Abstract
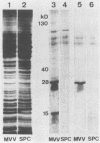
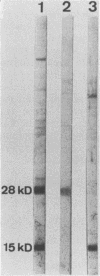
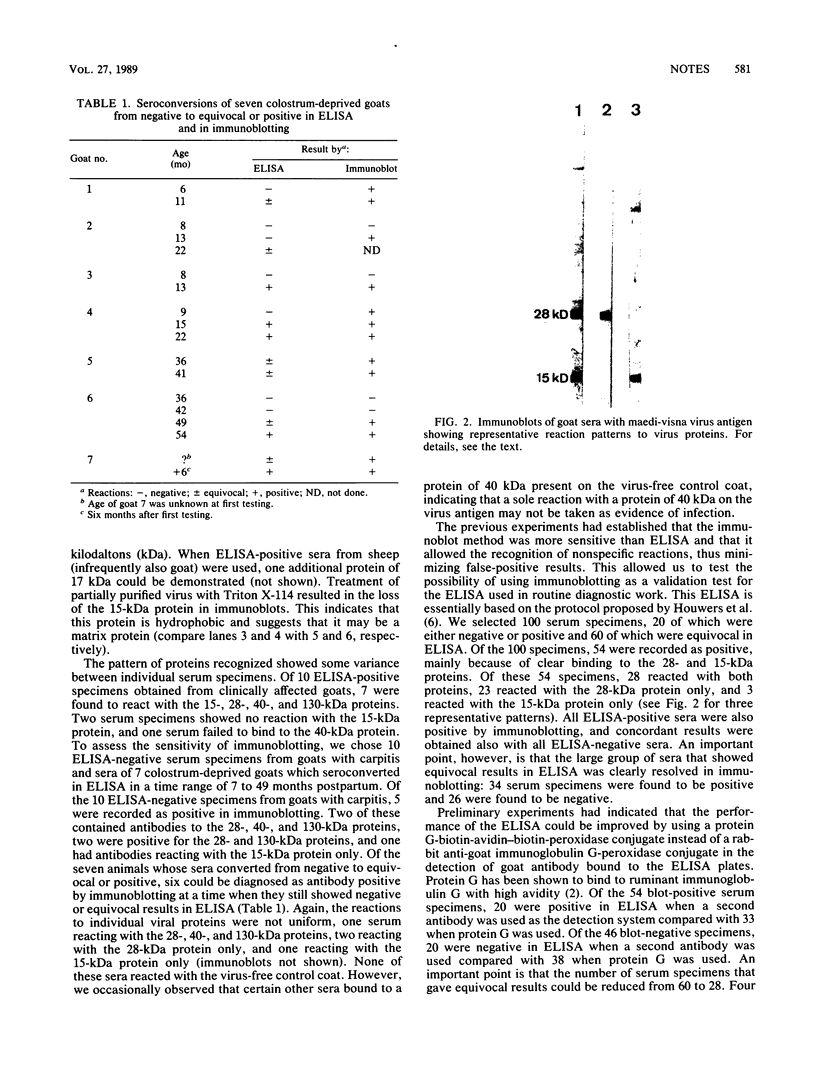
Sera from goats suffering from caprine arthritis-encephalitis contained antibodies to virus proteins of 15, 17, 28, 40, and 130 kilodaltons in immunoblots of maedi-visna virus. We propose to use immunoblotting as a validation test for enzyme-linked immunosorbent assay and demonstrate that the specificity of indirect enzyme-linked immunosorbent assay can be improved by replacing second antibody by a protein G-avidin-biotin conjugate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Gogolewski R. P., Barbet A. F., Cheevers W. P. Identification of caprine arthritis-encephalitis retrovirus proteins in immunodiffusion precipitin lines. J Gen Virol. 1985 May;66(Pt 5):1139–1143. doi: 10.1099/0022-1317-66-5-1139. [DOI] [PubMed] [Google Scholar]
- Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985 Oct;135(4):2589–2592. [PubMed] [Google Scholar]
- Archambault D., East N., Perk K., Dahlberg J. E. Development of an enzyme-linked immunosorbent assay for caprine arthritis-encephalitis virus. J Clin Microbiol. 1988 May;26(5):971–975. doi: 10.1128/jcm.26.5.971-975.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coackley W., Smith V. W., Houwers D. J. Preparation and evaluation of antigens used in serological tests for caprine syncytial retrovirus antibody in sheep and goat sera. Vet Microbiol. 1984 Oct;9(6):581–586. doi: 10.1016/0378-1135(84)90020-8. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., Gielkens A. L., Schaake J., Jr An indirect enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to maedi-visna virus. Vet Microbiol. 1982 Jul;7(3):209–219. doi: 10.1016/0378-1135(82)90035-9. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., Schaake J., Jr An improved ELISA for the detection of antibodies to ovine and caprine lentiviruses, employing monoclonal antibodies in a one-step assay. J Immunol Methods. 1987 Apr 2;98(1):151–154. doi: 10.1016/0022-1759(87)90449-2. [DOI] [PubMed] [Google Scholar]
- Klein J. R., Martin J., Griffing S., Nathanson N., Gorham J., Shen D. T., Petursson G., Georgsson G., Palsson P. A., Lutley R. Precipitating antibodies in experimental visna and natural progressive pneumonia of sheep. Res Vet Sci. 1985 Mar;38(2):129–133. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin K., Katz B. Z., Miller G. AIDS and antibodies to human immunodeficiency virus (HIV) in children and their families. J Infect Dis. 1987 Jan;155(1):54–63. doi: 10.1093/infdis/155.1.54. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nishanian P., Taylor J. M., Korns E., Detels R., Saah A., Fahey J. L. Significance of quantitative enzyme-linked immunosorbent assay (ELISA) results in evaluation of three ELISAs and Western blot tests for detection of antibodies to human immunodeficiency virus in a high-risk population. J Clin Microbiol. 1987 Feb;25(2):395–400. doi: 10.1128/jcm.25.2.395-400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Haller O., Vogt M., Lüthy R., Joller H., Oelz O., Popovic M., Sarngadharan M. G., Gallo R. C. Antibodies to HTLV-III in Swiss patients with AIDS and pre-AIDS and in groups at risk for AIDS. N Engl J Med. 1985 Jan 31;312(5):265–270. doi: 10.1056/NEJM198501313120502. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwahlen R., Aeschbacher M., Balcer T., Stucki M., Wyder-Walther M., Weiss M., Steck F. Lentivirusinfektionen bei Ziegen mit Carpitis und interstitieller Mastitis. Schweiz Arch Tierheilkd. 1983 May;125(5):281–299. [PubMed] [Google Scholar]