Abstract
When choosing an electromagnetic tracking system (EMTS) for image-guided procedures several factors must be taken into consideration. Among others these include the system’s refresh rate, the number of sensors that need to be tracked, the size of the navigated region, the system interaction with the environment, whether the sensors can be embedded into the tools and provide the desired transformation data, and tracking accuracy and robustness. To date, the only factors that have been studied extensively are the accuracy and the susceptibility of EMTSs to distortions caused by ferromagnetic materials. In this paper the authors shift the focus from analysis of system accuracy and stability to the broader set of factors influencing the utility of EMTS in the clinical environment. The authors provide an analysis based on all of the factors specified above, as assessed in three clinical environments. They evaluate two commercial tracking systems, the Aurora system from Northern Digital Inc., and the 3D Guidance system with three different field generators from Ascension Technology Corp. The authors show that these systems are applicable to specific procedures and specific environments, but that currently, no single system configuration provides a comprehensive solution across procedures and environments.
Keywords: image-guided therapy, electromagnetic tracking, accuracy analysis, usability study
INTRODUCTION
Tracking systems are a key component in image-guided interventions.1 Of the several types of available systems, optical ones were the first to be widely adopted in the clinical environment, as they provide accurate measurements that are not significantly affected by environmental factors. These systems have two key characteristics that limit their use in many interventions. They require a direct line of sight between the tracking system and tracked tools, and they cannot track flexible instruments. Electromagnetic tracking systems (EMTSs) do not place such restrictions and are thus potentially applicable in a large number of interventions that utilize flexible instruments inside the body. On the other hand EMTSs are often less accurate than optical systems and are susceptible to distortions induced by ferromagnetic materials.
These challenges have led researchers to evaluate EMTSs using a focused approach, characterizing system accuracy and stability.2, 3, 4, 5, 6 More recently, researchers have realized that generalizing results obtained in one environment to others is not straightforward. As a result, current accuracy evaluation studies have focused on specific clinical settings.2, 5, 6 Thus, one should use the manufacturer specified accuracy numbers only as a guideline, most likely obtained in an ideal environment. While these studies address the two primary challenges associated with the use of an EMTS in the clinic they do not cover all aspects determining the utility of these systems.
In this paper we identify a comprehensive set of factors that influence the utility of an EMTS in the clinical environment. We then evaluate two commercially available systems based on these factors, the 3D Guidance system from Ascension Technology Corp. (Milton, VT) and the Aurora system from Northern Digital Inc. (Waterloo, ON, Canada).
The motivation for the current evaluation stems from our experience in developing image-guidance systems using the Aurora EMTS. While tracking accuracy and robustness are critical factors, they are not sufficient for making an informed decision with regard to the utility of EMTS tracking for a specific procedure performed in a specific environment. The procedures we have targeted include radio frequency ablation (RFA) of liver tumors,7 creation of a transjugular intrahepatic portosystemic shunt (TIPS),8 carotid stent deployment,9 vertebroplasty,10 needle biopsies of liver lesions,11 and transbronchial biopsies.12
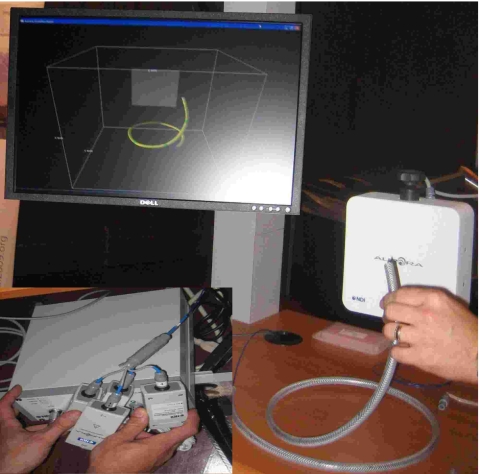
These procedures are performed in three different environments: an interventional radiology suite, a CT suite, and a pulmonology suite (Fig. 1). Our primary environment is the interventional radiology suite. It houses a floor mounted C-arm based cone beam CT (CBCT) system, the Siemens Axiom Artis dFA, which provides both volumetric images and projection images. The first four procedures, RFA, TIPS, carotid stent deployment, and vertebroplasty, are performed in this room. They utilize tracked needles, catheters, and vertebroplasty trochars. The liver lesion biopsies are performed in the CT suite and utilize needles. This suite houses a Siemens Somatom volume zoom CT machine that provides volumetric data and also real time single slice imaging CT fluoroscopy. Finally, the transbronchial biopsies are performed in the pulmonology suite using biopsy forceps inserted through the flexible bronchoscope’s working channel. The bronchoscope we use in this suite is the Pentax EB-1530T2. Fig. 2 shows the various tools used in these procedures.
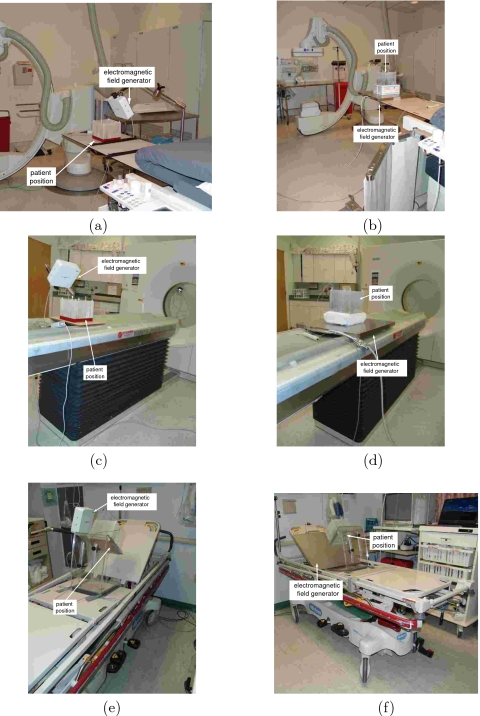
Figure 1.
Setup of electromagnetic tracking systems in the interventional environments in which we assess their usability [(a) and (b)] interventional radiology suite, [(c) and (d)] CT suite, and [(e) and (f)] pulmonology suite. Left column shows the setup with the Aurora system. 3D Guidance midrange and short-range field generator setups are similar. Right column shows the setup with the 3D Guidance flat-panel field generator.
Figure 2.
Tools used in the clinical procedures we study. From left to right: needle, catheter, vertebroplasty trochar, and biopsy forceps.
Ideally, we would like to have a single EMTS that is applicable across procedures and environments. Based on the set of procedures enumerated above we define the following requirements from this ideal EMTS:
-
(1)
Refresh rate: refresh rate of 100 Hz with a latency of less than 1 ms regardless of the number of deployed sensors, ensuring availability of up to date guidance information at a rate that is three times higher than current real time display.
-
(2)
Concurrency: tracks up to 30 sensors concurrently, potentially tracking multiple flexible tools with several sensors embedded in each tool.
-
(3)
Working volume: effective work volume of 53 m (room sized).
-
(4)
Obtrusiveness: sensors are wireless and can function for several hours, all hardware components can be positioned so that they do not restrict the physical access to the patient, and the system does not have any effect on other devices used during the procedure.
-
(5)
Completeness: sensors are small enough to embed in any tool and provide all six degrees of freedom (6DOF sensors).
-
(6)
Accuracy: resolution less than 0.1 mm and 0.1°, similar to current high end optical systems.
-
(7)
Robustness: not affected by the environment (light, sound, ferromagnetic materials, etc.).
Having established the requirements from the ideal EMTS we are now ready to evaluate existing EMTSs based on the way they address these requirements.
As far as we are aware, there are only three vendors for stand-alone EMTS, Ascension Technology Corp. (Milton, VT),13 Northern Digital Inc. (Waterloo, ON, Canada),14 and Polhemus (Burlington, VT).15 In this study we evaluated the 3D Guidance (Ascension Technology Corp.) and Aurora (Northern Digital Inc.) systems. We did not evaluate any of the tracking systems from Polhemus, as the sensor size used by these systems is on the scale of several centimeters which precludes embedding them in any of the medical devices which we need to track.
MATERIALS AND METHODS
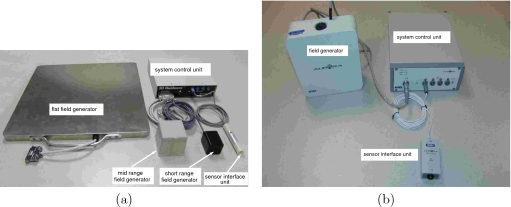
In this work we assess the 3D Guidance and Aurora EMTSs. Both systems consist of three basic components, the field generator, a system control unit that interfaces with a PC, and tracked sensor coils and their respective interfaces to the system control unit. Note that the 3D Guidance system is evaluated in three configurations, using the flat-panel, midrange, and short-range field generators. Figure 3 shows the system components for both EMTSs as used in this study.
Figure 3.
Electromagnetic tracking systems and their components, as used in this study: (a) 3D Guidance and (b) Aurora.
From our prior experience with assessing the accuracy of the Aurora EMTS,6 we have observed that performance is highly dependent on the interventional environment. The presence of medical apparatus distorts measurements in a manner unique to each setup. We thus believe that, while electromagnetic tracking is a viable option for tracking in the clinical environment its applicability should be evaluated per environment, and even more specifically on a per procedure basis. Hence, our evaluation is based on the way each system addresses the requirements described above in a set of specific environments or procedures where relevant.
Refresh rate
Refresh rate is important primarily when the data from the tracking system is used to provide quantitative guidance to the physician. This is the case in our needle biopsy application. As the physician inserts the needle towards the tumor, insertion depth is monitored using the distance between the needle tip and the target. This distance should be updated at a rate that does not limit the physicians actions.
The maximal refresh rate for the 3D Guidance system according to manufacturer specifications is 160 Hz when using the flat-panel field generator and 375 Hz when using the midrange and short-range field generators. The factory default refresh rate, set for optimal accuracy, for the flat-panel field generator is about 40 Hz and for the midrange and short-range field generators is about 68 Hz. We empirically evaluated the refresh rates for all three configurations using six degree of freedom (6DOF) sensors.
The maximal refresh rate for the Aurora system according to manufacturer specifications is 40 Hz. We empirically evaluated this system using five degree of freedom (5DOF) sensors.
Actual refresh rate was estimated as follows. All tools were manually translated inside the tracked region for a period of approximately 30 s. Time stamped data was acquired using a custom program from Ascension Corp. for the 3D Guidance system and a program developed in-house for the Aurora system. Both programs continuously request new measurements from the tracking system and record the returned measurement and a time stamp with millisecond resolution. The actual refresh rate is defined by the mean difference between consecutive time stamps. To test if the number of sensors affects the refresh rate the experiment was repeated using between one and four sensors.
System latency was only assessed qualitatively with respect to user perceived lag between moving a tracked sensor and the on screen motion of its simple mesh representation. This qualitative evaluation places an upper bound on the tracking system’s latency as it also incorporates the latency introduced by the graphics pipeline.
Concurrency
The 3D Guidance system supports tracking of up to 12 5DOF sensors and eight 6DOF sensors. The Aurora system supports tracking of up to eight 5DOF sensors or four 6DOF sensors. In the clinical setting, the number of tools that need to be tracked simultaneously is limited by the number of operating physicians and the complexity of the clinical procedure being performed. Therefore it is likely that most clinical procedures utilizing EMTS will rarely need to track more than a handful of tools at any given time. However, as the use of EMTS in the clinical environment proliferates, it is conceivable that flexible tools embedded with multiple sensors will require concurrent tracking of more than 12 sensors.
Working volume
The measurement space of an EMTS is defined as a volume around the field generator that is energized by AC or DC magnetic fields of a characterized nature. An EM sensor that is introduced within this volume responds with an induced voltage across the sensor that is proportional to the excitation field strength at that location. The drop-off in excitation signal power is inversely proportional to the fourth power of the distance from the field generator.16 Therefore, signal quality degrades the further away the sensor is from the field generator. For this reason, most manufacturers either specify measurement accuracy as a function of distance from the field generator (Aurora) or define an optimal subvolume wherein submillimetric or millimetric accuracy can be expected (3D Guidance).
We defined the ideal effective work volume as a tracking volume of 53 m (room sized). The rationale here is that in a clinical environment it is desirable to incorporate systems in as nonintrusive a manner as possible. With a room sized effective tracking volume, the positioning of the field generator relative to the patient is not an issue as the patient is always inside the tracked volume. In addition, the physical obtrusiveness of the system is greatly reduced as the generator can be positioned in the room in a flexible manner so that it does not restrict access to the patient or the mobility of medical staff and equipment. The reality, however, is that there are currently no systems that facilitate tracking of sensors smaller than 2 mm diameter within a volume larger than 1 m3. Based on the progression of EMTS since its development in the 1970s, it is conceivable that any system capable of tracking small sensors within a room-sized environment will be severely plagued by distortion and would most likely achieve this through stronger excitation signals that can potentially have an adverse effect on other medical apparatus. For practical purposes the use of an EMTS in a procedure is dependent on the active working area required for the intervention. That is, tracking must be possible in a volume that encompasses the anatomy of interest. In practice we have found that judicious positioning of the field generator does compensate for the currently available work volumes.
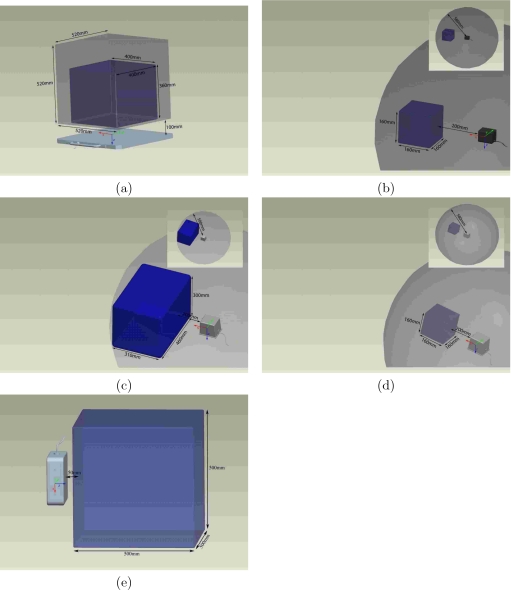
The 3D Guidance system’s working volume is dependent on the electromagnetic field generator. The optimal work volume (OWV) for each field generator is further defined by the manufacturer based on the type and size of the tracked sensor. With the flat-panel field generator the system’s working volume is a rectangular prism, 520×520×520 mm3 (x,y,z). The OWV is defined as a rectangular prism with sides of 400×400×360 mm3 irrespective of the sensor used. The work volumes for the midrange and short-range field generators are defined in terms of the radial distance from the center of the field generator. The origin for the midrange and short-range field generators is approximately located at the center of the field generator with the tracked volume being a sphere with a radius of 580 mm. The OWV for the midrange field generator is a rectangular prism with sides of 310×460×300 mm3 volume located 200 mm from the center of the field generator for a 1.8 mm 6DOF sensor and a 160×160×160 mm3 for the 1.3 mm 6DOF. The OWV for the short-range field generator is a 160×160×160 mm3 cube irrespective of the type of sensor used. The Aurora system provides a working volume of 500×500×500 mm3, which is its OWV. In further discussions with the Aurora manufacturer we were informed that there is an optional upgrade to the system that provides a silo shaped work volume that is larger than the one evaluated by us. That system was not included in this study. Figure 4 displays the work volumes of all the systems evaluated in this study.
Figure 4.
Work volumes for (a) 3D Guidance with flat-panel generator, (b) 3D Guidance with short-range generator, (c) 3D Guidance with midrange generator and 1.8 mm 6DOF sensor, (d) 3D Guidance with midrange generator and 1.3 mm 6DOF sensor, and (e) Aurora. Darker color volume denotes manufacturer defined optimal work volume.
The small size of the optimal work volume provided by the 3D Guidance system with the short-range field generator and with the midrange generator with the 1.3 mm sensors requires special consideration when positioning the field generator relative to the patient. For all of our procedures, except for carotid stent deployment, a work volume of 500×500×500 mm3 allows us to position the field generator with relative ease so that the tracked region encompasses the anatomical structures of interest. For carotid stent deployment, the initial part of the navigation starting at the femoral artery is performed using the current clinical approach, and once the tracked volume is reached the physician can use our navigation system.
Obtrusiveness
The obtrusiveness of an EMTS manifests itself in two ways: the physical presence of the system and the effect of its electronic components on medical apparatus. The physical obtrusiveness of a system stems from the fact that we introduce additional hardware into a clinical environment which is most often already cramped. Both the Aurora and 3D Guidance systems are wired. That is, the tracked tools are connected via wires to the control unit. While this does not preclude any of the procedures we are investigating, it does make them more cumbersome. More importantly, these additional wires require caution on the part of the medical staff when moving around the patient. In addition, the physical presence of an EMTS may interfere with imaging if system components are in the field of view of the imaging apparatus.
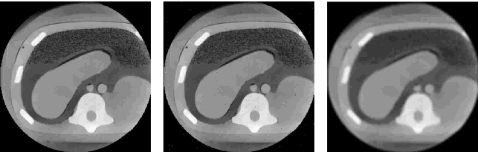
Electrical obtrusiveness is assessed based on the system’s influence on the imaging apparatus employed in each procedure. This is done by positioning the tracking system, acquiring images of a phantom while the system is still off, and then acquiring the same images while tracking. In some cases we have noticed that the images acquired during tracking are slightly degraded. We provide a qualitative analysis of the image quality. We do not perform quantitative analysis (e.g., peak signal to noise ratio) as it does not reflect the perceptual quality of the images which is the relevant quantity. In Fig. 5 we give an example of CBCT images of an abdominal phantom, showing that quantities such as peak signal to noise ratio do not correlate with perceptual quality. In this case the image is artificially corrupted once with impulse noise and once using a low pass filter resulting in the same peak signal to noise ratio (34.4 dB), which clearly does not reflect their perceptual quality. Finally, in a medical context, two images may have an overall similar perceptual quality but differ in their utility. For example, the location of small catheters can be obscured by localized impulse noise which otherwise does not detract from the overall perceptual quality of the image.
Figure 5.
Images of an abdominal phantom (CIRS, Norfolk, VA). From left to right, original CBCT slice, slice corrupted with impulse noise (salt and pepper), and slice corrupted with a low pass filter. Both corrupted slices have the same peak signal to noise ratio (34.4 dB) and are displayed using the same window and level values (1780,0). It is clear that the perceptual quality of the image corrupted by impulse noise is higher than the image corrupted via the low pass filter.
All of our imaging experiments in the interventional radiology and CT suites utilized an interventional 3D abdominal phantom (CIRS, Norfolk, VA). This phantom is composed of materials that mimic the x-ray attenuation of human tissue. All of the imaging experiments in the pulmonology suite utilized a pulmonary phantom, the AC9∕8 flourescent bronchial tree (Adam, Rouilly Ltd., Kent, UK).
Completeness
Both the Aurora and 3D Guidance systems support 5DOF and 6DOF sensors. The distinction between these two sensor types is that the 5DOF sensors do not resolve the rotation angle around the EM sensor’s main axis. For the Aurora system the smallest 5(6)DOF sensors have a diameter of 0.55 (1.8) mm. For the 3D Guidance system the smallest 5(6)DOF sensors have a diameter of 0.3 (1.3) mm. While we prefer to use 6DOF sensors these are not necessarily required for all applications. We evaluate the necessity of using 6DOF sensors on a per procedure basis with regard to the required guidance and the constraints imposed by the specific tool sizes.
Accuracy
The accuracy of each system was evaluated using a protocol similar to that described by Wilson et al.6 The protocol assesses the system’s static accuracy. A Plexiglass phantom is used to acquire data measurements at 225 locations. The phantom is a 180×180×180 mm3 cube with a 15×15 grid layout of holes on the top face. Each hole was drilled to a random known depth ranging from 10 to 140 mm. After construction, the depth of each hole was measured using an industrial depth gauge with a resolution of 0.01 mm and instrumentation error under 0.03 mm.
At each of the 225 locations, 100 transformations are sampled and a representative transformation is estimated. The translational part of this transformation is the arithmetic mean of the acquired translations. The rotational part is obtained as the arithmetic mean of the rotations, represented as unit quaternions, followed by normalization. While this estimate of the mean rotation initially ignores the fact that rotations belong to a nonlinear manifold, it has been shown that after renormalization this is an acceptable estimate.17
We also record the distance of each of these 100 samples from the EMTS origin and the range of the distance variability, reflecting the system’s stability. The phantom and EMTS coordinate systems are then registered using paired-point rigid registration.18 For each hole we compute the distance between the known point location and the reported one after applying the registration transformation. We also compute the angular difference between the known hole orientation and the reported one, again after applying the registration transformation.
For the registered data we provide the following descriptive statistics: maximal sample variation, rms error, mean error, standard deviation, error range, maximal error, and 95 percentile. It should be noted that from a clinical standpoint the mean and standard deviations of the errors are less meaningful as patient safety requires that we obtain bounds on the worst case behavior. We thus feel that the 95th percentile is the single most meaningful measure. The maximal sample variability describes the variability in the logged samples at each location and is defined as
where di is the distance from the EMTS origin for the ith acquisition at location j. The impetus for this measure is that we found some devices to offer excellent overall accuracy when averaged across 100 samples, but, within the samples there was a great deal of variability. Thus, for an accurate measurement the actual refresh rate is potentially reduced to one that is clinically unacceptable. We use the maximal sample variability to denote the overall stability of EM measurements when using the system’s default refresh rate.
Our goal is to assess the system accuracy in a setting that is as close as possible to the clinical one. We thus position our phantom so that its spatial location occupies the expected patient position, and its orientation is similar to the expected direction of tool insertion.
System accuracy was first assessed in an office, a ferromagnetically clean environment, and then in each of the clinical environments using the protocol described above. Figure 1 shows the experimental setup in the three clinical environments. In the interventional radiology suite the distance between the C arm and the center of the measurement space was approximately 200 cm. In the CT suite the distance between the CT gantry and the center of the measurement space was approximately 180 cm. In the pulmonology suite the distance between the patient stretcher and the center of the measurement space was approximately 35 cm.
Robustness
We define the robustness of an EMTS as its resilience to distortions arising from tools and imaging apparatus that are introduced and removed from the work volume during the procedure. Note that a system can be inaccurate but robust, consistently reporting the same measurements in a dynamic environment. More importantly, inaccuracies of a robust system can be corrected using static correction schemes. Currently, most correction schemes19, 20, 21 are static. This means that they assume either that no distorters are introduced or removed from the environment during the procedure or that the system is robust. This approach views the EMTS as an open loop system whose measurements can be corrected using constant correction values. In many clinical procedures one cannot guarantee that distorters will not be introduced or removed from the interventional site. It is clear that the robustness assumption is an important aspect of all static correction schemes. In our case we are primarily interested in the effects of the imaging apparatus as it is the only nonstationary instrumentation that can have a significant impact on the EMTS.
Robustness is quantified by analyzing the variability in EM measurements from two sensors during image acquisition. This setup is motivated by the use of dynamic reference frames in image-guided interventions, where all spatial data are transferred relative to a patient mounted reference frame. In our experiments we tracked two sensors placed on our anatomical phantoms at a fixed distance, of a few centimeters, from each other. We then acquired images with the specific imaging apparatus in each of our environments. If the separation between the two sensors remains constant throughout the test then imaging has no effect on tracking and the EMTS is deemed robust for our purposes.
To establish a ground truth, we first evaluate the stability of the measurements with the setup we expect to use during navigation, with all imaging apparatus far from the patient (the home position). Robustness was evaluated in each of the environments as follows.
Interventional radiology suite
In the interventional radiology suite the device that is potentially most disruptive to electromagnetic tracking is the C arm. To evaluate its effect on the stability of the tracking we performed the following experiment. We first acquired the ground truth measurements, tracking with the C arm at a distance of approximately 2 m from the patient location. The C arm was then moved into imaging position, approximately 50 cm from the sensor locations, and an x-ray fluoroscopy image and tracking data were simultaneously acquired for validation purposes as would be done in a navigated procedure. Finally, we acquired tracking data during a CBCT scan. The physical setup remained the same, with the only difference being the rotation of the C arm during the acquisition of the projection images. In our case, as we are interested in procedures performed in the thoracic-abdominal region we would potentially use the tracking data to acquire a respiratory gated CBCT image. For each of these experiments we computed the distance between the two sensors based on their respective transformations.
CT suite
In the CT suite, both the patient couch and CT gantry are potentially disruptive to electromagnetic tracking. Both these components are active while a CT scan is acquired. When CT fluoroscopy is used only the gantry rotates and the couch remains stationary. To evaluate the effect of these elements on the stability of the tracking we performed the following experiment. We first acquired ground truth measurements at the location where we expect to perform navigation, about 2 m away from the gantry. This was followed by acquisition of tracking data during CT fluoroscopy, simulating imaging performed for validation purposes in a navigated procedure. In this setup the bed was translated so that the sensors were inside the CT bore, approximately 35 cm away from the side of the bore. Finally we acquired tracking data during a CT scan, with the goal being to use the tracking data to acquire a gated CT scan for thoracic-abdominal interventions. For each of the data acquisitions we computed the distance between the two sensors based on their respective transformations.
Pulmonology suite
In the pulmonology suite the potentially problematic equipment is the patient’s stretcher itself and the endoscope with its CRT display. In this case we acquired data before and after turning the endoscope on. This is the only environment in which the setup used for evaluating robustness coincides with the setup we intend to use during navigation. In this setup the tracked sensors are approximately 1 m away from the CRT and 35 cm away from the stretcher.
Note that in our institute the procedure is performed with the patient lying on a stretcher. Unlike the interventional radiology and CT suites where the patient couches are fixed, the stretcher model varies, as the procedure is performed on whichever stretcher the patient is being transported with. This may result in performance variability based on the stretcher type. In our case we performed the evaluation with a single stretcher type, the Hill-Rom P8000 transport stretcher (Batesville, IN). Our conclusions with regard to this environment are thus valid only for this specific configuration.
EXPERIMENTAL RESULTS
We now describe how each of these systems addresses the requirements we have outlined above based on our experiments in the three clinical environments.
Refresh rate
The maximal refresh rate we obtained for the 3D Guidance system with the flat-panel field generator was approximately 160 Hz irrespective of the number of tracked sensors. The configurations with the midrange and short-range field generators were also unaffected by the number of tracked sensors, with an acquisition rate of 190 Hz.
The maximal refresh rate for the Aurora system was approximately 40 Hz, concurring with the manufacturer’s specifications. This was obtained with a single sensor. When an additional 5DOF sensor was attached the refresh rate dropped to 20–25 Hz. Attaching additional sensors after this initial decrease in refresh rate did not have any effect. In further discussions with the manufacturer we were informed that the newer version of the system is able to track multiple sensors at the maximal refresh rate of 40 Hz. That system was not included in this study.
Through our extensive work developing image-guidance applications with the Aurora system we have empirically found that a refresh rate of 25–30 Hz is sufficient for dynamic guidance of a human operator, with potentially higher refresh rates required for using an EMTS in conjunction with robotic devices.
System latency was assessed qualitatively using visual inspection. Latency of the 3D Guidance system in all three configurations and of the Aurora system was found to be insignificant. That is, none of the users perceived noticeable lag between action and on screen motion. Psychophysical experiments have shown that the average just-noticeable difference for latency discrimination in virtual reality environments for head or hand movement is approximately 15–20 ms.22 We thus conclude that the latency for all the evaluated systems is most likely less than 20 ms. As with refresh rate, using an EMTS in conjunction with a robotic device will potentially require lower latency.
Concurrency
For all of our applications we have found that we do not need to track more than eight sensors concurrently. More importantly, one of the constraints on the design of the procedure workflow was the number of tools that can be attached to the system at the same time, even when concurrent tracking is not required. Ideally, a technician attaches all tools to the control box at the beginning of the procedure and the physician utilizes them as is the case with nontracked instruments. This approach requires concurrent tracking of multiple tools even when most of them are not in use, as they should be automatically detected and tracked once the physician introduces them into the work volume.
Finally, flexible tools that contain more than a single sensor can potentially be useful for tracking internal organ motion, providing input for nonrigid registration. In this case the registration result would most likely improve if more tracked points are available. Figure 6 shows a recently introduced multisensor catheter tracked by the Aurora system. The catheter has eight embedded 5DOF sensors and a diameter of approximately 2 mm. An interesting observation with regard to multisensor tools is that the minimal tool diameter is not necessarily determined by the sensor diameter, which has been the limiting factor to date. Rather, the diameter of all the wires leading from the sensors to the control box may be larger than that of a single sensor.
Figure 6.
A catheter embedded with eight 5DOF sensors tracked by the Aurora system. The catheter is placed inside a large plastic tube for better visibility. Note that no other tool can be tracked when the catheter is being used (inset shows that all control box ports are in use).
Working volume
The size of the manufacturer specified optimal work volume places constraints on the positioning of the field generator. When positioning the generator relative to the patient we desire an optimal setup, maximizing the overlap between the optimal work volume and the anatomical structures of interest. As the work volume is not visible, positioning the field generator is often a trial and error process for an inexperienced user.
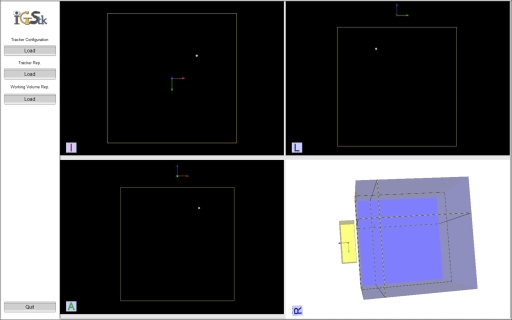
To position the field generator a single sensor or multiple tracked sensors are placed in the expected patient location and the field generator is moved so that all of the sensors are inside the measurement volume. This can be achieved using a simple program that reports sensor locations as obtained by the tracking system with a human operator comparing them to the known optimal values or with a program displaying a graphical representation of the work volume as shown in Fig. 7. It should be noted that this challenge is not unique to EM systems, as the working region of all tracking systems is not visible. It is simply that their small work volumes require additional care during system setup.
Figure 7.
Work volume visualization program showing the Aurora work volume and a tracked tool (sphere). The program facilitates setup of various tracking systems and is part of the IMAGE-GUIDED SURGERY TOOLKIT (Ref. 23). The display shows the xy, xz, and yz planes and a three dimensional translucent view of the work volume.
Obtrusiveness
The obtrusiveness of each EMTS was evaluated in our work environments with regard to its physical and electronic effects on the procedure, primarily the constraints introduced by the physical presence of the equipment and the electronic effect on imaging.
Interventional radiology suite
The interventional radiology suite is the only environment in which one has to exercise additional caution when positioning the EMTS’s field generator and wires. This is primarily due to the dynamic nature of this environment, where the EMTS is positioned while the C arm is stationary. From our experience, we have found that it is not immediately evident which spatial locations will be in the path of the rotating arm during CBCT acquisitions.
In all procedures performed in the interventional radiology suite the 3D Guidance configured with flat-panel field generator does not change the access to the patient, as the field generator is placed underneath the mattress [Fig. 1b]. The 3D Guidance configured with the midrange and short-range generators is more obtrusive. In these configurations the field generator is mounted on a passive mechanical arm positioned approximately 150 mm from the patient. This restricts physical access from certain directions but has not been a limiting factor in any of our procedures. The Aurora system is positioned similarly [Fig. 1a]. It is slightly more intrusive than the midrange and short-range configurations due to the larger form factor of the field generator.
In this environment all of our navigated procedures utilize either preoperative CT or in situ CBCT for navigation and x-ray fluoroscopy for intraoperative imaging, validating the information presented by the navigation system.
The only system that always influences the quality of the images due to its physical presence is the 3D Guidance with the flat-panel field generator. We have found that the field generator cannot be easily moved in and out of the tracking position as it is placed underneath the patient. Thus, even if tracking is not required during image acquisition the field generator remains in the field of view of the C arm. This resulted in imaging artifacts both in the x-ray fluoroscopy images and in the CBCT data, as it is reconstructed using multiple projection images. For all other systems the presence of the field generator, mounted on the passive arm, precludes imaging from certain C-arm poses. This is not a limiting factor as long as imaging and tracking are not required simultaneously, since the field generator is easily moved out of the way.
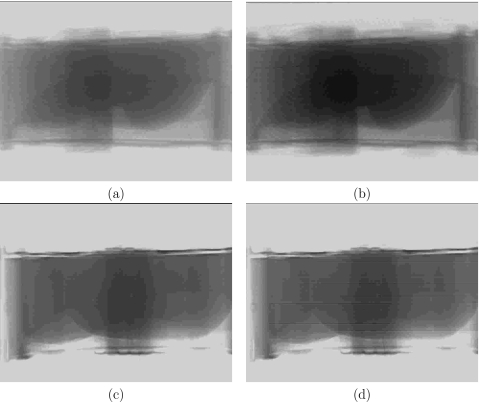
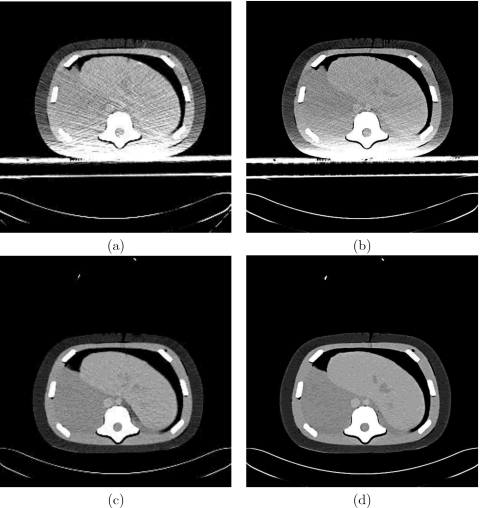
For the 3D Guidance with flat-panel field generator no noticeable differences were visually detected when comparing the same images, x-ray and CBCT, acquired with the system turned off and during tracking. It is possible that the system electronics does add additional distortion but it is hard to detect given the existing image distortion due to the physical presence of the field generator. The 3D Guidance with midrange field generator exhibited very minor horizontal line artifacts in x-ray fluoroscopy when images were acquired during tracking. No visually noticeable artifacts were detected in the CBCT data. The 3D Guidance system with the short-range field generator exhibited horizontal line artifacts in x-ray fluoroscopy when images were acquired in conjunction with tracking. In turn, the CBCT data also exhibited visible artifacts as it is reconstructed using multiple x-ray images. The Aurora system was equivalent to the 3D Guidance with midrange field generator. Figure 8 shows example x-ray fluoroscopy images acquired for the 3D Guidance system with the flat-panel and short-range field generators. Figure 9 shows axial slices from CBCT reconstructions for both systems.
Figure 8.
Effect of 3D Guidance flat-panel [(a) and (b)] and short-range [(c) and (d)] field generators on x-ray fluoroscopy image quality; all images are of the CIRS abdominal phantom. Left column: images acquired with the tracking system off; right column: images acquired while tracking.
Figure 9.
Effect of 3D Guidance flat-panel [(a) and (b)] and short-range [(c) and (d)] field generators on CBCT reconstruction quality; all images are of the CIRS abdominal phantom. Left column: axial slice reconstructed from data acquired with the tracking system off: right column: reconstruction from data acquired while tracking. Ellipse delineates streaking artifacts due to electronic interference caused by the short-range field generator.
CT suite
In the needle biopsy procedures performed in the CT suite, the 3D Guidance system’s flat-panel field generator is placed underneath the patient [Fig. 1d]. This requires that the standard mattress be replaced with one which will support the patient. The curved shape of the patient couch means that using the flat-panel field generator raises the patient position, reducing the effective bore size. This is likely to cause concerns with obese patients. Both the midrange and short-range field generators are positioned using a passive mechanical arm positioned approximately 150 mm from the patient. This restricts physical access to one side of the patient but has not been a limiting factor in this procedure as the mechanical arm and field generator are on the opposite side of the CT bed from the physician. As was the case in the interventional radiology suite, the Aurora system is similar in terms of physical obtrusiveness.
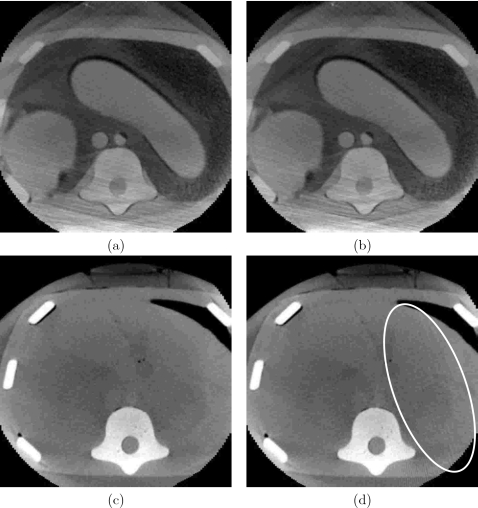
In the needle biopsy procedures there are two modes of imaging, acquisition of a preoperative CT scan and intraoperative CT fluoroscopy, real time single slice imaging. When using the 3D Guidance system with the flat-panel field generator the images always included the field generator as it is placed underneath the patient. This results in degraded images. The 3D Guidance with midrange and short-range field generators and the Aurora system are not in the field of view when imaging and thus their physical presence does not affect image quality.
In this environment image quality, both CT and CT fluoroscopy, with tracking and when the systems were off was visually similar for all EMTSs. Figure 10 shows typical CT fluoroscopy and CT images concurrently acquired with tracking for the 3D Guidance system with flat panel and for the Aurora system.
Figure 10.
Effect of 3D Guidance flat-panel [(a) and (b)] and Aurora [(c) and (d)] field generators on CT fluoroscopy and CT reconstruction quality. Images acquired with and without tracking were equivalent (examples are with tracking). Left column: CT fluoroscopy; right column: CT. The 3D Guidance configured with the midrange and short-range field generators resulted in images similar to those acquired with the Aurora system.
Pulmonology suite
In the transbronchial biopsy procedure the patient lies on a stretcher at a 45° upright angle. The 3D Guidance flat-panel field generator is physically unobtrusive as it is placed under the patient’s back [Fig. 1f]. When using the midrange and short-range field generators they are positioned using a passive mechanical arm attached to the stretcher’s rail. This restricts physical access to one side of the patient but is not a limiting factor since the physician stands on the opposite side of the stretcher facing the bronchoscopy monitor. The Aurora setup is similar, see Fig. 1e, leading to the same limitations.
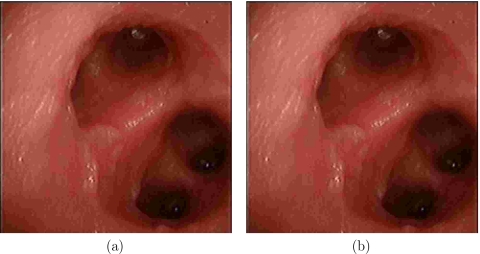
In this environment we have found that none of the systems had any visible effect on the video images. Figure 11 shows example images acquired with and without tracking using the Aurora system.
Figure 11.
Effect of Aurora system on bronchoscopic video images: (a) image acquired without tracking and (b) image acquired with tracking. Images acquired when using the 3D Guidance system in all three configurations are similar.
Completeness
In each of the environments we evaluate the use of 5DOF or 6DOF sensors based on the required information and the constraints on the sensor size as imposed by the specific tools used in the procedure.
Interventional radiology suite
The tools that require tracking in our procedures are 18 gauge (diameter of 1.02 mm) needles for RFA and TIPS, 22 gauge (diameter of 0.6 mm) needles and a trochar with a diameter of 4.2 mm for vertebroplasty, and a catheter with a diameter of 2.3 mm for stent deployment. Given the tool and sensor sizes, some of these procedures can only utilize 5DOF sensors. Luckily, all of the tools are similar in that they are used in a way that allows us to model them as cylinders. That is, the rotation around the tool axis is not required for navigation, enabling the use of 5DOF sensors.
CT suite
The tools required for the biopsy procedures performed in the CT suite are 18 gauge needles as used in the procedures performed in the interventional radiology suite.
Pulmonology suite
The tool that we need to track for the transbronchial biopsy is a forcep that must fit through the bronchoscope’s working channel. In our case, the working channel has a diameter of 2 mm, and the forceps have a sheath diameter of 1.8 mm. For this procedure we cannot use the same cylindrical model as done for other procedures. Navigation is performed using a preoperative CT in which a virtual camera is positioned in the same pose as the bronchscope’s camera which is tracked relative to the forceps. The tracked forceps are thus required to provide all six degrees of freedom, as the unknown rotation around the sensor axis is similar to an unknown camera rotation around the view direction. It should be noted that while a 6DOF sensor is the first choice for such procedures, it is possible to use a 5DOF sensor to perform the tracking in combination with video-to-CT registration to compensate for the unknown rotation.24
Accuracy
The accuracy of each of the tracking systems was evaluated as described in Sec. 2F. Accuracy is presented using descriptive statistics of error location and spread. Note that in some cases this concise description of the error distribution may not convey the distribution’s complexity.25 That is why for our primary work environment, the interventional radiology suite, we also provide a more detailed description of the positional error distribution in the form of histograms and plots displaying the error as a function of the distance from the field generator origin. It should be noted that in the tools we use the sensor is embedded near (5–10 mm) the tip. That is, our point of interest is close to the center of rotation. This construction mitigates errors in orientation as their effect on localizing the tip is minimized.
In the office environment the 3D Guidance systems with the midrange and short-range field generators were the most accurate with comparable performance. It should be noted that the measurements acquired using the short-range generator with its default settings vary considerably; this is mitigated by the fact that we are averaging over 100 transformations. The 3D Guidance with the flat-panel generator and the Aurora system were also comparable to each other but less accurate than the former systems. Table 1 summarizes the accuracy evaluation in the office environment.
Table 1.
Results of accuracy experiments in office and ferromagnetically clean environment. All error values are in millimeters and degrees.
| 3DG flat panel | 3DG short range | 3DG midrange | Aurora | |||||
|---|---|---|---|---|---|---|---|---|
| Position | Angle | Position | Angle | Position | Angle | Position | Angle | |
| Maximal sample Distance variable | 0.71 | 3.63 | 0.48 | 4.67 | ||||
| rms | 1.19 | 9.45 | 0.55 | 1.59 | 0.39 | 2.64 | 1.39 | 2.87 |
| Mean | 1.02 | 9.35 | 0.50 | 0.99 | 0.36 | 2.39 | 1.15 | 2.36 |
| Standard deviation | 0.61 | 1.35 | 0.21 | 1.25 | 0.16 | 1.12 | 0.78 | 1.62 |
| Range | 2.92 | 9.97 | 1.25 | 5.20 | 0.73 | 4.95 | 5.97 | 7.98 |
| Max. | 3.11 | 14.72 | 1.30 | 5.23 | 0.80 | 6.14 | 6.20 | 8.27 |
| 95 percentile | 2.33 | 11.11 | 0.89 | 4.23 | 0.63 | 5.64 | 2.41 | 8.03 |
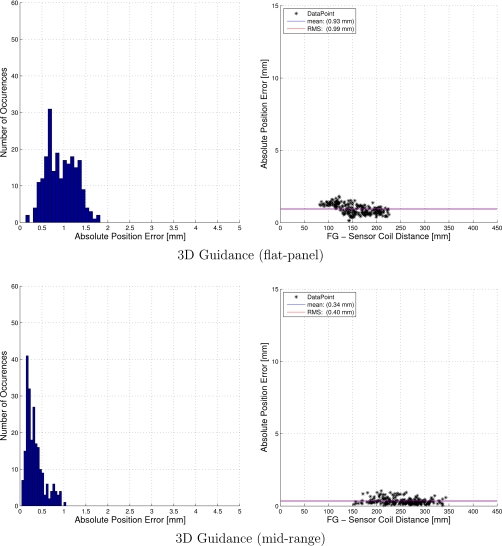
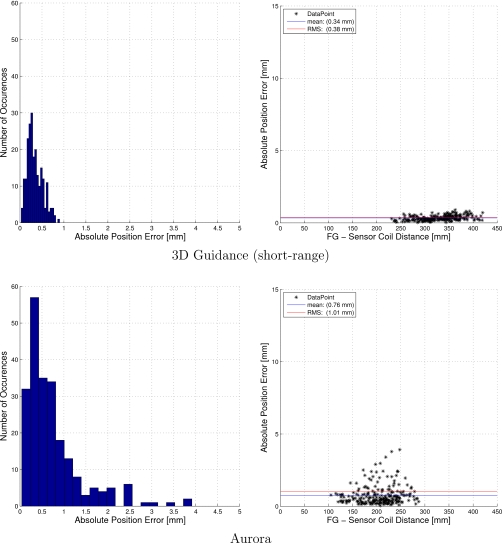
The interventional radiology suite was found to be the optimal environment for tracking. Surprisingly, the tracking accuracy in this environment is equivalent or even better than the accuracy obtained in our office environment. The reason that all the systems performed well in this environment is that it can be controlled so that the distortion of the EMTS magnetic field is minimal. The C arm, which is a potential cause for distortion, can be placed in its home position, about 2 m away from the patient and EMTS location. In addition, the region of the patient table over which the EMTS work volume is positioned is suspended in midair. Table 2 summarizes the accuracy evaluation in the interventional radiology suite. The 3D Guidance with short-range and midrange field generators were the most accurate in this environment, with the Aurora and 3D Guidance with flat-panel field generator having comparable accuracy. On the other hand, the 3D Guidance with short-range field generator was the least stable system. That is, measurements fluctuated considerably but when averaged gave excellent accuracy. Figures 1213 provide a more detailed description of the positional error distribution in the form of histograms and plots displaying the error as a function of the distance from the field generator origin.
Table 2.
Results of accuracy experiments in interventional radiology suit. All error values are in millimeters and degree.
| 3DG flat panel | 3DG short range | 3DG midrange | Aurora | |||||
|---|---|---|---|---|---|---|---|---|
| Position | Angle | Position | Angle | Position | Angle | Position | Angle | |
| Maximal sample Distance variability | 0.5 | 4.48 | 0.36 | 0.49 | ||||
| rms | 0.99 | 5.22 | 0.38 | 2.21 | 0.4 | 1.75 | 1.01 | 1.54 |
| Mean | 0.93 | 5.07 | 0.34 | 1.86 | 0.34 | 1.07 | 0.76 | 1.07 |
| Standard deviation | 0.34 | 1.25 | 0.18 | 1.21 | 0.21 | 1.39 | 0.67 | 1.11 |
| Range | 1.69 | 6.62 | 0.87 | 5.39 | 1.01 | 5.64 | 3.87 | 4.74 |
| Max. | 1.82 | 9.63 | 0.90 | 6.14 | 1.05 | 5.69 | 3.91 | 4.82 |
| 95 percentile | 1.47 | 8.65 | 0.69 | 5.19 | 0.80 | 5.12 | 2.20 | 4.37 |
Figure 12.
Accuracy evaluation in the interventional radiology suite: positional error histogram and error as a function of the distance from the field generator origin.
Figure 13.
Accuracy evaluation in the interventional radiology suite: positional error histogram and error as a function of the distance from the field generator origin.
In the CT suite there are two primary causes of electromagnetic distortion, the patient couch and the CT gantry. In practice, navigation is performed as far as possible from the CT gantry, so that the couch is the primary reason for degraded accuracy. Table 3 summarizes the accuracy evaluation in the CT suite. In this environment the 3D Guidance with flat panel was the most accurate. Note that the main cause of the distortion is the couch mechanism that is underneath the field generator, which complies with the assumptions underlying the use of the flat-panel generator. The 3D Guidance with short-range field generator and the Aurora system had comparable accuracy. The 3D Guidance with midrange provided slightly more accurate results.
Table 3.
Results of accuracy experiments in CT suite. All error values are in millimeters and degrees.
| 3DG flat panel | 3DG short range | 3DG midrange | Aurora | |||||
|---|---|---|---|---|---|---|---|---|
| Position | Angle | Position | Angle | Position | Angle | Position | Angle | |
| Maximal sample Distance variability | 0.30 | 2.54 | 0.48 | 0.54 | ||||
| rms | 1.08 | 4.03 | 6.49 | 34.82 | 3.64 | 3.51 | 5.76 | 2.31 |
| Mean | 1.02 | 3.82 | 5.67 | 33.43 | 3.18 | 3.13 | 5.13 | 2.05 |
| Standard deviation | 0.36 | 1.29 | 3.17 | 9.77 | 1.78 | 1.61 | 2.62 | 1.07 |
| Range | 1.76 | 6.18 | 17.34 | 48.11 | 10.09 | 7.25 | 18.33 | 5.47 |
| Max. | 1.96 | 7.94 | 18.14 | 54.79 | 10.56 | 7.35 | 19.24 | 5.67 |
| 95 percentile | 1.56 | 7.35 | 12.29 | 47.28 | 5.88 | 6.55 | 10.83 | 4.05 |
In the pulmonology suite the primary source of distortion is the patient stretcher. In our case we evaluated accuracy with the Hill-Rom (Batesville, IN) P8000 transport stretcher. Table 4 summarizes the accuracy evaluation in the pulmonology suite. In this environment the 3D Guidance with short-range and midrange field generators and the Aurora were comparable and more accurate than the 3D Guidance with flat-panel field generator.
Table 4.
Results of accuracy experiments in pulmonology suite. All error values are in millimeters and degrees.
| 3DG flat panel | 3DG short range | 3DG midrange | Aurora | |||||
|---|---|---|---|---|---|---|---|---|
| Position | Angle | Position | Angle | Position | Angle | Position | Angle | |
| Maximal sample Distance variability | 2.43 | 1.85 | 0.35 | 0.38 | ||||
| rms | 3.14 | 3.14 | 1.00 | 2.36 | 0.92 | 1.86 | 1.16 | 1.11 |
| Mean | 2.78 | 6.37 | 0.89 | 1.99 | 0.79 | 1.25 | 0.95 | 0.80 |
| Standard deviation | 1.47 | 1.36 | 0.45 | 1.26 | 0.47 | 1.37 | 0.67 | 0.78 |
| Range | 10.02 | 7.16 | 3.76 | 6.06 | 3.55 | 5.95 | 5.70 | 3.76 |
| Max. | 10.40 | 11.66 | 3.92 | 6.48 | 3.59 | 6.06 | 5.83 | 3.78 |
| 95 percentile | 5.78 | 10.40 | 1.54 | 5.77 | 1.65 | 5.37 | 2.19 | 3.3 |
Robustness
Robustness of the EMTS was evaluated in all three clinical environments as described in Sec. 2G. As we are only interested in the effect the imaging apparatus has on tracking, we start the data acquisition several seconds before image acquisition. The data are then demeaned using the mean distance computed from the first 2 s. This removes the effect of the specific sensor to sensor distance from the experiments, as the actual distance between sensors varied between experiments.
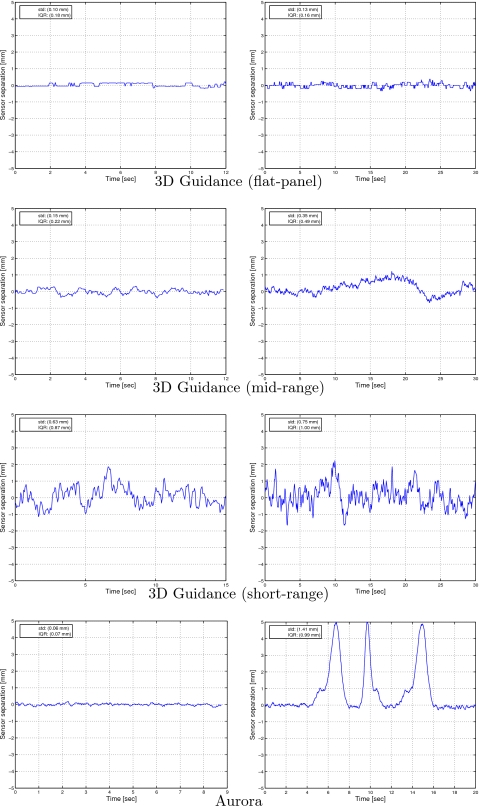
We first evaluated robustness in the interventional radiology suite. At the home position the C arm is approximately 2 m away form the measurement location. The standard deviation of the distances between the two sensors as measured by the 3D Guidance system with flat-panel, midrange, and short-range field generators were 0.12, 0.13, and 0.61 mm, respectively. The standard deviation obtained with the Aurora system was 0.05 mm. We then acquired data for the two sensors during acquisition of a fluoroscopy image and during a 20 s CBCT scan as described in Sec. 2G. Figure 14 presents the demeaned distance plots for all systems. We conclude that the 3D Guidance with flat-panel field generator is overall the most robust system, resulting in stable results even during a CBCT scan. The 3D Guidance midrange generator and Aurora are robust to x-ray fluoroscopy imaging but not to CBCT acquisition, exhibiting measurement fluctuations. The 3D Guidance system with short-range field generator exhibited fluctuations for all experimental setups. This is consistent with its accuracy evaluation. That is, the measurements fluctuate considerably when using the default settings even in the home position but when averaged are accurate.
Figure 14.
Effect of image acquisition on tracking as measured by the variation in distance between two fixed sensors (Interventional radiology suite). Left column: during x-ray fluoroscopy; right column: during CBCT scan. Data are demeaned using the mean of the first 2 s.
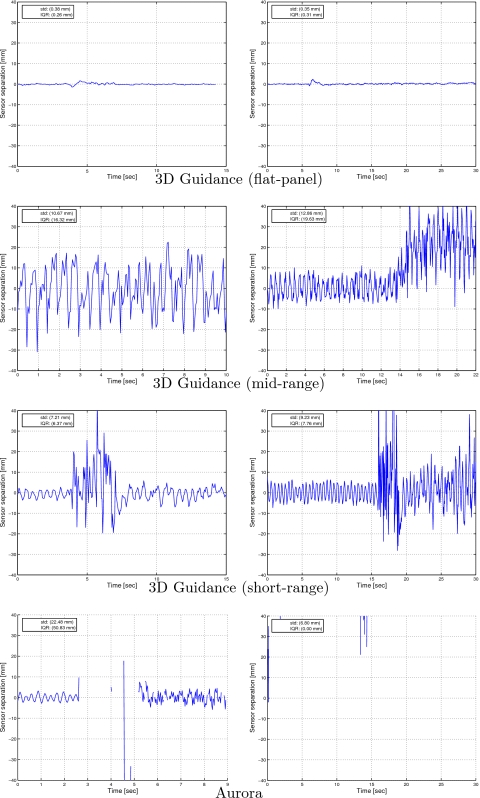
We then repeated the experiment in the CT suite. At the home position, the measurement space is approximately 2–3 m away from the CT gantry. The standard deviations of the distance between the two sensors as measured by the 3D Guidance system with flat-panel, midrange, and short-range field generators are 0.10, 0.17, and 0.38 mm, respectively. The standard deviation obtained with the Aurora system was 0.04 mm. We then acquired data for the two sensors during acquisition of a CT fluoroscopy image and during a CT scan as described in Sec. 2G. Figure 15 presents the demeaned distance plots for all systems. From these experiments we see that the only system that is robust in all setups is the 3D Guidance with flat-panel field generator. All other systems were only stable at home position, the region away (2–3 m) from the CT gantry in which we perform navigated procedures. It should be noted that the majority of data acquisition attempts with the Aurora system, in the vicinity of the gantry, failed.
Figure 15.
Effect of image acquisition on tracking as measured by the variation in distance between two fixed sensors (CT suite). Left column; during CT fluoroscopy; right column: during CT scan. Data are demeaned using the mean of the first 2 s.
Finally, we evaluated the robustness in the pulmonology suite. We acquired tracking data with the endoscope initially turned off and then turned it on. All systems were stable except for a single outlying measurement acquired by the 3D Guidance with the short-range field generator. This is consistent with its fluctuations as seen in all other experiments. The standard deviations of the distance between the two sensors as measured by the 3D Guidance system with flat-panel, midrange, and short-range field generators were 0.07, 0.06, and 0.13 mm, respectively. The standard deviation obtained with the Aurora system was 0.03 mm.
DISCUSSION AND CONCLUSIONS
In the past electromagnetic tracking system evaluation has focused on the system’s accuracy and ability to deal with the presence of electromagnetic field distorting objects. This type of assessment alone is insufficient for the clinical environment.
Based on our experience using electromagnetic tracking in the clinical environment we have compiled a more comprehensive list of requirements. These should be assessed on a per procedure basis and include (1) the system’s refresh rate, (2) the number of sensors that can be tracked concurrently, (3) the size of the tracking volume, (4) the obtrusiveness of the system, (5) the sensor size and the information provided by the sensors (5DOF or 6DOF), (6) the tracking accuracy, and (7) the system robustness.
In this work we assessed the 3D Guidance EMTS with flat-panel, midrange, and short-range field generators and the Aurora EMTS. Each system was found to have certain strengths and weaknesses, but no single system was optimal across environments and procedures.
All systems provided a sufficient refresh rate for our purposes. The number of concurrently tracked sensors was also sufficient for our procedures. The tracking volumes provided by the systems was found to be sufficient for our procedures, although the 3D Guidance with flat panel and the Aurora system were easier to position as their optimal work volumes are larger than the 3D Guidance with midrange and short-range generators. Both the 3D Guidance and Aurora systems are wired, requiring care on the part of the medical staff when moving around the patient, so they do not lose their footing. Note that this is no different than wired optical systems that are in common use in image-guided interventions. From an imaging standpoint we have found that the 3D Guidance with flat panel is very obtrusive for x-ray based imaging, as the field generator is always in the field of view. We have also found that the other systems do reduce the quality of x-ray projection images but to a much lesser extent. The sensor sizes available with both the 3D Guidance and the Aurora system and the information they provide, 5DOF or 6DOF, are sufficient for most of our procedures. The only case where we are limited is in our bronchoscopy procedure where we combine a 5DOF with image based registration to obtain the full 6DOF. Both the 3D Guidance and Aurora systems were found to be sufficiently accurate for our purposes. This is primarily due to our ability to control the environment so that the guided intervention is performed in a region with minimal distortion. It should be noted that the measurements acquired with the 3D Guidance with short-range field generator fluctuated considerably, but that the system was deemed accurate using our protocol, as we average 100 consecutive samples. Finally, we evaluated the robustness of each system by concurrently acquiring data and imaging with apparatus that introduce severe distortions to the magnetic field. The only system that was found to be robust in all environments is the 3D Guidance with flat-panel field generator.
From this work it is clear that a holistic evaluation of EMTS is necessary. This is exemplified by the assessment of the 3D Guidance system with the flat-panel field generator. In the CT suite, this system proved to be the most accurate and robust; however, it was also the most obtrusive with regard to imaging. Thus the clinical utility of this system can only be determined based on all of the factors we have enumerated. In this case it is up to the physician to approve the use based on their perception of the image quality.
We have also shown that generalizing conclusions with regard to system utility is hard, as the requirements and constraints of the various clinical environments and procedures vary considerably. Often ones expectations are not met, with evaluation performed in one environment not reflecting the system’s behavior in another one. In our case we were favorably surprised to find that navigation accuracy was higher in the interventional radiology suite than in the office environment which we initially considered the optimal environment for EM navigation.
While none of the systems we evaluated was optimal across environments and procedures they are applicable to specific interventions in specific environments. We have found that minor modifications in current clinical practice can considerably improve system utility. This is exemplified by our institutional review board approved clinical trial for navigated lung biopsy procedures in the CT suite environment using the Aurora EMTS. In this case standard clinical practice is to perform the biopsy with the patient in the CT bore using CT fluoroscopy. By simply moving the patient bed away from the CT gantry after data acquisition we are able to perform the navigated procedure, whereas close to the gantry, the Aurora system fails to perform tracking. We thus conclude that judicious use of EMTS can benefit many clinical interventions.
ACKNOWLEDGMENTS
The authors would like to thank Sebástián Ordas for developing the work volume visualization program, Dr. Stefan Kirsch for helpful discussions with regard to the Aurora navigation system, and Jeff Stanley from NDI and Patricia Scott from Ascension Corp. for technical support. This work was funded by U.S. Army Grant No. W81XWH-04-1-0078 and NIH∕NCI Grant No. 5R01CA124377-03. The content of this manuscript does not necessarily reflect the position or policy of the U.S. Government.
References
- Birkfellner W., Hummel J., Wilson E., and Cleary K., “Tracking devices,” Image-Guided Interventions Technology and Applications, edited by Peters T. and Cleary K. (Springer-Verlag, New York, 2008). [Google Scholar]
- Atuegwu N. C. and Galloway R. L., “Volumetric characterization of the aurora magnetic tracker system for image-guided transorbital endoscopic procedures,” Phys. Med. Biol. 53, 4355–4368 (2008). [DOI] [PubMed] [Google Scholar]
- Hummel J. B., Bax M. R., Figl M. L., Kang Y., C. R.Maurer, Jr., Birkfellner W. W., Bergmann H., and Shahidi R., “Design and application of an assessment protocol for electromagnetic tracking systems,” Med. Phys. 10.1118/1.1944327 32, 2371–2379 (2005). [DOI] [PubMed] [Google Scholar]
- Nafis C., Jensen V., Beauregard L., and Anderson P., “Method for estimating dynamic EM tracking accuracy of surgical navigation tools,” Proc. SPIE 10.1117/12.653448 6141, 61410K-1–61410K-16 (2006). [DOI] [Google Scholar]
- Nafis C., Jensen V., and von Jakoc R., “Method for evaluating compatibility of commercial electromagnetic (EM) catheter tracking systems with surgical and imaging tools,” Proc. SPIE 10.1117/12.769513 6918, 691820-1–691820-15 (2008). [DOI] [Google Scholar]
- Wilson E., Yaniv Z., Zhang H., Nafis C., Shen E., Shechter G., Wiles A. D., Peters T., Lindisch D., and Cleary K., “A hardware and software protocol for the evaluation of electromagnetic tracker accuracy in the clinical environment: a multi-center study,” Proc. SPIE 10.1117/12.712701 6509, 65092T-1–65092T-11 (2007). [DOI] [Google Scholar]
- Banovac F., Tang J., Xu S., Lindisch D., Chung H. Y., Levy E. B., Chang T., McCullough M. F., Yaniv Z., Wood B. J., and Cleary K., “Precision targeting of liver lesions using a novel electromagnetic navigation device in physiologic phantom and swine,” Med. Phys. 10.1118/1.1992267 32, 2698–2705 (2005). [DOI] [PubMed] [Google Scholar]
- Levy E. B., Tang J., Lindisch D., Glossop N., Banovac F., and Cleary K., “Implementation of an electromagnetic tracking system for accurate intrahepatic puncture needle guidance: Accuracy results in an in vitro model,” Acad. Radiol. 14, 344–354 (2007). [DOI] [PubMed] [Google Scholar]
- Banovac F., Wood B., Popa T., Lindisch D., Zhang H., Cleary K., and Glossop N., “Feasibility of carotid stent deployment in swine using an electromagnetic navigation device for catheter guidance,” Proceedings of the Computer Assisted Radiology and Surgery, 2005, p. 1308, Berlin, Germany.
- Ding J., Khan N., Cheng P., Wilson E., Watson V., Cleary K., and Yaniv Z., “Accuracy analysis of an image-guided system for vertebroplasty spinal therapy based on electromagnetic tracking of instruments,” Proc. SPIE 10.1117/12.772794 6918, 69181K-1–69181K-7 (2008). [DOI] [Google Scholar]
- Banovac F., Wilson E., Zhang H., and Cleary K., “Needle biopsy of anatomically unfavorable liver lesions with an electromagnetic navigation assist device in a computed tomography environment,” J. Vasc. Interv. Radiol. 17, 1671 (2006). [DOI] [PubMed] [Google Scholar]
- Choi J., Gruionu L., Popa T., Anderson E., and Cleary K., “Transbronchial biopsy based on electromagnetic tracked biopsy forceps,” Int. J. Computer Assisted Radiology and Surgery 2, S143–S145 (2007). [Google Scholar]
- Ascension Technology Corporation (Milton, VT) (accessed April 7, 2008) (URL http://www.ascension-tech.com/).
- Northern Digital Inc. (Waterloo, ON, Canada) (accessed April 7, 2008) (URL http://www.ndigital.com/).
- Polhemus (Burlington, VT) (accessed April 7, 2008) (URL http://www.polhemus.com/).
- Nixon M. A., McCallum B. C., Fright R. W., and Price B. N., “The effects of metals and interfering fields on electromagnetic trackers,” Presence: Teleoperators & Virtual Environments 7, 204–218 (1998). [Google Scholar]
- Gramkow C., “On averaging rotations,” Int. J. Comput. Vis. 42, 7–16 (2001). [Google Scholar]
- Horn B. K. P., “Closed-form solution of absolute orientation using unit quaternions,” J. Opt. Soc. Am. A 10.1364/JOSAA.4.000629 4, 629–642 (1987). [DOI] [Google Scholar]
- Chung A. J., Edwards P. J., Deligianni F., and Yang G. Z., “Freehand co-calibration of an optical and electromagnetic tracker,” Proceedings of the Second International Workshop: Medical Imaging and Augmented Reality, Lecture Notes in Computer Science, 3150, Springer, pp. 320–328, 2004.
- Fischer G. S. and Taylor R. H., “Electromagnetic tracker measurement error simulation and tool design,” Medical Image Computing and Computer-Assisted Intervention, Lecture Notes in Computer Science, 3750, Springer, pp. 73–80, 2005. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Nakada K., Sato Y., Konishi K., Hashizume M., and Tamura S., “Intraoperative magnetic tracker calibration using a magneto-optic hybrid tracker for 3-D ultrasound-based navigation in laparoscopic surgery,” IEEE Trans. Med. Imaging 27, 255–270 (2008). [DOI] [PubMed] [Google Scholar]
- Adelstein B. D., Lee T. G., and Ellis S. R., “Head tracking latency in virtual environments: Psychophysics and a model,” Human Factors and Ergonomics Society Annual Meeting Proceedings, 47, pp. 2083–2087, 2003.
- “The image-guided surgery toolkit (igstk)” (accessed October 31, 2008) (URL http://www.igstk.org/index.htm).
- Mori K., Deguchi D., Akiyama K., Kitasaka T., C. R.Maurer, Jr., Suenaga Y., Takabatake H., Mori M., and Natori H., “Hybrid bronchoscope tracking using a magnetic tracking sensor and image registration,” Proceedings of Medical Image Computing and Computer-Assisted Intervention, Lecture Notes in Computer Science, 3750, Springer, pp. 543–550, 2005. [DOI] [PubMed] [Google Scholar]
- Frantz D. D., Wiles A. D., Leis S. E., and Kirsch S. R., “Accuracy assessment protocols for electromagnetic tracking systems,” Phys. Med. Biol. 10.1088/0031-9155/48/14/314 48, 2241–2251 (2003). [DOI] [PubMed] [Google Scholar]