (p = 0.3039 and p = 0.6993, respectively).
Table 2.
Mitochondrial Free Matrix Calcium in Mature and Aged Male and Female Hearts
| [Ca2+]Mito |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
| Control | 217.0 (6.5) | 209.5 (7.2) | 202.7 (20.9) | 204.3 (18.5) | 0.6055 | 0.9511 | 0.8629 | 0.7257 | 0.9535 |
| GI | 619.3 (16.5) | 593.2 (17.6) | 773.2 (17.9) | 762.7 (19.4) | <0.0001 | 0.3178 | 0.6662 | 0.3039 | 0.6993 |
| K/Mg | 608.7 (13.8) | 602.5 (15.5) | 652.3 (19.1) | 668.0 (17.8) | 0.0039 | 0.7793 | 0.5213 | 0.7730 | 0.5630 |
| K/Mg + DZX | 483.5 (19.5) | 572.7 (22.6) | 572.3 (16.9) | 665.0 (15.5) | 0.004 | 0.004 | 0.9724 | 0.0122 | 0.0127 |
Mature and aged, male and female mitochondrial free matrix calcium (nM/mg mitochondrial protein/mg dry weight) for Control (after 75 minutes of perfusion), GI, K/Mg and K/Mg + DZX (after 30 minutes of equilibrium, 30 minutes of ischemia. 15 minutes of reperfusion). All results are shown as mean (SEM) for n = 6 animals for each group. P values for specific comparisons between male and female animals, within an age group were calculated using a t test. Significant differences at p < 0.05 are shown in bold face.
DZX = diazoxide; GI = global ischemia; K/Mg = potassium/magnesium.
K/Mg cardioplegia significantly decreased [Ca2+]Mito in hearts of aged males (p = 0.001 versus GI) and aged females (p = 0.024 versus GI), but not in hearts of mature males or mature females (p = 1.000 versus GI for each). No significant difference in [Ca2+]Mito between mature male and female hearts or aged male and female K/Mg hearts was observed (p = 0.7730 and p = 0.5630, respectively).
K/Mg + DZX cardioplegia significantly decreased [Ca2+]Mito in mature male (p < 0.001 versus GI), in aged male (p < 0.001 versus GI), and in mature female (p = 0.019 versus GI), but not in aged female hearts (p = 1.000 versus GI). [Ca2+]Mito was significantly increased in mature female hearts compared with mature male K/Mg + DZX hearts and in the aged female hearts compared with the aged male K/Mg + DZX hearts (Table 2).
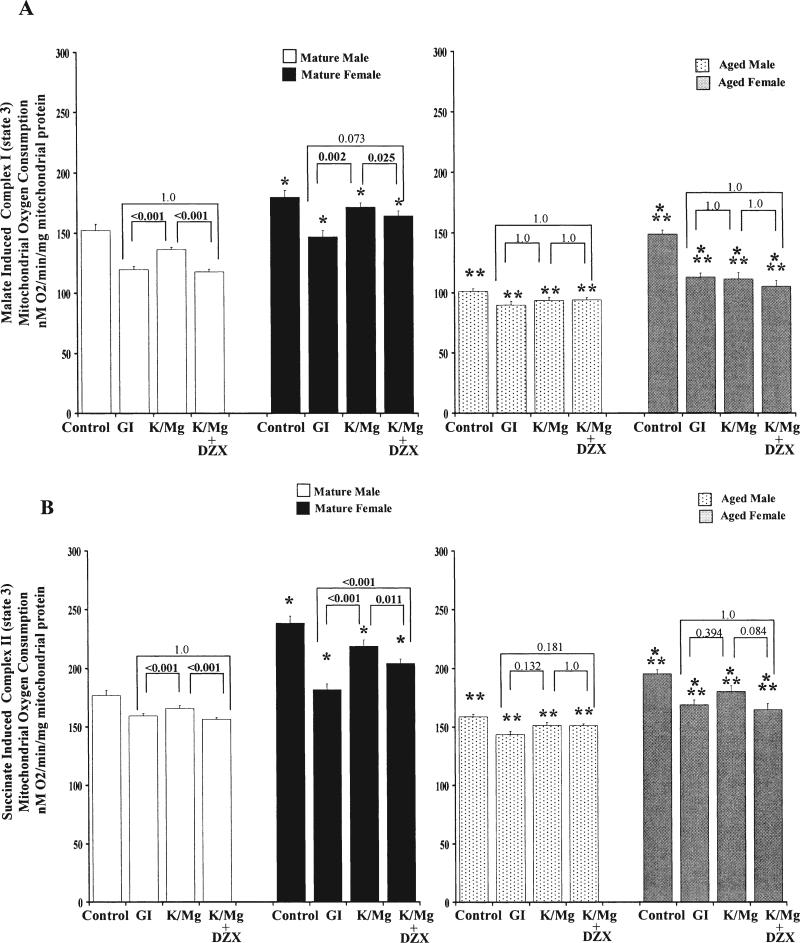
state 3 (active) mitochondrial oxygen consumption. Malate and succinate state 3 (active) mitochondrial oxygen consumption was significantly increased in both mature and aged female hearts compared with male hearts (Fig 3; Table 3). Age significantly decreased both malate and succinate state 3 mitochondrial oxygen consumption.
Fig 3.
State 3 (active) oxygen consumption (adenosine diphosphate-stimulated respiration) in (A) malate-induced (complex I) and (B) succinate induced (complex II) energized mitochondria (nM O2/min/mg mitochondrial protein) in mature male (white bars), mature female (black bars) and aged male (dotted bars) and aged female (gray bars), control (after 75 minutes perfusion) global ischemia (GI), potassium/magnesium (K/Mg), and K/Mg + diazoxide (DZX) energized mitochondria after 30 minutes of global ischemia and 15 minutes of reperfusion. All results are shown as the mean ± standard error of the mean (7 to 8 animals for each group). *Significant differences at p < 0.001 versus male treatment group. **Significant differences at p < 0.001 versus mature treatment group.
Table 3.
Malate Induced, Complex I and Succinate Induced, Complex II State 3, Active, Mitochondrial Oxygen Consumption in Mature and Aged Male and Female Hearts
| Malate Induced Complex I |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
Effects |
Comparisons |
|||||||
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
| Control | 152.1 ± 5.61 (44) | 180.0 ± 5.5 (48) | 101.3 ± 2.2 (47) | 148.7 ± 3.3 (31) | <0.0001 | <0.0001 | 0.0388 | 0.0006 | <0.0001 |
| GI | 119.7 ± 2.6 (55) | 146.8 ± 5.3 (44) | 89.9 ± 2.9 (46) | 113.0 ± 3.6 (34) | <0.0001 | <0.0001 | 0.6026 | <0.0001 | <0.0001 |
| K/Mg | 136.3 ± 1.9 (61) | 171.5 ± 4.0 (45) | 93.8 ± 2.4 (48) | 111.6 ± 5.2 (38) | <0.0001 | <0.0001 | 0.0083 | <0.0001 | 0.0033 |
| K/Mg + DZX | 117.8 ± 2.2 (57) | 164.4 ± 4.3 (44) | 94.0 ± 2.1 (46) | 105.6 ± 4.9 (40) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0344 |
| Succinate Induced Complex II |
Probability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group |
Effects |
Comparisons |
|||||||
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Male vs Female | Male vs Female | |
| Control | 176.9 ± 4.3 (39) | 238.5 ± 5.8 (47) | 158.6 ± 2.2 (50) | 195.2 ± 3.8 (29) | <0.0001 | <0.0001 | 0.0053 | <0.0001 | <0.0001 |
| GI | 159.4 ± 1.8 (32) | 181.7 ± 5.0 (38) | 143.3 ± 2.9 (48) | 168.9 ± 4.1 (32) | <0.0001 | <0.0001 | 0.6886 | <0.0001 | <0.0001 |
| K/Mg | 165.8 ± 2.4 (32) | 218.7 ± 5.2 (48) | 151.4 ± 2.9 (48) | 180.3 ± 4.7 (32) | <0.0001 | <0.0001 | 0.0048 | <0.0001 | <0.0001 |
| K/Mg + DZX | 156.3 ± 1.8 (40) | 204.1 ± 3.6 (48) | 151.0 ± 1.5 (48) | 164.9 ± 5.0 (32) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0117 |
Mature and aged, male and female malate induced, complex I and succinate induced, complex II, active mitochondrial oxygen consumption (nM O2/min/mg mitochondrial protein) and RCI (state 3/state 4) for Control (after 75 minutes of perfusion), GI, K/Mg and K/Mg + DZX (after 30 minutes equilibrium, 30 minutes of ischemia. 15 minutes reperfusion). All results are shown as mean (SEM) for n = 7−8 animals for each group. The number of individual observations for each value is shown in parentheses. P values for specific comparisons between male and female animals, within an age group, were calculated using a t test. Significant differences at p < 0.05 are shown in bold face.
ATP = adenosine triphosphate; DZX = diazoxide; GI = global ischemia; K/Mg = potassium/magnesium.
Both malate and succinate state 3 mitochondrial oxygen consumption were significantly decreased in GI hearts compared with control. K/Mg cardioplegia significantly increased both malate and succinate mitochondrial oxygen consumption compared with GI in mature male and female hearts; however, no significant difference compared with GI was observed in aged male or aged female hearts (Fig 3).
K/Mg + DZX significantly decreased succinate state 3 mitochondrial oxygen consumption in mature male and female hearts compared with K/Mg but had no affect in aged male or aged female hearts (Fig 3; Table 3). K/Mg + DZX significantly increased succinate state 3 mitochondrial oxygen consumption only in mature female hearts; in all others, no significant difference compared with GI was observed (Fig 3).
A significant difference between K/Mg and K/Mg + DZX malate state 3 mitochondrial oxygen consumption was observed in mature male hearts (p < 0.001). No significant difference was observed in any other experimental treatments (p = 1.000; Table 3).
Both malate and succinate state 3 mitochondrial oxygen consumption were affected by age, significantly decreased in aged compared with mature and gender, significantly increased in female hearts compared with male and by age by gender, significantly increased in mature female hearts compared with male, and significantly increased in aged female hearts compared with aged male, except for GI (Table 3). Significant differences between mature male and female hearts and aged male and female hearts were observed in all experimental groups (Table 3).
Comment
Our data indicate that mitochondrial oxygen consumption is significantly increased in both the mature and aged female heart compared with the male heart. We also show that aging significantly decreases mitochondrial oxygen consumption in both the male and female heart. These data agree with previous studies demonstrating that mitochondrial oxygen consumption decreases with age [12, 15]. The mechanism relating to the gender differences observed in our study remains to be elucidated; however, substrate utilization may play a role. In vivo studies have demonstrated that compared with men, women oxidize proportionately more fat and less carbohydrate during endurance exercise performed in the fasted state [19, 20]. Recent studies have also observed altered citrate levels between men and women and have suggested that estrogen may play an essential role in the regulation of protein and lipid biosynthesis by controlling pH in mitochondria and the cytoplasm [21]. Whether these differences alter isolated mitochondrial oxygen consumption requires more in depth study.
Our data show that after unprotected global ischemia, there is a significant decrease in tissue ATP content, a significant decrease in both complex I and complex II mitochondrial oxygen consumption, and a significant increase in [Ca2+]Mito. In global ischemia, increased [Ca2+]Mito accumulation has been shown to destabilize the inner mitochondrial membrane and cause the inner membrane pore to open and permit further cation movement [11, 12, 14, 22]. This is an energy-dependent process requiring ATP to transport calcium against the electrochemical gradient out of the mitochondrion, thus reducing tissue ATP content, a process called futile calcium cycling [10].
In the aged heart, [Ca2+]Mito is significantly increased and would result in increased futile calcium cycling and decreased tissue ATP content, which in turn would limit postischemic functional recovery and cell viability. Support for this mechanism is seen in our earlier observations demonstrating that global ischemia significantly decreases postischemic functional recovery and significantly increases infarct size in the GI heart compared with the control heart and that these effects are exacerbated in the aged compared with the mature heart [2].
Our data further indicate that the cardioprotection afforded by cardioplegia ± diazoxide modulates tissue ATP content, mitochondrial oxygen consumption, and [Ca2+]Mito. The proportionate role of each of these mechanisms is affected by age and gender, however. In the mature male and female heart, K/Mg cardioplegia had significantly increased tissue ATP content and significantly increased both complex I and complex II mitochondrial oxygen consumption but had no effect on [Ca2+]Mito compared with GI. The increase in both complex I and complex II mitochondrial oxygen consumption would allow for increased high-energy phosphate resynthesis, which would allow for proper mitochondrial function in the presence of increased [Ca2+]Mito concentration.
In the aged male and female heart, K/Mg cardioplegia did not enhance either complex I or complex II mitochondrial oxygen consumption but significantly decreased [Ca2+]Mito levels. This action would decrease futile calcium cycling, thus maintaining high-energy phosphate stores for cell survival and function.
The addition of diazoxide to K/Mg cardioplegia further decreased [Ca2+]Mito compared with K/Mg alone in both the mature and aged male heart and allowed for decreased complex I and complex II mitochondrial oxygen consumption. In the mature and aged female heart, however, the addition of diazoxide to K/Mg cardioplegia had no effect on [Ca2+]Mito compared with K/Mg alone, and both complex I and complex II mitochondrial oxygen consumption were significantly increased compared with mature and aged males, respectively. This increase in [Ca2+]Mito levels, which are significantly increased in the aged compared with the mature heart, would be expected to increase futile calcium cycling and significantly decrease tissue ATP content, as evidenced in our results. These events would further impact negatively on postischemic functional recovery and infarct size as we demonstrated previously [2].
It has been proposed that increased [Ca2+]Mito destabilizes the mitochondrial inner membrane, causing the inner membrane pores to open and thus permitting further movement of cations across the mitochondrial membrane [22]. The opening of the inner membrane pores renders the mitochondrion incapable of synthesizing ATP and has been suggested to be a key event in the process leading to myocardial cell death.
We have suggested that the early opening of the mitoKATP channels would allow for K+ entry into the mitochondria and result in the depolarization of the mitochondrion, which would reduce Ca2+ entry through the mitochondrial calcium uniporter and thus decrease [Ca2+]Mito during global ischemia. Subsequently, these events would result in ATP preservation and reduce the requirement for high-energy resynthesis during reperfusion and reduce energy flux through the electron transport with resultant and reactive oxygen species generation. These events would putatively allow for enhanced postischemic functional recovery and the limitation of infarct size. The inability of diazoxide to decrease [Ca2+]Mito in the mature and aged female heart would increase futile calcium cycling and decrease the high-energy phosphates required for cell maintenance and cell survival. These results are in agreement with our previous studies demonstrating that the addition of diazoxide to cardioplegia is less effective in females compared with males and significantly less effective in the aged female compared with the aged male [2].
The mechanism(s) which differentiate female from male heart response to cardioplegia ± diazoxide remain to be fully elucidated. Our data demonstrate that mitochondrial oxygen consumption is significantly increased in the mature and aged female compared with the male. The reason for these differences is at present unknown; however, we speculate that estradiol may play an important role.
Previous studies have shown that the mechanism of action of 17β-estradiol is modulated by the mitoKATP channels and that the infarct-size-limiting effects of 17β-estradiol are abolished by 5-hydroxydecanoate, a mitoKATP channel-blocker [23-25]. Whether these changes are related to hormonal changes or other factors remains at the level of speculation and more involved studies are required.
It is important to note that our results have been obtained in an isolated perfused heart and require further studies using an in situ blood perfused model. Our data do demonstrate, however, that mitochondrial oxygen consumption and [Ca2+]Mito are modulated by age and gender and are associated with observed age-related and gender-related differences in the response to global ischemia and the cardioprotection afforded by cardioplegia ± diazoxide.
Acknowledgments
This study was supported by the National Institutes of Health (HL29077).
References
- 1.Toyoda Y, Levitsky S, McCully JD. Opening of mitochondrial ATP-sensitive potassium channels enhances cardioplegic protection. Ann Thorac Surg. 2001;71:1281–9. doi: 10.1016/s0003-4975(00)02667-9. [DOI] [PubMed] [Google Scholar]
- 2.McCully JD, Toyoda Y, Wakiyama H, Rousou AJ, Parker RA, Levitsky S. Age and gender related differences in ischemia/reperfusion injury and cardioprotection: effects of diazoxide. Ann Thorac Surg. 2006;82:117–23. doi: 10.1016/j.athoracsur.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterworth J, Prielipp JR, Cerese J, Livingston J, Burnett D. Female gender associates with increased duration of intubation and length of stay after coronary artery surgery. CABG Clinical Benchmarking Database Participants. Anesthesiology. 2000;92:414–24. doi: 10.1097/00000542-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Abramov D, Tamariz MG, Sever JY, et al. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg. 2000;70:800–5. doi: 10.1016/s0003-4975(00)01563-0. [DOI] [PubMed] [Google Scholar]
- 5.Edwards FH, Carey JS, Grover FL, Bero JW, Hartz RS. Impact of gender on coronary bypass operative mortality. Ann Thorac Surg. 1998;66:125–31. doi: 10.1016/s0003-4975(98)00358-0. [DOI] [PubMed] [Google Scholar]
- 6.Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–56. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Boucher F, Tanguy S, Toufektsian MC, Besse S, Tressallet N, Favier A. Age-dependent changes in myocardial susceptibility to zero flow ischemia and reperfusion in isolated perfused rat hearts: relation to antioxidant status. Mech Ageing Dev. 1998;103:301–16. doi: 10.1016/s0047-6374(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 8.Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–81. doi: 10.1161/hc1002.105133. [DOI] [PubMed] [Google Scholar]
- 9.Faulk EA, McCully JD, Tsukube T, Hadlow NC, Krukenkamp IB, Levitsky S. Myocardial mitochondrial calcium accumulation modulates nuclear calcium accumulation and DNA fragmentation. Ann Thorac Surg. 1995;60:338–44. doi: 10.1016/0003-4975(95)00446-r. [DOI] [PubMed] [Google Scholar]
- 10.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Diazoxide and cardioplegia ameliorate ischemia/reperfusion cell death through the modulation of mitochondrial volume and calcium accumulation and mitochondrial respiratory control index. Am J Physiol Heart Circ Physiol. 2004;287:H1967–76. doi: 10.1152/ajpheart.00338.2004. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol. 1996;28:S1–10. doi: 10.1097/00005344-199600003-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–97. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Bak MI, Wei JY, Ingwall JS. Interaction of hypoxia and aging in the heart: analysis of high energy phosphate content. J Mol Cell Cardiol. 1998;30:661–72. doi: 10.1006/jmcc.1997.0633. [DOI] [PubMed] [Google Scholar]
- 14.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemiareperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–89. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 15.Pepe S. Mitochondrial function in ischemia and reperfusion of the aging heart. Clin Exper Pharm Phys. 2000;27:745–50. doi: 10.1046/j.1440-1681.2000.03326.x. [DOI] [PubMed] [Google Scholar]
- 16.Suckow MA, Brammer Dw, Rush HG, Chrisp CE. Biology and diseases of rabbits. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine. 2nd ed. Academic Press; Toronto: 2002. pp. 329–64. [Google Scholar]
- 17.Lowry OH, Passonneao JV. A flexible system of enzymatic analysis. Academic Press; New York, San Francisco, London: 1972. pp. 151–6. [Google Scholar]
- 18.McCully JD, Wakiyama H, Hsieh Y-J, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–35. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 19.Riddell MC, Partington SL, Stupka N, Armstrong D, Rennie C, Tarnopolsky MA. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance trained athletes. Int J Sport Nutr Exerc Metab. 2003;13:407–21. doi: 10.1123/ijsnem.13.4.407. [DOI] [PubMed] [Google Scholar]
- 20.Knechtle B, Muller G, Willmann F, Kotteck K, Eser P, Knecht H. Fat oxidation in men and women endurance athletes in running and cycling. Int J Sports Med. 2004;25:38–44. doi: 10.1055/s-2003-45232. [DOI] [PubMed] [Google Scholar]
- 21.Kochhar S, Jacobs DM, Ramadan Z, Berruex F, Fuerholz A, Fay LB. Probing gender-specific metabolism differences in humans by nuclear magnetic resonance-based metabonomics. Anal Biochem. 2006;352:274–81. doi: 10.1016/j.ab.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Frolkis VV, Frolkis RA, Mkhitarian LS, et al. Contractile function and Ca2+ transport system of myocardium in ageing. Gerontology. 1988;34:64–74. doi: 10.1159/000212932. [DOI] [PubMed] [Google Scholar]
- 23.Hale SL, Birnbaum Y, Kloner RA. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J. 1996;132:258–62. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CH, Su FS, Chou TF, Lee TM. Differential effects of sarcolemmal and mitochondrial channels activated by 17β-estradiol on reperfusion arrhythmias and infarct size in canine hearts. J Pharmacol Exp Ther. 2002;301:234–40. doi: 10.1124/jpet.301.1.234. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto O, Asanuma H, Kim J, et al. A role of opening of mitochondrial ATP-sensitive potassium channels in the infarct size-limiting effect of ischemic preconditioning via activation of protein kinase C in the canine heart. Biochem Biophys Res Commun. 2005;338:1460–6. doi: 10.1016/j.bbrc.2005.10.109. [DOI] [PubMed] [Google Scholar]