Abstract
The roles of physicists in medical imaging have expanded over the years, from the study of imaging systems (sources and detectors) and dose to the assessment of image quality and perception, the development of image processing techniques, and the development of image analysis methods to assist in detection and diagnosis. The latter is a natural extension of medical physicists’ goals in developing imaging techniques to help physicians acquire diagnostic information and improve clinical decisions. Studies indicate that radiologists do not detect all abnormalities on images that are visible on retrospective review, and they do not always correctly characterize abnormalities that are found. Since the 1950s, the potential use of computers had been considered for analysis of radiographic abnormalities. In the mid-1980s, however, medical physicists and radiologists began major research efforts for computer-aided detection or computer-aided diagnosis (CAD), that is, using the computer output as an aid to radiologists—as opposed to a completely automatic computer interpretation—focusing initially on methods for the detection of lesions on chest radiographs and mammograms. Since then, extensive investigations of computerized image analysis for detection or diagnosis of abnormalities in a variety of 2D and 3D medical images have been conducted. The growth of CAD over the past 20 years has been tremendous—from the early days of time-consuming film digitization and CPU-intensive computations on a limited number of cases to its current status in which developed CAD approaches are evaluated rigorously on large clinically relevant databases. CAD research by medical physicists includes many aspects—collecting relevant normal and pathological cases; developing computer algorithms appropriate for the medical interpretation task including those for segmentation, feature extraction, and classifier design; developing methodology for assessing CAD performance; validating the algorithms using appropriate cases to measure performance and robustness; conducting observer studies with which to evaluate radiologists in the diagnostic task without and with the use of the computer aid; and ultimately assessing performance with a clinical trial. Medical physicists also have an important role in quantitative imaging, by validating the quantitative integrity of scanners and developing imaging techniques, and image analysis tools that extract quantitative data in a more accurate and automated fashion. As imaging systems become more complex and the need for better quantitative information from images grows, the future includes the combined research efforts from physicists working in CAD with those working on quantitative imaging systems to readily yield information on morphology, function, molecular structure, and more—from animal imaging research to clinical patient care. A historical review of CAD and a discussion of challenges for the future are presented here, along with the extension to quantitative image analysis.
Keywords: computer-aided detection, computer-aided diagnosis, quantitative image analysis, image processing, CAD
INTRODUCTION
Research and development of methodology and instrumentation for diagnostic or therapeutic applications are among the major responsibilities of medical physicists. In medical diagnosis, physicists have been contributing to the development of imaging techniques since the discovery of x-rays by W. C. Roentgen. The roles of physicists in medical imaging have expanded in all directions over the years, from the study of imaging systems (sources and detectors) to the assessment of image quality and perception, the development of image processing techniques, and the development of image analysis methods to assist in detection and diagnosis, to name a few. The latter is a natural extension of medical physicists’ goals in developing imaging or other techniques to help physicians acquire diagnostic information and improve clinical decisions.
The benefit of a medical imaging exam is dependent both on the physical quality of the medical images and on the ability of the radiologist interpreting them. Studies indicate that radiologists do not detect all abnormalities on images that are visible on retrospective review, and they do not always correctly characterize abnormalities that are found. In the clinical interpretation of medical images, limitations in the human eye-brain visual system, reader fatigue, distraction, the presence of overlapping structures that camouflage disease in images, and the vast number of normal cases seen in screening programs provide cause for detection and interpretation errors.1, 2, 3, 4, 5, 6
Lusted discussed the use of computers in the analysis of radiographic abnormalities in the mid-1950s.7 In the 1960s and 1970s, researchers including physicists and clinicians started to investigate computerized image analysis aimed at automated detection or classification of abnormalities,8, 9, 10, 11, 12, 13, 14, 15, 16 including analyses on breast images11 and chest radiographs.12, 13 However, limited computer power and quality of the image digitization equipment at that time may have limited the chance of success for these early attempts. The goal of stand alone, automated computerized detection or diagnosis also made it difficult to achieve the accuracy and the acceptance required for clinical use. In the 1970s and 1980s, with the advent of digital subtraction angiography and the application of other digital images, various investigators started developing computer-based quantitative analysis of angiographic vasculature.17, 18
In the mid-1980s, a team of medical physicists and radiologists in the Kurt Rossmann Laboratories in the Department of Radiology at the University of Chicago started their research efforts for computer-aided detection or computer-aided diagnosis (CAD), that is, using the computer output as an aid to radiologists—as opposed to a completely automatic computer interpretation—focusing initially on methods for the detection of lesions on chest radiographs and mammograms.19, 20, 21, 22 In this usage, CAD can be defined as a diagnosis made by a radiologist who uses the output from a computer analysis of the image data in their decision making process. The final medical decision is made by the radiologist, not the computer. Note that with CAD, the role of the computer analysis is not to replace the radiologist but rather to aid the radiologist in his∕her image interpretation and∕or decision making. For more than the past 20 years, investigations of computerized image analysis for detection or diagnosis of abnormalities in a variety of 2D and 3D medical images have been conducted through collaborations between medical physicists and radiologists. Radiologists were expected to ultimately use the output from computerized analysis of medical images as a “second opinion,” like a spellchecker, in detecting and characterizing lesions as well as in making diagnostic decisions, as schematically shown in Fig. 1. Many reviews and chapters have already been written on the development and implementation of CAD methods.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 It is important to note that success in CAD required knowledge of imaging physics (i.e., image acquisition method) as well as knowledge of various computer vision and artificial intelligence techniques. Because of the numerous works that have been conducted, this brief review is by no means exhaustive, but only serves as a historical perspective of the importance of CAD research in diagnostic imaging and medical physics, and reports on the various roles played by medical physicists in the evaluation and understanding of CAD and its limitations.
Figure 1.
Schematic diagram of a CAD system for medical image interpretation.
The growth of CAD over the past 20 years has been tremendous—from the early days of time-consuming film digitization and CPU-intensive computations on a limited number of cases to its current status in which developed CAD approaches are evaluated rigorously on large clinically relevant databases. Figure 2 illustrates the growth of CAD research in terms of number of publications in Medical Physics. CAD research by medical physicists includes many aspects—collecting relevant normal and pathological cases; developing computer algorithms appropriate for the medical interpretation task including those for segmentation, feature extraction, and classifier design (Fig. 3); developing methodology for assessing CAD performance; validating the algorithms using appropriate cases to measure performance and robustness; conducting observer studies with which to evaluate radiologists in the diagnostic task without and with the use of the computer aid; and ultimately assessing performance with a clinical trial. Currently, CAD has been extended to include image analysis of various disease types—breast cancer, lung cancer, interstitial disease, colon cancer, osteoporosis, osteolysis, vascular plaque, aneurysms, and others—on various modalities, including analog and digital radiography, ultrasound, CT, PET, MRI, and others.
Figure 2.
Publications on or related to CAD in Medical Physics.
Figure 3.
Components within the “black box” of a CAD system.
CAD techniques and systems can broadly be categorized into two types—computer-aided detection (CADe) and computer-aided diagnosis (CADx). CADe implies that radiologists use computer outputs of the locations of suspect regions, leaving the characterization, diagnosis, and patient management to the radiologist. CADe is basically a detection task, i.e., a localization task. CADx extends the computer analyses to yield output on the characterization of a region or lesion, initially located by either a human or a computerized detection system. The computer might output mathematical descriptors to characterize the lesion and∕or estimate the probability of malignancy (or other abnormality), leaving the final diagnosis and patient management to the physician. CADx is a classification task for differential diagnosis. Ultimately, the goal of CAD is to reduce search errors, reduce interpretation errors, and reduce variation between and within observers.
There is strong synergy between CAD and quantitative image analysis. With continued growth in CAD techniques and the associated increase in accuracies, quantitative image analysis is a natural extension of the new algorithmic methods to help extract quantitative features and absolute measures of morphology and function to improve medical diagnosis. Conversely, quantitative imaging accentuates the need for highly robust and efficient computer-assisted image analysis tools and stimulates the development of CADe and CADx for the new imaging applications.
COMPUTER-AIDED DETECTION
Computer-aided detection entails the use of a computer output that only yields the location of suspect lesions. Characterization and diagnosis of the abnormality as well as patient management are left for the radiologist. Such systems are most beneficial in imaging examinations in which many cases need to be interpreted with most being normal—such as in screening programs—e.g., screening mammography, low-dose thoracic CT for smokers, and colon cancer screening.
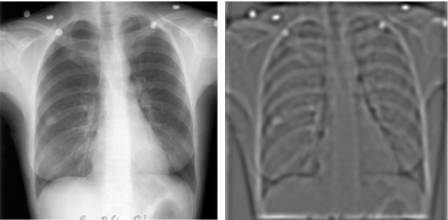
Medical diagnostic imaging lends much of its scientific development to the adaptation of signal detection theory38, 39 to guide its technological evolution and performance evaluation. Medical physicists play an important role in this process.40, 41, 42, 43, 44, 45, 46 One fundamental concept is the relationship between image quality measures such as the signal-to-noise ratio (SNR) and the detectability of signals in an image.47, 48, 49, 50, 51, 52, 53 The development of various medical imaging modalities centered around the goal of improving image quality and SNR of the lesion of interest, which is important for both human observers and machine vision. To achieve this goal for CADe systems, CAD researchers proposed the difference image technique19, 20, 21, 22 in which the input image was processed to generate a SNR-enhanced image and a SNR-suppressed image, as demonstrated on a chest radiograph in Fig. 4a, which shows the processing of the nodule prior to additional computer vision techniques for nodule detection. Subsequently, the difference of the two processed images is obtained to yield an image in which the conspicuity of the lesion is greatly increased [Fig. 4b]. This method of enhancing the SNR was an extension of the prior research of these medical physicists. Although the implementation differs and depends on the lesion of interest, many CADe systems to date follow a similar approach of enhancing the SNR as a first step.
Figure 4.
Difference-image approach to detecting nodule candidates on chest radiographs. The approach aimed to enhance the nodule with one processing filter and to suppress the anatomical background with another processing filter, with the difference resulting in an image for further analysis. Reprinted with permission from Giger et al. 1988 (Ref. 21).
CADe in mammography
Breast cancer detection is one of the principal research areas that has been studied since the early days of CAD research. Mammographic interpretation is a difficult task because mammographic signs of breast cancer such as microcalcifications and soft tissue masses can be very subtle and often obscured by dense fibroglandular breast tissue. The recommended annual screening mammography for women over 40 years of age results in a large volume of mammograms to be read by radiologists. Studies indicate that the false-negative rate of mammography ranges from 10% to 30%.54, 55, 56, 57, 58, 59, 60 In a study that reviewed retrospectively prior mammograms of breast cancer patients, it was found that 67% of the cancers were visible on the prior mammograms.61 CADe, therefore, potentially can be very useful for mammography.
Computerized analysis systems for mammography usually are focused on the detection of either clustered microcalcifications or mass lesions, with more recent methods on architectural distortions. These methods have been reviewed extensively.23, 24, 25, 26, 27, 28, 29, 31, 33, 62, 63
A number of investigators have reported computerized methods for detection of microcalcifications.16, 19, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84 Although the specific techniques used in different systems varied, they generally contained several major steps. The breast region is first extracted by boundary detection. The mammogram may then be processed by image enhancement methods to increase the SNR of the microcalcifications. The signal candidates are identified and segmented based on their SNR difference or gray level contrast from the surrounding background tissue. Features that characterize the shape, size, contrast of the individual microcalcifications, and of the cluster are extracted and used as input to classifiers for differentiation of true and false signals. Additional false-positive (FP) reduction techniques, such as artificial neural networks, may be trained to further distinguish between true signal patterns (i.e., the lesion) and normal anatomic background. The clustering property of significant microcalcifications is used to further reduce FPs, and the remaining clusters are flagged as suspicious lesion locations.
Soft tissue masses are imaged as focal densities on mammograms. Masses with well-circumscribed margins are more likely to be fibroadenoma or a benign cyst whereas masses with ill-defined or spiculated borders have a high likelihood of being malignant. However, there is large overlap between the border characteristics of malignant and benign masses. Initially, a few investigators developed automatic algorithms for detection of masses on mammograms11, 64, 85, 86 comparing regions between the left and right breast images. The development of mass detection systems evolved more rapidly since the late 1980s.86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 The overall scheme of these systems generally contains several major steps similar to those in a microcalcification detection system. The breast region is first segmented from the mammogram. The mammogram may be preprocessed with a spatial filter or nonlinear technique to enhance the suspicious regions. The mass candidates are segmented from the breast image based on their gray level contrast, gradient orientation, or spicule information. Feature descriptors are extracted from the segmented objects. Rule-based classifiers or other linear, nonlinear, or neural network classifiers are then trained to classify the mass candidates as true mass or FPs.
While many analyses of mammograms include the specific stages of lesion segmentation and feature extraction, some investigators have focused on extracting information directly from the image data. Zhang et al. trained a shift-invariant neural network to detect individual microcalcifications in a background-corrected region.74 Tourassi et al. used information theory in developing a content-based retrieval and detection system that took as input regions throughout the mammogram in the detection of masses.113
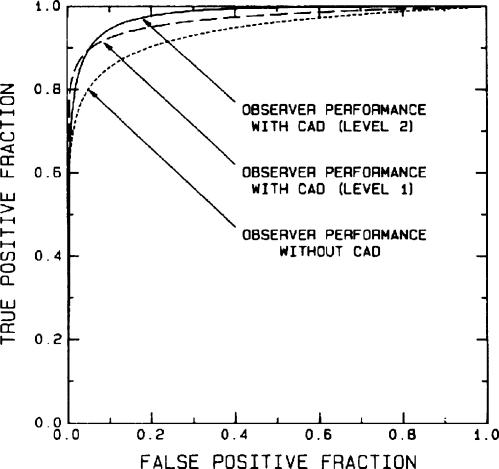
In 1990, Chan et al.114 reported on the first observer study to compare radiologists’ detection of microcalcifications with and without the aid of a computer-aided detection (CADe) system using receiver operating characteristic (ROC) methodology and demonstrated that the radiologists’ performance was improved significantly with CADe (Fig. 5). This study established the potential usefulness of CADe as a second opinion. It also revealed the important concept that it is not necessary for the CADe system performance to be as high as or higher than that of the radiologists in order to provide a useful second opinion, as long as it can provide information complementary to what radiologists may have. Additional studies followed for both clustered calcifications and mass lesions.92
Figure 5.
ROC curves illustrating statistically significant improvement in radiologists’ detection of microcalcification clusters when a computer aid is used. Level 1 corresponds to use of the computer having a performance level of 87% true-positive rate and an average of four false-positive clusters per image. Level 2 corresponds to use a computer aid with the same 87% true-positive rate but a simulated average false-positive cluster rate of only one false-positive cluster per image. Reprinted with permission from Chan et al. (Ref. 114).
Figures 6a, 6b show the first prototype CAD system (circa 1994), along with an example output on thermal paper, which was developed and applied to screening mammography at the University of Chicago. The system received as input a screen∕film mammogram, which was subsequently digitized and automatically analyzed by the computer. The output annotation from the system would indicate suspect locations (clustered microcalcifications or mass lesions) on a thermal paper printout or monitor.
Figure 6.
(a) First prototype CADe system—developed for screening mammography at the University of Chicago (circa 1994); (b) system annotated output on thermal paper.
The first commercial CADe system for screening mammography was approved by the Food and Drug Administration (FDA) in 1998. Other systems for mammography have obtained FDA approval since then and approval of CADe system for digital mammography also followed. A large number of CADe systems are being used clinically in screening screen film and digital mammography both in the United States and overseas. Several reports have been published on the performance of some of the commercial systems in clinical practice, as summarized in Tables 1, 2.387, 388, 389, 390, 391, 392, 393, 394, 395, 396, 397 The results indicated that the cancer detection rate in general increased with an accompanied increase in the recall rate, as can be expected. The design of these clinical studies can be separated into two major groups: (i) a sequential reading design in which interpretations by the same radiologist without CADe are immediately followed by interpretation with CADe and (ii) a longitudinal in time (historical) design in which a statistical comparison is made of a group of radiologists over two periods of time before and after CADe is implemented in the practice. The former design, therefore, collected without and with CADe data from the same patient cohorts and the same radiologists, whereas the latter design collected data from different patient cohorts and the radiologists may not be the same. The rationale and biases of these designs have been discussed by CAD researchers.36, 115, 116 The latter design may introduce additional variabilities from factors such as differences in the patient characteristics and the radiologists’ experiences in the two periods of time. The larger variances may make it more difficult to observe the incremental gain in sensitivity with CADe compared to without CADe. The different biases and variances may account for part of the differences in the observed effects of CADe on the sensitivity and specificity in these prospective studies.
Table 1.
Prospective clinical trial of commercial CADe systems for screening mammography. These studies used a sequential reading design in which the interpretations by the same radiologist without CADe immediately followed by with CADe were recorded for individual cases. The results were, therefore, collected from the same patient cohorts and the same radiologists.
| Investigators | Number of cases | Change incancer detectionrate (%) | Change inrecall rate(%) |
|---|---|---|---|
| Freer et al. (Ref. 387) | 12 860 | +19.5 | +18.5 |
| Birdwell et al. (Ref. 388) | 8682 | +7.4 | +8 |
| Khoo et al. (Ref. 389) | 6111 | +1.4 | +5.8 |
| Dean et al. (Ref. 390) | 9520 | +11.4 | +26 |
| Morton et al. (Ref. 391) | 18 096 | +7.6 | +9.5 |
| Ko et al. (Ref. 392) | 5016 | +4.7 | +14.9 |
Table 2.
Prospective clinical trial of commercial CADe systems for screening mammography. These studies compared the statistical results by a group of radiologists over two periods of time before and after CADe was implemented in the practice. The patient cohorts were different and the radiologists may not be the same.
| Investigators | Number ofexams(unaided) | Number ofexams(aided) | Change incancerdetection rate (%) | Change inrecallrate (%) |
|---|---|---|---|---|
| Gur et al. (Ref. 393) | 56 432 | 59 139 | +1.7 | +0.1 |
| (24 radiologists) | ||||
| 82 129 | 44 629 | −3.3 | −4.9 | |
| (7 high-volume radiologists) | ||||
| Feig et al. (Ref. 394) | 11 803 | 21 639 | +19.7 | +14.1 |
| (17 low-volume radiologistsin Guret al.study) | ||||
| Cupples et al. (Ref. 395) | 7872 | 19 402 | +16.1 | +8.1 |
| Fenton et al. (Ref. 396)1 | 398 159 | 31 186 | +4.5 | +30.7 |
| Gromet et al. (Ref. 397) | 112 413 | 118 808 | +11.1 | +3.9 |
From a survey study of 43 facilities, seven of which implemented CAD during the study period between 1998 and 2002.
The effects of CADe can be expected to depend on many other factors, including the level of expertise and vigilance of the radiologist and how the radiologist utilizes the CADe marks. Current CADe systems for screening mammography are designed to be used as a second reader, not as a concurrent reader. The radiologist should first interpret the case thoroughly as if there is no CADe, and should not reduce their level of suspicion at locations where there are no CADe marks. It is well known that CADe systems can miss lesions that radiologists detect routinely and mark many FPs. The benefits of CADe often rely on its detection of some lesions that radiologists may overlook and the willingness of radiologists to work up some of the CADe marks. If CADe is used as it is designed, the sensitivity will never decrease and the recall rate is expected to increase. For radiologists with low false negative rates, the incremental gain by CADe will likely be small. Furthermore, the incremental gain in sensitivity will not be realized if radiologists become too dependent on the CADe system and reduce their vigilance in interpreting the mammograms themselves, or if they ignore the CADe marks because of too many FPs. It is important that the user understands the capability and the limitations of the CADe system and uses it properly in order to take advantage of CADe.
The relatively large number of FPs in current CADe systems is a major drawback of using CADe for some radiologists. Continued efforts are needed to improve the sensitivity and the specificity of the systems. Most current CADe systems concentrate on detection in a single mammogram. One promising approach to improving the performances of CAD systems is to incorporate multiple image information, including correlation of two mammographic views (CC and MLO views) of the same breast, comparison of current and prior mammograms, or comparison of bilateral mammograms. These strategies emulate those routinely performed by radiologists in mammographic interpretation to detect new lesions and reduce FPs.54, 117, 118
Studies have been conducted to incorporate information from multiple mammographic views of the same breast, such as the CC and MLO views, for lesion detection and the reduction of FPs.84, 119, 120, 121, 122 Radiologists compare the left and right mammograms to detect asymmetry in the density patterns of the breasts. Thus, researchers used digital bilateral comparison techniques, including methods for image registration, to incorporate information from both breasts and identify asymmetries.64, 89, 91, 123, 124, 125 These studies indicate that multiview information fusion has a strong potential for improving the performance of CADe systems.
Radiologists routinely compare the current and prior mammograms, if available, for detection of newly developed mammographic abnormalities. Automated analysis of interval changes in serial mammograms requires identification of corresponding locations on two mammograms of the same view. The deformability of the breast and lack of invariant “landmarks” make it difficult to correctly register two breast images using conventional registration techniques. Various investigators have developed methods for use in temporal subtraction using automatically delineated skin line and nipple positions,126 as well as regional registration techniques to localize corresponding lesion locations on mammograms of the same view to within a small search region of the true location.124, 127, 128
Multimodality imaging is a promising approach to improving breast cancer detection. There is strong interest in developing a combined full breast 3D ultrasound and digital mammography system in which the ultrasound scanning will be performed automatically in the same compression as the digital mammogram so that the corresponding lesions between the two can be correlated geometrically.129 To facilitate the implementation of such a system in screening mammography, ideally one will have a CADe system that can automatically detect suspicious masses on the digital mammogram and initiate the ultrasound scanning, if needed, while the breast is still under compression. After image acquisition, the CADe system will automatically detect the lesions in the 3D ultrasound volume and correlate the lesions with those detected on the digital mammograms. The combined information from the two modalities can be used to improve cancer detection and reduce recalls.
With the advent of direct digital mammography systems, a number of new breast imaging techniques are under development, including digital breast tomosynthesis,130, 131, 132, 133, 134 and single-energy or dual-energy contrast-enhanced digital subtraction mammography135, 136 and breast computed tomography (CT).137, 138, 139 Tomosynthesis mammography and breast CT hold the promise of improving breast cancer detection and diagnosis, especially in dense breasts. Combined tomosynthesis mammography and 3D ultrasound scanning is also being developed.129 These new modalities or multimodality images drastically increase the number of images that radiologists have to interpret for each case. If CADe systems are available to assist radiologists in the analysis of the new modalities efficiently and in integrating the information from different modalities effectively, it may facilitate the introduction of the new techniques to clinical practice. Development of CADe systems for tomosynthesis mammography is underway.140, 141 It can be expected that CAD development for the other modalities will also be initiated when image databases become available for design of the CAD systems.
CADe in thoracic imaging
CADe systems for various lung diseases have been reported in the literature. Chest radiography is the most commonly performed procedure in medical imaging, however, interpretation of chest radiographs is a difficult task because of the overlapping ribs and its low contrast sensitivity for subtle abnormalities. CAD of lung disease was attempted in the 1970s.12, 14 Dedicated efforts in the 1980s revived the interests in development of CADe systems for chest radiographs.20, 21, 142 Over the last two decades, a large number of studies have been conducted to develop computerized methods for analysis of various abnormalities in chest radiographs, including detection of lung nodules,20, 21, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152 detection and classification of interstitial diseases,142, 153 detection of pneumothorax,154 and temporal subtraction of chest radiographs to detect interval changes.155, 156, 157 The effects of CADe for lung nodule detection on radiologists were evaluated by a number of observer performance studies.144, 158, 159, 160, 161 Similar to CADe for breast cancer detection in mammography, these studies indicated that the detection accuracy for lung nodules in chest radiographs could be significantly improved with the use of CADe. A commercial lung nodule CADe system for chest radiography was approved by the FDA in 2001 but no large-scale prospective clinical trials have been reported to date.
The Early Lung Cancer Action Project (ELCAP) study showed that thoracic CT has higher sensitivity for detection of early stage lung cancer than chest x-rays.162 However, it is not known whether early detection can actually reduce the mortality rate or increase the chance of survival for lung cancer patients. An NCI-sponsored randomized, controlled study, National Lung Screening Trial (NLST), was conducted to compare the mortality rate of lung cancer patients using helical CT or chest x-rays but the results are not yet available. Thoracic CT, especially helical CT, produces a large number of slices for each case. There will be a dramatic increase in radiologists’ workload if CT is recommended for lung cancer screening in the future. The potential usefulness of CT for lung cancer screening has stimulated interest in the development of CADe systems for lung nodule detection on thoracic CT scans. A number of research groups have reported CADe methods in this area.163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174 The performances of these systems vary, and the performances were evaluated on data sets using different CT scan protocols and having cases of different nodule characteristics. The NCI recognized the need for CAD techniques for lung CT interpretation and supported the Lung Imaging Database Consortium (LIDC) to collect a standard database of lung CT images for this purpose.175 The first commercial CADe system for thoracic CT was approved by the FDA in 2004. Although no prospective clinical trial of lung CADe has been reported to date, retrospective observer performance studies indicated that observers’ accuracy in detection of lung nodules on chest CT scans can be significantly improved with the use of CADe,176, 177, 178, 179, 180, 181 indicating the potential for CADe to assist in radiologists’ reading in clinical practice.
CADe in colon imaging
CT colonography is another important area of application for CADe. Colon cancer is the third leading cause of cancer deaths for men and women in the United States. Colon cancer screening involves detection of polyps, which can be the precursor of colon cancer, and cancerous growths on the walls of the large intestine. Currently the most reliable procedure for colon cancer screening is a colonoscopy. CT colonography (CTC) is being studied as an alternative procedure. Interpretation of CTC is time consuming and difficult even with the help of the virtual colonoscopic view that helps the radiologist fly through the entire colon to search for abnormalities. The radiologist’s sensitivity of polyp detection in CTC varies over a wide range as reported in the literature, which was attributed to many factors such as the variability in CT scanning techniques, colon preparation methods, size of the polyps in the studied patient cohort, and the radiologists’ experience with CTC.
CADe may be a useful adjunct to CTC to reduce false negatives and reader variability. A number of research groups have reported CADe methods for analysis of CTC in the past few years.182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193 The current CTC CADe systems have sensitivity ranging from 80% to 100% at an FP rate of 2 to 15 per scan. Most of the studies used a small data set for evaluation so that the variances of the results may be high. In addition, the performance of CADe depends strongly on the data set characteristics, including the polyp size in the data set and the CTC scanning protocol, as well as the method used for scoring of the true positives and FPs of the CADe algorithm. It is still unknown how these performances would generalize to unknown cases in prospective studies. Several retrospective observer studies have been conducted to evaluate the effects of CADe on radiologists’ interpretation of CTC.185, 194, 195, 196 These studies indicate that radiologists reading with CADe outperformed radiologists alone. The usefulness of CADe for CTC has yet to be evaluated in prospective clinical trials.
COMPUTER-AIDED DIAGNOSIS—FOR DIFFERENTIAL DIAGNOSIS
Once a lesion is detected, for example, such as in a screening program, further imaging of the abnormality may be necessary in order to justify subsequent patient management such as invasive evaluations (e.g., a biopsy) and∕or therapeutic interventions. Thus, the role of a CADx system is to aid in the characterization of an already-found lesion or other abnormality in terms of its morphological or functional attributes, and in the estimation of its probability of malignancy or other disease state. Such a computer system is expected to aid a radiologist in his∕her differential diagnosis and improve the positive predictive value (PPV) of the interpretation. The input to a CADx algorithm could be either a radiologist-detected or a computer-detected lesion or region. This input could be in the form of an indication of the approximate center of the lesion or an actual delineation of the lesion outline. As clinical CADe systems begin to give more information beyond just localization, CADx is slowly being introduced.
Just as radiologists use multiple modalities in the work up of a patient’s case, so can a computer system. Medical physicists, armed with their knowledge of the physics of the various imaging modalities, such as x-ray radiography, special radiographic views, sonography, and MRI, are able to develop CADx for the various modalities and use the information individually or in combinations. Radiologists’ use of the output of a CADx system is expected to improve the sensitivity for cancer diagnosis, reduce the number of benign biopsies, and reduce variability between and within radiologists. Extensions of such systems will potentially be developed for assessing prognosis, assessment of tumor growth rate, and response to treatment.
CADx in breast imaging
Medical physicists have played key roles in developing CADx methods in breast imaging across the modalities. Computerized classification systems can be designed to take as input either human-perceived lesion features or computer-extracted features. Note that a diagnostic task involves both the extraction of lesion characteristics and the subsequent merging of these characteristics into a diagnosis. In 1988, Getty et al. demonstrated that radiologists’ performances improved when using a CADx system that merged the lesion characteristics that the radiologists had indicated via a checklist.197 Although such human-perceived lesion features, e.g., BI-RADS rating, can be subjective and may vary between radiologists, the usefulness of merging such features by computer systems has been demonstrated.197, 198, 199, 200, 201, 202, 203, 204, 205, 206 Computer-extracted features, i.e., mathematical descriptors, can characterize the lesion using features either that radiologists can perceive such as mass spiculation or distribution of microcalcifications, or those that are not so visually intuitive to the eye, such as those obtained with co-occurrence matrices.62, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248 These computer-extracted features can be obtained from standard mammographic views (CC and MLO), special view mammogram, prior mammographic exams, or from tomosynthesis mammograms, as well as from sonograms and∕or breast MR images. Note that computer-extracted lesion features can be obtained from either radiologist-delineated lesion margins or from computer-segmented lesion margins. Various methods have been proposed for this important segmentation stage in CADx systems.217, 230, 242, 249, 250, 251, 252, 253 A poor segmentation of the lesion margin would subsequently yield erroneous mathematical descriptors (features) of the lesion. As with radiologists, computer performance in diagnosing lesions improves for special-view mammograms, as opposed to standard views,228 and also when prior mammograms are included in the overall analysis.226, 247
With the advent of FFDM systems, investigations have been conducted to understand the necessary conversions of a CADx system when going from digitized screen∕film images to FFDM images.254, 255 For example, investigators have shown that a mammographic CADx system developed for characterizing clustered microcalcifications on screen∕film mammograms as malignant or benign can also be used for FFDM images; the system appeared to maintain consistently high performance without requiring substantial modification from its initial development on screen-film mammography.246, 256 While the underlying concepts regarding malignant features remain, the importance of the different features on a correct output may be dependent on the physical image quality of acquisition system, and, thus, retraining (calibration) may be necessary.
The understanding of the imaging physics of breast sonography allows for the development of additional lesion features, such as posterior acoustic shadowing, and, thus, their corresponding mathematical descriptors. CADx systems for ultrasound include mathematical descriptors of texture, margin, and posterior acoustic shadowing criteria.208, 220, 232, 236, 257, 258, 259, 260 Medical physicists have led the field in robustness evaluation studies across institutions and across manufacturers on sonographic CADx,236, 240 and in extending the analysis to 3D images.239
The use of dynamic contrast-enhanced MRI continues to increase in diagnostic work up and preoperative staging for breast cancer. Assessment of contrast medium uptake and washout are related to tumor blood flow, and, thus, the associated kinetics are related to angiogenesis and likelihood of malignancy.261 There is a need for standardization of MR breast imaging as protocols can vary greatly between and within institutions, and, thus, standardized lexicon are being developed.262 Success in MRI analysis depends on knowledge of the underlying biology, the physics of the acquisition, and computer vision techniques. Some commercial systems focus on just the kinetic aspects of breast MRI and plot the kinetic curve (uptake and washout of the contrast agent) of regions of interest on the display workstation. CADx systems being developed for MRI yield morphological features, kinetic features, or combinations.215, 223, 231, 235, 243, 399 MRI CADx systems have the potential to improve both the accuracy and efficiency of interpretation.
Medical physicists have led various observer studies demonstrating CADx as an aid to radiologists in the task of distinguishing between malignant and benign lesions,222, 237, 244, 263, 398 demonstrating that radiologists’ performance in classification of malignant and benign microcalcifications or masses could be improved significantly by use of CADx. Others showed that improvement in performance with the use of CADx can be obtained by both expert mammographers and community-based radiologists with the performance of the aided nonexperts reaching the levels of the unaided experts.263 Use of computer output has also been shown to reduce the variability among radiologists’ interpretations.264 Medical physicists have also conducted observer studies for serial mammographic exams,265 sonographic CADx including those for both 2D237 and 3D ultrasound systems,239 and for multimodality breast CADx in which the CADx system outputs analyses of both mammography and ultrasound.244
Effective and efficient communication of the computer output to the radiologist is a necessary step in the overall CADx protocol. During residency, radiologists learn through the review of cases from conferences, teaching files, and atlases, and, thus, the access to online cases of known pathology during a radiologist’s daily practice may be helpful for continuous learning. Searching an online image atlas can be based on individual features, on likelihood of malignancy, or on psychophysical measures of similarity. One of the first display systems for computer analysis output, by Sklansky et al.,266 used a graphical method to show a chosen number of similar malignant lesions and the same number of similar benign lesions. Swett et al. utilized an expert system to control the display of similar cases.267, 268 Giger et al. developed a CADx workstation interface that includes mathematical descriptors of lesion characteristics as well as an estimate of the probability that the suspect lesion is abnormal or not, with the output given in terms of a numerical estimate of the probability of malignancy, a retrieval of similar lesions from an online database, and∕or a graphical representation of the case in question relative to the distributions of normals and abnormals in a given population.269, 270, 244 This interface, shown in Fig. 7, displays similar images and uses color coding to indicate whether the similar images are malignant or benign (red outlines=malignant, green outlines=benign lesions). It searches either via the computer-estimated probability of malignancy or by way of specific lesion characteristics, and shows a specific number of the closest similar lesions—whether they are all malignant, all benign, or a mixture.269, 270, 244 Investigators using the psychophysical aspects of similarity have combined the computer-extracted lesion characteristics with subjective similarity measures obtained from observers reviewing pairs of images271, 272, 273 or from observers giving subjective perceived ratings of lesion features.274
Figure 7.
Computer∕human interface for a multimodality workstation with computer outputs in numerical, pictorial, and graphical modes for both (a) mammography CADx output and (b) sonography CADx output.
Multimodality CADx output can be given separately for each modality or as a combined output that includes features from each modality, both of which have been shown to improve performance.275, 241 In addition, medical physicists are investigating the appropriate output in terms of an estimate of the probability of malignancy, knowing that the specific cancer prevalence in the training database may affect the actual output value.276
CADx in thoracic imaging
Due to that range of potential diseases present in the thorax, various types of computer-aided diagnosis methods are being developed for both chest radiography and CT, and include computer-aided diagnosis algorithms for pulmonary nodules and interstitial lung diseases.
Use of computers for the differential diagnosis of lung nodules in chest radiographs and thoracic CTs has advanced in recent years. Candidate nodules detected on thoracic CT may be categorized as malignant or benign, or as actionable or not. Research parallels that for breast lesions in that characteristic features of the nodules are extracted from chest radiographs and merged using classifiers to yield a likelihood of malignancy.277, 278, 279 Others have developed classification methods for nodules detected on CT—both conventional and thin-section CT.280, 281, 282, 283, 284, 285 This characterization of lung nodules on CT has been enhanced with the advent of PET∕CT systems, allowing for characteristics from both modalities to be used in the computer classification.
QUANTITATIVE IMAGE ANALYSIS
Quantitative metrics in anatomical imaging
While CAD is a quantitative tool that appears to its radiologist users as a qualitative tool, radiologists also make use of physically relevant quantitative measures under limited circumstances. These quantitative values have physical meaning in the radiographic interpretation. The use of distance and angular measurements using rulers or protractors is the most ubiquitous example, and clinical applications include the ultrasound-determined crown-rump length for fetal aging,286 angular measurements for scoliosis,287 and measuring tumor width.288 Medical physicists were not needed for radiologists to capitalize on simple length measurements for diagnosis, but radiologists do use the fact that the Hounsfield unit (HU) in CT is proportional to the linear attenuation coefficient, and medical physicist Godfrey Hounsfield (also a Nobel Prize recipient) developed the normalization procedure that made this possible. Lung nodules that exceed a certain HU value are considered benign due to their calcification, while nodules under this value have a higher probability of malignancy.289 Medical physicists have played a role in understanding the limitation of quantitative CT.290 Dual energy x-ray absorptometry (DEXA) is capable of accurately determining the projected bone mineral density (mg∕cm2), and has been used to assess fracture risk for over two decades.291 CT can measure the bone mineral density292 in three dimensions (mg∕cm3), and has also been used to quantify fracture risk.293 The 3D capabilities of QCT provide the additional benefit of discriminating between trabecular and cortical bone density,294 and here again the calibration methods necessary for accurate bone mineral quantitation were developed by medical physicists.294 The use of digital subtraction angiography allows interventional radiologists to assess the anatomical constriction of a vessel, and from the DSA procedure, a quantitative measure of stenosis can be easily derived. DSA was developed by Mistretta and colleagues,295, 296, 297 and is the worldwide standard for peripheral angiography today. Stenosis can be repeatedly measured during a revascularization procedure such as stent placement or angioplasty to monitor the success of the intervention and provide guidance to the interventionalist as to whether or not they have successfully dilated the vessel lumen.
Quantitative metrics in functional imaging
Imaging modalities used in the traditional nuclear medicine department, including planar imaging298 and SPECT,299 compensate for their comparatively low spatial resolution by providing unique functional information concerning metabolism, pharmacokinetic uptake, and other biodistribution information. Nuclear medicine procedures became faster with the development of the Anger camera,300 developed by medical physicist Hal Anger, and faster imaging in turn gave rise to quantitative kinetic studies. Physicists played an important role as nuclear medicine hardware started to incorporate computers,301 a process that enabled quantitative imaging in nuclear medicine. These images, both in 2D (planar) and 3D (SPECT), can be quantitative302, 303, 304 when calibrated appropriately and used for kinetic measurements of the heart including the ejection fraction, myocardial perfusion, and ventricular volume.305 The vascular dynamics of SPECT imaging can also be applied in neuroradiology for brain perfusion in acute stroke. Medical physicists, working in collaboration with radiologists (e.g. Ref. 306), helped develop specific nuclear imaging procedures. Commercially available SPECT∕CT systems are inspired by the early work of Hasegawa and colleagues307, 308 in building dual modality scanners.
Positron emission tomography (PET) was invented by medical physicists309, 310 and used in the research setting for many years, but enjoyed widespread clinical assimilation in the United States only when reimbursement mechanisms became established. In the present form of PET∕CT, developed by Townsend and colleagues,311, 312 this hybrid modality has revolutionized oncologic imaging and allows metabolic information (PET) to be evaluated along with high resolution anatomic information (CT). Furthermore, the inclusion of CT in the PET examination allows the PET image to be corrected for photon attenuation,313 transforming PET imaging into a more quantitative modality.
Magnetic resonance imaging (MRI) was developed initially by spectroscopist Paul Lauterbaur,314 but most of the hardware development and refinement of pulse sequences was performed by medical physicists.315, 316, 317, 318 The biological effects of MRI were also studied early on by medical physicists.319 Functional MRI (fMRI) is a tool widely used by psychiatrists and neurophysiologists to study the spatial and temporal aspects of cognition and emotion. Blood oxygenation level dependent320 (BOLD) techniques are used to monitor blood flow in the brain while simultaneously providing an audible, visual, or other sensorial stimulus to the patient. These techniques were developed by medical physicists working with other scientists.321, 322 The techniques are quantitative because the activity maps that are generated from these studies use correlation and other more sophisticated statistical measures to map spatiotemporal patterns of brain activity specific to the sensorial stimulus. While such techniques are used more for fundamental research than clinical evaluation, clinical applications such as mapping epileptic foci and surgical planning are becoming more common.
EVALUATION OF CAD AND QUANTITATIVE IMAGE ANALYSIS SYSTEMS
Medical physicists have played important roles in developing methodology for evaluating image analysis systems, assessing variations between such systems, and conducting observer studies. In CAD evaluations, performance levels can be determined for the computer alone or for radiologists when they are using the system output as an aid in their interpretations.323 CADe methods typically employ FROC curves to understand sensitivity versus average false-positive detections per image in assessing performance of the computer analysis, whereas CADx methods are evaluated using ROC analysis to assess computer performance in the task of distinguishing between malignant and benign lesions.115, 324, 325, 326, 327 As decision making extends beyond two-class diagnoses, n-class classifiers will require appropriate measures of performance, and these efforts are also being led by medical physicists.328, 329, 330, 331 Furthermore, during Jiang et al.’s research on CADx of clustered microcalcifications, the investigators realized the need for a more relevant measure of performance—beyond the area under the ROC curve (AUC)—in situations such as diagnostic workup in which a high level of sensitivity is crucial, and, thus, the partial area index was developed as demonstrated in Fig. 8.332
Figure 8.
Three empirical ROC curves with area under the ROC curve (AUC) values of 0.917, 0.825, and 0.862. The partial area values are 0.817, 0.484, and 0.261, respectively, for a sensitivity threshold 0.90. It is apparent that ROC (1), with its high partial area index, corresponds to a high sensitivity at high specificity (1-FPF). Reprinted with permission from Jiang et al. (Ref. 323).
The database characteristics, for example, in terms of size, lesion distribution, difficulty, as well as the integrity of the truth, can greatly influence the training and testing of a CAD algorithm. Various investigators have demonstrated the effect of different databases on mass or microcalcification detection performance using FROC analysis,333, 334 the effects of differences in scoring methods on the sensitivity and specificity of a CAD system,62, 335 and the potential biases resulting from insufficient sample size and improper feature selection methods,336, 337, 338, 339, 340, 341, 342, 343 the effect of dominant features in the training of artificial neural networks,221 and effect from training and testing with similar images.344 Studies of robustness in which CAD systems are evaluated across institutions and across manufacturers are necessary in the translation of the research to the clinical arena.225, 236, 240, 345 Additional investigations have focused on the computer performance on lesions not initially detected in screening programs.61, 346
Medical physicists have led the efforts of the LIDC (lung imaging database consortium) initiated by NCI. The LIDC has demonstrated and provided methods for the careful and necessary collection of images and relevant diagnostic information to enable CAD research. It has also considered various issues including the integrity of expert-defined “truth,” radiologist variability in the identification of lung nodules on CT scans, and a comparison of different size metrics for pulmonary nodule measurements.175, 347, 348, 349, 350, 351 Databases are only as good as the associated truth about the abnormality, whether it be the location of the lesion, biopsy results on malignancy∕benignity, or consensus opinions. In the development of CADe systems for lung nodule detection, for example, different investigators have used different “truths,” and have trained and evaluated systems with images of cancerous lung nodules, with all types of lung nodules (both malignant and benign), and∕or with any “actionable regions,” i.e., a region that is suspicious enough to cause further examinations or diagnostic actions.
Ultimate evaluation of CAD involves evaluating the performance of radiologists using the computer output as an aid (i.e., in observer studies or clinical trials). Various observer studies have been cited throughout this article. With observer studies, researchers aim to mimic the interpretation task on a database that might be enriched with a higher prevalence of cancer cases. Radiologists’ performance with CAD systems has been compared to double reading by humans.352, 353, 354 While results on performances are obtained from observer studies, ultimately clinical trials need to demonstrate efficacy of CAD systems.
It is important to realize that with CADe systems, which are focused on screening programs in which most cases will be normal, a large number of cases will be necessary for there to be sufficient power to demonstrate an actual improvement. Jiang et al. have reported that to detect an increase of one additional cancer per reader per 1000 screening mammograms with 80% power, a trial with a new modality (such as CADe) would need at least 25 radiologists, who would each read at least 8,000 screening mammograms.355 In addition, the measure of performance selected may also affect the overall conclusion, as noted by Horsch et al. in the analysis of performances in terms of radiologists’ reported probability of malignancy, in terms of BI-RAD ratings, and in terms of the patient management decision to biopsy or not.356
Evaluation studies on quantitative image analysis include additional needs since the absolute metrics such as tumor volume or blood flow must be correlated with actual physical conditions. Studies, such as clinical trials for therapeutic response or drug discovery, require careful standardization of the imaging protocol. In an effort to develop consistent and quality-controlled imaging protocols, uniform protocols for imaging in clinical trials (UPICT) (http:∕@upict.acr.org∕) was formed. Validation of quantitative imaging metrics have similar requirements as those for CAD methods in that verification of “truth” and adequate statistics are necessary.
CHALLENGES, LESSONS LEARNED, AND THE FUTURE
The first FDA-approved CAD system in 1998 was for computer-aided detection in screening mammography. While the detection of many types of cancers lend themselves to CADe due to the potential of oversight “errors” in a screening population of many normal cases, mammography was a good imaging exam for commercialization of CADe, since screening mammography is basically a dedicated imaging protocol, i.e., there is no other major “disease” or “incidental findings” for which the exam is ordered. The multitude of potential diseases∕conditions presenting on a chest radiograph combined with the inconvenience of film digitization for just one CAD task, slowed the research on CAD for lung cancer. However, CADe on thoracic CT (and on digital radiography) appears to be thriving, as image data are now primarily digital and the display of the computer output can be activated by a software button.
The potential for CADe is being explored for many other modalities and diseases. Examples include detection of pulmonary embolism357, 358 and hepatocellular carcinoma on CT scans,359 coronary artery diseases on cardiac CT angiograms,360 urinary tract cancer in CT images,361 masses on breast ultrasound images,236 vertebrate fracture on lateral chest radiographs,362 brain tumor or intracranial aneurysm on MR angiograms,363, 364 retinography,365 and detection of tumor change on whole-body nuclear medicine scans.366 Although CADe developments in these and other areas are still at an early stage, it can be seen that researchers will continue to expand their interests and efforts in CADe to various areas of applications.
Although the clinical community is accepting CADe to their practice, challenges exist for the current CADe systems and the development of new CADe systems. CADe systems may suffer from high FP rates. Most FPs might be dismissed by radiologists easily but some might require unnecessary work up. Some radiologists are concerned with the medicolegal consequences that the CAD marks that are not worked up may turn out to be malignant. Improving the CADe algorithms to reduce FPs is a constant goal for CAD developers. To develop CADe for a new area, the most difficult step is often the collection of a large database, representative of the patient population, for training and testing the CADe algorithms. Furthermore, whether a CADe system is useful as an aid to radiologists may be evaluated in prospective clinical trials. As discussed above, the outcomes of a clinical trial may be influenced by the study design and other human factors such as radiologists’ experience and vigilance, and their response to the CAD marks, in addition to the performance of the CADe system. Understanding these issues will be important for the study of the impact of CADe on medical diagnosis and for motivating radiologists to take best advantage of CADe in clinical practice.
The incorporation of CAD into new imaging modalities will become commonplace. Just as computers continue to be an integral part of our lives—so will they grow in medical imaging. CAD is now an integral component in most major medical imaging meetings and numerous CAD papers are published in journals such as Medical Physics each year. CAD will play an important role in the process of medical image interpretation and become an indispensable component in diagnostic imaging in the not-too-distant future. Various medical physicists are continuing to expand the role of CAD beyond computerized detection and diagnosis, such as in assessing cancer risk,367, 368, 369, 370 risk of osteoporosis,371, 372, 373, 374, 375 and potential occurrence of osteolysis.376 Extension of techniques developed for CAD are expected to also play a role in measurements of response to therapy, such as in assessing changes in tumors following chemotherapy377, 378 and in the quantitative analysis on thoracic CT in the assessment of mesothelioma.379 Furthermore, as new imaging modalities make available more and more data for interpretation, inclusion of CAD will be a necessity. Examples include assessment of multiple disease states in thoracic∕abdominal CT380, 381, 382, 383 and improved analysis of cardiac CT.384, 385
As emphasized elsewhere,386 the use of quantitative information that is accessible through imaging is predicted to dramatically increase over the next decade. Several factors lead to this observation. (1) We are in the postdigital image era, and virtually all image data are interpreted in digital format by radiologists using an imaging workstation (a computer). Thus, the image data are readily available for computerized assessment by interpreting physicians. (2) Radiography and other planar imaging modalities are slowly giving way to tomographic imaging, in CT, PET, SPECT, ultrasound, and MRI. Tomographic images provide a much richer data set in which spatial and other geometric parameters can be quantified. (3) The scan times for most modalities are steadily decreasing, giving rise to temporal imaging protocols (which provide image data at two or more time points) for the assessment of time-dependent physiological parameters such as blood flow, perfusion, permeability, and other velocity-based metrics. (4) Medicine is in a state of transition from a practice-based specialty to diagnoses and treatment decisions, which are evidence-based. This change will place more emphasis on quantitative parameters as diagnostic endpoints.
Medical physicists will have a very important role to play in this future landscape of quantitative imaging, by validating the quantitative integrity of scanners and developing imaging techniques, and image processing tools, which provide quantitative data in a more automated and accurate fashion. While the medical physicist played an essential and undeniable role in the development of imaging systems over the past 50 years, as imaging systems become more complex and the need for better and more accurate quantitative information from images grows, the role of the physicist will be even more important in the next 50 years.
The future includes the combined research efforts from physicists working in CAD with those working on quantitative imaging systems to readily yield information on morphology, function, molecular structure, and more—from animal imaging research to clinical patient care.
ACKNOWLEDGMENTS
Maryellen Giger is grateful for the many fruitful discussions with the faculty and research staff in the Department of Radiology and Committee on Medical Physics at the University of Chicago. Certain parts of the chapter are the result of research supported in parts by USPHS grants from NCI, NIBIB, and NIAMS, as well as from the U.S. Army Breast Cancer Research Program, the American Cancer Society, the Whitaker Foundation, and The University of Chicago Cancer Research Center. M. Giger is a stockholder in R2 Technology, a Hologic Company (Sunnyvale, CA). It is the University of Chicago conflict-of-interest policy that investigators disclose publicly actual or potential significant financial interests that may appear to be affected by the research activities. Heang-Ping Chan is grateful for the efforts by the faculty and researchers in the Department of Radiology and the CAD Research Laboratory at the University of Michigan. Certain parts of the chapter are the result of research supported in parts by USPHS grants from NCI and NIBIB, as well as from the U.S. Army Breast Cancer Research Program. John Boone was funded in part by a grant from the NIH (R01 EB002138).
References
- Kundel H. L. and Wright D. J., “The influence of prior knowledge on visual search strategies during the viewing of chest radiographs,” Radiology 93, 315–320 (1969). [DOI] [PubMed] [Google Scholar]
- Kundel H. L., Revesz G., Ziskin M. C., and Shea F. J., “The image and its influence on quantitative radiological data,” Invest. Radiol. 7, 187–205 (1972). [DOI] [PubMed] [Google Scholar]
- Kundel H. L. and Revesz G., “Lesion conspicuity, structured noise, and film reader error,” AJR, Am. J. Roentgenol. 126, 1233–1238 (1976). [DOI] [PubMed] [Google Scholar]
- Carmody D. P., Nodine C. F., and Kundel H. L., “An analysis of perceptual and cognitive factors in radiographic interpretation,” Perception 9, 339–344 (1980). [DOI] [PubMed] [Google Scholar]
- Kundel H. L. and Hendee W. R., “The perception of radiologic image information. Report of an NCI workshop on April 15-16, 1985,” Invest. Radiol. 20, 874–877 (1985). [DOI] [PubMed] [Google Scholar]
- Nodine C. F., Kundel H. L., Mello-Thoms C., Weinstein S. P., Orel S. G., Sullivan D. C., and Conant E. F., “How experience and training influence mammography expertise,” Acad. Radiol. 6, 575–585 (1999). [DOI] [PubMed] [Google Scholar]
- Lusted L. B., “Medical electronics,” N. Engl. J. Med. 252, 580–585 (1955). [DOI] [PubMed] [Google Scholar]
- Lodwick G. S., Keats T. E., and Dorst J. P., “The coding of roentgen images for computer analysis as applied to lung cancer,” Radiology 81, 185–200 (1963). [DOI] [PubMed] [Google Scholar]
- Becker H. C., Nettleton N. J., Meyers P. H., Sweeney J. W., and Nice C. M., “Digital computer determination of a medical diagnostic index directly from chest x-ray images,” IEEE Trans. Biomed. Eng. 11, 67–72 (1964). [DOI] [PubMed] [Google Scholar]
- Meyers P. H., Nice C. M., Becker H. C., Nettleton W. J., Sweeney J. W., and Mechstroth G. R., “Automated computer analysis of radiographic images,” Radiology 83, 1029–1033 (1964). [DOI] [PubMed] [Google Scholar]
- Winsberg F., Elkin M., Macy J., Bordaz V., and Weymouth W., “Detection of radiographic abnormalities in mammograms by means of optical scanning and computer analysis,” Radiology 89, 211–215 (1967). [Google Scholar]
- F.Roellinger, Jr., Kahveci A., Chang J., Harlow C., Dwyer III S., and Lodwick G., “Computer analysis of chest radiographs,” Comput. Graph. Image Process. 2, 232–251 (1973). [Google Scholar]
- Toriwaki J., Suenaga Y., Negoro T., and Fukumura T., “Pattern recognition of chest x-ray images,” Comput. Graph. Image Process. 2, 252–271 (1973). [Google Scholar]
- Kruger R., Thompson W., and Turner A., “Computer diagnosis of pneumoconiosis,” IEEE Trans. Syst. Man Cybern. SMC-4, 40–50 (1974). [Google Scholar]
- Kimme C., O’Laughlin B. J., and Sklansky J., Automatic Detection of Suspicious Abnormalities in Breast Radiographs (Academic Press, New York, 1975), pp. 427–447. [Google Scholar]
- Spiesberger W., “Mammogram inspection by computer,” IEEE Trans. Biomed. Eng. 26, 213–219 (1979). [DOI] [PubMed] [Google Scholar]
- Reiber J., Kooijman C., Slager C., Gerbrands J., Schuurbiers J., den Boer A., Wijns W., and Hugenholta S. P., “Coronary artery dimensions from cineangiograms—Methodology and validation of a computer-assisted analysis procedure,” IEEE Trans. Med. Imaging 3, 131–141 (1984). [DOI] [PubMed] [Google Scholar]
- Fujita H., Doi K., Fencil L. E., and Chua K. G., “Image feature analysis and computer-aided diagnosis in digital radiography. 2. Computerized determination of vessel sizes in digital subtraction angiography,” Med. Phys. 10.1118/1.596066 14, 549–556 (1987). [DOI] [PubMed] [Google Scholar]
- Chan H.-P., Doi K., Galhotra S., Vyborny C. J., MacMahon H., and Jokich P. M., “Image feature analysis and computer-aided diagnosis in digital radiography. 1. Automated detection of microcalcifications in mammography,” Med. Phys. 10.1118/1.596065 14, 538–548 (1987). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Doi K., and MacMahon H., “Computerized detection of lung nodules in digital chest radiographs,” Proc. SPIE 767, 384–386 (1987). [Google Scholar]
- Giger M. L., Doi K., and MacMahon H., “Image feature analysis and computer aided diagnosis in digital radiography. 3. Automated detection of nodules in peripheral lung fields,” Med. Phys. 10.1118/1.596247 15, 158–166 (1988). [DOI] [PubMed] [Google Scholar]
- Doi K., Chan H.-P., and Giger M., “Method and system for enhancement and detection of abnormal anatomic regions in a digital image.” University of Chicago, U. S. Pat. No. 4907156, March 1990.
- Giger M. L., “Computer-aided diagnosis,” in Syllabus: A Categorical Course in Physics. Technical Aspects of Breast Imaging, Haus A. G. and Yaffe M. J., eds. (RSNA Publications, Oak Brook, IL, 1993), pp. 272–298. [Google Scholar]
- Vyborny C. J. and Giger M. L., “Computer vision and artificial intelligence in mammography,” AJR, Am. J. Roentgenol. 162, 699–708 (1994). [DOI] [PubMed] [Google Scholar]
- Giger M. and MacMahon H., “Image processing and computer-aided diagnosis,” Radiol. Clin. North Am. 34, 565–596 (1996). [PubMed] [Google Scholar]
- Doi K., MacMahon H., Giger M., and Hoffmann K., Proceedings of the First International Workshop on Computer-Aided Diagnosis in Medical Imaging (Elsevier, New York, 1999).
- Giger M. L., Huo Z., Kupinski M. A., and Vyborny C. J., “Computer-aided diagnosis in mammography,” in Handbook of Medical Imaging, Sonka M. and Fitzpatrick J. M., eds. (The Society of Photo-Optical Instrumentation Engineers, Bellingham, WA, 2000), pp. 915–1004. [Google Scholar]
- Jiang Y., “Classification of breast lesions in mammograms.” in Handbook of Medical Imaging, Processing and Analysis, Bankman I., ed. (Academic Press, New York, 2000), pp. 341–358. [Google Scholar]
- Vyborny C. J., Giger M. L., and Nishikawa R. M., “Computer-aided detection and diagnosis of breast cancer,” Radiol. Clin. North Am. 10.1016/S0033-8389(05)70197-4 38, 725–740 (2000). [DOI] [PubMed] [Google Scholar]
- van Ginneken B., ter Haar Romeny B. M., and Viergever M. A., “Computer-aided diagnosis in chest radiography: A survey,” IEEE Trans. Med. Imaging 10.1109/42.974918 20, 1228–1241 (2001). [DOI] [PubMed] [Google Scholar]
- Giger M., “Computerized image analysis in breast cancer detection and diagnosis,” Seminars in Breast Disease 5, 199–210 (2002). [Google Scholar]
- Krupinski E., “The future of image perception in radiology: Synergy between humans and computers,” Acad. Radiol. 10, 1–3 (2003). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Sahiner B., and Hadjiiski L. M., “Computer-aided diagnosis in screening mammography,” in Advances in Breast Imaging: Physics, Technology, and Clinical Applications—Categorical Course in Diagnostic Radiology Physics, Karellas A. and Giger M. L., eds. (RSNA, Oak Brook, IL, 2004), pp. 191–204. [Google Scholar]
- Sluimer I., Schilham A., Prokop M., and van Ginneken B., “Computer analysis of computed tomography scans of the lung: A survey,” IEEE Trans. Med. Imaging 10.1109/TMI.2005.862753 25, 385–405 (2006). [DOI] [PubMed] [Google Scholar]
- Doi K., “Computer-aided diagnosis in medical imaging: historical review, current status and future potential,” Comput. Med. Imaging Graph. 10.1016/j.compmedimag.2007.02.002 31, 198–211 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa R. M., “Current status and future directions of computer-aided diagnosis in mammography,” Comput. Med. Imaging Graph. 10.1016/j.compmedimag.2007.02.009 31, 224–235 (2007). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Hadjiisk L., Zhou C., and Sahiner B., “Computer-aided diagnosis of lung cancer and pulmonary embolism in computed tomography—A review,” Acad. Radiol. 15, 535–555 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. M. and Swets J. A., Signal Detection Theory and Psychophysics (Wiley, New York, 1966). [Google Scholar]
- Swets J. A. and Pickett R. M., Evaluation of Diagnostic Systems: Methods from Signal Detection Theory (Academic Press, New York, 1982). [Google Scholar]
- Rossmann K., “Comparison of several methods for evaluation image quality of radiographic screen-film system,” Am. J. Roentgenol., Radium Ther. Nucl. Med. 97, 772–775 (1966). [DOI] [PubMed] [Google Scholar]
- Rossmann K., “Image quality,” Radiol. Clin. North Am. 7, 419–433 (1969). [PubMed] [Google Scholar]
- Goodenough D. J., Rossmann K., and Lusted L. B., “Radiographic applications of signal detection theory,” Radiology 105, 199–200 (1972). [DOI] [PubMed] [Google Scholar]
- Dainty J. C. and Shaw R., Image Science (Academic Press, New York, 1974). [Google Scholar]
- Wagner R. F., “Toward a unified view of radiological imaging systems. Part II: Noisy images,” Med. Phys. 10.1118/1.594362 4, 279–296 (1977). [DOI] [PubMed] [Google Scholar]
- Metz C. E., “ROC methodology in radiologic imaging,” Invest. Radiol. 10.1097/00004424-198609000-00009 21, 720–733 (1986). [DOI] [PubMed] [Google Scholar]
- Barrett H. H. and Myers K., Foundations of Image Science (Wiley, New York, 2004). [Google Scholar]
- Wagner R. F., Weaver K. E., Denny E. W., and Bostrom R. G., “Toward a unified view of radiological imaging systems. Part I: Noiseless images,” Med. Phys. 10.1118/1.1637272 1, 11–24 (1974). [DOI] [PubMed] [Google Scholar]
- Sandrik J. M. and Wagner R. F., “Absolute measures of physical image quality: Measurement and application to radiographic magnification,” Med. Phys. 10.1118/1.595099 9, 540–549 (1982). [DOI] [PubMed] [Google Scholar]
- Giger M. L. and Doi K., “Investigation of basic imaging properties of digital radiography. Part 1: Modulation transfer function,” Med. Phys. 10.1118/1.595629 11, 287–295 (1984). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Doi K., and Metz C. E., “Investigation of basic imaging properties of digital radiography. Part 2: Noise Wiener spectrum,” Med. Phys. 10.1118/1.595583 11, 797–805 (1984). [DOI] [PubMed] [Google Scholar]
- Loo L.-N. D., Doi K., and Metz C. E., “A comparison of physical image quality indices and observer performance in the radiographic detection of nylon beads,” Phys. Med. Biol. 10.1088/0031-9155/29/7/007 29, 837–856 (1984). [DOI] [PubMed] [Google Scholar]
- Giger M. L. and Doi K., “Investigation of basic imaging properties in digital radiography. 3. Effect of pixel size on SNR and threshold contrast,” Med. Phys. 10.1118/1.595708 12, 201–208 (1985). [DOI] [PubMed] [Google Scholar]
- Tapiovaara M. J. and Wagner R. J., “SNR and DQE analysis of broad-spectrum x-ray imaging,” Phys. Med. Biol. 10.1088/0031-9155/30/6/002 30, 519–529 (1985). [DOI] [Google Scholar]
- Bassett L. W., Bunnell D. H., Jahanshahi R., Gold R. H., Arndt R. D., and Linsman J., “Breast cancer detection: One versus two views,” Radiology 165, 95–97 (1987). [DOI] [PubMed] [Google Scholar]
- Hillman B. J., Fajardo L. L., Hunter T. B., Mockbee B., Cook C. E., Hagman R. M., Bjelland J. C., Frey C. S., and Harris C. J., “Mammogram interpretation by physician assistants,” AJR, Am. J. Roentgenol. 149, 907–911 (1987). [DOI] [PubMed] [Google Scholar]
- Wallis M. G., Walsh M. T., and Lee J. R., “A review of false negative mammography in a symptomatic population,” Clin. Radiol. 10.1016/S0009-9260(05)80218-1 44, 13–15 (1991). [DOI] [PubMed] [Google Scholar]
- Bird R. E., Wallace T. W., and Yankaskas B. C., “Analysis of cancers missed at screening mammography,” Radiology 184, 613–617 (1992). [DOI] [PubMed] [Google Scholar]
- Harvey J. A., Fajardo L. L., and Innis C. A., “Previous mammograms in patients with impalpable breast carcinomas: Retrospective vs blinded interpretation,” AJR, Am. J. Roentgenol. 161, 1167–1172 (1993). [DOI] [PubMed] [Google Scholar]
- Beam C. A., Layde P. M., and Sullivan D. C., “Variability in the interpretation of screening mammograms by US radiologists—Findings from a national sample,” Arch. Intern Med. 10.1001/archinte.156.2.209 156, 209–213 (1996). [DOI] [PubMed] [Google Scholar]
- Elmore J. G., Nakano C. Y., Koepsell T. D., Desnick L. M., D’Orsi C. J., and Ransohoff D. F., “International variation in screening mammography interpretations in community-based programs,” J. Natl. Cancer Inst. 95, 1384–1393 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdwell R. L., Ikeda D. M., O’Shaughnessy K. F., and Sickles E. A., “Mammographic characteristics of 115 missed cancers later detected with screening mammography and the potential utility of computer-aided detection,” Radiology 219, 192–202 (2001). [DOI] [PubMed] [Google Scholar]
- Giger M., Huo Z., Kupinski M., and Vyborny C., “Computer-aided diagnosis in mammography,” in Handbook of Medical Imaging Volume II: Medical Imaging Processing and Analysis, Sonka M. and Fitzpatrick M. eds. (SPIE, Bellingham, WA, 2000). [Google Scholar]
- Nishikawa R., “Current status and future directions of computer-aided diagnosis in mammography,” Comput. Med. Imaging Graph. 10.1016/j.compmedimag.2007.02.009 31, 224–235 (2007). [DOI] [PubMed] [Google Scholar]
- Semmlow J. L., Shadagopappan A., Ackerman L. V., Hand W., and Alcorn F. S., “A fully automated system for screening mammograms,” Comput. Biomed. Res. 10.1016/0010-4809(80)90027-0 13, 350–362 (1980). [DOI] [PubMed] [Google Scholar]
- Fam B. W., Olson S. L., Winter P. F., and Scholz F. J., “Algorithm for the detection of fine clustered calcifications on film mammograms,” Radiology 169, 333–337 (1988). [DOI] [PubMed] [Google Scholar]
- Davies D. H. and Dance D. R., “Automatic computer detection of clustered calcifications in digital mammograms,” Phys. Med. Biol. 10.1088/0031-9155/35/8/007 35, 1111–1118 (1990). [DOI] [PubMed] [Google Scholar]
- Astley S., Hutt I., Adamson S., Miller P., Rose P., Boggis C., Taylor C., Valentine T., Davies J., and Armstrong J., “Automation in mammography: Computer vision and human perception,” Proc. SPIE 10.1117/12.148683 1905, 716–730 (1993). [DOI] [Google Scholar]
- Bankman I. N., Christens-Barry W. A., Kim D. W., Weinberg I. N., Gatewood O. B., and Brody W. R., “Automated recognition of microcalcification clusters in mammograms,” Proc. SPIE 10.1117/12.148684 1905, 731–738 (1993). [DOI] [Google Scholar]
- Karssemeijer N., “Recognition of clustered microcalcifications using a random field model,” Proc. SPIE 10.1117/12.148689 1905, 776–786 (1993). [DOI] [Google Scholar]
- Nishikawa R. M., Giger M. L., Doi K., Vyborny C. J., Schmidt R. A., Metz C. E., Wu Y., Yin F.-F., Jiang Y., Huo Z., Lu P., Zhang W., Ema T., Bick U., Papaioannou J., and Nagel R. H., “Computer-aided detection and diagnosis of masses and clustered microcalcifications from digital mammograms,” Proc. SPIE 10.1117/12.148655 1905, 422–432 (1993). [DOI] [Google Scholar]
- Shen L., Rangayyan R. M., and Desautels J. E. L., “Automatic detection and classification system for calcifications in mammograms,” Proc. SPIE 10.1117/12.148691 1905, 799–805 (1993). [DOI] [Google Scholar]
- Clarke L. P., Kallergi M., Qian W., Li H. D., Clark R. A., and Silbiger M. L., “Tree-structured non-linear filter and wavelet transform for microcalcification segmentation in digital mammography,” Cancer Lett. 77, 173–181 (1994). [DOI] [PubMed] [Google Scholar]
- Qian W., Clarke L. P., Kallergi M., and Clark R. A., “Tree-structured nonlinear filters in digital mammography,” IEEE Trans. Med. Imaging 10.1109/42.276142 13, 25–36 (1994). [DOI] [PubMed] [Google Scholar]
- Zhang W., Doi K., Giger M. L., Wu Y., Nishikawa R. M., and Schmidt R. A., “Computerized detection of clustered microcalcifications in digital mammograms using a shift-invariant artificial neural network,” Med. Phys. 10.1118/1.597177 21, 517–524 (1994). [DOI] [PubMed] [Google Scholar]
- Chan H.-P., Lo S.-C. B., Sahiner B., Lam K. L., and Helvie M. A., “Computer-aided detection of mammographic microcalcifications: Pattern recognition with an artificial neural network,” Med. Phys. 10.1118/1.597428 22, 1555–1567 (1995). [DOI] [PubMed] [Google Scholar]
- Zheng B., Chang Y.-H., Staiger M., Good W., and Gur D., “Computer-aided detection of clustered microcalcifications in digitized mammograms,” Acad. Radiol. 2, 655–662 (1995). [DOI] [PubMed] [Google Scholar]
- Strickland R. N. and Hahn H., “Wavelet transforms for detecting microcalcifications in mammograms,” IEEE Trans. Med. Imaging 10.1109/42.491423 15, 218–229 (1996). [DOI] [PubMed] [Google Scholar]
- Yoshida H., Doi K., Nishikawa R. M., Giger M. L., and Schmidt R. A., “An improved computer-assisted diagnostic scheme using wavelet transform for detecting clustered microcalcifications in digital mammograms,” Acad. Radiol. 3, 621–627 (1996). [DOI] [PubMed] [Google Scholar]
- Gavrielides M. A., Lo J. Y., Vargas-Voracek R., and Floyd J. C. E., “Segmentation of suspicious clustered microcalcifications in mammograms,” Med. Phys. 10.1118/1.598852 27, 13–22 (2000). [DOI] [PubMed] [Google Scholar]
- Yu S. and Guan L., “A CAD system for the automatic detection of clustered microcalcifications in digitized mammograms,” IEEE Trans. Med. Imaging 10.1109/42.836371 19, 115–126 (2000). [DOI] [PubMed] [Google Scholar]
- El-Naqa I., Yang Y., Nishikawa R. M., and Wernick M. N., “A support vector machine approach for detection of microcalcifications,” IEEE Trans. Med. Imaging 10.1109/TMI.2002.806569 21, 1552–1563 (2002). [DOI] [PubMed] [Google Scholar]
- Salfity M., Nishikawa R., Jiang Y., and Papaioannou J., “The use of a priori information to improve the detection of microcalcifications on mammograms,” Med. Phys. 10.1118/1.1559884 30, 823–831 (2002). [DOI] [PubMed] [Google Scholar]
- Ge J., Sahiner B., Hadjiiski L. M., Chan H.-P., Wei J., Helvie M. A., and Zhou C., “Computer aided detection of clusters of microcalcifications on full field digital mammograms,” Med. Phys. 10.1118/1.2211710 33, 2975–2988 (2006). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H.-P., Hadjiiski L. M., Helvie M. A., Paramagul C., Ge J., Wei J., and Zhou C., “Joint two-view information for computerized detection of microcalcifications on mammograms,” Med. Phys. 10.1118/1.2208919 33, 2574–2585 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimme C., O’Loughlin B. J., and Sklansky J., “Automatic detection of suspicious abnormalities in breast radiographs,” in Data Structures, Computer Graphics, and Pattern Recognition, Fu K. S., Kunii T. L., and Klinger A., eds. (Academic Press, New York, 1975), pp. 427–447. [Google Scholar]
- Lai S. M., Li X., and Bischof W. F., “On techniques for detecting circumscribed masses in mammograms,” IEEE Trans. Med. Imaging 10.1109/42.41491 8, 377–386 (1989). [DOI] [PubMed] [Google Scholar]
- Brzakovic D., Luo X. M., and Brzakovic P., “An approach to automated detection of tumors in mammograms,” IEEE Trans. Med. Imaging 10.1109/42.57760 9, 233–241 (1990). [DOI] [PubMed] [Google Scholar]
- Lau T. K. and Bischof W. F., “Automated detection of breast tumors using the asymmetry approach,” Comput. Biomed. Res. 10.1016/0010-4809(91)90049-3 24, 273–295 (1991). [DOI] [PubMed] [Google Scholar]
- Yin F.-F., Giger M. L., Doi K., Metz C. E., Vyborny C. J., and Schmidt R. A., “Computerized detection of masses in digital mammograms: Analysis of bilateral subtraction images,” Med. Phys. 10.1118/1.596610 18, 955–963 (1991). [DOI] [PubMed] [Google Scholar]
- Ng S. L. and Bischof W. F., “Automated detection and classification of breast tumors,” Comput. Biomed. Res. 10.1016/0010-4809(92)90040-H 25, 218–237 (1992). [DOI] [PubMed] [Google Scholar]
- Yin F.-F., Giger M. L., Vyborny C. J., Doi K., and Schmidt R. A., “Comparison of bilateral-subtraction and single-image processing techniques in the computerized detection of mammographic masses,” Invest. Radiol. 28, 473–481 (1993). [DOI] [PubMed] [Google Scholar]
- Kegelmeyer W. P., Pruneda J. M., Bourland P. D., Hillis A., Riggs M. W., and Nipper M. L., “Computer-aided mammographic screening for spiculated lesions,” Radiology 191, 331–337 (1994). [DOI] [PubMed] [Google Scholar]
- Laine A., Schuler S., Fan J., and Huda W., “Mammographic feature enhancement by multiscale analysis,” IEEE Trans. Med. Imaging 10.1109/42.363095 13, 725–740 (1994). [DOI] [PubMed] [Google Scholar]
- Li H. D., Kallergi M., Clarke L. P., Jain V. K., and Clarke R. A., “Markov random field for tumor detection in digital mammograms,” IEEE Trans. Med. Imaging 10.1109/42.414622 14, 565–576 (1995). [DOI] [PubMed] [Google Scholar]
- Zheng B., Chang Y. H., and Gur D., “Computerized detection of masses in digitized mammograms using single-image segmentation and a multilayer topographic feature analysis,” Acad. Radiol. 2, 959–966 (1995). [DOI] [PubMed] [Google Scholar]
- Karssemeijer N. and te Brake G., “Detection of stellate distortions in mammograms,” IEEE Trans. Med. Imaging 10.1109/42.538938 15, 611–619 (1996). [DOI] [PubMed] [Google Scholar]
- Petrick N., Chan H. P., Wei D., Sahiner B., Helvie M. A., and Adler D. D., “Automated detection of breast masses on mammograms using adaptive contrast enhancement and texture classification,” Med. Phys. 10.1118/1.597756 23, 1685–1696 (1996). [DOI] [PubMed] [Google Scholar]
- Wei D., Chan H. P., Petrick N., Sahiner B., Helvie M. A., Adler D. D., and Goodsitt M. M., “False-positive reduction technique for detection of masses on digital mammograms: Global and local multiresolution texture analysis,” Med. Phys. 10.1118/1.598011 24, 903–914 (1997). [DOI] [PubMed] [Google Scholar]
- Mendez A. J., Tahoces P. G., Lado M. J., Souto M., and Vidal J. J., “Computer-aided diagnosis: Automatic detection of malignant masses in digitized mammograms,” Med. Phys. 10.1118/1.598274 25, 957–964 (1998). [DOI] [PubMed] [Google Scholar]
- Kobatake H., Murakami M., Takeo H., and Nawano S., “Computer detection of malignant tumors on digital mammograms,” IEEE Trans. Med. Imaging 10.1109/42.774164 18, 369–378 (1999). [DOI] [PubMed] [Google Scholar]
- Petrick N., Chan H. P., Sahiner B., and Helvie M. A., “Combined adaptive enhancement and region-growing segmentation of breast masses on digitized mammograms,” Med. Phys. 10.1118/1.598658 26, 1642–1654 (1999). [DOI] [PubMed] [Google Scholar]
- te Brake G. M. and Karssemeijer N., “Single and multiscale detection of masses in digital mammograms,” IEEE Trans. Med. Imaging 10.1109/42.790462 18, 628–639 (1999). [DOI] [PubMed] [Google Scholar]
- te Brake G. M., Karssemeijer N., and Hendriks J., “An automatic method to discriminate malignant masses from normal tissue in digital mammograms,” Phys. Med. Biol. 10.1088/0031-9155/45/10/308 45, 2843–2857 (2000). [DOI] [PubMed] [Google Scholar]
- Hatanaka Y., Hara T., Fujita H., Kasai S., Endo T., and Iwase T., “Development of an automated method for detecting mammographic masses with a partial loss of region,” IEEE Trans. Med. Imaging 10.1109/42.974916 20, 1209–1214 (2001). [DOI] [PubMed] [Google Scholar]
- Mudigonda N. R., Rangayyan R. M., and Desautels J. E. L., “Detection of breast masses in mammograms by density slicing and texture flow-field analysis,” IEEE Trans. Med. Imaging 10.1109/42.974917 20, 1215–1227 (2001). [DOI] [PubMed] [Google Scholar]
- Lo S. C. B., Li H., Wang Y., Kinnard L., and Freedman M. T., “A multiple circular path convolution neural network system for detection of mammographic masses,” IEEE Trans. Med. Imaging 10.1109/42.993133 21, 150–158 (2002). [DOI] [PubMed] [Google Scholar]
- Petrick N., Chan H. P., Sahiner B., Helvie M. A., Paquerault S., and Hadjiiski L. M., “Breast cancer detection: Evaluation of a mass detection algorithm for computer-aided diagnosis: Experience in 263 patients,” Radiology 10.1148/radiol.2241011062 224, 217–224 (2002). [DOI] [PubMed] [Google Scholar]
- Baydush A. H., Catarious D. M., Abbey C. K., and Floyd C. E., “Computer aided detection of masses in mammography using subregion Hotelling observers,” Med. Phys. 10.1118/1.1582011 30, 1781–1787 (2003). [DOI] [PubMed] [Google Scholar]
- Tourassi G. D., Vargas-Voracek R., David M.Catarious, Jr., and Carey E.Floyd, Jr., “Computer-assisted detection of mammographic masses: A template matching scheme based on mutual information,” Med. Phys. 10.1118/1.1589494 30, 2123–2130 (2003). [DOI] [PubMed] [Google Scholar]
- Wei J., Chan H.-P., Sahiner B., Hadjiiski L. M., Helvie M. A., Roubidoux M. A., Zhou C., and Ge J., “Dual system approach to computer-aided detection of breast masses on mammograms,” Med. Phys. 10.1118/1.2357838 33, 4157–4168 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Hadjiiski L. M., Sahiner B., Chan H., Ge J., Roubidoux M. A., Helvie M. A., Zhou C., Wu Y., Paramagul C., and Zhang Y., “Computer aided detection systems for breast masses: Comparison of performances on full-field digital mammograms and digitized screen-film mammograms,” Acad. Radiol. 6, 659–669 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampat M. P., Bovik A. C., Whitman G. J., and Markey M. K., “A model-based framework for the detection of spiculated masses on mammography,” Med. Phys. 10.1118/1.2890080 35, 2110–2123 (2008). [DOI] [PubMed] [Google Scholar]
- Tourassi G. D., Harrawood B., Singh S., Lo J. Y., and Floyd C. E., “Evaluation of information-theoretic similarity measures for content-based retrieval and detection of masses in mammograms,” Med. Phys. 10.1118/1.2401667 34, 140–150 (2007). [DOI] [PubMed] [Google Scholar]
- Chan H.-P., Doi K., Vyborny C. J., Schmidt R. A., Metz C. E., Lam K. L., Ogura T., Wu Y., and MacMahon H., “Improvement in radiologists’ detection of clustered microcalcifications on mammograms: The potential of computer-aided diagnosis,” Invest. Radiol. 10.1097/00004424-199010000-00006 25, 1102–1110 (1990). [DOI] [PubMed] [Google Scholar]
- Metz C., “Fundamental ROC analysis,” in Handbook of Medical Imaging, Volume I: Physics and Psychophysics, Beutel J., Kundel H., and Van Metter R., eds. (SPIE Press, Bellingham, 2000), pp. 751–769. [Google Scholar]
- Helvie M. A., Hadjiiski L., Makariou E., Chan H.-P., Petrick N., Sahiner B., Lo S.-C. B., Freedman M., Adler D., Bailey J., Blane C., Hoff D., Hunt K., Joynt L., Klein K., Paramagul C., Patterson S. K., and Roubidoux M. A., “Sensitivity of noncommercial computer-aided detection system for mammographic breast cancer detection,” Radiology 10.1148/radiol.2311030429 231, 208–214 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurfjell E., Taube A., and Tabar L., “One-view versus 2-view mammography screening—A prospective population-based study,” Acta Radiol. 35, 340–344 (1994). [PubMed] [Google Scholar]
- Blanks R. G., Wallis M. G., and Given-Wilson R. M., “Observer variability in cancer detection during routine repeat (incident) mammographic screening in a study of two versus one view mammography,” J. Med. Screen 6, 152–158 (1999). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Petrick N., Chan H. P., Paquerault S., Helvie M. A., and Hadjiiski L. M., “Recognition of lesion correspondence on two mammographic views—A new method of false-positive reduction for computerized mass detection,” Proc. SPIE 10.1117/12.431139 4322, 649–655 (2001). [DOI] [Google Scholar]
- Paquerault S., Petrick N., Chan H. P., Sahiner B., and Helvie M. A., “Improvement of computerized mass detection on mammograms: Fusion of two-view information,” Med. Phys. 10.1118/1.1446098 29, 238–247 (2002). [DOI] [PubMed] [Google Scholar]
- Zheng B., Leader J. K., Abrams G. S., Lu A. H., Wallace L. P., Maitz G. S., and Gur D., “Multiview-based computer-aided detection scheme for breast masses,” Med. Phys. 10.1118/1.2237476 33, 3135–3143 (2006). [DOI] [PubMed] [Google Scholar]
- v. Engeland S., and Karssemeijer N., “Combining two mammographic projections in a computer aided mass detection method,” Med. Phys. 10.1118/1.2436974 34, 898–905 (2007). [DOI] [PubMed] [Google Scholar]
- Yin F.-F., Giger M. L., Doi K., Vyborny C. J., and Schmidt R. A., “Computerized detection of masses in digital mammograms: Automated alignment of breast images and its effect on bilateral-subtraction technique,” Med. Phys. 10.1118/1.597307 21, 445–452 (1994). [DOI] [PubMed] [Google Scholar]
- Hadjiiski L. M., Chan H. P., Sahiner B., Petrick N., and Helvie M. A., “Automated registration of breast lesions in temporal pairs of mammograms for interval change analysis—Local affine transformation for improved localization,” Med. Phys. 10.1118/1.1376134 28, 1070–1079 (2001). [DOI] [PubMed] [Google Scholar]
- Wu Y.-T., Wei J., Hadjiiski L. M., Sahiner B., Zhou C., Ge J., Shi J., Zhang Y., and Chan H. P., “Bilateral analysis based false positive reduction for computer-aided mass detection,” Med. Phys. 10.1118/1.2756612 34, 3334–3344 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouras W., Giger M., Lu P., Wolverton D., Vyborny C., and Doi K., “Investigation of temporal subtraction scheme for computerized detection of breast masses.” Proceedings of Digital Mammography ‘96 Annual Meeting (1996). [Google Scholar]
- Sanjay-Gopal S., Chan H. P., Wilson T., Helvie M., Petrick N., and Sahiner B., “A regional registration technique for automated interval change analysis of breast lesions on mammograms,” Med. Phys. 10.1118/1.598806 26, 2669–2679 (1999). [DOI] [PubMed] [Google Scholar]
- Timp S., Van Engeland S., and Karssemeijer N., “A regional registration method to find corresponding mass lesions in temporal mammogram pairs,” Med. Phys. 10.1118/1.1984323 32, 2629–2638 (2005). [DOI] [PubMed] [Google Scholar]
- Carson P. L., LeCarpentier G. L., Roubidoux M. A., Erkamp R. Q., Fowlkes J. B., and Goodsitt M. M., “Physics and technology of breast US imaging including automated three-dimensional US,” in Advances in Breast Imaging: Physics, Technology, and Clinical Applications—Categorical Course in Diagnostic Radiology Physics, Karellas A. and Giger M. L., eds. (RSNA, Oak Brook, IL, 2004), pp. 223–232. [Google Scholar]
- Niklason L. T., Christian B. T., Niklason L. E., Kopans D. B., Castleberry D. E., Opsahl-Ong B. H., Landberg C. E., Slanetz P. J., Giardino A. A., Moore R., Albagli D., DeJule M. C., Fitzgerald F. C., Fobare D. F., Giambattista B. W., Kwasnick R. F., Liu J., Lubowski S. J., Possin G. E., Richotte J. F., Wirth C.-Y., and Wirth R. F., “Digital tomosynthesis in breast imaging,” Radiology 205, 399–406 (1997). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., Vedantham S., Baker S. P., Glick S. J., D’Orsi C. J., and Webber R. L., “Evaluation of linear and nonlinear tomosynthetic reconstruction methods in digital mammography,” Acad. Radiol. 8, 219–224 (2001). [DOI] [PubMed] [Google Scholar]
- Rafferty E. A., Georgian-Smith D., Kopans D. B., Hall D. A., Moore R., and Wu T., “Comparison of full-field digital tomosynthesis with two view conventional film screen mammography in the prediction of lesion malignancy,” Radiology 225(P), 268–268 (2002). [Google Scholar]
- Wu T., Stewart A., Stanton M., McCauley T., Phillips W., Kopans D. B., Moore R. H., Eberhard J. W., Opsahl-Ong B., Niklason L., and Williams M. B., “Tomographic mammography using a limited number of low-dose cone-beam projection images,” Med. Phys. 10.1118/1.1543934 30, 365–380 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chan H.-P., Sahiner B., Wei J., Goodsitt M. M., Hadjiiski L. M., Ge J., and Zhou C., “A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis,” Med. Phys. 10.1118/1.2237543 33, 3781–3795 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong R. A., Yaffe M. J., Skarpathiotakis M., Shumak R. S., Danjoux N. M., Gunesekara A., and Plewes D. B., “Contrast-enhanced digital mammography: Initial clinical experience,” Radiology 10.1148/radiol.2283020961 228, 842–850 (2003). [DOI] [PubMed] [Google Scholar]
- Lewin J. M., Isaacs P. K., Vance V., and Larke F. J., “Dual-energy contrast-enhanced digital subtraction mammography: Feasibility,” Radiology 10.1148/radiol.2291021276 229, 261–268 (2003). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Nelson T. R., Lindfors K. K., and Seibert J. A., “Dedicated breast CT: Radiation dose and image quality evaluation,” Radiology 10.1148/radiol.2213010334 221, 657–667 (2001). [DOI] [PubMed] [Google Scholar]
- Chen B. and Ning R., “Cone-beam volume CT breast imaging: Feasibility study,” Med. Phys. 10.1118/1.1461843 29, 755–770 (2002). [DOI] [PubMed] [Google Scholar]
- Kwan A. L. C., Boone J. M., Yang K., and Huang S.-Y., “Evaluation of the spatial resolution characteristics of a cone-beam breast CT scanner,” Med. Phys. 10.1118/1.2400830 34, 275–281 (2007). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Wei J., Sahiner B., Rafferty E. A., Wu T., Roubidoux M. A., Moore R. H., Kopans D. B., Hadjiiski L. M., and Helvie M. A., “Computerized detection of masses on digital tomosynthesis mammograms—A preliminary study,” Proceedings of IWDM, 2004, pp. 199–202.
- Reiser I., Nishikawa R. M., Giger M. L., Wu T., Rafferty E. A., Moore R., and Kopans D. B., “Computerized mass detection for digital breast tomosynthesis directly from the projection images,” Med. Phys. 10.1118/1.2163390 33, 482–491 (2006). [DOI] [PubMed] [Google Scholar]
- Katsuragawa S., Doi K., and MacMahon H., “Image feature analysis and computer-aided diagnosis in digital radiography. Detection and characterization of interstitial lung disease in digital chest radiographs,” Med. Phys. 10.1118/1.596224 15, 311–319 (1988). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Ahn N., Doi K., MacMahon H., and Metz C. E., “Computerized detection of pulmonary nodules in digital chest images: Use of morphological filters in reducing false-positive detections,” Med. Phys. 10.1118/1.596478 17, 861–865 (1990). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Doi K., MacMahon H., Metz C. E., and Yin F.-F., “Computer-aided detection of pulmonary nodules in digital chest images,” Radiographics 10, 41–52 (1990). [DOI] [PubMed] [Google Scholar]
- Lo S. C., Lou S. L., Lin J. S., Freedman M. T., and Mun S. K., “Artificial convolution neural network techniques and applications to lung nodule detection,” IEEE Trans. Med. Imaging 10.1109/42.476112 14, 711–718 (1995). [DOI] [PubMed] [Google Scholar]
- Yue Z., Goshtasby A., and Ackerman L., “Automatic detection of rib borders in chest radiographs,” IEEE Trans. Med. Imaging 10.1109/42.414618 14, 525–536 (1995). [DOI] [PubMed] [Google Scholar]
- Xu X. W., Doi K., Kobayashi T., MacMahon H., and Giger M. L., “Development of an improved CAD scheme for automated detection of lung nodules in digital chest images,” Med. Phys. 10.1118/1.598028 24, 1395–1403 (1997). [DOI] [PubMed] [Google Scholar]
- Mao F., Qian W., Gaviria J., and Clarke L. P., “Fragmentary window filtering for multiscale lung nodule detection: Preliminary study,” Acad. Radiol. 5, 306–311 (1998). [DOI] [PubMed] [Google Scholar]
- Penedo M. G., Carreira M. J., Mosquera A., and Cabello D., “Computer-aided diagnosis: A neural-network-based approach to lung nodule detection,” IEEE Trans. Med. Imaging 10.1109/42.746620 17, 872–880 (1998). [DOI] [PubMed] [Google Scholar]
- Bae K. T., Kim J. S., Na Y. H., Kim K. G., and Kim J. H., “Pulmonary nodules: Automated detection on CT images with morphologic matching algorithm—Preliminary results,” Radiology 236, 286–293 (2005). [DOI] [PubMed] [Google Scholar]
- Suzuki K., Shiraishi J., Abe H., MacMahon H., and Doi K., “False-positive reduction in computer-aided diagnostic scheme for detecting nodules in chest radiographs by means of massive training artificial neural network,” Acad. Radiol. 12, 191–201 (2005). [DOI] [PubMed] [Google Scholar]
- Shiraishi J., Li F., and Doi K., “Computer-aided diagnosis for improved detection of lung nodules by use of PA and lateral chest radiographs,” Acad. Radiol. 14, 28–37 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Katsuragawa S., Kobayashi T., MacMahon H., and Doi K., “Computerized analysis of interstitial disease in chest radiographs: Improvement of geometric-pattern feature analysis,” Med. Phys. 10.1118/1.598012 24, 915–924 (1997). [DOI] [PubMed] [Google Scholar]
- Sanada S., Doi K., and MacMahon H., “Image feature analysis and computer-aided diagnosis in digital radiography. Automated detection of pneumothorax in chest images,” Med. Phys. 10.1118/1.596790 19, 1153–1160 (1992). [DOI] [PubMed] [Google Scholar]
- Kano A., Doi K., MacMahon H., Hassell D. D., and Giger M. L., “Digital image subtraction of temporally sequential chest images for detection of interval change,” Med. Phys. 10.1118/1.597308 21, 453–461 (1994). [DOI] [PubMed] [Google Scholar]
- Ishida T., Katsuragawa S., Nakamura K., MacMahon H., and Doi K., “Iterative image warping technique for temporal subtraction of sequential chest radiographs to detect interval change,” Med. Phys. 10.1118/1.598627 26, 1320–1329 (1999). [DOI] [PubMed] [Google Scholar]
- Armato S. G., Doshi D. J., Engelmann R., Caligiuri P., and MacMahon H., “Temporal subtraction of dual-energy chest radiographs,” Med. Phys. 10.1118/1.2163387 33, 1911–1919 (2006). [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Xu X.-W., MacMahon H., Metz C. E., and Doi K., “Effect of a computer-aided diagnosis scheme on radiologists’ performance in detection of lung nodules on radiographs,” Radiology 199, 843–848 (1996). [DOI] [PubMed] [Google Scholar]
- MacMahon H., Engelmann R., Behlen F. M., Hoffmann K. R., Ishida T., Roe C., Metz C. E., and Doi K., “Computer-aided diagnosis of pulmonary nodules: Results of a large-scale observer test,” Radiology 213, 723–726 (1999). [DOI] [PubMed] [Google Scholar]
- Kakeda S., Moriya J., Sato H., Aoki T., Watanabe H., Nakata H., Oda N., Katsuragawa S., Yamamoto K., and Doi K., “Improved detection of lung nodules with aid of computerized detection method: Evaluation of a commercial computer-aided diagnosis system,” AJR, Am. J. Roentgenol. 182, 505–510 (2004). [DOI] [PubMed] [Google Scholar]
- Sakai S., Soeda H., Takahashi N., Okafuji T., Yoshitake T., Yabuuchi H., Yoshino I., Yamamoto K., Honda H., and Doi K., “Computer-aided nodule detection on digital chest radiography: Validation test on consecutive T1 cases of resectable lung cancer,” J. Digit Imaging 19, 376–382 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke C. I., McCauley D. I., Yankelevitz D. F., Naidich D. P., McGuinness G., Miettinen O. S., Libby D. M., Pasmantier M. W., Koizumi J., Altorki N. K., and Smith J. P., “Early lung cancer action project: Overall design and findings from baseline screening,” Lancet 10.1016/S0140-6736(99)06093-6 354, 99–105 (1999). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Bae K. T., and MacMahon H., “Computerized detection of pulmonary nodules in computed tomography images,” Invest. Radiol. 10.1097/00004424-199404000-00013 29, 459–465 (1994). [DOI] [PubMed] [Google Scholar]
- Armato S. G., Giger M. L., and MacMahon H., “Automated detection of lung nodules in CT scans: Preliminary results,” Med. Phys. 10.1118/1.1387272 28, 1552–1561 (2001). [DOI] [PubMed] [Google Scholar]
- Brown M. S., McNitt-Gray M. F., Goldin J. G., Suh R. D., Sayre J. W., and Aberle D. R., “Patient-specific models for lung nodule detection and surveillance in CT images,” IEEE Trans. Med. Imaging 10.1109/42.974919 20, 1242–1250 (2001). [DOI] [PubMed] [Google Scholar]
- Armato S., Li F., Giger M., MacMahon H., Sone S., and Doi K., “Lung cancer: Performance of automated lung nodule detection applied to cancers missed in a CT screening program,” Radiology 225, 685–692 (2002). [DOI] [PubMed] [Google Scholar]
- Gurcan M. N., Sahiner B., Petrick N., Chan H. P., Kazerooni E. A., Cascade P. N., and Hadjiiski L., “Lung nodule detection on thoracic computed tomography images: Preliminary evaluation of a computer-aided diagnosis system,” Med. Phys. 10.1118/1.1515762 29, 2552–2558 (2002). [DOI] [PubMed] [Google Scholar]
- Suzuki K., S. G.ArmatoIII, Li F., Sone S., and Doi K., “Massive training artificial neural network (MTANN) for reduction of false positives in computerized detection of lung nodules in low-dose computed tomography,” Med. Phys. 10.1118/1.1580485 30, 1602–1617 (2003). [DOI] [PubMed] [Google Scholar]
- Sensakovic W., S.ArmatoIII, Starkey A., and Ogarek J., “Automated matching of temporally sequential CT sections,” Med. Phys. 10.1118/1.1812611 31, 3417–3424 (2004). [DOI] [PubMed] [Google Scholar]
- Ge Z., Sahiner B., Chan H. P., Hadjiiski L. M., Cascade P. N., Bogot N., Kazerooni E. A., Wei J., and Zhou C., “Computer aided detection of lung nodules: False positive reduction using a 3D gradient field method and 3D ellipsoid fitting,” Med. Phys. 10.1118/1.1944667 32, 2443–2454 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peldschus K., Herzog P., Wood S. A., Cheema J. I., Costello P., and Schoepf U. J., “Computer-aided diagnosis as a second reader—Spectrum of findings in CT studies of the chest interpreted as normal,” Chest 10.1378/chest.128.3.1517 128, 1517–1523 (2005). [DOI] [PubMed] [Google Scholar]
- Rubin G. D., Lyo J. K., Paik D. S., Sherbondy A. J., Chow L. C., Leung A. N., Mindelzun R., Schraedley-Desmond P. K., Zinck S. E., Naidich D. P., and Napel S., “Pulmonary nodules on multi-detector row CT scans: Performance comparison of radiologists and computer-aided detection,” Radiology 10.1148/radiol.2341040589 234, 274–283 (2005). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Ge Z., Chan H., Hadjiiski L. M., Bogot N., Cascade P., and Kazerooni E., “False positive reduction using Hessian features in computer-aided detection of pulmonary nodules on thoracic CT images.” Proceedings of the SPIE—Medical Imaging Annual Meeting 2005.
- Marten K. and Engelke C., “Computer-aided detection and automated CT volumetry of pulmonary nodules,” Eur. Radiol. 17, 888–901 (2007). [DOI] [PubMed] [Google Scholar]
- Armato S. G., McLennan G., McNitt-Gray M. F., Meyer C. R., Yankelevitz D., Aberle D. R., Henschke C. I., Hoffman E. A., Kazerooni E. A., MacMahon H., Reeves A. P., Croft B. Y., and Clarke L. P., “Lung image database consortium: Developing a resource for the medical imaging research community,” Radiology 10.1148/radiol.2323032035 232, 739–748 (2004). [DOI] [PubMed] [Google Scholar]
- Awai K., Murao K., Ozawa A., Komi M., Hayakawa H., Hori S., and Nishimura Y., “Pulmonary nodules at chest CT: Effect of computer-aided diagnosis on radiologists’ detection performance,” Radiology 10.1148/radiol.2302030049 230, 347–352 (2004). [DOI] [PubMed] [Google Scholar]
- Lee J. W., Goo J. M., Lee H. J., Kim J. H., Kim S., and Kim Y. T., “The potential contribution of a computer-aided detection system for lung nodule detection in multidetector row computed tomography,” Invest. Radiol. 10.1097/00004424-200411000-00001 39, 649–655 (2004). [DOI] [PubMed] [Google Scholar]
- Marten K., Seyfarth T., Auer F., Wiener E., Grillhösl A., Obenauer S., Rummeny E. J., and Engelke C., “Computer-assisted detection of pulmonary nodules: Performance evaluation of an expert knowledge-based detection system in consensus reading with experienced and inexperienced chest radiologists,” Eur. Radiol. 14, 1930–1938 (2004). [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldin J. G., Rogers S., Kim H. J., Suh R. D., McNitt-Gray M. F., Shah S. K., Truong D., Brown K., and Sayre J. W., “Computer-aided lung nodule detection in CT results of large-scale observer test,” Acad. Radiol. 12, 681–686 (2005). [DOI] [PubMed] [Google Scholar]
- Li F., Li Q., Engelmann R., Aoyama M., Sone S., MacMahon H., and Doi K., “Improving radiologists’ recommendations with computer-aided diagnosis for management of small nodules detected by CT,” Acad. Radiol. 13, 943–950 (2006). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Hadjiiski L. M., Chan H. P., Shi J., Cascade P. N., Kazerooni E. A., Zhou C., Wei J., Chughtai A. R., Poopat C., Song T., Stojanovska J., Frank L., and Attili A., “Effect of CAD on radiologists’ detection of lung nodules on thoracic CT scans: Observer performance study,” Proc. SPIE 6515, 1D1–1D7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acar B., Beaulieu C. F., Gokturk S. B., Tomasi C., Paik D. S., Jeffrey R. B., Yee J., and Napel S., “Edge displacement field-based classification for improved detection of polyps in CT colonography,” IEEE Trans. Med. Imaging 10.1109/TMI.2002.806405 21, 1461–1467 (2002). [DOI] [PubMed] [Google Scholar]
- Nappi J. and Yoshida H., “Automated detection of polyps with CT colonography: Evaluation of volumetric features for reduction of false-positive findings,” Acad. Radiol. 9, 386–397 (2002). [DOI] [PubMed] [Google Scholar]
- Yoshida H., Nappi J., MacEneaney P., Rubin D., and Dachman A., “Computer-aided diagnosis scheme for detection of polyps at CT colonography,” Radiographics 22, 963–979 (2002). [DOI] [PubMed] [Google Scholar]
- Summers R. M., Jerebko A. K., Franaszek M., Malley J. D., and Johnson C. D., “Colonic polyps: Complementary role of computer-aided detection in CT colonography,” Radiology 10.1148/radiol.2252011619 225, 391–399 (2002). [DOI] [PubMed] [Google Scholar]
- Nappi J. J., Frimmel H., Dachman A. H., and Yoshida H., “Computerized detection of colorectal masses in CT colonography based on fuzzy merging and wall-thickening analysis,” Med. Phys. 10.1118/1.1668591 31, 860–872 (2004). [DOI] [PubMed] [Google Scholar]
- Summers R. M., Yao J. H., and Johnson C. D., “CT colonography with computer-aided detection: Automated recognition of ileocecal valve to reduce number of false-positive detections,” Radiology 10.1148/radiol.2331031326 233, 266–272 (2004). [DOI] [PubMed] [Google Scholar]
- Bogoni L., Cathier P., Dundar M., Jerebko A., Lakare S., Liang J., Periaswamy S., Baker M. E., and MacAri M., “Computer-aided detection (CAD) for CT colonography: A tool to address a growing need,” Br. J. Radiol. 78, S57–S62 (2005). [DOI] [PubMed] [Google Scholar]
- Wang Z. G., Liang Z. R., Li L. H., Li X., Li B., Anderson J., and Harrington D., “Reduction of false positives by internal features for polyp detection in CT-based virtual colonoscopy,” Med. Phys. 10.1118/1.2122447 32, 3602–3616 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Schraedley-Desmond P., Napel S., Olcott E. W., Jeffrey R. B., Yee J., Zalis M. E., Margolis D., Paik D. S., Sherbondy A. J., Sundaram P., and Beaulieu C. F., “CT colonography: Influence of 3D viewing and polyp candidate features on interpretation with computer-aided detection,” Radiology 239, 768–776 (2006). [DOI] [PubMed] [Google Scholar]
- Suzuki K., Yoshida H., Nappi J., and Dachman A., “Massive-training artificial neural network (MTANN) for reduction of false positives in computer-aided detection of polyps: Suppression of rectal tubes,” Med. Phys. 10.1118/1.2349839 33, 3814–3824 (2006). [DOI] [PubMed] [Google Scholar]
- Taylor S. A., Halligan S., Burling D., Roddie M. E., Honeyfield L., McQuillan J., Amin H., and Dehmeshki J., “Computer-assisted reader software versus expert reviewers for polyp detection on CT colonography,” AJR, Am. J. Roentgenol. 186, 696–702 (2006). [DOI] [PubMed] [Google Scholar]
- Zhao L. X., Botha C. P., Bescos J. O., Truyen R., Vos F. M., and Post F. H., “Lines of curvature for polyp detection in virtual colonoscopy,” IEEE Trans. Vis. Comput. Graph. 10.1109/TVCG.2006.158 12, 885–892 (2006). [DOI] [PubMed] [Google Scholar]
- Mani A., Napel S., Paik D. S., Jeffrey R. B., Yee J., Olcott E. W., Prokesch R., Davila M., Schraedley-Desmond P., and Beaulieu C. F., “Computed tomography colonography: Feasibility of computer-aided polyp detection in a ‘first reader’ paradigm,” J. Comput. Assist. Tomogr. 10.1097/00004728-200405000-00003 28, 318–326 (2004). [DOI] [PubMed] [Google Scholar]
- Okamura A., Dachman A. H., Parsad N., Näppi J., and Yoshida H., “Evaluation of the effect of CAD on observers’ performance in detection of polyps in CT colonography,” in CARS 2004. Computer Assisted Radiology and Surgery, Proc. 18th International Congress and Exhibition, Lemke H. U., Vannier M. W., Inamura K., Farman A. G., Doi K., and Reiber J. H. C., eds. (Elsevier, Chicago, IL, 2004), pp. 989–992.
- Halligan S., Altman D. G., Mallett S., Taylor S. A., Burling D., Roddie M., Honeyfield L., McQuillan J., Amin H., and Dehmeshki J., “Computed tomographic colonography: Assessment of radiologist performance with and without computer-aided detection,” Gastroenterology 131, 1690–1699 (2006). [DOI] [PubMed] [Google Scholar]
- Getty D. J., Pickett R. M., D’Orsi C. J., and Swets J. A., “Enhanced interpretation of diagnostic images,” Invest. Radiol. 10.1097/00004424-198804000-00002 23, 240–252 (1988). [DOI] [PubMed] [Google Scholar]
- D’Orsi C. J., Getty D. J., Swets J. A., Pickett R. M., Seltzer S. E., and McNeil B. J., “Reading and decision aids for improved accuracy and standardization of mammographic diagnosis,” Radiology 184, 619–622 (1992). [DOI] [PubMed] [Google Scholar]
- Wu Y., Giger M. L., Doi K., Vyborny C. J., Schmidt R. A., and Metz C. E., “Artificial neural networks in mammography: Application to decision making in the diagnosis of breast cancer,” Radiology 187, 81–87 (1993). [DOI] [PubMed] [Google Scholar]
- Floyd C. E. J., Lo J., Yun A. J., Sullivan D. C., and Kornguth P. J., “Prediction of breast cancer malignancy using an artificial neural network,” Cancer 74, 2944–2948 (1994). [DOI] [PubMed] [Google Scholar]
- Baker J. A., Kornguth P. J., Lo J. Y., Williford M. E., and C. E.Floyd, Jr., “Breast cancer: Prediction with artificial neural network based on BI-RADS standardized lexicon,” Radiology 196, 817–822 (1995). [DOI] [PubMed] [Google Scholar]
- Lo J. Y., Baker J. A., Kornguth P. J., Iglehart J. D., and C. E.Floyd, Jr., “Predicting breast cancer invasion with artificial neural networks on the basis of mammographic features,” Radiology 203, 159–163 (1997). [DOI] [PubMed] [Google Scholar]
- C. E.Floyd, Jr., Lo J. Y., and Tourassi G. D., “Case-based reasoning computer algorithm that uses mammographic findings for breast biopsy decisions,” AJR, Am. J. Roentgenol. 175, 1347–1352 (2000). [DOI] [PubMed] [Google Scholar]
- Markey M. K., Lo J. Y., and C. E.Floyd, Jr., “Differences between computer-aided diagnosis of breast masses and that of calcifications,” Radiology 10.1148/radiol.2232011257 223, 489–493 (2002). [DOI] [PubMed] [Google Scholar]
- D’Orsi C.et al. , Breast Imaging Reporting and Data System (BI-RADS), 4th ed. (American College of Radiology, Reston, VA, 2003). [Google Scholar]
- Gupta S., Chyn P. F., and Markey M. K., “Breast cancer CADx based on BI-RAds descriptors from two mammographic views,” Med. Phys. 10.1118/1.2188080 33, 1810–1817 (2006). [DOI] [PubMed] [Google Scholar]
- Stavros A. et al. , “Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions,” Radiology 196, 123–134 (1995). [DOI] [PubMed] [Google Scholar]
- Garra B., Krasner B., Horii S., Ascher S., Mun S., and Zeman R., “Improving the distinction between benign and malignant breast lesions: The value of sonographic texture analysis,” Ultrason. Imaging 10.1006/uimg.1993.1017 15, 267–285 (1993). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Vyborny C. J., and Schmidt R. A., “Computerized characterization of mammographic masses: Analysis of spiculation,” Cancer Lett. 77, 201–211 (1994). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Vyborny C. J., Bick U., Lu P., Wolverton D. E., and Schmidt R. A., “Analysis of spiculation in the computerized classification of mammographic masses,” Med. Phys. 10.1118/1.597626 22, 1569–1579 (1995). [DOI] [PubMed] [Google Scholar]
- Jiang Y., Nishikawa R. M., Wolverton D. E., Giger M. L., Doi K., Vyborny C. J., and Schmidt R. A., “Automated feature analysis and classification of malignant and benign clustered microcalcifications,” Radiology 198, 671–678 (1996). [DOI] [PubMed] [Google Scholar]
- Kocur C., Rogers S., Myers L., Burns T., Kabrisky M., Hoffmeister J., Bauer K., and Steppe J., “Using neural networks to select wavelet features for breast cancer diagnosis,” IEEE Eng. Med. Biol. Mag. 15, 94–102 (1996). [Google Scholar]
- Viton J.-L., Rasigni M., Rasigni G., and Llebaria A., “Method for characterizing masses on digital mammograms,” Opt. Eng. (Bellingham) 10.1117/1.601107 35, 3453–3459 (1996). [DOI] [Google Scholar]
- Chan H. P., Sahiner B., Petrick N., Helvie M. A., Lam K. L., Adler D. D., and Goodsitt M. M., “Computerized classification of malignant and benign microcalcifications on mammograms: Texture analysis using an artificial neural network,” Phys. Med. Biol. 10.1088/0031-9155/42/3/008, 42, 549–567 (1997). [DOI] [PubMed] [Google Scholar]
- Gilhuijs K. G., Giger M. L., and Bick U., “Computerized analysis of breast lesions in three dimensions using dynamic magnetic-resonance imaging,” Med. Phys. 10.1118/1.598345 25, 1647–1654 (1998). [DOI] [PubMed] [Google Scholar]
- Huo Z. et al. , “Automated computerized classification of malignant and benign mass lesions on digitized mammograms,” Acad. Radiol. 5, 155–168 (1998). [DOI] [PubMed] [Google Scholar]
- Kupinski M. and Giger M. L., “Automated seeded lesion segmentation on digital mammograms,” IEEE Trans. Med. Imaging 10.1109/42.730396 17, 510–517 (1998). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H. P., Petrick N., Helvie M. A., and Goodsitt M. M., “Computerized characterization of masses on mammograms: The rubber band straightening transform and texture analysis,” Med. Phys. 10.1118/1.598228 25, 516–526 (1998). [DOI] [PubMed] [Google Scholar]
- Chen D. R., Chang R. F., and Huang Y. L., “Computer-aided diagnosis applied to US of solid breast nodules by using neural networks,” Radiology 213, 407–412 (1999). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Al-Hallaq H., Huo Z., Moran C., Wolverton D. E., Chan C. W., and Zhong W., “Computerized analysis of lesions in US images of the breast,” Acad. Radiol. 6, 665–674 (1999). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., and Metz C. E., “Effect of dominant features on neural network performance in the classification of mammographic lesions,” Phys. Med. Biol. 10.1088/0031-9155/44/10/315 44, 2579–2595 (1999). [DOI] [PubMed] [Google Scholar]
- Jiang Y., Nishikawa R. M., Schmidt R. A., Metz C. E., Giger M. L., and Doi K., “Improving breast cancer diagnosis with computer-aided diagnosis,” Acad. Radiol. 6, 22–33 (1999). [DOI] [PubMed] [Google Scholar]
- Penn A. I., Bolinger L., Schnall M. D., and Loew M. H., “Discrimination of MR images of breast masses with fractal-interpolation function models,” Acad. Radiol. 6, 156–163 (1999). [DOI] [PubMed] [Google Scholar]
- Taylor P., Fox J., and Pokropek A. T., “The development and evaluation of CADMIUM: A prototype system to assist in the interpretation of mammograms,” Med. Image Anal. 3, 321–337 (1999). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Vyborny C. J., Wolverton D. E., and Metz C. E., “Computerized classification of benign and malignant masses on digitized mammograms: A study of robustness,” Acad. Radiol. 7, 1077–1084 (2000). [DOI] [PubMed] [Google Scholar]
- Hadjiiski L., Sahiner B., Chan H. P., Petrick N., Helvie M. A., and Gurcan M., “Analysis of temporal changes of mammographic features: Computer-aided classification of malignant and benign breast masses,” Med. Phys. 10.1118/1.1412242 28, 2309–2317 (2001). [DOI] [PubMed] [Google Scholar]
- Horsch K., Giger M. L., Venta L. A., and Vyborny C. J., “Automatic segmentation of breast lesions on ultrasound,” Med. Phys. 10.1118/1.1386426 28, 1652–1659 (2001). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., and Vyborny C. J., “Computerized analysis of multiple-mammographic views: Potential usefulness of special view mammograms in computer-aided diagnosis,” IEEE Trans. Med. Imaging 10.1109/42.974923 20, 1285–1292 (2001). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H. P., Petrick N., Helvie M. A., and Hadjiiski L. M., “Improvement of mammographic mass characterization using spiculation meausures and morphological features,” Med. Phys. 10.1118/1.1381548 28, 1455–1465 (2001). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Petrick N., Chan H. P., Hadjiiski L. M., Paramagul C., Helvie M. A., and Gurcan M. N., “Computer-aided characterization of mammographic masses: Accuracy of mass segmentation and its effects on characterization,” IEEE Trans. Med. Imaging 10.1109/42.974922 20, 1275–1284 (2001). [DOI] [PubMed] [Google Scholar]
- Gilhuijs K., Deurloo E., Muller S., Peterse J., and Schultze Kool L., “Breast MR imaging in women at increased lifetime risk of breast cacer: Clinical system for computerized assessment of breast lesions—Initial results,” Radiology 10.1148/radiol.2253011582 225, 907–916 (2002). [DOI] [PubMed] [Google Scholar]
- Horsch K., Giger M. L., Venta L. A., and Vyborny C. J., “Computerized diagnosis of breast lesions on ultrasound,” Med. Phys. 10.1118/1.1429239 29, 157–164 (2002). [DOI] [PubMed] [Google Scholar]
- Drukker K., Giger M. L., and Mendelson E. B., “Computerized analysis of shadowing on breast ultrasound for improved lesion detection,” Med. Phys. 10.1118/1.1584042 30, 1833–1842 (2003). [DOI] [PubMed] [Google Scholar]
- Sajda P., Spence C., and Parra L., “A multi-scale probabilistic network model for detection, synthesis and compression in mammographic image analysis,” Med. Image Anal. 7, 187–204 (2003). [DOI] [PubMed] [Google Scholar]
- Chen W., Giger M. L., Lan L., and Bick U., “Computerized interpretation of breast MRI: Investigation of enhancement-variance dynamics,” Med. Phys. 10.1118/1.1695652 31, 1076–1082 (2004). [DOI] [PubMed] [Google Scholar]
- Drukker K., Giger M. L., Vyborny C. J., and Mendelson E. B., “Computerized detection and classification of cancer on breast ultrasound,” Acad. Radiol. 11, 526–535 (2004). [DOI] [PubMed] [Google Scholar]
- Horsch K., Giger M. L., Vyborny C. J., and Venta L. A., “Performance of computer-aided diagnosis in the interpretation of lesions on breast sonography,” Acad. Radiol. 11, 272–280 (2004). [DOI] [PubMed] [Google Scholar]
- Kallergi M., “Computer-aided diagnosis of mammographic microcalcification clusters,” Med. Phys. 10.1118/1.1637972 31, 314–326 (2004). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H. P., Roubidoux M. A., Helvie M. A., Hadjiiski L. M., Ramachandran A., Paramagul C., LeCarpentier G. L., Nees A., and Blane C., “Computerized characterization of breast masses on three-dimensional ultrasound volumes,” Med. Phys. 10.1118/1.1649531 31, 744–754 (2004). [DOI] [PubMed] [Google Scholar]
- Drukker K., Giger M., and Metz C., “Robustness of computerized lesion detection and classification scheme across different breast ultrasound platforms,” Radiology 10.1148/radiol.2373041418 237, 834–840 (2005). [DOI] [PubMed] [Google Scholar]
- Drukker K., Horsch K., and Giger M., “Multimodality computerized diagnosis of breast lesions using mammography and sonography,” Acad. Radiol. 12, 970–979 (2005). [DOI] [PubMed] [Google Scholar]
- Chen W., Giger M. L., and Bick U., “A fuzzy c-means (FCM)-based approach for computerized segmentation of breast lesions in dynamic contrast-enhanced MR images,” Acad. Radiol. 13, 63–72 (2006). [DOI] [PubMed] [Google Scholar]
- Chen W., Giger M. L., Bick U., and Newstead G. M., “Automatic identification and classification of characteristic kinetic curves of breast lesions on DCE-MRI,” Med. Phys. 10.1118/1.2210568 33, 2878–2887 (2006). [DOI] [PubMed] [Google Scholar]
- Horsch K., Giger M. L., Vyborny C. J., Lan L., Mendelson E. B., and Hendrick R. E., “Classification of breast lesions with multimodality computer-aided diagnosis: Observer study results on an independent clinical data set,” Radiology 10.1148/radiol.2401050208 240, 357–368 (2006). [DOI] [PubMed] [Google Scholar]
- Varela C., Timp S., and Karssemeijer N., “Use of border information in the classification of mammographic masses,” Phys. Med. Biol. 10.1088/0031-9155/51/2/016 51, 425–441 (2006). [DOI] [PubMed] [Google Scholar]
- Rana R. S., Jiang Y., Schmidt R. A., Nishikawa R. M., and Liu B., “Independent evaluation of computer classification of malignant and benign calcifications in full-field digital mammograms,” Acad. Radiol. 14, 363–370 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timp S., Varela C., and Karssemeijer N., “Temporal change analysis for characterization of mass lesions in mammography,” IEEE Trans. Med. Imaging 10.1109/TMI.2007.897392 26, 945–953 (2007). [DOI] [PubMed] [Google Scholar]
- Shi J., Sahiner B., Chan H. P., Ge J., Hadjiisk L., Helvie M. A., Nees A., Wu Y. T., Wei J., Zhou C., Zhang Y., and Cui J., “Characterization of mammographic masses based on level set segmentation with new image features and patient information,” Med. Phys. 10.1118/1.2820630 35, 280–290 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssemeijer N., “A relaxation method for image segmentation using a spatially dependent stochastic-model,” Pattern Recogn. Lett. 11, 13–23 (1990). [Google Scholar]
- te Brake G. M. and Karssemeijer N., “Segmentation of suspicious densities in digital mammograms,” Med. Phys. 10.1118/1.1339884 28, 259–266 (2001). [DOI] [PubMed] [Google Scholar]
- Catarious D. J., Baydush A., and Floyd C. J., “Incorporation of an iterative, linear segmentation routine into a mammographic mass CAD system,” Med. Phys. 10.1118/1.1738960 31, 1512–1520 (2004). [DOI] [PubMed] [Google Scholar]
- Timp S. and Karssemeijer N., “A new 2D segmentation method based on dynamic programming applied to computer aided detection in mammography,” Med. Phys. 10.1118/1.1688039 31, 958–971 (2004). [DOI] [PubMed] [Google Scholar]
- Yuan Y., Giger M., Li H., Suzuki K., and Sennett C., “A dual-stage method for lesion segmentation on digital mammograms,” Med. Phys. 10.1118/1.2790837 34, 4180–4193 (2007). [DOI] [PubMed] [Google Scholar]
- Yarusso L. M., Nishikawa R. M., Papaioannou J., Nagel R., Jokich P., and Venta L. A., “Application of computer-aided diagnosis to full-field digital mammography,” in Digital Mammography 2000, Yaffe M. J., ed. (Medical Physics Publishing, Madison, WI, 2000), pp. 421–426. [Google Scholar]
- Li H., Giger M., Yuan Y., Lan L., Suzuki K., Jamieson A., Yarusso L., Nishikawa R. M., and Sennett C., Comparison of Computerized Image Analyses for Digitized Screen-Film Mammograms and Full-Field Digital Mammography Images (Springer, Berlin, Germany, 2006), pp. 569–575. [Google Scholar]
- Hadjiiski L., Filev P., Chan H. P., Ge J., Sahiner B., Helvie M. A., and Roubidoux M. A., “Computerized detection and classification of malignant and benign microcalcifications on full field digital mammograms,” in Digital Mammography (Springer, Berlin, Germany, 2008), pp. 336–342. [Google Scholar]
- Chen D.-R., Chang R.-F., and Huang Y.-L., “Computer-aided diagnosis applied to US of solid breast nodules by using neural networks,” Radiology 213, 407–412 (1999). [DOI] [PubMed] [Google Scholar]
- Chang R.-F., Wu W.-J., Moon K. M., and Chen D.-R., “Improvement in breast tumor discrimination by support vector machines and speckle-emphasis texture analysis,” Ultrasound Med. Biol. 10.1016/S0301-5629(02)00788-3 29, 679–686 (2003). [DOI] [PubMed] [Google Scholar]
- Gefen S., Tretiak O., Piccoli C., Donohue K., Petropulu A., Shankar P., Huang L., Kutay M., Genis V., Forsberg F., Reid J., and Goldberg B., “ROC analysis of ultrasound tissue characterization lassifiers for breast cancer diagnosis,” IEEE Trans. Med. Imaging 10.1109/TMI.2002.808361 22, 170–177 (2003). [DOI] [PubMed] [Google Scholar]
- Huang Y.-L. and Chen D.-R., “Watershed segmentation for breast tumor in 2-D sonography,” Ultrasound Med. Biol. 10.1016/j.ultrasmedbio.2003.12.001 30, 625–632 (2004). [DOI] [PubMed] [Google Scholar]
- Brix G. et al. , “Microcirculation and microvasculature in breast tumors: Pharmacokinetic analysis of dynamic breast MR images series,” Magn. Reson. Med. 52, 420–429 (2004). [DOI] [PubMed] [Google Scholar]
- Ikeda D. et al. , “Develpment, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic resonance imaging studies,” J. Magn. Reson Imaging 13, 889–895 (2001). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Vyborny C. J., and Metz C. E., “Effectiveness of computer-aided diagnosis—Observer study with independent database of mammograms,” Radiology 10.1148/radiol.2242010703 224, 560–568 (2002). [DOI] [PubMed] [Google Scholar]
- Jiang Y., Nishikawa R. M., Schmidt R. A., Toledano A. Y., and Doi K., “Potential of computer-aided diagnosis to reduce variability in radiologists’ interpretations of mammograms depicting microcalcifications,” Radiology 10.1148/radiol.220001257 220, 787–794 (2001). [DOI] [PubMed] [Google Scholar]
- Hadjiiski L., Chan H. P., Sahiner B., Helvie M. A., Roubidoux M. A., Blane C., Paramagul C., Petrick N., Bailey J., Klein K., Foster M., Patterson S., Adler D., Nees A., and Shen J., “Improvement in radiologists’ characterization of malignant and benign breast masses on serial mammograms with computer-aided diagnosis: An ROC study,” Radiology 10.1148/radiol.2331030432 233, 255–265 (2004). [DOI] [PubMed] [Google Scholar]
- Sklansky J., Tao E., Ornes C., and Disher A., A Visualized Mammographic Database in Computer-Aided Diagnosis (Elsevier, New York, 1999). [Google Scholar]
- Swett H. A. and Fisher P. R., “ICON: A computer-based approach to differential diagnosis in radiology,” Radiology 163, 555–558 (1987). [DOI] [PubMed] [Google Scholar]
- Swett H. A., Fisher P. R., Cohn A. I., Miller P. I., and Mutalik P. G., “Expert system controlled image display,” Radiology 172, 487–493 (1989). [DOI] [PubMed] [Google Scholar]
- Giger M. L., Huo Z., Lan L., and Vyborny C., “Intelligent search workstation for computer-aided diagnosis,” Proc. CARS, pp. 822–827 (2000).
- Giger M. L., Huo Z., Vyborny C. J., Lan L., Nishikawa R. M., and Rosenbourgh I. “Results of an observer study with an intelligent mammographic workstation for CAD,” in Digital Mammography IWDM 2002, Peitgen H.-O., ed. (Springer-Verlag, Berlin, Germany, 2003), pp. 297–303. [Google Scholar]
- Swets J. A., Getty D. J., Pickett R. M., D’Orsi C. J., Seltzer S. E., and McNeil B. J., “Enhancing and evaluating diagnostic accuracy,” Med. Decis Making 11, 9–18 (1991). [DOI] [PubMed] [Google Scholar]
- Li Q., Li F., Shiraishi J., Katsuragawa S., Sone S., and Doi K., “Investigation of new psychophysical measures for evalution of similar images on thoracic CT for distinction between benign and malignant nodules,” Med. Phys. 10.1118/1.1605351 30, 2584–2593 (2003). [DOI] [PubMed] [Google Scholar]
- Muramatsu C., Li Q., Suzuki K., Schmidt R. A., Shiraishi J., Newstead G. M., and Doi K., “Investigation of psychophysical measure for evaluation of similar images for mammographic masses: Preliminary results,” Med. Phys. 10.1118/1.1944913 32, 2295–2304 (2005). [DOI] [PubMed] [Google Scholar]
- Zheng B., Lu A., Hardesty L. A., Sumkin J. H., Hakim C. M., Ganott M. A., and Gur D., “A method to improve visual similarity of breast masses for an interactive computer-aided diagnosis environment,” Med. Phys. 10.1118/1.2143139 33, 111–117 (2006). [DOI] [PubMed] [Google Scholar]
- Sahiner B.et al. , “The effect of multi-modality computer classifier on radiologists’ accuracy in characterizing breast masses,” RSNA Abstract Book (RSNA, Oak Brook, IL, 2004). [Google Scholar]
- Horsch K., Giger M. L., and Metz C. E., “Prevalence scaling: Applications to an intelligent workstation for the diagnosis of breast cancer,” Acad. Radiol. 15, 1446–1457 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney J. and Swensen S., “Solitary pulmonary nodules: Determining the likelihood of malignancy with neural network analysis,” Radiology 196, 823–829 (1995). [DOI] [PubMed] [Google Scholar]
- Nakamura K. et al. , “Computerized analysis of the likelihood of malignancy in solitary pulmonary nodules by use of artificial neural networks,” Radiology 214, 823–830 (2000). [DOI] [PubMed] [Google Scholar]
- Aoyama M., Li Q., Katsuragawa S., MacMahon H., and Doi K., “Automated computerized scheme for distinction between benign and malignant solitary pulmonary nodules on chest images,” Med. Phys. 10.1118/1.1469630 29, 701–708 (2002). [DOI] [PubMed] [Google Scholar]
- Kawata Y. et al. , “Quantitative surface characterization of pulmonary nodules based on thin-section CT images,” IEEE Trans. Med. Imaging 45, 2132–2138 (1998). [Google Scholar]
- McNitt-Gray M. F., Hart E., Wyckoff N., Sayre J., Goldin J., and Aberle D. R., “A pattern classification approach to characterizing solitary pulmonary nodules imaged on high resolution CT: Preliminary restuls,” Med. Phys. 10.1118/1.598603 26, 880–888 (1999). [DOI] [PubMed] [Google Scholar]
- Aoyama M., Li Q., Katsuragawa S., Li F., Sone S., and Doi K., “Computerized scheme for determinaion of the likelihood measure of malignancy for pulmonary nodules on low-dose CT images,” Med. Phys. 10.1118/1.1543575 30, 387–394 (2003). [DOI] [PubMed] [Google Scholar]
- Li F. et al. , “Improvement in radiologists’ performance for differentiating small benign from malignant lung nodules on high-resolution CT by using computer-estimated likelihood of malignancy,” AJR, Am. J. Roentgenol. 183, 1209–1215 (2004). [DOI] [PubMed] [Google Scholar]
- Mori K. et al. , “Development of a novel computer-aided diagnosis system for automatic discrimination of malignant from benign solitary nodules on thin-section dynamic computed tomography,” J. Comput. Assist. Tomogr. 29, 215–222 (2005). [DOI] [PubMed] [Google Scholar]
- Shah S. et al. , “Computer aided characterization of solitary pulmonary nodules using volumetric and contrast enhancement features,” Acad. Radiol. 12, 1310–1319 (2005). [DOI] [PubMed] [Google Scholar]
- Kurjak A., Cecuk S., and Breyer B., “Prediction of maturity in first trimester of pregnancy by ultrasonic measurement of fetal crown-rump length,” J. Clin. Ultrasound 4, 83–84 (1976). [DOI] [PubMed] [Google Scholar]
- Merlino A. F., “A protractor for measuring scoliosis by the Cobb technique,” J. Bone Jt. Surg., Am. Vol. 55, 1098–1099 (1973). [PubMed] [Google Scholar]
- Therasse P., Arbuck E. A. E. S. G., Wanders J., Kaplan R. S., Rubinstein L., Verweij J., Van G. M., van Oosterom A. T., Christian M. C., and Gwyther S. G., “New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada,” J. Natl. Cancer Inst. 10.1093/jnci/92.3.205 92, 205–216 (2000). [DOI] [PubMed] [Google Scholar]
- Zerhouni E., Spivey R. H. M. J. F., Leo F. P., Stitik F. P., and Siegelman S. S., “Factors influencing quantitative CT measurements of solitary pulmonary nodules,” J. Comput. Assist. Tomogr. 6, 1075–1087 (1982). [DOI] [PubMed] [Google Scholar]
- Goodsitt M., Chan T. W. W. H. P., Larson S. C., Christodoulou E. G., and Kim J., “Accuracy of the CT numbers of simulated lung nodules imaged with multi-detector CT scanners,” Med. Phys. 10.1118/1.2219332 33, 3006–3017 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders J., Kerr D. J. S. E., Stein J. A., Ramos E., Moscona A. A., and Resnick D., “Quantitative dual-energy radiographic absorptiometry of the lumbar spine: in vivo comparison with dual-photon absorptiometry,” Radiology 170, 129–131 (1989). [DOI] [PubMed] [Google Scholar]
- Cann C. E., “Low-dose CT scanning for quantitative spinal mineral analysis,” Radiology 140, 813–815 (1981). [DOI] [PubMed] [Google Scholar]
- Cann C., Genant F. O. K. H. K., and Ettinger B., “Quantitative computed tomography for prediction of vertebral fracture risk,” Bone (N.Y.) 10.1016/8756-3282(85)90399-0 6, 1–7 (1985). [DOI] [PubMed] [Google Scholar]
- Cann C. E., “Quantitative CT for determination of bone mineral density: A review,” Radiology 166, 509–522 (1988). [DOI] [PubMed] [Google Scholar]
- Mistretta C. and Crummy C. M. S. A. B., “Digital angiography: A perspective,” Radiology 139, 273–276 (1981). [DOI] [PubMed] [Google Scholar]
- Mistretta C. and Crummy A., “Diagnosis of cardiovascular disease by digital subtraction angiography,” Science 214, 761–765 (1981). [DOI] [PubMed] [Google Scholar]
- Mistretta C. and Crummy A., “Basic concepts of digital angiography,” Prog. Cardiovasc. Dis. 28, 245–255 (1986). [DOI] [PubMed] [Google Scholar]
- Aebersold P., “The development of nuclear medicine,” Am. J. Roentgenol., Radium Ther. Nucl. Med. 75, 1027–1039 (1956). [PubMed] [Google Scholar]
- Jaszczak R., Whitehead C. B. L. F. R., and Coleman R. E., “Lesion detection with single-photon emission computed tomography (SPECT) compared with conventional imaging,” J. Nucl. Med. 23, 97–102 (1982). [PubMed] [Google Scholar]
- Anger H., “Scintillation camera with multichannel collimators,” J. Nucl. Med. 5, 515–531 (1964). [PubMed] [Google Scholar]
- Weber D. A., “Computers in nuclear medicine: Introductory concepts,” Semin Nucl. Med. 8, 107–112 (1978). [DOI] [PubMed] [Google Scholar]
- Tsui B., Zhao E. C. F. X., and McCartney W. H., “Quantitative single-photon emission computed tomography: Basics and clinical considerations,” Semin Nucl. Med. 24, 38–65 (1994). [DOI] [PubMed] [Google Scholar]
- King M. and Tsui T. S. P. B. M., “Attenuation compensation for cardiac single-photon emission computed tomographic imaging: Part 1. Impact of attenuation and methods of estimating attenuation maps,” J. Nucl. Cardiol. 2, 513–524 (1995). [DOI] [PubMed] [Google Scholar]
- King M., Tsui T. S. P. B. M., Glick S. J., and Soares E. J., “Attenuation compensation for cardiac single-photon emission computed tomographic imaging: Part 2. Attenuation compensation algorithms,” J. Nucl. Cardiol. 3, 55–64 (1996). [DOI] [PubMed] [Google Scholar]
- Pretorius P., Xia M. A. K. W., Tsui B. M., Pan T. S., and Villegas B. J., “Evaluation of right and left ventricular volume and ejection fraction using a mathematical cardiac torso phantom,” J. Nucl. Med. 38, 1528–1535 (1997). [PubMed] [Google Scholar]
- Katzberg R., O’Mara R. H. T. R. E., and Weber D. A., “Radionuclide skeletal imaging and single photon emission computed tomography in suspected internal derangements of the temporomandibular joint,” J. Oral Maxillofac Surg. 42, 782–787 (1984). [DOI] [PubMed] [Google Scholar]
- Liew S. and Hasegawa B., “Noise, resolution, and sensitivity considerations in the design of a single-slice emission-transmission computed tomographic system,” Med. Phys. 10.1118/1.596643 18, 1002–1015 (1991). [DOI] [PubMed] [Google Scholar]
- Lang T., Hasegawa S. C. L. B. H., Brown J. K., Blankespoor S. C., Reilly S. M., Gingold E. L., and Cann C. E., “Description of a prototype emission-transmission computed tomography imaging system,” J. Nucl. Med. 33, 1881–1887 (1992). [PubMed] [Google Scholar]
- Hoffman E. and Phelps M., “Positron emission tomography,” Med. Instrum. 13, 147–151 (1979). [PubMed] [Google Scholar]
- Raichle M., R. L. G. M. J.Welch, Jr., Larson K. B., Laux B. E., and Ter-Pogossian M. M., “Regional cerebral oxygen utilization with positron emission tomography,” Trans. Am. Neurol. Assoc. 104, 154–156 (1979). [PubMed] [Google Scholar]
- Beyer T., Townsend T. B. D. W., Kinahan P. E., Charron M., Roddy R., Jerin J., Young J., Byars L., and Nutt R., “A combined PET∕CT scanner for clinical oncology,” J. Nucl. Med. 41, 1369–1379 (2000). [PubMed] [Google Scholar]
- Townsend D. and Cherry S., “Combining anatomy and function: The path to true image fusion,” Eur. Radiol. 11, 1968–1974 (2001). [DOI] [PubMed] [Google Scholar]
- Kinahan P., Townsend T. B. D. W., and Sashin D., “Attenuation correction for a combined 3D PET∕CT scanner,” Med. Phys. 10.1118/1.598392 25, 2046–2053 (1998). [DOI] [PubMed] [Google Scholar]
- Lauterbur P. C., “Progress in n.m.r. zeugmatography imaging,” Philos. Trans. R. Soc. London, Ser. B 289, 483–487 (1980). [DOI] [PubMed] [Google Scholar]
- Gore J., Emery J. S. O. E. W., and Doyle F. H., “Medical nuclear magnetic resonance imaging: I. Physical principles,” Invest. Radiol. 16, 269–274 (1981). [DOI] [PubMed] [Google Scholar]
- Bottomley P. A., “NMR imaging techniques and applications: A review,” Rev. Sci. Instrum. 10.1063/1.1137180 53, 1319–1337 (1982). [DOI] [PubMed] [Google Scholar]
- Glover G. and Pelc N., “A rapid-gated cine MRI technique,” Magn. Reson. Med. 24, 299–333 (1988). [PubMed] [Google Scholar]
- Glover G. and Pauly J., “Projection reconstruction techniques for reduction of motion effects in MRI,” Magn. Reson. Med. 10.1002/mrm.1910280209 28, 275–289 (1992). [DOI] [PubMed] [Google Scholar]
- Bottomley P. and Edelstein W., “Power deposition in whole-body NMR imaging,” Med. Phys. 10.1118/1.595000 8, 510–512 (1981). [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee A. R. K. T. M., and Tank D. W., “Brain magnetic resonance imaging with contrast dependent on blood oxygenation,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.87.24.9868 87, 9868–9872 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham G., Zhong O. A. P. J., Constable R. T., Prichard J. W., and Gore J. C., “BOLD MRI monitoring of changes in cerebral perfusion induced by acetazolamide and hypercarbia in the rat,” Magn. Reson. Med. 31, 557–560 (1994). [DOI] [PubMed] [Google Scholar]
- Menon R., Ogawa X. H. S., Strupp J. P., Anderson P., and Ugurbil K., “BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: Echo-planar imaging correlates with previous optical imaging using intrinsic signals,” Magn. Reson. Med. 10.1002/mrm.1910330323 33, 453–459 (1995). [DOI] [PubMed] [Google Scholar]
- Wagner R. F., Beiden S. V., Campbell G., Metz C. E., and Sacks W. M., “Assessment of medical imaging and computer-assisted systems: Lessons from recent experience,” Acad. Radiol. 9, 1264–1277 (2002). [DOI] [PubMed] [Google Scholar]
- Metz C. E., “Basic principles of ROC analysis,” Semin Nucl. Med. 8, 283–298 (1978). [DOI] [PubMed] [Google Scholar]
- Wagner R. F., Chan H. P., Mossoba J. T., Sahiner B., and Petrick N., “Components of variance in RoC analysis of CADx classifier performance,” Proc. SPIE 10.1117/12.310896 3338, 859–875 (1998). [DOI] [Google Scholar]
- Beiden S. V., Wagner R. F., Doi K. Nishikawa R. M., Freedman M., Lo S.-C., and Xu X.-W., “Independent versus sequential reading in ROC studies of computer-assisted modalities: Analysis of components of variance,” Acad. Radiol. 9, 1026–1043 (2002). [DOI] [PubMed] [Google Scholar]
- Wagner R., Metz C. E., and Campbell G., “Assessment of medical imaging systems and computer aids: A tutorial review,” Acad. Radiol. 14, 723–748 (2007). [DOI] [PubMed] [Google Scholar]
- Edwards D. C., Lan L., Metz C. E., Giger M. L., and Nishikawa R. M., “Estimating three-class ideal observer decision variables for computerized detection and classification of mammographic mass lesions,” Med. Phys. 10.1118/1.1631912 31, 81–90 (2004). [DOI] [PubMed] [Google Scholar]
- Edwards D. C., Metz C. E., and Kupinski M. A., “Ideal observers and optimal ROC hypersurfaces in N-class classification,” IEEE Trans. Med. Imaging 10.1109/TMI.2004.828358 23, 891–895 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Metz C. E., Tsui B. M. W., Links J. M., and Frey E. C., “Three-class ROC analysis—A decision theoretic approach under the ideal observer framework,” IEEE Trans. Med. Imaging 10.1109/TMI.2006.871416 25, 571–581 (2006). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H. P., and Hadjiisk L., “Performance analysis of 3-class classifiers: Properties of the 3D ROC surface and the normalized volume under the surface for the ideal observer,” IEEE Trans. Med. Imaging 27, 215–227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. L., Metz C. E., and Nishikawa R. M., “A receiver operating: Characteristic partial area index for highly sensitive diagnostic tests,” Radiology 201, 745–750 (1996). [DOI] [PubMed] [Google Scholar]
- Nishikawa R. M., Giger M. L., Doi K., Metz C. E., Yin F.-F., Vyborny C. J., and Schmidt R. A., “Effect of case selection on the performance of computer-aided detection schemes,” Med. Phys. 10.1118/1.597287 21, 265–269 (1994). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Lo S. C. B., Sahiner B., Lam K. L., and Helvie M. A., “Computer-aided detection of mammographic microcalcifications: Pattern recognition with an artificial neural network,” Med. Phys. 10.1118/1.597428 22, 1555–1567 (1995). [DOI] [PubMed] [Google Scholar]
- Giger M. L., “Current issues in CAD for mammography,” in Digital Mammography ‘96, Doi K., Giger M. L., Nishikawa R. M., and Schmidt R. A., eds. (Elsevier, Amsterdam, 1996), pp. 53–59. [Google Scholar]
- Fukunaga K. and Hayes R. R., “Effects of sample size on classifier design,” IEEE Trans. Pattern Anal. Mach. Intell. 10.1109/34.31448 11, 873–885 (1989). [DOI] [Google Scholar]
- Jain A. and Zongker D., “Feature selection: Evaluation, application, and small sample size performance,” IEEE Trans. Pattern Anal. Mach. Intell. 10.1109/34.574797 19, 153–158 (1997). [DOI] [Google Scholar]
- Chan H. P., Sahiner B., Wagner R. F., and Petrick N., “Classifier design for computer-aided diagnosis: Effects of finite sample size on the mean performance of classical and neural network classifiers,” Med. Phys. 10.1118/1.598805 26, 2654–2668 (1999). [DOI] [PubMed] [Google Scholar]
- Kupinski M. A. and Giger M. L., “Feature selection with limited datasets,” Med. Phys. 10.1118/1.598821 26, 2176–2182 (1999). [DOI] [PubMed] [Google Scholar]
- Sahiner B., Chan H. P., Petrick N., Wagner R. F., and Hadjiiski L., “Feature selection and classifier performance in computer-aided diagnosis: The effect of finite sample size,” Med. Phys. 10.1118/1.599017 27, 1509–1522 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourassi G. D., Frederick E. D., Markey M. K., and C. E.Floyd, Jr., “Application of the mutual information criterion for feature selection in computer-aided diagnosis,” Med. Phys. 10.1118/1.1418724 28, 2394–2402 (2001). [DOI] [PubMed] [Google Scholar]
- Lee G. and Bottema M., “Significance of classification scores subsequent to feature selection,” Pattern Recogn. Lett. 27, 1702–1709 (2006). [Google Scholar]
- Sahiner B., Chan H. P., and Hadjiisk L., “Classifier performance prediction for computer-aided diagnosis using a limited data set,” Med. Phys. 10.1118/1.2868757 35, 1559–1570 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. and Doi K., “Analysis and minimization of overtraining effect in rule-based classifiers for computer-aided diagnosis,” Med. Phys. 10.1118/1.1999126 33, 320–328 (2006). [DOI] [PubMed] [Google Scholar]
- Lo J. Y., Markey M. K., Baker J. A., and C. E.Floyd, Jr., “Cross-institutional evaluation of BI-RADS predictive model for mammographic diagnosis of breast cancer,” AJR, Am. J. Roentgenol. 178, 457–463 (2002). [DOI] [PubMed] [Google Scholar]
- te Brake G. M., Karssemeijer N., and Hendriks J. H., “Automated detection of breast carcinomas not detected in a screening program,” Radiology 207, 465–471 (1998). [DOI] [PubMed] [Google Scholar]
- Clarke L. P., Croft B. Y., Staab E., Baker H., and Sullivan D. C., “National Cancer Institute initiative: Lung image database resource for imaging research,” Acad. Radiol. 8, 447–450 (2001). [DOI] [PubMed] [Google Scholar]
- Meyer C. R. et al. , “The lung image database consortium: Evaluation of lung MDCT nodule annotations across radiologists and methods,” Acad. Radiol. 13, 1254–1265 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- S. G.Armato, III, Roberts R. Y., McNitt-Gray M. F., Meyer C. R., Reeves A. P., McLennan G., Engelmann R. M., Bland P. H., Aberle D. R., Kazerooni E. A., MacMahon H., van Beek E. J., Yankelevitz D., Croft B. Y., and Clarke L. P., “The lung image database consortium (LIDC): Ensuring the integrity of expert-defined ‘truth’,” Acad. Radiol. 14, 1455–1463 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNitt-Gray M. F., S. G.ArmatoIII, Meyer C. R., Reeves A. P., McLennan G., Pais R. C., Freymann J., Brown M. S., Engelmann R. M., Bland P. H., Laderach G. E., Piker C., Guo J., Towfic Z., Qing D. P., Yankelevitz D. F., Aberle D. R., van Beek E. J., MacMahon H., Kazerooni E. A., Croft B. Y., and Clarke L. P., “The lung image database consortium (LIDC) data collection process for nodule detection and annotation,” Acad. Radiol. 14, 1464–1474 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves A. P. et al. , “The lung image database consortium: A comparison of different size metrics for pulmonary nodule measurements,” Acad. Radiol. 14, 1475–1485 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssemeijer N., Otten J. D. M., Verbeek A. L. M., Groenewoud J. H., de Koning H. J., Hendriks J., and Holland R., “Computer-aided detection versus independent double reading of masses on mammograms,” Radiology 10.1148/radiol.2271011962 227, 192–200 (2003). [DOI] [PubMed] [Google Scholar]
- Gilbert F. J., Astley S. M., McGee M. A., Gillan M. G., Boggis C. R., Griffiths P. M., and Duffy S. W., “Single reading with computer-aided detection and double reading of screening mammograms in the United Kingdom National Breast Screening Program,” Radiology 10.1148/radiol.2411051092 241, 47–53 (2006). [DOI] [PubMed] [Google Scholar]
- Skaane P., Kshirsagar A., Stapleton S., Young K., and Castellino R. A., “Effect of computer-aided detection on independent double reading of paired screen-film and full-field digital screening mammograms,” AJR, Am. J. Roentgenol. 10.2214/AJR.05.2207 188, 377–384 (2007). [DOI] [PubMed] [Google Scholar]
- Jiang Y., Miglioretti D. L., Metz C. E., and Schmidt R. A., “Designing imaging trials to demonstrate improvements in breast cancer detection rate,” Radiology 10.1148/radiol.2432060253 243, 360–367 (2007). [DOI] [PubMed] [Google Scholar]
- Horsch K., Giger M., and Metz C. E., “Potential effect of different radiologist reporting methods on studies showing benefit of CAD,” Acad. Radiol. 15, 139–152 (2008). [DOI] [PubMed] [Google Scholar]
- Masutani Y., MacMahon H., and Doi K., “Computerized detection of pulmonary embolism in Spiral CT angiography based on volumetric image analysis,” IEEE Trans. Med. Imaging 10.1109/TMI.2002.806586 21, 1517–1523 (2002). [DOI] [PubMed] [Google Scholar]
- Zhou C., Chan H. P., Patel S., Cascade P. N., Sahiner B., Hadjiiski L. M., and Kazerooni E. A., “Preliminary investigation of computer-aided detection of pulmonary embolism in 3D computed tomographic pulmonary angiography (CTPA) images,” Acad. Radiol. 12, 782–792 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima T., Zhang X., Kitagawa T., Kanematsu M., Zhou X., Hara T., Fujita H., Yokoyama R., Kondo H., Hoshi H., Nawano S., and Shinozaki K., “Computer-aided detection (CAD) of hepatocellular carcinoma on multiphase CT images,” Proc. SPIE 6514, 2Q1–2Q10 (2007). [Google Scholar]
- Zhou C., Chan H. P., Chughtai A., Patel S., Kazerooni E. A., Sahiner B., and Hadjiiski L. M., “Computerized analysis of coronary artery plaque disease: Early experience of automated segmentation of coronary arteries in ECG-gated cardiac CT,” 93rd Scientific Assembly and Annual Meeting of the Radiological Society of North America (2007).
- Hadjiiski L. M., Sahiner B., Caoili E. M., Cohan R. H., and Chan H. P., “Automated detection of ureter abnormalities on multidetector row CT urography,” Proc. SPIE 6144, 1W1–1W7 (2006). [Google Scholar]
- Kasai S., Li F., Shiraishi J., Li Q., and Doi K., “Computerized detection of vertebral compression fractures on lateral chest radiographs: Preliminary results of a tool for early detection of osteoporosis,” Med. Phys. 10.1118/1.2364053 33, 4664–4676 (2006). [DOI] [PubMed] [Google Scholar]
- Arimura H., Li Q., Korogi Y., Hirai T., Katsuragawa S., Yamashita Y., Tsuchiya K., and Doi K., “Computerized detection of intracranial aneurysms for three-dimensional MR angiography: Feature extraction of small protrusions based on a shape-based difference image technique,” Med. Phys. 10.1118/1.2163389 33, 394–401 (2006). [DOI] [PubMed] [Google Scholar]
- Kobashi S., Kondo K., and Hata Y., “Computer-aided diagnosis of intracranial aneurysms in MRA images with case-based reasoning,” IEICE Trans. Inf. Syst. E89-D(1), 340–350 (2006). [Google Scholar]
- Niemeijer M., van Ginneken B., Staal J., Suttorp-Schulten M., and Abramoff M., “Automatic detection of red lesions in digital color fundus photographs,” IEEE Trans. Med. Imaging 24, 584–592 (2005). [DOI] [PubMed] [Google Scholar]
- Shiraishi J., Li Q., Appelbaum D., Pu Y., and Doi K., “Development of a computer-aided diagnostic scheme for detection of interval changes in successive whole-body scans,” Med. Phys. 10.1118/1.2401044 34, 25–36 (2006). [DOI] [PubMed] [Google Scholar]
- Byng J. W., Boyd N. F., Fishell E., Jong R. A., and Yaffe M. J., “Automated analysis of mammographic densities,” Phys. Med. Biol. 10.1088/0031-9155/41/5/007 41, 909–923 (1996). [DOI] [PubMed] [Google Scholar]
- Byng J. W., Yaffe M. J., Lockwood G. A., Little L. E., Tritchler D. L., and Boyd N. F., “Automated analysis of mammographic densities and breast carcinoma risk,” Cancer 80, 66–74 (1997). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Wolverton D. E., Zhong W., Cumming S., and Olopade O. I., “Computerized analysis of mammographic parenchymal patterns for breast cancer risk assessment: Feature selection,” Med. Phys. 10.1118/1.598851 27, 4–12 (2000). [DOI] [PubMed] [Google Scholar]
- Huo Z., Giger M. L., Olopade O. I., Wolverton D. E., Weber B. L., Metz C. E., Zhong W., and Cummings S. A., “Computerized analysis of digitized mammograms of BRCA1 and BRCA2 gene mutation carriers,” Radiology 225, 519–526 (2002). [DOI] [PubMed] [Google Scholar]
- Caligiuri P., Giger M., and Favus M., “Multifractal radiographic analysis of osteoporosis,” Med. Phys. 10.1118/1.597390 21, 503–508 (1994). [DOI] [PubMed] [Google Scholar]
- Southard T. and Southard K., “Detection of simulated osteoporosis in maxillae using radiographic texture analysis,” IEEE Trans. Biomed. Eng. 43, 123–132 (1996). [DOI] [PubMed] [Google Scholar]
- Majumdar S., Lin J., Link T., Millard J., Augat P., Ouyang X., Newitt D., Gould R., Kothari M., and Genant H., “Fractal analysis of radiographs: Assessment of trabecular bone structure and prediction of elastic modulus and strength,” Med. Phys. 10.1118/1.598628 26, 1330–1340 (1999). [DOI] [PubMed] [Google Scholar]
- Chinander M. R., Giger M., Martell J., and Favus M., “Computerized analysis of radiographic bone patterns: Effect of imaging conditions on performance,” Med. Phys. 10.1118/1.598858 27, 75–85 (2000). [DOI] [PubMed] [Google Scholar]
- Vokes T. J., Giger M., Chinander M. R., Tg K., Favus M., and Dixon L., “Radiographic texture analysis of densitometer-generated calcaneus images differentiates postmenopausal women with and without fractures,” Osteoporosis Int. 10.1007/s00198-006-0089-y 17, 1472–1482 (2006). [DOI] [PubMed] [Google Scholar]
- Wilkie J. R., Giger M. L., Chinander M. R., Engh C., Hopper R., and Martell J., “Temporal radiographic texture analysis in the detection of periprosthetic osteolysis,” Med. Phys. 3, 377–387 (2008). [DOI] [PubMed] [Google Scholar]
- DeMartini W., Lehman C., Peacock S., and Russell M., “Computer-aided detection applied to breast MRI: Assessment of CAD-generated enhancement and tumor sizes in breast cancers before and after neoadjuvant chemotherapy,” Acad. Radiol. 12, 806–814 (2005). [DOI] [PubMed] [Google Scholar]
- Street E., Hadjiisk L., Sahiner B., Gujar S., Ibrahim M., Mukerji S., and Chan H. P., “Automated volume analysis of head lesions on CT scans using 3D level set segmentation,” Med. Phys. 10.1118/1.2794174 34, 4399–4408 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- S. G.ArmatoIII, Oxnard G., Kocherginsky M., Vogelzang N., Kindler H., and MacMahon H., “Evaluation of semi-automated measurements of mesothelioma tumor thickness on CT scans,” Acad. Radiol. 12, 1301–1309 (2005). [DOI] [PubMed] [Google Scholar]
- Abe C., Kahn C. E., Doi K., and Katsuragawa S., “Quantitative analysis of liver texture in ultrasound images: A preliminary study,” Invest. Radiol. 27, 71–77 (1992). [DOI] [PubMed] [Google Scholar]
- Bae K. T., Giger M. L., Chen C. T., and Kahn C. E., “Automatic segmentation of 3-D liver structure from CT data,” Med. Phys. 10.1118/1.597064 20, 71–78 (1993). [DOI] [PubMed] [Google Scholar]
- Hoffmann K. R., Chen S. Y., Kormano M., and Coulden R. A., “Segmentation and display of hepatic vessels and metastases,” Proc. SPIE 1898, 263–270 (1993). [Google Scholar]
- Garra B. S., Insana M. F., Sesterhenn I. A., Hall T. J., Wagner R. F., Rotellar C., Winchester J., and Zeman R. K., “Quantitative ultrasonic detection of parenchymal structural change in diffuse renal disease,” Invest. Radiol. 29, 134–140 (1994). [DOI] [PubMed] [Google Scholar]
- King M., Giger M. L., Suzuki K., Bardo D. M., Greenberg B., Lan L., and Pan X., “Computerized assessment of motion-contaminated calcified plaques in cardiac multidetector CT,” Med. Phys. 10.1118/1.2804718 34, 4876–4889 (2007). [DOI] [PubMed] [Google Scholar]
- King M., Giger M. L., Suzuki K., and Pan X., “Feature-based characterization of motion-contaminated calcified plaques in cardiac multidetector CT,” Med. Phys. 10.1118/1.2794172 34, 4860–4875 (2007). [DOI] [PubMed] [Google Scholar]
- Boone J. M., “Radiological interpretation 2020: Toward quantitative image assessment,” Med. Phys. 10.1118/1.2789501 34, 4173–4179 (2007). [DOI] [PubMed] [Google Scholar]
- Freer T. W. and Ulissey M. J., “Screening mammography with computer-aided detection: Prospective study of 12,860 patients in a community breast center,” Radiology 10.1148/radiol.2203001282 220, 781–786 (2001). [DOI] [PubMed] [Google Scholar]
- Birdwell R. L., Bandodkar P., and Ikeda D. M., “Computer-aided detection with screening mammography in a university hospital setting,” Radiology 10.1148/radiol.2362040864 236, 451–457 (2005). [DOI] [PubMed] [Google Scholar]
- Khoo L. A. L., Taylor P., and Given-Wilson R. M., “Computer-aided detection in the United Kingdom national breast screening programme: Prospective study,” Radiology 10.1148/radiol.2372041362 237, 444–449 (2005). [DOI] [PubMed] [Google Scholar]
- Dean J. C. and Ilvento C. C., “Improved cancer detection using computer-aided detection with diagnostic and screening mammography: Prospective study of 104 cancers,” AJR, Am. J. Roentgenol. 10.2214/AJR.05.0111 187, 20–28 (2006). [DOI] [PubMed] [Google Scholar]
- Morton M. J., Whaley D. H., Brandt K. R., and Amrami K. K., “Screening mammograms: Interpretation with computer-aided detection—Prospective evaluation,” Radiology 10.1148/radiol.2392042121 239, 375–383 (2006). [DOI] [PubMed] [Google Scholar]
- Ko J. M., Nicholas M. J., Mendel J. B., and Slanetz P. J., “Prospective assessment of computer-aided detection in interpretation of screening mammography,” AJR, Am. J. Roentgenol. 10.2214/AJR.05.1582 187, 1483–1491 (2006). [DOI] [PubMed] [Google Scholar]
- Gur D., Sumkin J. H., Rockette H. E., Ganott M. A., Hakim C., Hardesty L. A., Poller W. R., Shah R., and Wallace L., “Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system,” J. Natl. Cancer Inst. 96, 185–190 (2004). [DOI] [PubMed] [Google Scholar]
- Feig S. A., Sickles E. A., Evans W. P., and Linver M. N., “Re: Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system,” J. Natl. Cancer Inst. 96, 1260–1261 (2004). [DOI] [PubMed] [Google Scholar]
- Cupples T. E., Cunningham J. E., and Reynolds J. C., “Impact of computer-aided detection in a regional screening mammography program,” AJR, Am. J. Roentgenol. 10.2214/AJR.04.1300 185, 944–950 (2005). [DOI] [PubMed] [Google Scholar]
- Fenton J. J., Taplin S. H., Carney P. A., Abraham L., Sickles E. A., D’Orsi C., Berns E. A., Cutter G., Hendrick R. E., Barlow W. E., and Elmore J. G., “Influence of computer-aided detection on performance of screening mammography,” N. Engl. J. Med. 10.1056/NEJMoa066099 356, 1399–1409 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromet M., “Comparison of computer-aided detection to double reading of screening mammograms: Review of 231,221 mammograms,” AJR, Am. J. Roentgenol. 190, 854–859 (2008). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Sahiner B., Helvie M. A., Petrick N., Roubidoux M. A., Wilson T. E., Adler D., Paramagul C., Newman J., and Sanjay-Gopal S., “Improvement of radiologists’ characterization of mammographic masses by using computer-aided diagnosis: An ROC study,” Radiology 212, 817–827 (1999). [DOI] [PubMed] [Google Scholar]
- Behrens S., Laue H., Althaus M., Boehler T., Kuemmerlen B., Hahn H. K., and Peitgen H.-O., “Computer assistance for MR based diagnosis of breast cancer: Present and future challenges,” Comput. Med. Imaging Graph. 31, 236–247 (2007). [DOI] [PubMed] [Google Scholar]