Abstract
The authors report interim clinical results from an ongoing NIH-sponsored trial to evaluate digital chest tomosynthesis for improving detectability of small lung nodules. Twenty-one patients undergoing computed tomography (CT) to follow up lung nodules were consented and enrolled to receive an additional digital PA chest radiograph and digital tomosynthesis exam. Tomosynthesis was performed with a commercial CsI∕a-Si flat-panel detector and a custom-built tube mover. Seventy-one images were acquired in 11 s, reconstructed with the matrix inversion tomosynthesis algorithm at 5-mm plane spacing, and then averaged (seven planes) to reduce noise and low-contrast artifacts. Total exposure for tomosynthesis imaging was equivalent to that of 11 digital PA radiographs (comparable to a typical screen-film lateral radiograph or two digital lateral radiographs). CT scans (1.25-mm section thickness) were reviewed to confirm presence and location of nodules. Three chest radiologists independently reviewed tomosynthesis images and PA chest radiographs to confirm visualization of nodules identified by CT. Nodules were scored as: definitely visible, uncertain, or not visible. 175 nodules (diameter range 3.5–25.5 mm) were seen by CT and grouped according to size: <5, 5–10, and >10 mm. When considering as true positives only nodules that were scored definitely visible, sensitivities for all nodules by tomosynthesis and PA radiography were 70%(±5%) and 22%(±4%), respectively, (p<0.0001). Digital tomosynthesis showed significantly improved sensitivity of detection of known small lung nodules in all three size groups, when compared to PA chest radiography.
Keywords: tomosynthesis, pulmonary nodules, chest radiography
INTRODUCTION
Digital tomosynthesis is a type of limited angle tomography that allows reconstruction of multiple image planes from a set of projection data acquired over a limited range of x-ray tube movement. It offers the potential for improved diagnostic performance over conventional radiography by eliminating the visual clutter of overlying anatomy. While it does not have the depth resolution of computed tomography (CT), it provides high-resolution images in the sagittal or coronal planes at a dose and cost that are expected to be lower than with CT.
Digital tomosynthesis was first proposed several decades ago,1 but has only recently become practical with the advent of high-speed, self-scanned flat-panel detectors. We have investigated its application to the detection of pulmonary nodules, which are often subtle and obscured by overlying anatomy. A prototype system has been constructed in our laboratory for chest imaging, and we have previously reported on experiments to optimize the practical elements of image acquisition2 as well as the impulse response3 and noise power properties.4
We are currently conducting a NIH-funded clinical trial to evaluate digital tomosynthesis for improving the detection of pulmonary nodules. This article reports interim results from the initial cohort of subjects in that ongoing trial. In this initial report, detection sensitivity for pulmonary nodules was measured for digital tomosynthesis relative to conventional chest radiography over a range of nodule sizes.
METHODS
Image acquisition was performed using a prototype system constructed of a commercial-grade CsI∕a-Si flat-panel detector [equivalent to the detector in the Revolution XQ∕i system (GE Healthcare, Milwaukee, WI)], and a custom-built x-ray tube mover that moved the tube along a vertical path while articulating it to point at the center of the detector (SID=180 cm). Image acquisition parameters were chosen according to optimum values determined from previous experiments: 71 projection images, 20° of total tube angular motion, and 5 mm plane spacing for reconstruction. The set of 71 projection images was acquired in 11 s, which was easily within a breath hold of human subjects in this trial. Image reconstruction was accomplished using the matrix inversion tomosynthesis algorithm previously described,3, 5, 6, 7 and a sliding average of seven adjacent planes was formed to reduce noise and low-contrast tomosynthesis artifacts. The resulting images demonstrated subjectively excellent rendition of objects in each slice of interest, with minimal degradation from structures outside that slice.
Each human subject in this IRB-approved study was selected from the caseload of patients returning for follow-up CT evaluation of known lung nodules. Subjects with noncalcified nodules in the range of 3–15 mm diameter were targeted for inclusion in the study. In addition to the medically required CT, each consented subject also received a PA∕lateral conventional chest exam (although only the PA images were used in the results in this interim report) acquired on a commercial flat-panel system (Revolution XQ∕i, GE Healthcare) and a tomosynthesis imaging exam acquired on the prototype system described above. The phototimed mA s required for the PA radiograph was used to manually set the mA s on the subsequent tomosynthesis image acquisition. The total exposure for the tomosynthesis image acquisition was approximately that of 11 PA radiographs (about equal to a conventional screen-film lateral radiograph or two digital lateral radiographs), resulting in total tomosynthesis exposures of 68–135 mR for the subjects in this study.
CT images of subjects included in this report were reconstructed at 1.25-mm section thickness. Some additional initial human subjects had CT data reconstructed at 5-mm section thickness only and were not included in this analysis. Data from the first 21 human subjects (11 male, 10 female; ages 48–77) who had CT images reconstructed at 1.25-mm CT section thickness were analyzed and form the basis of this report.
Two chest radiologists reviewed all CT data at standard lung window settings in a consensus panel and noted the size and location of all nodules ≥3 mm in diameter for each subject. This data set was regarded as the “truth” set for nodule presence, but the nodules were not confirmed by pathology as benign or malignant. Three chest radiologists then independently reviewed tomosynthesis images, and at separate sessions at least 2 weeks apart, the PA radiograph of each subject to confirm visualization of nodules identified by CT. The readers knew the exact location of each target nodule based on the information provided by the CT image and assessed whether a nodule was visible at that specified location in the tomosynthesis and PA images. Nodules were scored as “definitely visible,” “uncertain,” or “not visible” in the tomosynthesis images and PA radiographs. Nodules scores were grouped according to size: Group A (<5 mm diameter), Group B (5–10 mm diameter), and Group C (>10 mm diameter).
Statistical comparisons of sensitivities of nodule detection by PA radiography and tomosynthesis (relative to the reference standard CT) were conducted by means of z score tests with standard errors estimated by the method proposed by Rao and Scott.8 Sensitivity of detection was compared by size group and for the overall cohort of all nodules. Sensitivity results were computed individually for each of the three observers and averaged for the composite results.
RESULTS
In total, 175 nodules (≥3 mm diameter) were found in the 21 subjects, with the number of nodules per subject ranging from 1–22. The minimum and maximum nodule diameters were 3.5 and 25.5 mm, respectively, and the mean diameter was 7.3 mm.
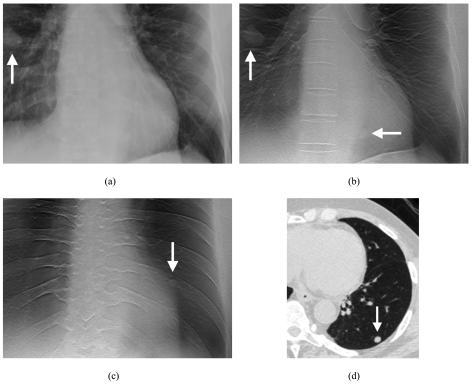
Figure 1 depicts the appearance of pulmonary nodules in one of the human subjects. A nodule that is clearly seen in the PA radiograph [Fig. 1a] is also seen in the tomosynthesis images [Fig. 1b], but there is an additional nodule that is seen only in the tomosynthesis images [Fig. 1c] and confirmed by CT [Fig. 1d].
Figure 1.
Images of nodules in one of the human subjects. (a) Coned view of digital PA radiograph shows one clearly visible right lung nodule (arrow). (b) Tomosynthesis image shows the same nodule (vertical arrow) as seen on the PA radiograph in (a). A second nodule (horizontal arrow) is also visible that was not seen in the PA radiograph in (a). (c) Tomosynthesis image at a more posterior level shows an additional left lung nodule (arrow) not seen in the PA radiograph in (a). (d) CT image (lung window) confirms left lower lobe nodule seen in (c).
Sensitivity results are presented in Table 1 as true-positive percentages (percentages of CT-confirmed nodules that were visible), using a score of “definitely visible” as a true positive response. A highly statistically significant improvement in detection sensitivity was noted for tomosynthesis over conventional PA radiography at all nodule sizes (p≤0.004 in all cases). Although the purpose of this interim study was not to evaluate reader effects, pairwise tests between readers for detection sensitivity indicated no statistically significant difference between readers at the 0.05 level.
Table 1.
Detection sensitivity (true positive percentage) when considering nodules scored “definitely visible” as true positive responses, averaged over three observers.
| Nodule size | No. of nodules | Tomosynthesis | PA radiography | p value |
|---|---|---|---|---|
| All | 175 | 70%(±5%) | 22%(±4%) | <0.0001 |
| 3−<5 mm | 40 | 53%(±8%) | 7%(±5%) | <0.0001 |
| 5−10 mm | 106 | 71%(±5%) | 20%(±3%) | <0.0001 |
| >10 mm | 29 | 90%(±6%) | 53%(±7%) | 0.004 |
For the stringent criterion of counting only “definitely visible” as true positive responses, more than half of the smallest nodules (3−5 mm) were visible in the tomosynthesis images, compared with only 7% in the PA radiographs. Thus, tomosynthesis extends the detection limit to substantially smaller nodules than with PA radiography. Considering only the nodules that would be actionable by the criteria endorsed by the Fleischner Society (>4 mm in diameter),9 74% were visible in the tomosynthesis images compared with 25% in the PA radiographs, indicating a threefold increase in detection sensitivity.
DISCUSSION
These results demonstrate for the first time with a flat-panel based chest tomosynthesis system that tomosynthesis has the potential for significantly improved detection sensitivity of known small lung nodules, when compared to PA chest radiography. These results will be followed up with the larger cohort of subjects in the ongoing NIH-sponsored trial.
Two observations are important when considering these results. First, it will be important to compare these findings acquired under a somewhat artificial viewing paradigm to the detection accuracy results from the ongoing study that will use ROC analysis. The current viewing paradigm allowed the radiologists to know the exact target location of each nodule to evaluate in the PA and tomosynthesis images. Of course, under normal clinical viewing, the locations of any nodules are not known a priori. Thus, the current results are likely to overestimate the detection sensitivities of both tomosynthesis and PA radiography and represent an upper bound of relative detection sensitivities between the two methods. Nonetheless, the large difference in sensitivity between tomosynthesis and PA radiography suggests that tomosynthesis will likely demonstrate improved sensitivity under more clinically realistic conditions, although the magnitude of the difference may not be as great.
A second point to consider is that the current interim results measured sensitivity only. It is also important to evaluate specificity when comparing two imaging modalities. Measures of specificity of pulmonary nodule detection will not be available until the conclusion of the ongoing NIH-sponsored trial.
One possible criticism of this study is that it did not measure detection of nodules <3 mm in diameter. These smaller nodules were not considered because earlier work suggested that they would be difficult to visualize with tomosynthesis, they are numerous, and they would not be considered actionable by the criteria of the Fleischner Society.9
While preliminary, these results indicate the likelihood that tomosynthesis will demonstrate improved performance for pulmonary nodule detection relative to conventional chest radiography in clinical practice.
ACKNOWLEDGMENTS
Grant support for this project was provided by the National Institutes of Health (R01 CA080490) and GE Healthcare. Duke University and GE Healthcare jointly hold a patent on tube movement strategy in tomosynthesis. The authors gratefully acknowledge and appreciate the expertise of Annette Rich, RT(R), Brenda Prince, RT(R), Melissa Jenkins, RT(R)(CT), and Anne Jarvis, RT(R)(M) in the collection of clinical human subject data.
References
- Grant D. G., “Tomosynthesis: A three-dimensional radiographic imaging technique,” IEEE Trans. Biomed. Eng. 10.1109/TBME.1972.324154 BME-19, 20–28 (1972). [DOI] [PubMed] [Google Scholar]
- Godfrey D. J., Rader A., and J. T.DobbinsIII, “Practical strategies for the clinical implementation of matrix inversion tomosynthesis (MITS),” Proc. SPIE 10.1117/12.480352 5030, 379–390 (2003). [DOI] [Google Scholar]
- Godfrey D. J., McAdams H. P., and J. T.DobbinsIII, “Optimization of the matrix inversion tomosynthesis (MITS) impulse response and modulation transfer function characteristics for chest imaging,” Med. Phys. 10.1118/1.2170398 33, 655–667 (2006). [DOI] [PubMed] [Google Scholar]
- Godfrey D. J., “Optimization and clinical implementation of matrix inversion tomosynthesis (MITS) for the detection of subtle pulmonary nodules,” Ph.D. dissertation, Duke University, 2005. [Google Scholar]
- Warp R. J., Godfrey D. J., and J. T.DobbinsIII, “Applications of matrix inverse tomosynthesis,” Proc. SPIE 10.1117/12.384512 3977, 376–383 (2000). [DOI] [Google Scholar]
- J. T.DobbinsIII, Godfrey D. J., and McAdams H. P., “Chest tomosynthesis,” in Advances in Digital Radiography: RSNA Categorical Course in Digital Radiography, edited by Samei E. and Flynn M. J. (Radiological Society of North America, Oak Brook, Illinois, 2003). [Google Scholar]
- J. T.DobbinsIII and Godfrey D. J., “Digital x-ray tomosynthesis: current state of the art and clinical potential,” Phys. Med. Biol. 10.1088/0031-9155/48/19/R01 48, R65–R106 (2003). [DOI] [PubMed] [Google Scholar]
- Rao J. N. K. and Scott A. J., “A simple method for the analysis of clustered binary data,” Biometrics 48, 577–585 (1992). [PubMed] [Google Scholar]
- MacMahon H., Austin J. H. M., Gamsu G., Herold C. J., Jett J. R., Naidich D. P., E. F.Patz, Jr., and Swensen S. J., “Guidelines for management of small pulmonary nodules detected on CT scans: A statement from the Fleischner Society,” Radiology 10.1148/radiol.2372041887 237, 395–400 (2005). [DOI] [PubMed] [Google Scholar]