Abstract
Phantoms are widely used during the development of new imaging systems and algorithms. For development and optimization of new imaging systems such as tomosynthesis, where conventional image quality metrics may not be applicable, a realistic phantom that can be used across imaging systems is desirable. A novel anthropomorphic lung phantom was developed by plastination of an actual pig lung. The plastinated phantom is characterized and compared with reference to in vivo images of the same tissue prior to plastination using high resolution 3D CT. The phantom is stable over time and preserves the anatomical features and relative locations of the in vivo sample. The volumes for different tissue types in the phantom are comparable to the in vivo counterparts, and CT numbers for different tissue types fall within a clinically useful range. Based on the measured CT numbers, the phantom cardiac tissue experienced a 92% decrease in bulk density and the phantom pulmonary tissue experienced a 78% decrease in bulk density compared to their in vivo counterparts. By-products in the phantom from the room temperature vulcanizing silicone and plastination process are also identified. A second generation phantom, which eliminates most of the by-products, is presented. Such anthropomorphic phantoms can be used to evaluate a wide range of novel imaging systems.
Keywords: phantom, anthropomorphic phantom, lung phantom, plastinated phantom
INTRODUCTION
Phantoms are used extensively during imaging system development and optimization to provide a representation of the real and complex imaging task for which the system will be used. One category of such imaging system analysis is the measurement of observer-independent metrics, such as the MTF and the DQE. For such measurements, simplified phantoms with geometric components that are known exactly a priori are more desirable.1 Another category of imaging system analysis is the measurement of metrics based on the real and complex imaging task of interest, e.g., task-based observer studies. In such system analysis, a more complex phantom that closely represents the in vivo anatomy, such as the anthropomorphic phantoms introduced in Refs. 2, 3, 4, 5, 6, 7, 8, is desired to minimize the transitional gap from phantom studies to clinical studies caused by an overly simplified phantom.
According to Ref. 4, anthropomorphic (radiotherapy) phantoms have been commercially available since the 1960s with the introduction of the Alderson Research Laboratories “RANDO” phantom. With respect to anthropomorphic chest phantoms, the majority of the commercially available phantoms is targeted to represent the most widely used application, chest radiography. For new chest imaging system developments, such as digital tomosynthesis, a more anatomically accurate and complex phantom could potentially be of use.
To meet these requirements, a novel anthropomorphic lung phantom was developed by plastination of an actual pig lung from a 42 kg pig. Plastination, invented in 1978 by Von Hagens9 as a unique preservation method for biological tissue, was chosen as an aid to develop the phantom. Plastination is the replacement of tissue fluid and some lipid with a curable polymer. The steps of plastination are specimen preparation, dehydration, impregnation, and curing. Impregnation is the replacement of a volatile solvent (the dehydrant in this instance) with a curable polymer (silicone in this instance).9, 10 Hence, a biological specimen is transformed from biodegradable to eternal preservation. Therefore, plastination process yields stable phantom when compared with fresh or formalin fixed phantoms,11 ensuring repeatability and reproducibility.
An analysis of the plastinated anthropomorphic phantom is provided by comparing its anatomical features and the imaging characteristics to its in vivo counterpart. High resolution 3D CT reconstructions of the in vivo pig lungs and of the plastinated phantom were prepared and used. In Sec. 2, the in vivo and postplastination CT data acquisition parameters and the anthropomorphic phantom plastination process are described. Also described are the three comparison metrics used for the analysis: anatomical features, volume estimates, and CT numbers. In Sec. 3, the in vivo and the phantom reconstructions are compared using the three metrics introduced. A second generation phantom is also presented that is free of most of the by-products identified in the first generation phantom, therefore providing an accurate representation of the in vivo anatomical structures and complexity. In Sec. 4, the room temperature vulcanizing (RTV) silicone and plastination process by-products in the phantom are identified and difficulties in segmentation are discussed.
MATERIALS AND METHODS
A three-step process was needed in order to compare the in vivo pig’s lungs and heart with the plastinated phantom of the same organs. First, the pig’s thorax was scanned in vivo on a clinical CT system. Next, the lungs and the heart were harvested from the pig. After cleaning of extraneous mediastinal tissue from the heart and lungs, the specimen was plastinated to generate a phantom using the novel method described in detail below. Finally, the plastinated phantom was scanned on a clinical CT system. The phantom reconstructions were then compared with the in vivo reconstructions.
In vivo acquisition
A 42 kg female Yorkshire pig was obtained through our lab animal facility’s procurement office from a California vendor. After 1 week acclimation, the animal was fasted 18 h prior to the study. The pig was administered a single intramuscular injection of Telazol® for sedation and Atropine, an anticholinergic. After a supplemental dose of Isoflurane inhalant gas via face mask, the pig was intubated then maintained on Isoflurane for general anesthesia for the duration of the study. Monitoring and vitals were recorded by a licensed veterinary nurse every 15–20 min including: blood gas levels, heart rate, respiration rate, body temperature, oxygen saturation, and activated clotting times. Eight French sheaths were placed percutaneously in both the femoral artery and vein for blood sampling, wire∕catheter placement, and contrast delivery.
The pig’s thorax was scanned in vivo at a known partial pressure (end-inspiration and end-expiration) on a multidetector clinical CT system (Siemens Sensation 16, Siemens Medical Solutions, Forchheim, Germany, 120 kVp, 100 mA s). The acquired projection images were reconstructed to a 3D volume using the lung kernel (B60f) at an in-plane resolution of 0.54 mm×0.54 mm and 0.75 mm slice thickness with two adjacent slices having an overlap of 0.35 mm. In anticipation of better visualization of the vascular structures, intra-arterial contrast agent (Conray 282 mg∕mL, Mallinckrodt, Inc., St. Louis, MO) enhanced in vivo data were acquired and reconstructed to a 3D volume using the soft tissue kernel (B30f) at an in-plane resolution of 0.82 mm×0.82 mm and 0.75 mm slice thickness with two adjacent slices having an overlap of 0.35 mm.
At the end of the study, the animal was euthanized with an intravenous injection of Beuthanasia solution. The animal’s husbandry, care, and well-being was provided by the veterinary staff and technicians and followed the Institutional Animal Care and Use Committee approved protocol, as well as university, state, and federal policies.
Plastination
After harvesting the viscera from the pig’s thoracic cavity, the viscera were processed using the classic four-step phantom plastination procedure. Initially, one phantom was made from the in vivo scanned heart and lungs.
Specimen preparation
After the lungs, heart, and great vessels were extracted, the lungs were inflated and fixed by an intratracheal perfusion of 10% formalin. The formalin fixative incorporates into the cell membranes and molecular structure, making the specimen firm and minimizing shrinkage. The fixing process denatures any tissue enzymes which may remain active after dehydration and plastination and cause tissue decomposition or interfere with polymerization after impregnation of the polymer. The pulmonary arteries and veins and the atria and ventricles were filled with RTV silicone. Only short remnants of the aorta and the venae cavae remained with the specimen block. After hardening overnight, the prepared specimen was rinsed with running tap water before being dehydrated.
Dehydration
The dehydration step replaces the tissue fluid and some lipid with an organic solvent (acetone) which is miscible with water.10 The specimen was submerged in acetone for 3 weeks. The acetone bath was monitored weekly for purity and changed weekly to thoroughly dehydrate the cardiac and pulmonary tissue (i.e., all tissue fluid replaced with acetone), including the heart and vessel walls.
Impregnation
The impregnation of a biological specimen with a curable polymer is based on the difference in the boiling point of the volatile solvent (acetone) and the polymer mix.10 Generally, the solvent within the specimen is “boiled away” as pressure is decreased, leaving a tissue void such that the polymer mix is drawn into the tissue (cells and interstitium). The fully dehydrated specimen was submerged in liquid silicone polymer mix and impregnated with the silicone mix by slowly decreasing the pressure over an extended period of about 6 weeks. After the impregnation was complete, low pressure air was connected to the trachea to inflate the lungs and empty the airways of the liquid silicone.
Curing (polymerization or hardening)
Once a specimen is impregnated with liquid silicone, the polymer should remain in the specimen, and this is accomplished by curing the polymer. After all excess polymer had been removed from the specimen’s extracellular and airways, a cross-linking hardening chemical was vaporized in and around the lungs to harden the plastination silicone.
Plastinated phantom image acquisition
The plastinated phantom was scanned on a multidetector clinical CT system (Siemens Sensation 64, Siemens Medical Solutions, Forchheim, Germany) with acquisition parameters of 120 kVp and 200 mA s. The acquired projection images were reconstructed to a 3D volume using the lung kernel (B60f) and the soft tissue kernel (B30f) at an in-plane resolution of approximately 0.43 mm×0.43 mm and 0.75 mm slice thickness with two adjacent slices having an overlap of 0.45 mm
Comparison metrics
The 3D CT reconstructions of the in vivo anatomy and the plastinated phantom were compared using three different metrics.
First, the geometric accuracy of the plastinated phantom was examined since the in vivo anatomy went through a complex plastination process. The anatomical features were visually compared on a slice-by-slice basis over a multitude of locations within the two 3D reconstructions using the same display window and level. Original data as well as segmented data (using GE Advantage Windows Volume Viewer, version 4.2_07) were used in the comparisons. Appropriate voxel size compensations were applied for the slice displays since the two 3D reconstructions had different voxel sizes. This enabled physical dimension measurements and comparisons of the in vivo and the plastinated phantom anatomical features. Qualitative assessment of the preserved anatomical features as well as the discrepancies arising from the complex plastination process were possible through the comparisons.
Second, quantitative volume estimates of different anatomical features in the two 3D reconstructions, such as the pulmonary tissue and the airways, were computed and compared. The phantom volume estimates were compared to the in vivo volume estimates at both inspiration and expiration. Volume estimates of the heart and the vessels in the contrast-enhanced in vivo reconstruction were computed. The volumes were computed using a threshold-based segmentation algorithm and a magnitude-based region-growing algorithm (GE Advantage Windows Volume Viewer, version 4.2_07). The segmented volume generated by the algorithm depended on the chosen thresholds (applied globally to the volume) and the region-growing tolerance and sensitivity (applied locally within the volume). The two parameters were iteratively controlled throughout the segmentation process to minimize erroneous inclusions of known unwanted regions in the final segmentation. Finally, 3D volume visualizations of the segmented anatomy were generated for comparison.
Third, CT numbers were measured at ROIs within different anatomical features, such as the airway and tissue, of the in vivo and the phantom reconstructions. Multiple 3D ROIs within the pulmonary tissue, airway, and cardiac tissue in the phantom and the in vivo reconstructions were chosen such that: (1) the number of voxels in each of the ROI ranged from at least 1000 to as high as 760000, (2) each ROI had as homogeneous as possible distribution of the constituent material, and (3) the ROIs were nonoverlapping and spread out over a wide range of valid regions. The CT number mean and standard deviation for each ROI were computed. Computed values were compared between the phantom and the in vivo reconstructions, as well as between anatomical structures.
Second generation phantom
Subsequent phantoms were created following the same four-step plastination procedure to try and improve phantom quality. Whereas the analyzed phantom experienced a transit time from one site to the next between the harvesting and the plastination, the subsequent phantoms did not experience such gap in time, resulting in a better controlled environment for the plastination procedure. These phantoms were imaged using the same acquisition parameters as the first analyzed phantom.
RESULTS
Anatomical features
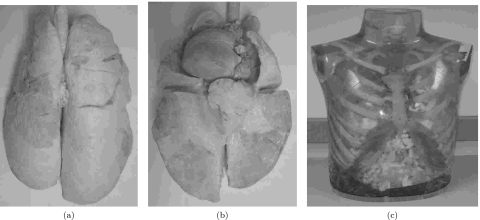
A stable plastinated phantom was produced which maintained the anatomical relationship of the original in vivo lung [Figs. 1a, 1b]. Typical coronal and axial slices of the phantom and of the segmented in vivo reconstructions are demonstrated (Fig. 2). The coronal slices were chosen where the main airway bifurcation occurs. Displayed on an one-to-one scale, the phantom lung is observed to be larger in size than the in vivo lung. In terms of the finer details, an ideal one-to-one correspondence between the two volumes is not readily achieved, but the relative in vivo anatomical features, such as the airways and the vessels, are well preserved in the phantom.
Figure 1.
Plastinated anthropomorphic lung phantom external: (a) dorsal∕posterior view and (b) ventral∕anterior view. (c) Sample chest cavity with ribs that can be used in conjunction with the phantom to generate more realistic data. (The phantom and the chest cavity are not shown to scale.)
Figure 2.
Plastinated anthropomorphic lung phantom: (a) coronal slice and (c) axial slice. Segmented in vivo lung: (b) coronal slice and (d) axial slice. The dotted lines in the coronal slices indicate the location of the axial slices, and vice-versa. All four images are shown at the same scale, window, and level.
3D visualizations of the airways (visualized by air contained within the airways) and vessels segmented from the phantom and the in vivo reconstructions are demonstrated for comparison. Airway visualizations of the phantom reconstruction [Fig. 3a] and the in vivo reconstruction [Fig. 4a] show the fine anatomical details in both. The orientation, location, and the shape of the airway branches in the phantom matches well with the in vivo airway scans. After the phantom reconstruction [Fig. 3b] and the contrast-enhanced in vivo reconstruction [Fig. 4b], vessels also show similarities, but at the same time, noticeable discrepancies exist. The in vivo pulmonary arteries, aorta, and caudal∕inferior vena cava are visualized. The length of the pulmonary arteries and the short stump of the aorta are identified in the phantom only. The caudal∕inferior vena cava is missing in the phantom, which is visible in the contrast-enhanced in vivo data. Visualization of the airway and the vessels in the phantom lung [Fig. 3c] shows a detailed preservation of the two in vivo anatomical features.
Figure 3.
3D visualizations of the segmented anatomical features in the phantom CT data. (a) Ventral∕anterior and left lateral views of the airway (open volume of the airways). (b) Ventral∕anterior and left lateral views of the heart and vessels. (c) Ventral∕anterior view of the airway (open volume of the airways) and the vessels.
Figure 4.
3D visualizations of the segmented anatomical features in the in vivo inspiration CT data. (a) Ventral∕anterior and left lateral views of the airway (open volume of the airways). (b) Ventral∕anterior and left lateral views of the heart and vessels in the contrast-enhanced CT data. The dotted circles indicate the vascular structures missing in the phantom. (c) Outer hull of the segmented pulmonary tissue. The empty region in the middle is where the heart is located in the in vivo CT data.
For the in vivo lung, the chest cavity and diaphragm constraints defined the maximum limits of the lung. Without the same constraints, the phantom lung parenchyma exhibits a different shape from the in vivo parenchyma [Figs. 25]. The phantom lung parenchyma extends vertically (in the z direction) up to 274 mm; it extends horizontally as much as 190 mm [Fig. 5a]. On the other hand, the in vivo lung parenchyma extends vertically up to 168 mm near the diaphragm and approximately 202 mm along the periphery near the ribs [Fig. 5c]. The in vivo lung parenchyma extends horizontally as much as 206 mm. The diaphragm confines the in vivo lung parenchyma resulting in compressed pulmonary tissue, especially near the diaphragm, corresponding to smaller lung volume and higher CT numbers. As for the phantom heart, it was observed to be smaller in size when compared to the in vivo heart. Finally, the phantom heart exhibited a horizontally elliptical shape [Fig. 5b]; whereas, the in vivo heart exhibited a vertically elliptical shape [Fig. 5d].
Figure 5.
Phantom: (a) coronal slice of the lung parenchyma maximum vertical extent, (b) coronal slice where the heart has the largest area. In vivo: (c) coronal slice of the lung parenchyma maximum vertical extent, (d) coronal slice where the heart has the largest area. All images shown at the same scale. The display window and level for (a) and (b) are equal as are the window and level for (c) and (d).
Volume estimates
Table 1 shows the estimated volumes of the different anatomical features in the phantom and in the in vivo reconstructions. The volume of the phantom pulmonary tissue ranges from 1.06 to 1.50 times that of the in vivo inspiration pulmonary tissue depending on the segmentation method. The in vivo inspiration pulmonary tissue volume is more than twice the in vivo expiration pulmonary tissue volume. Due to difficulties in the phantom pulmonary tissue segmentation, only the 3D visualization of the segmented in vivo pulmonary tissue at inspiration is shown [Fig. 4c].
Table 1.
Volume estimates (cm3) of the pulmonary tissue, air within the airways, and pulmonary vessels and heart.
| Phantom | In vivo | ||
|---|---|---|---|
| Inspiration | Expiration | ||
| Pulmonary tissue | 1700–2400a | 1600 | 700 |
| Air within the airways | 40 | 60 | 40 |
| Pulmonary vessels and heart | 350b | 500 | Inconclusive |
Range of estimates dependent on the segmentation parameters.
Incomplete as a result of the RTV curing process and air pockets∕bubbles inside the heart.
The estimated volume of the phantom airways (air within the airways) was 2∕3 that of the in vivo airways (air within the airways) at inspiration, but equal to the volume of the in vivo airways (air within the airways) at expiration. The volume difference between the phantom and the in vivo inspiration airways could be explained by the thicker in vivo airways visible in the 3D visualizations. The estimated volume of the in vivo reconstruction at inspiration is 1.5 times that at expiration.
The estimated volume of the phantom vessels∕heart was smaller than that of the in vivo inspiration vessels∕heart. Estimated volume using the contrast-enhanced reconstruction was not computed because of ambiguities when attempting to segment nonvascular tissue from contrast-filled vessels.
CT numbers
The phantom cardiac tissue mean CT number (162 HU) is approximately 126 HU higher than the in vivo cardiac tissue mean CT numbers at inspiration and expiration [Fig. 6a]. On the other hand, the in vivo cardiac tissue mean CT numbers at inspiration (35 HU) and expiration (37 HU) are different by 2 HU. The phantom cardiac tissue ROI CT number variance is smaller than in vivo (inspiration and expiration) cardiac tissue ROI CT number variance.
Figure 6.
Mean (◼) and the ±1 standard deviation (vertical bar) of CT numbers at different ROIs within the cardiac tissue and the pulmonary tissue of the phantom and the in vivo inspiration and expiration. (a) Cardiac tissue and (b) Pulmonary tissue.
The phantom pulmonary tissue mean CT number (−844 HU) is 17 HU lower than the in vivo inspiration pulmonary mean CT number (−827 HU) [Fig. 6b]. The in vivo expiration pulmonary tissue mean CT number (−640 HU) is approximately 187 HU higher than the in vivo inspiration pulmonary tissue mean CT number (Table 2). The in vivo expiration mean CT numbers are spread over a large range depending on the ROI, whereas the phantom and the in vivo inspiration mean CT numbers are distributed over a limited range. The CT number difference between the phantom cardiac and pulmonary tissue (Δp=1006 HU) is 144 HU larger than the CT number difference between the in vivo inspiration cardiac and pulmonary tissue (Δi=862 HU).
Table 2.
Cardiac and pulmonary tissue mean CT numbers and corresponding linear attenuation coefficients.
| Mean CT number (HU) | Linear attenuation coefficienta (cm−1) | ||
|---|---|---|---|
| Phantom | 162 | 0.2637 | |
| Cardiac tissue | In vivo inspiration | 35 | 0.2348 |
| In vivo expiration | 37 | 0.2353 | |
| Phantom | −844 | 0.0354 | |
| Pulmonary tissue | In vivo inspiration | −827 | 0.0393 |
| In vivo expiration | −640 | 0.0817 |
Using Eq. A3 in the Appendix.
Although not shown, the phantom air within the airway mean CT number (−971 HU) and the in vivo inspiration air within the airway mean CT number (−972 HU) are different by only 1 HU, as expected.
Finally, simulated projections of the phantom [Fig. 7a] and of the segmented in vivo anatomy [Fig. 7b] were demonstrated. For better comparison, the segmented in vivo reconstruction had the bones and body tissue removed to match the phantom reconstruction. Anatomical complexity visible in the projection of the phantom closely resembles the complexity in the projection of the in vivo reconstruction, although it is not an exact one-to-one correspondence. The arrows in the figure indicate pulmonary tissue regions where the anatomical complexity show resemblance such as the branching of vessels. The simulated projections also confirm the elongated phantom lung parenchyma and the different heart orientations between the phantom and the in vivo heart.
Figure 7.
(a) Simulated projection of the phantom. (b) Simulated projection of the segmented in vivo inspiration data. The images are displayed in identical scale. The display windows and levels were adjusted so that the anatomical complexity of the lung can be seen. (c) Simulated projection of the second generation phantom. (d) Simulated projection of the second generation phantom shown in different window and level to enhance the visualization of the anatomical features inside the lung parenchyma.
A second generation phantom manufactured with a tighter control over the plastination procedure are nearly free of the RTV silicone and plastination by-products discussed in Sec. 4. Sample coronal and axial slices of a second generation phantom are shown [Figs. 8a, 8b, 8c]. Few of the by-products still exist in the second generation phantom, such as partial filling of the vessels. A simulated projection of the second generation phantom is shown [Figs. 7c, 7d] demonstrating the anatomical complexity visible in projection images.
Figure 8.
Second generation phantom: (a) coronal slice, (b) axial slice, and (c) axial slice. The dotted lines indicate the slice locations with respect to each other. (d) First generation phantom axial slice with the RTV and plastination process by-products indicated: (1) air pockets, (2) dense pulmonary tissue, (3) unfilled vessels, (4) partially filled vessel, and (5) collapsed airway.
DISCUSSION
One major benefit of the plastinated phantom is its stability compared with that of fresh or formalin fixed tissues because of the construction process ensuring reproducibility of imaging studies. In addition to its stability, the plastinated phantom maintains the anatomical complexity of the original in vivo anatomy. The complex anatomical preservation has been evaluated through the comparisons presented between the in vivo and the plastinated lung from the same animal. At the same time, some variations from the in vivo reconstruction have been identified upon detailed inspection. The majority of the variations can be attributed to the normal by-products of the RTV silicone and plastination processes.
The sample phantom axial slice [Fig. 8d] demonstrates the variations. Air pockets exist at numerous locations within the lung parenchyma. Dense pulmonary tissue, mostly at the periphery of the lung parenchyma, which may have been caused by incomplete emptying of the alveoli before curing, was observed to exhibit higher CT numbers than the surrounding pulmonary tissue. The phantom vessel thickness is thinner than the in vivo vessel thickness due to incomplete filling of the vessels with RTV silicone and shrinkage due to dehydration. Additionally, unfilled vessels in the phantom are evident from the single set of main vessel branch in the phantom [Fig. 3b] compared to two sets of main vessel branches in the in vivo reconstruction [Fig. 4b]. Air bubbles, by-products of the RTV silicone curing process, were visible. These RTV silicone curing process by-products are the cause of the lower volume estimate compared to the in vivo reconstruction (Table 1). The phantom airways (air within the airways) have less detailed structures at the periphery and their thickness are thinner than the in vivo airway [Figs. 4a, 3a]. This is likely due to the shrinkage∕consolidation of cartilage during dehydration.
The phantom pulmonary tissue segmentation was complicated by the lack of contrasting material outside the phantom and by the plastination by-products, i.e., air pockets and dense pulmonary tissue regions, resulting in a range of volume estimates. A region-growing segmentation algorithm with tight control parameters resulted in a 6% larger volume estimate than the in vivo volume estimate; an algorithm with overly relaxed control parameters provided an upper bound for the phantom pulmonary tissue (Table 1). In contrast, the in vivo (inspiration and expiration) pulmonary tissue volumes were easier to segment and resulted in more stable volume estimates. For example, the difference in volume estimates between the in vivo inspiration and the expiration volume estimates is as expected.
Mean CT numbers of the airways (air within the airways) inside the phantom and the in vivo lung parenchyma were measured as reference values. The phantom airway mean CT number (−971 HU) was equivalent to the in vivo inspiration airway mean CT numbers (−972 HU), as expected. The outlier in the in vivo inspiration pulmonary tissue mean CT numbers [Fig. 6b] corresponds to the lung parenchyma near the diaphragm exhibiting compressed tissue. The wide distribution of the in vivo expiration pulmonary tissue mean CT numbers [Fig. 6b] can be attributed to the large variation of tissue compression at different ROIs. The in vivo expiration pulmonary tissue mean CT number (−640 HU) is higher than the in vivo inspiration pulmonary tissue mean CT number (−827 HU) as expected, reflecting the pulmonary tissue compression at expiration.
To analyze the difference in the mean CT numbers between the in vivo and the phantom tissues, mass attenuation coefficients (MACs) for the four different tissue types (in vivo cardiac tissue, in vivo pulmonary tissue, phantom cardiac tissue, and phantom pulmonary tissue) were investigated. To compute the MACs, a two-element mixture model was assumed. Each in vivo tissue type was modeled as mixtures of water and air, and each phantom tissue type was modeled as a mixture of plastination silicone and air. Therefore, the MAC for each tissue type can be expressed as a weighted sum of water MAC and air MAC (for the in vivo tissue) or plastination silicone MAC and air MAC (for the phantom tissue). We referenced the in vivo cardiac∕pulmonary tissue densities, water MAC, and air MAC from the literature. We used the referenced values to compute the fractional weights of water and air for the in vivo cardiac and pulmonary tissues such that the resulting MACs would match the observed mean CT numbers. Details are provided in the Appendix, and only the results are stated here.
Assuming the water and air mixture model, the in vivo cardiac tissue is comprised of 83% water and 17% air, whereas the in vivo pulmonary tissue is comprised of 60% water and 40% air. For comparison with the phantom cardiac and pulmonary tissues, we assumed a complete replacement of water by plastination silicone after plastination. In other words, the phantom cardiac tissue is assumed to be comprised of 83% plastination silicone and 17% air; the phantom pulmonary tissue is assumed to be comprised of 60% plastination silicone and 40% air. Therefore, plastination silicone MAC is needed to compute the phantom tissue MACs using the plastination silicone and air mixture model. We computed the plastination silicone MAC by measuring the CT number of a pure plastination sample and using the density value provided by the plastination silicone manufacturer.
After having computed the phantom cardiac and pulmonary tissue MACs using the two-element mixture model, the bulk (or packing) densities were estimated from the relationship between the MAC and the linear attenuation coefficient (LAC) of a material, i.e., (LAC)=(MAC)×(bulk density). The estimated bulk densities of the phantom cardiac and pulmonary tissues were compared with those of the in vivo cardiac and pulmonary tissues (Table 3). The phantom cardiac tissue bulk density is 92% of the in vivo cardiac tissue bulk density, and the phantom pulmonary tissue bulk density is 78% of the in vivo pulmonary tissue bulk density. In other words, for the same mass of plastination silicone and water, phantom cardiac tissue occupies a 1.09 times larger volume than the in vivo cardiac tissue. On the other hand, for the same mass of plastination silicone and water, phantom pulmonary tissue occupies a 1.28 times larger volume than the in vivo pulmonary tissue. The median value, 2050 cm3, for the range of phantom pulmonary tissue volume measurements (Table 1) matches well with the increased volume, 1.28×1600=2048 cm3, of the hypothesized in vivo pulmonary tissue volume after plastination. This comparison indicates an overinflation of the phantom pulmonary tissue, which has gone through an inflation step during the plastination process.
Table 3.
In vivo and phantom cardiac and pulmonary tissue bulk densities.
CONCLUSION
A novel anthropomorphic lung phantom plastinated from a pig with the vascular system filled with RTV silicone followed by plastination was developed which is stable when compared with fresh or formalin fixed phantoms. This will ensure repeatable and reproducible acquisition of images across various x-ray imaging modalities. High resolution 3D CT reconstructions from the in vivo pig lungs and from the plastinated phantom were prepared. The anatomical features and the imaging characteristics of the plastinated phantom and in vivo lungs were compared. Using volume estimates, 3D visualizations of the anatomical features, and simulated projections, the preservation of the in vivo anatomical features∕locations in the phantom was confirmed. Different anatomical structures within the two reconstructions exhibit the expected range of CT numbers corresponding to the constituent material. The phantom and the in vivo reconstructions do not exhibit an exact one-to-one correspondence in shape since the phantom was constructed without the constraint of the chest cavity.
RTV silicone and plastination process by-products in the plastinated phantom, such as dense peripheral pulmonary tissue, collapsed airways, air pockets inside the lung and heart, and missing∕partial vessel filling, were identified. Nonmatching CT number differences between the cardiac and pulmonary tissues for the phantom and the in vivo data have been identified. By assuming a two-element mixture model, different fractional weights of water and air for the in vivo cardiac and pulmonary tissues have been presented; 83% water for the cardiac tissue and 60% water for the pulmonary tissue. Assuming that the fractional weights are preserved in the phantom tissue, the reason behind the observed mean CT numbers for the phantom cardiac and pulmonary tissues has been explained in terms of the bulk density differences (from the in vivo counterparts). The differences in bulk densities correspond to a 1.09 times larger volume per mass for the phantom cardiac tissue and a 1.28 times larger volume per mass for the phantom pulmonary tissue. The larger increase in volume (or smaller density) of the phantom pulmonary tissue can be attributed to the inflation step in the plastination process. A second generation phantom has been constructed which suffers from almost none of the by-products observed in the previous phantom. These novel anthropomorphic phantoms are accurate representations of the in vivo anatomical structures and complexity.
Based on our analysis, further fine adjustments can be made in future phantom constructions to incorporate the subtle differences in the imaging characteristics of the in vivo anatomy. Further plastination improvement include better controlled inflation of the lung and construction of the phantom inside a chest cavity-like structure. These anthropomorphic plastinated phantoms enable the research and development of novel imaging systems by providing a repeatable and stable phantom that accurately represents the real anatomy with comparable complexity.
ACKNOWLEDGMENTS
The authors thank Laura Pierce (3D Laboratory, Stanford University) for assistance with segmentation. This work was supported in part by The Whitaker Foundation, the Baxter Foundation, NIH NIBIB Grant No. EB002352, and the Lucas Foundation.
APPENDIX: TWO-ELEMENT MIXTURE MODEL ANALYSIS
A simple two elemental mixture model for the MACs of the in vivo cardiac, in vivo pulmonary, plastinated cardiac, plastinated pulmonary tissues is used for analysis. The MAC for each in vivo tissue type is modeled as a two elemental mixture: mixture of water and air. The mixture model can be expressed as
| (A1) |
| (A2) |
where (μ∕ρ)IC is the in vivo cardiac tissue MAC, (μ∕ρ)IP is the in vivo pulmonary tissue MAC, (μ∕ρ)w is the water MAC, (μ∕ρ)a is the air MAC, w1 is the fractional weight of water MAC for the in vivo cardiac tissue, and w2 is the fractional weight of water MAC for the in vivo pulmonary tissue.
The in vivo cardiac and pulmonary tissue MACs can be computed from the measured LACs and densities. From the definition of a CT number, LAC can be found using
| (A3) |
where μ is the LAC, HU is the measured CT number, and μw is the water LAC. Because the measured mean CT number for the in vivo cardiac tissue was 35 HU, the in vivo cardiac tissue LAC equals 0.2348 cm−1. Similarly, because the measured mean CT number for the in vivo pulmonary tissue was −827 HU, the in vivo pulmonary tissue LAC equals 0.0393 cm−1. In order to find the MAC for each of the two tissue types, in vivo cardiac and pulmonary tissue densities are needed. The in vivo cardiac tissue bulk density was assumed to be the same as skeletal muscle bulk density, ρIC=1.050 g∕cm3, found in Ref. 12. The in vivo pulmonary tissue bulk density was assumed to be the median value for the average bulk density at inspiration, ρIP=0.179 g∕cm3, found in Ref. 13. The resulting MAC values for the in vivo cardiac and pulmonary tissue were (μ∕ρ)IC=0.2237 cm2∕g and (μ∕ρ)IP=0.2193 cm2∕g (Table 4).
Table 4.
Referenced MAC and density values.
| Value | Reference | |
|---|---|---|
| In vivo cardiac tissue bulk density | ρIC=1.050 g∕cm3 | 12 |
| In vivo inspiration pulmonary tissue bulk density | ρIP=0.179 g∕cm3 | 13 |
| Water MAC @ 50 keV mean photon energy | (μ∕ρ)w=0.2269 cm2∕g | 12 |
| Air MAC @ 50 keV mean photon energy | (μ∕ρ)a=0.2080 cm2∕g | 12 |
| Water density | ρw=1.00 g∕cm3 | 12 |
| Plastination silicone density | ρs=0.95 g∕cm3 | Manufacturer data sheet |
Substituting (μ∕ρ)IC, (μ∕ρ)IP, (μ∕ρ)w, and (μ∕ρ)a into Eqs. A1, A2, we can find the fractional weights of water, w1 and w2. The values for (μ∕ρ)w and (μ∕ρ)a were referenced from Ref. 12, and because the CT data were acquired at 120 kVp with a typical filtration, we assumed a mean photon energy of 50 keV. At 50 keV, the MAC values from Ref. 12 were (μ∕ρ)w=0.2269 cm2∕g and (μ∕ρ)a=0.2080 cm2∕g. Therefore, the fractional weights of water for the in vivo cardiac and pulmonary tissue are w1=0.828 and w2=0.598, respectively. Under the water and air mixture model, the in vivo cardiac tissue is comprised of 83% water and 17% air, whereas, the in vivo pulmonary tissue is comprised of 60% water and 40% air.
To compare the in vivo tissue measurements to the phantom tissue measurements, we use the fact that water in the in vivo tissues is replaced by the plastination silicone during the plastination process. Assuming a complete replacement of water with plastination silicone after plastination, the plastinated cardiac and pulmonary tissue MACs can be expressed as
| (A4) |
| (A5) |
where (μ∕ρ)PC is the plastinated cardiac tissue MAC, (μ∕ρ)PP is the plastinated pulmonary tissue MAC, and (μ∕ρ)s is the plastination silicone MAC. Because a complete replacement of water with plastination silicone was assumed, the fractional weights, w1=0.828 and w2=0.598, are unchanged for the phantom tissues. In order to compute the plastination silicone MAC, plastination silicone LAC and its density are needed. Plastination silicone LAC (0.2721 cm−1) was computed from measured mean CT number of pure plastination silicone samples (199 HU), and its density (ρs=0.95 g∕cm3) was found in the data sheet provided by the plastination silicone manufacturer. Substituting (μ∕ρ)s=0.2864 cm2∕g, w1=0.828, and w2=0.598 into Eqs. A4, A5, we get (μ∕ρ)PC=0.2729 cm2∕g and (μ∕ρ)PP=0.2548 cm2∕g.
The measured mean CT numbers for the phantom cardiac and pulmonary tissue were 162 and −844 HU, respectively. Using Eq. A3, the phantom cardiac tissue LAC equals 0.2637 cm−1 and the phantom pulmonary tissue LAC equals 0.0354 cm−1. We use the relationship between LAC and MAC, i.e., μ=(μ∕ρ)×ρ, to infer the bulk (or packing) densities of the two tissue types in the phantom. Therefore, the computed bulk density of the phantom cardiac tissue is ρPC=0.966 g∕cm3 to match the measured CT number 162 HU; the computed bulk density of the phantom pulmonary tissue is ρPP=0.139 g∕cm3 to match the measured CT number −844 HU. When compared to the in vivo densities (Table 4), ρIC and ρIP, the phantom cardiac tissue bulk density is 92% of the in vivo bulk density, and the phantom pulmonary tissue bulk density is 78% of the in vivo bulk density. In other words, for the same mass of material, phantom cardiac tissue occupies a 1.09 times larger volume than the in vivo cardiac tissue. On the other hand, for the same mass of material, phantom pulmonary tissue occupies a 1.28 times larger volume than the in vivo pulmonary tissue. This comparison indicates an overinflation of the phantom pulmonary tissue, which has gone through an inflation step during the plastination process.
We have so far assumed a complete replacement of water with plastination silicone after the plastination process. If we assume an incomplete replacement of water with plastination silicone and assume that the portion not replaced by plastination silicone is replaced with air, the fractional weights w1 and w2 are altered. For example, if only 0.9 of water is replaced by plastination silicone in the cardiac and the pulmonary tissues, then the altered fractional weights become and . The resulting MACs for the phantom cardiac and pulmonary tissues are and . To exhibit the measured CT numbers for the phantom cardiac and pulmonary tissues, the densities for the phantom cardiac and pulmonary tissue have to be and , respectively.
Phantom cardiac tissue MAC was computed from experimentally measured mass and volume to determine the amount of incomplete water replacement in the analyzed phantom. A sample of the phantom cardiac tissue was cutoff from the phantom heart, and its mass and volume were measured. The mass was measured to be 0.229 g, using an analytical balance. The volume was measured to be approximately 0.24 cm3, using a piston-driven air displacement pipette. Because the graduation of the pipette was limited to 0.01 cm3 and a larger phantom cardiac tissue sample could not be taken, the computed bulk density, , is sensitive to the small changes in the volume. For example, if the volume is assumed to equal 0.235 cm3, which is at the limit of the error margin, the computed bulk density is .
Given the measurement precision limitation, a fractional range from 0.96 to 1.00 of water in the in vivo cardiac tissue can be assumed to have been replaced by plastination silicone. Therefore, we can infer that at least 0.96 fraction of water has been replaced by plastination silicone even when considering the margin of error in the volume measurement. In such as case, the phantom cardiac tissue bulk density is 93% of the in vivo bulk density. Assuming that the 0.96 fractional replacement of water also holds true for the phantom pulmonary tissue, the phantom pulmonary tissue bulk density is 78% of the in vivo bulk density. Even if the margin of error is taken into account, the density ratios between in vivo and phantom tissues, i.e., ∼92% for the cardiac tissue and 78% for the pulmonary tissue, do not change much. Therefore, experimentally measured phantom cardiac tissue MAC confirms the overinflation of the phantom pulmonary tissue during the plastination process.
References
- Baydush A. H., Ghem W. C., and C. E.Floyd, Jr., “Anthropomorphic versus geometric chest phantoms: A comparison of scatter properties,” Med. Phys. 10.1118/1.598954 27(5), 894–897 (2000). [DOI] [PubMed] [Google Scholar]
- Madsen E. L., Zagzebski J. A., and Ghilardi-Netto T., “An anthropomorphic torso section phantom for ultrasonic imaging,” Med. Phys. 10.1118/1.594657 7(1), 43–50 (1980). [DOI] [PubMed] [Google Scholar]
- Caldwell C. B. and Yaffe M. J., “Development of an anthropomorphic breast phantom,” Med. Phys. 10.1118/1.596506 17(2), 273–280 (1990). [DOI] [PubMed] [Google Scholar]
- Kleck J. H., Smathers J. B., Holly F. E., and Myers L. T., “Anthropomorphic radiation therapy phantoms: A quantitative assessment of tissue substitutes,” Med. Phys. 10.1118/1.596552 17(5), 800–806 (1990). [DOI] [PubMed] [Google Scholar]
- Muramatsu Y., Tsuda Y., Nakamura Y., Kubo M., Takayama T., and Hanai K., “The development and use of a chest phantom for optimizing scanning techniques on a variety of low-dose helical computed tomography devices,” J. Comput. Assist. Tomogr. 10.1097/00004728-200305000-00012 27(3), 364–374 (2003). [DOI] [PubMed] [Google Scholar]
- Bencomo J. A., Chu C., Tello V. M., Cho S. H., and Ibbott G. S., “Anthropomorphic breast phantoms for quality assurance and dose verification,” J. Appl. Clin. Med. Phys. 5(1), 36–49 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarot C. B., Siewerdsen J. H., Haycocks T., Moseley D. J., and Jaffray D. A., “An innovative phantom for quantitative and qualitative investigation of advanced x-ray imaging technologies,” Phys. Med. Biol. 10.1088/0031-9155/50/21/N01 50(2), N287–N297 (2005). [DOI] [PubMed] [Google Scholar]
- Tornai M. P., McKinley R. L., Bryzmialkiewicz C. N., Cutler S. J., and Crotty D. J., “Anthropomorphic breast phantoms for preclinical imaging evaluation with transmission or emission imaging,” Proc. SPIE 10.1117/12.596094 5746(1), 825–834 (2005). [DOI] [Google Scholar]
- Von Hagens G., “Impregnation of soft biological specimens with thermosetting resins and elastomers,” Anat. Rec. 194(2), 247–55 (1979). [DOI] [PubMed] [Google Scholar]
- de Jong K. and Henry R. W., “Silicone plastination of biological tissue: Cold-temperature technique, Biodur s10∕s15 technique and products,” J. Int. Soc. Plastination 22, 2–14 (2007). [Google Scholar]
- Brizzi E., Sgambati E., Capaccioli L., Giurovich E., and Montigiani L., “A radiological-anatomical comparison between formalin-preserved organs and “plastinated” ones,” Ital. J. Anat. Embryol. 99(3), 145–155 (1994). [PubMed] [Google Scholar]
- Hubbell J. and Seltzer S., “Tables of x-ray mass attenuation coefficients and mass energy-absorption coefficients (version 1.4),” Published online: http://physics.nist.gov/PhysRefData/XrayMassCoef/cover.html, National Institute of Standards and Technology, Gaithersburg, MD, 2004.
- Van Dyk J., Keane T., and Rider W., “Lung density as measured by computerized tomography: Implications for radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 8(8), 1363–1372 (1982). [DOI] [PubMed] [Google Scholar]