Abstract
During human pregnancy, monocytes recruited to the uterus (decidua) are modified to promote immune defense and semiallogeneic pregnancy. The purpose of this study was to identify decidual factors involved in programming of monocytes into decidual macrophages by comparing the surface and secretory phenotypes of resting and interferon-γ (IFN-γ)–activated monocytes, unfractionated decidual cells, purified term decidual macrophages, and monocyte-derived macrophages. Surface markers for antigen presentation (HLA-DR, CD86), a membrane-bound cytokine interleukin (IL)–15, leukocyte immunoglobulin-like receptors (LILRB1, LILRB2), and secreted anti-inflammatory cytokines (transforming growth factor [TGF]–β1 and IL-10) were assessed. The results demonstrate that differentiated, activated monocytes closely resemble but are not identical to decidual macrophages. In addition to differential IFN-γ responsiveness, decidual macrophages were smaller than monocyte-derived macrophages and produced IL-10, which monocyte-derived macrophages did not. Only the unfractionated decidual cells secreted TGF-β1. These results suggest that activation, differentiation, and decidual signals cooperate to program monocytes into the decidual macrophage phenotype.
Keywords: Activation, cell surface markers, cytokines, differentiation, leukocyte immunoglobulin-like receptors (LILR)
Multiple factors and conditions attract circulating blood monocytes and drive the cells to become tissue macrophages, which then exhibit tissue- and organ-specific subpopulation markers.1,2 Chemokines, cytokines, and their receptors are important to the movement of the cells from blood into tissues, where organ- or tissue-specific environmental signals induce specific phenotypic profiles.
Macrophages in the mammalian decidua, a specialized tissue of pregnancy derived from the uterine endometrial lining, have been of considerable interest.3,4 Programming occurs in this setting, where much is required of the macrophage. These cells (1) have been implicated in phagocytosis and tissue remodeling4; (2) defend against infections3-5; (3) may influence the functions of neighboring cells such as invasive cytotrophoblast cells, glandular epithelial cells, and arterial endothelial cells6-8; (4) present antigens9,10; (5) produce cytokines such as interleukin (IL)–10 and IL-1511,12; and (6) play a role in the conclusion of pregnancy.13
Many potential conditioning factors, which may be responsible for monocyte/macrophage recruitment and activity, have been described in the human uterus.14-18 One factor may include the well-described monocyte/macrophage activation factor, interferon-γ (IFN-γ), which is a product of uterine natural killer (NK) cells. IFN-γ is present in the pregnant uterus of mice and humans,19-21 and macrophages in human decidua are activated, as shown by a display of human leukocyte antigen (HLA) class II antigens.12,22,23 Another factor that may contribute to the development of the phenotypic profile of the macrophage in the pregnant uterus is macrophage colony-stimulating factor (M-CSF; also known as CSF-1), high levels of which are produced in pregnant mouse and human uterine epithelial cells.24-26
In this study, we investigated the hypothesis that activation, driven by IFN-γ, and differentiation, driven by M-CSF, as well as uteroplacental factors are required to drive monocytes into a decidual macrophage phenotype. We here report phenotypic markers at each step and present evidence in support of the concept that all 3 conditions are essential to arriving at the phenotype exhibited by macrophages in the decidua during the final trimester of pregnancy.
Materials and Methods
Isolation, Culture, and Activation of Blood Monocytes
Human monocytes were obtained by venipuncture and collection of peripheral blood from healthy male volunteer donors. These acquisitions were done in accordance with protocols approved by the institutional Human Subjects Committee. Male, rather than female, peripheral blood was used to avoid potential effects of estrogen and progesterone on monocyte/macrophage function. Peripheral blood mononuclear cells (PBMCs) were first isolated by density gradient centrifugation on Histopaque-1077 (1.077 g/mL; Sigma-Aldrich, St Louis, MO), and the monocytes were then purified from PBMCs using anti-CD14–coated magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The cells were maintained in serum-free macrophage medium (Gibco; Invitrogen, Carlsbad, CA) alone or supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA) at 37°C, 5% CO2. To induce activation, cells were cultured for 48 hours in the presence of 100 U/mL IFN-γ (R&D Systems, Minneapolis, MN).
Generation of Monocyte-Derived Macrophages
The method for generating monocyte-derived macrophages has been published.27 In brief, monocytes were isolated as described above and differentiated by culturing for 5 days in medium containing 150 IU/mL M-CSF (R&D Systems). To induce activation, the cells were cultured for 48 hours in the presence of 100 U/mL IFN-γ (R&D Systems).
Tissue Dissection and Isolation of Unfractionated Decidual Cells and Purified Decidual Macrophages
Reflected extraplacental membranes were taken from normal, scheduled, cesarean deliveries at term (n = 10) in the absence of labor. All tissues were collected in accordance with protocols approved by the institutional Human Subjects Committee. Decidual macrophages were isolated using previously reported methods.13,28 In brief, decidual tissue was separated from the chorionic membrane by manual scraping. The tissue was thoroughly rinsed to remove blood and minced extensively. The tissue was then subjected to enzymatic digestion using 200 U/mL collagenase (type IV; Sigma-Aldrich), 1 mg/mL hyaluronidase (type 1-S; Sigma-Aldrich), and 150 μg/mL DNase I (type IV; Sigma-Aldrich) in Hank's Balanced Salt Solution (Mediatech, Herndon, VA) containing 20 mM HEPES (Sigma-Aldrich), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco; Invitrogen), 30 mM sodium bicarbonate (Mediatech), and 10 mg/mL bovine serum albumin (low endotoxin; Sigma-Aldrich) for 1 hour in a 37°C shaking water bath. The digested tissue and supernatant were filtered though sterile gauze followed by filtering through sterile 100-μm and 7-μm nylon membranes. The resulting cell suspension was fractionated over Histopaque-1077, and the macrophage-containing interface was washed thoroughly. Purified macrophages were obtained from the cell suspension using anti-CD14–coated magnetic beads (Miltenyi Biotec) according to the manufacturer's instructions. For some experiments, unfractionated decidual cells were also tested. Decidual cell mixtures and purified macrophages were counted, and viability was determined by trypan blue dye exclusion. The cells were cultured in serum-free macrophage medium (Gibco; Invitrogen) alone or supplemented with 10% FBS at 37°C, 5% CO2.
Immunohistochemistry
For immunohistological analysis, sections of reflected amniochorion membranes were rolled and embedded in tissue-freezing medium and then stored at −80°C until sectioned by cryostat. Five-micrometer serial sections were cut from the frozen membranes, fixed in acetone, and evaluated immunohistochemically by staining with a mouse monoclonal antibody to CD14 (IgG2b; Zymed, South San Francisco, CA; 5 μg/mL). An isotype-matched mouse antibody (BD Pharmingen, San Jose, CA) was used at the same concentration as a negative control. Binding was detected using biotinylated horse antimouse IgG (10 μg/mL; Vector Laboratories, Burlingame, CA), followed by a streptavidin peroxidase conjugate (Zymed) and the substrate 3-amino-9-ethylcarbozole in N,N-dimethylformamide (Zymed), which produces a red color where the antibody has bound. Tissue sections were counterstained with Mayer's hematoxylin and coverslipped for examination by light microscopy.
Cell Surface Enzyme-Linked Immunosorbent Assays
To compare relative levels of surface markers, cell surface enzyme-linked immunosorbent assays (ELISAs) were performed on approximately 80% confluent monolayers of monocytes (n = 3 blood donors), purified decidual macrophages (n = 3 placentas), and monocyte-derived macrophages (n = 3 to 4 blood donors) grown in triplicate in 96-well microplates using a protocol adapted from Roby et al.29 For some experiments, values for unfractionated decidual cells (2 to 3 different placentas) were compared with values for purified decidual macrophages. Cells were grown in serum-free macrophage media (Gibco) supplemented with 10% FBS in the absence or presence of 100 U/mL IFN-γ (Atlanta Biologicals) for 48 hours at 37°C, 5% CO2. Upon termination of the culture period, supernatant media were aspirated, and the monolayers were fixed in 1% paraformaldehyde at 4°C for 15 minutes. Following washing with phosphate-buffered saline, the monolayers were incubated with blocking mixture (5% milk, 2% normal horse serum, and 2% normal human serum [Sigma-Aldrich]) at room temperature for 30 minutes. Mouse monoclonal antibodies to the HLA class II antigen (IgG2a, HLA-DR; BD Pharmingen, San Jose, CA; 1.25 μg/mL), CD86 (IgG1, anti-B7-2; BD Pharmingen; 1.25 μg/mL), IL-15 (IgG1; R&D Systems; 2 μg/mL), leukocyte immunoglobulin-like receptor (LILR)B1 (IgG1, clone M401; Amgen, Thousand Oaks, CA; 1 μg/mL) and LILRB2 (IgG1, clone M422;Amgen; 1 μg/mL), and isotype-matched controls at the same concentrations (BD Pharmingen) were used. Binding of primary and control antibodies was detected using peroxidase-conjugated horse antimouse IgG (Vector Laboratories; 1 μg/mL), followed by color development with SureBlue TMB 1-Component substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). The reactions were terminated after 15 minutes using 1 N HCl. Absorbance was read at 450 nm using the Synergy HT Multi-Detection Microplate Reader and KC4 Software (Biotek Instruments, Winooski, VT). Controls consisted of (1) media alone plus secondary antibody only and (2) isotype-specific control antibodies followed by secondary antibody.
Supernatant ELISA
To determine IL-10 levels, supernatant culture media from monocytes, unfractionated decidual cells, purified decidual macrophages, and monocyte-derived macrophages were cultured in 96-well microplates in serum-free macrophage media (Gibco) supplemented with 10% FBS for 48 hours or 72 hours at 37°C, 5% CO2. Duplicate wells were established for each sample. For determination of transforming growth factor (TGF)–β1 levels, culture conditions were the same except that media did not include FBS so as to avoid cross-reactivity with bovine TGF-β1. IL-10 and TGF-β1 were measured using Quantikine colorimetric sandwich ELISA kits (R&D Systems), which included the use of a standard curve for quantification as well as blank wells for testing culture media that had not been exposed to cells. Absorbance was read at 450 nm using the Synergy HT Multi-Detection Microplate Reader and KC4 Software (BioTek). Average values obtained from the blank wells were subtracted from standard- and sample-containing wells to derive specific values.
Data Analysis
To calculate cellular responses to IFN-γ treatment by cell surface ELISA, background values obtained with media alone were first subtracted from primary antibody and isotype-specific antibody control values, and then absorbance values obtained from cells cultured in the absence of IFN-γ were compared for significant differences with values obtained for cells cultured in the presence of IFN-γ. The resulting data were subjected to repeated-measures ANOVA followed by Tukey posttesting using SPSS version 11.0.
Results
In the following experiments, characteristics of 4 types of cells were compared: monocytes, unfractionated decidual cells containing macrophages, primary cultures of purified decidual macrophages, and in vitro–generated monocyte-derived macrophages. Potential conditions associated with driving monocytes into the decidual macrophage phenotype that were tested included activation, differentiation, and environmental conditioning.
Figure 1A shows the locations of CD14pos macrophages in term extraplacental membranes. Fetal macrophages (open arrowheads) are located in the connective tissue between the amnion epithelium and the chorion membrane. These fetal cells are eliminated during dissection of the tissue. Maternal macrophages are scattered randomly thoughout the decidua and are clustered near the chorion membrane. As shown in Figure 1B, the isotype-specific control antibody failed to yield positive signals.
Figure 1.
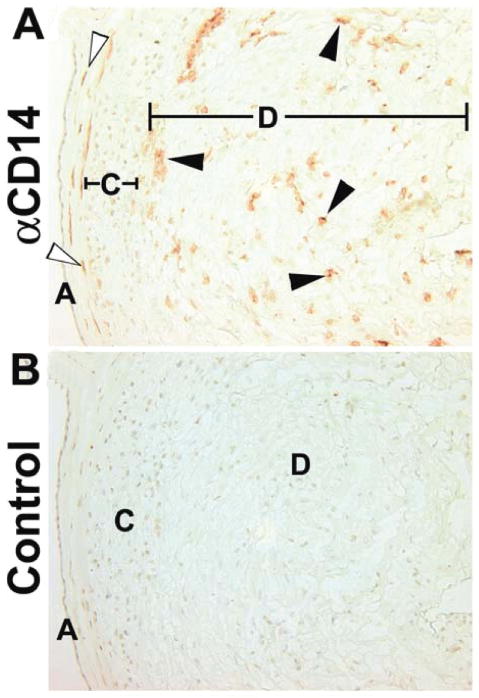
Immunohistochemical localization of macrophages in term extraplacental membranes. (A) Anti-CD14. (B) Isotype matched control. Open arrows mark fetal macrophages between the amnion and chorion membranes. Closed arrows mark decidual macrophages close to the chorion membrane and scattered randomly through the decidua. A, amnion epithelium; C, chorion cytotrophoblast cell layer; D, decidua. Original magnifications, 200×.
Decidual Macrophages Maximally Display Markers for Activation
In the first set of experiments, cell ELISA assays were used to assess the ability of a macrophage activation factor, IFN-γ, and a macrophage differentiation factor, M-CSF, to drive monocytes into a phenotype similar to that of the decidual macrophage. Levels of 2 IFN-γ–responsive markers involved in antigen presentation, HLA-DR and CD86 (B7-2), were compared using a cell ELISA. A growth factor for NK cells11 that has been implicated in decidualization30,31 and is displayed on monocyte cell surfaces,32 IL-15, was investigated by the same method.
As shown in Figure 2, monocytes, purified decidual macrophages, and monocyte-derived macrophages displayed the 3 surface markers. IFN-γ is known to enhance mononuclear phagocyte display of Fc receptors, which could cause misinterpretation of data in these assays. Therefore, binding of isotype control antibodies to cells treated with or without IFN-γ was calculated. For both IgG1 (control for anti-CD86, -IL-15, -LILRB1, -LILRB2) and IgG2a (control for anti-HLA-DR), the absorbance values (0.07 for untreated, 0.08 for IFN-γ treated) were not significantly different when comparing IFN-γ–treated and -untreated groups.
Figure 2.
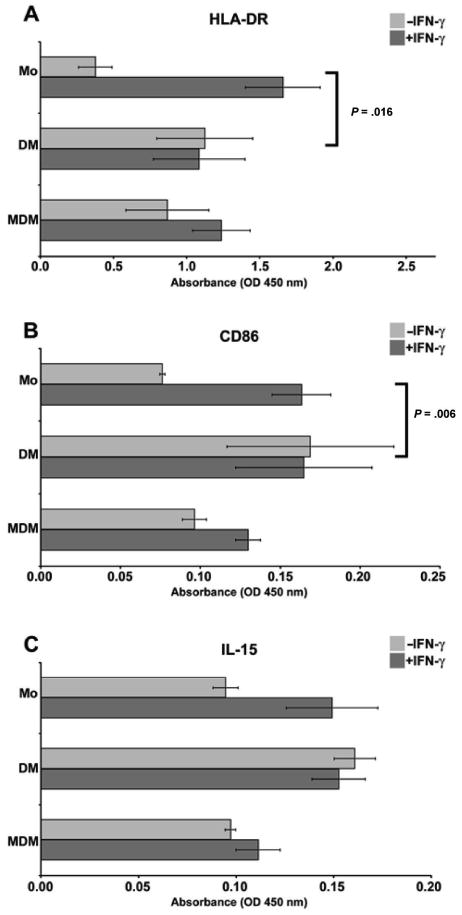
Analysis of activation markers on monocytes, purified decidual macrophages, and monocyte-derived macrophages using cell enzyme-linked immunosorbent assay (ELISA). Antibodies used to obtain the results were (A) anti-HLA-DR, (B) anti-CD86, and (C) anti-IL-15. In each panel, markers on blood monocytes (Mo), purified decidual macrophages (DM), and monocyte-derived macrophages (MDM) cultured in the absence (−IFN-γ) or presence (+IFN-γ) of 100 U/mL interferon-γ (IFN-γ) for 48 hours are shown. OD indicates optical density. Data shown are the means of values obtained in at least 3 separate experiments ± SEM. Brackets and P values indicate statistical significance between fold changes in response to IFN-γ treatment.
Activation
Figures 2A, 2B, and 2C show that monocyte responsiveness to IFN-γ treatment was significantly greater than purified decidual macrophage responsiveness. Following exposure to IFN-γ, levels of markers in monocytes were increased by 4.4-, 2.1-, and 1.6-fold for HLA-DR, CD86, and IL-15, respectively. Brackets and P values indicate statistical significance between fold changes in response to IFN-γ treatment. When monocyte induction was compared with decidual macrophage induction for HLA-DR, the P value was .0016. For induction of CD86 between the same 2 populations, the P value was .006. Although the trend persisted, statistical significance was not reached for differences between monocytes and decidual macrophages in induction of IL-15.
These data imply that activation by IFN-γ differentially increased similarities between monocytes and purified decidual macrophages, enhancing molecules involved in antigen presentation (HLA-DR, CD86) but not expression of a surface cytokine involved in lymphocyte activation (IL-15).
Activation and differentiation
As shown in Figures 2A to 2C, in the resting stage, levels of HLA-DR on monocytes that had been differentiated (ie, monocyte-derived macrophages) were similar to levels in purified decidual macrophages, and levels of CD86 and IL-15 appeared slightly lower, although the differences were not statistically significant. Monocyte-derived macrophages were responsive to IFN-γ activation, showing 1.4-, 1.3-, and 1.1-fold induction of HLA-DR, CD86, and IL-15, respectively. Although consistent among the 3 preparations of cells tested, none of the comparisons between monocyte-derived macrophages and decidual macrophages were statistically significant with regard to levels of markers expressed or responsiveness to IFN-γ.
This lack of statistical significance indicates that M-CSF treatment increased the resemblances between monocytes and primary decidual macrophages.
Decidual Macrophages and Monocyte-Derived Macrophages Display Similar Cellular Morphologies
In the next set of experiments, we compared the morphologies of activated decidual macrophages and activated monocyte-derived macrophages. Figure 3A shows the morphological characteristics of decidual macrophages following the IFN-γ activation protocol. Activated decidual macrophages were plastic adherent and demonstrated heterogeneous morphologies that include elongated cells with fine extensions, small rounded cells, large flattened cells, and cells containing cytoplasmic vesicles.
Figure 3.
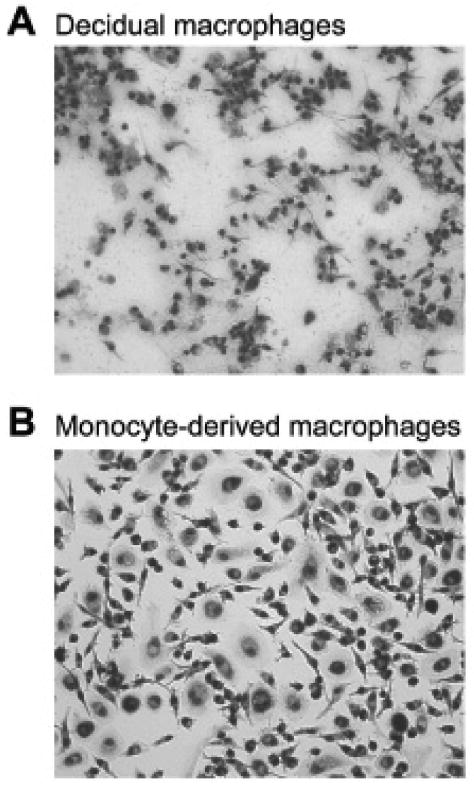
Morphologic appearances of (A) decidual macrophages cultured for 48 hours in medium containing interferon-γ (IFN-γ) and (B) monocyte-derived macrophages, which had been cultured for 5 days in medium containing 150 IU/mL macrophage colony-stimulating factor followed by 48 hours in medium containing 100 U/mL IFN-γ. Original magnifications, 200×.
Differentiation of monocytes with M-CSF induced an increase in size, adherence to plastic, and ruffling of the membrane, but the cells remained rounded (not shown). As illustrated in Figure 3B, when the activation factor, IFN-γ, was added, the monocyte-derived macrophages exhibited heterogenous morphologies with elongated, round, and flattened cells in the cultures that were similar to those of decidual macrophages. However, neither differentiation nor activation induced the smaller cell size that characterized the decidual macrophage.
Thus, these experiments indicate that as with expression of the surface markers tested above, both differentiation and activation induce decidual macrophage morphology. However, reduction in cell size was uniquely associated with residency in tissue.
Comparison of Inhibitory Receptors for HLA-G
HLA-G is composed of a group of related proteins produced by cytotrophoblast cells in human placentas.33 Primary decidual macrophages in early and late gestation decidua contain mRNAs encoding 2 receptors for HLA-G, LILRB1 (ILT2), and LILRB2 (ILT4).34 Binding of HLA-G to these receptors would be expected to block activation signals and influence their production of proinflammatory and anti-inflammatory cytokines. In this group of experiments, we tested the expression of the receptor proteins by cell ELISA to predict whether activation and/or differentiation of monocytes would induce the decidual macrophage phenotype.
Figure 4 shows that the expression of LILRB1 and LILRB2 was similar in the 3 cell populations, monocytes, purified decidual macrophages, and monocyte-derived macrophages, prior to stimulation with IFN-γ. Activation effectively increased levels of both LILRB1 and LILRB2 on monocytes but not decidual macrophages. Brackets and P values indicate statistical significance between fold changes in response to IFN-γ treatment, with statistically significant differences of P = .044 and P = .024, respectively, between the responses of the 2 populations. Differentiation obviated this induction, with no statistically significant changes in monocyte-derived macrophages.
Figure 4.
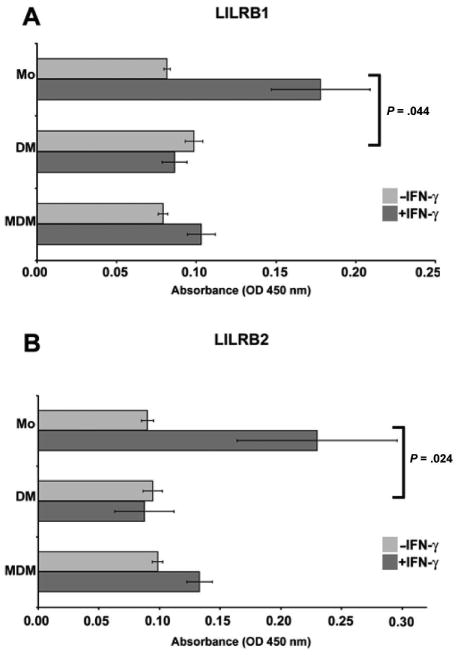
Analysis of HLA-G receptors on monocytes, purified decidual macrophages, and monocyte-derived macrophages by cell surface enzyme-linked immunosorbent assay. (A) Leukocyte immunoglobulin-like receptor (LILR)B1 and (B) LILRB2. Blood monocytes (Mo), purified decidual macrophages (DM), and monocyte-derived macrophages (MDM) were cultured in the absence (−IFN-γ) or presence (+IFN-γ) of 100 U/mL interferon-γ (IFN-γ) for 48 hours. OD indicates optical density. Data shown are the mean values obtained in 3 separate experiments ± SEM. Brackets and P values indicate statistical significance between fold changes in response to IFN-γ treatment.
Thus, unlike expression of HLA-DR, CD86, and IL-15 as well as decidual cell morphology, neither activation by IFN-γ nor differentiation by M-CSF appeared to be involved in regulating levels of expression of the receptors for HLA-G displayed by the purified decidual macrophages taken from term extraplacental membranes.
Differentiation and Activation Do Not Recapitulate Decidual Macrophage Production of IL-10
The previous experiments suggest that activation and differentiation program monocytes into a decidual macrophage phenotype that is mirrored in many but not all respects by exposing monocytes to differentiation by M-CSF and activation by IFN-γ in vitro. In the next set of experiments, we compared the production of 2 cytokines, TGF-β1 and IL-10, in the 3 cell populations. Both cytokines are known to be products of mononuclear phagocytes, and both could support an immunosuppressive environment in the decidua. Cell culture supernatants were collected from 3 sets each of monocytes, freshly isolated decidual macrophages, and monocyte-derived macrophages for IL-10 and TGF-β1 assays.
When evaluated by using an ELISA assay, TGF-β1 was undetectable in supernatant culture media from monocytes, purified decidual macrophages, and monocyte-derived macrophages regardless of time of incubation (data not shown).
By contrast, IL-10 was absent from the culture supernatants of monocytes and monocyte-derived macrophages (data not shown) but was readily detected in culture supernatants from purified decidual macrophages (see Table 1). This cytokine was identified after overnight culture, and levels increased after 48 hours and 72 hours of culture. Absolute values differed in the 3 preparations, although temporal profiles were similar, with peak production reached by culturing the cells for 48 hours.
Table 1.
Interleukin-10 Production by Purified Decidual Macrophages
| Cell Preparation No. | Culture | ||
|---|---|---|---|
| Overnight | 48 h | 72 h | |
| 0816 | 89.70a | 136.55 | 135.84 |
| 1101 | 15.14 | 44.53 | 54.45 |
| 1206 | 66.93 | 136.14 | 137.68 |
Interleukin-10 (pg/mL), measured by supernatant enzyme-linked immunosorbent assay.
These data indicate that as with reduction in cell size, programming of monocytes (TGF-β1neg, IL-10neg) into the cytokine secretory profile of decidual macrophages (TGF-β1neg, IL-10pos) is not achieved by activation or differentiation, suggesting that the uteroplacental environment is engaged in this programming step.
Comparisons of Purified Decidual Macrophages and Unfractionated Decidual Cells
In the final group of experiments, the goal was to learn whether the expression patterns described above for purified decidual macrophages would be the same or different in cultures of unfractionated decidual cells.
As shown in Figure 5, activation marker (HLA-DR, CD86) levels were significantly higher in unfractionated decidual cells (n = 3) when compared with purified decidual macrophages (P = .018). By contrast, neither IL-15 levels (n = 3; Figure 5) nor LILRB1 and LILRB2 levels (n = 2, data not shown) were different. Brackets and P values indicate statistical significance between absorbance values regardless of IFN-γ treatment. In 1 experiment, the unfractionated and purified preparations were matching (ie, from the same placenta), whereas in 2 experiments, they were not. The outcomes were the same for all preparations.
Figure 5.

Analysis of (A) HLA-DR, (B) CD86, and (C) IL-15 on resting (−IFN-γ) and activated (+IFN-γ) purified decidual macrophages and unfractionated decidual cells using a cell enzyme-linked immunosorbent assay. OD indicates optical density. Data shown are the mean values obtained in 3 separate experiments ± SEM. Brackets and P values indicate statistical significance between absorbance values regardless of interferon-γ (IFN-γ) treatment.
IL-10 levels in supernatant culture media from purified decidual macrophages (n = 3) and mixed decidual cell cultures (n = 4) were similar in the 2 populations (data not shown), whereas TGF-(β1 was present only in the supernatant culture media from the unfractionated decidual cells (n = 6; Table 2). After overnight incubation, TGF-β1 was detectable in 1 of the 6 preparations (85 pg/mL). After 48 hours of culture, the cytokine was detectable in 3 of the 6 preparations (range, 34-1131 pg/mL), and after 72 hours of culture, TGF-β1 was present in 5 of the 6 cultures (range, 27-1251 pg/mL).
Table 2.
Transforming Growth Factor–β1 Production by Unfractionated Decidual Cells
| Cell Preparation No. | Culture | ||
|---|---|---|---|
| Overnight | 48 h | 72 h | |
| 0816 | 85.44a | 122.08 | 144.37 |
| 1206 | 0.00 | 33.70 | 75.20 |
| 1214 | 0.00 | 1131.30 | 1251.30 |
| 0914 | 0.00 | 0.00 | 135.84 |
| 0829 | 0.00 | 0.00 | 26.92 |
| 0909 | 0.00 | 0.00 | 0.00 |
Transforming growth factor–β1 (pg/mL), measured by supernatant enzyme-linked immunosorbent assay.
These results indicate that unfractionated decidual cells exhibit a higher density of antigen presentation molecules (HLA-DR, CD86) and increased secretion of a multipurpose cytokine with immunosuppressive properties, TGF-β1, than purified decidual macrophages. By contrast, the display of IL-15, expression of HLA-G receptors, and secretion of IL-10 were not different when values for purified decidual macrophages and unfractionated decidual cells were compared.
Summary of Results
Figure 6 illustrates the markers that characterize monocytes during in vitro activation and differentiation. The phenotype of the final product of in vitro conditioning, the activated monocyte-derived macrophage, is compared with that of the decidual macrophage.
Figure 6.
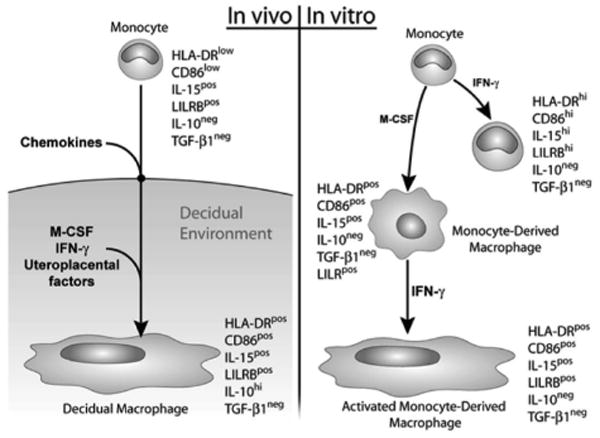
Schematic illustration showing marker expression of decidual macrophages (left panel) in comparison with in vitro–activated monocytes, differentiated monocytes, and monocytes that were both differentiated and activated (right panel). IFN, interferon; IL, interleukin; M-CSF, macrophage colony-stimulating factor; TGF, transforming growth factor.
Discussion
The results of this study show that activation and differentiation as well as additional programming by the uteroplacental environment are required to achieve the phenotype of the human decidual macrophage, particularly with regard to cell size and secretion of the anti-inflammatory cytokine IL-10.
The criteria we used for comparisons of monocytes and monocyte-derived macrophages with primary decidual macrophages were based on evidence from other studies for expression of certain markers and secreted products by decidual macrophages. In general, our results were in agreement with those presented earlier. Earlier studies include publications showing that human decidual macrophages express HLA-DR,22,23 CD86,35 IL-15,11 and LILRB.34 TGF-β1 is present in decidua and is induced in activated mononuclear phagocytes by exposure to HLA-G.36 IL-10 is a well-described product of decidual macrophages12 that is not induced in an activated mononuclear phagocyte tumor cell line by exposing them to HLA-G.36 Thus, the scientific literature holds evidence that the markers we tested are known to be expressed by decidual macrophages and that TGF-β1 and IL-10 are generated through different pathways of stimulation.
A major finding in this study was that differentiation and activation are required to develop many aspects of the decidual macrophage phenotype, including appropriate levels of HLA-DR, CD86, and IL-15. Differentiation of monocytes into monocyte-derived macrophages using M-CSF resulted in similar morphology and surface marker expression to that of the decidual macrophage. The importance of local production of M-CSF is supported by these findings and by reports of dramatic induction of M-CSF production during murine and human pregnancy.24-26 In addition, the reported phenotype of the M-CSF null mouse (op/op) clearly demonstrates the necessity of M-CSF for normal fertility and development of the appropriate uterine macrophage phenotype in the mouse model.37
With regard to activation, monocytes were highly responsive to IFN-γ induction of all of the surface markers exhibited by decidual macrophages and monocyte-derived macrophages. By contrast, decidual macrophages were entirely refractory to this activating agent, and monocyte-derived macrophages were largely but not completely resistant. A reasonable explanation for the failure of decidual macrophages to respond is that the cells are maximally activated in situ, and consistent exposure to IFN-γ could result in lower levels of IFN-γ receptor expression, although this was not tested in our study. Alternatively, poor responsiveness could be due to the presence in the decidua of inhibitory factors that prevent stimulation of IFN-γ–responsive genes.
By contrast, activation was not required for monocytes to reach decidual macrophage levels of LILRB1 and LILRB2. Our data suggest that monocytes, decidual macrophages, and monocyte-derived macrophages express similar levels of these receptors for HLA-G and would be expected to engage in signal transduction following exposure to placental HLA-G. However, significantly higher levels were obtained in monocytes but not the other 2 cell populations following treatment with IFN-γ consistent with the idea that monocytes are exquisitely sensitive to activation signals but may revert to an appropriate functional level after migrating into tissues/organs such as the decidua.
A second major finding was that, as suggested by many studies such as those by Lidstrom et al,38 decidual macrophages are both activated and phenotypically immunosuppressive. Early studies showed that decidual macrophages in mice produce the immune inhibitor prostaglandin-E2,39 and Heikkinen et al12 have shown that these cells spontaneously produce IL-10 and indoleamine 2,3-dioxygenase, both of which are known to mediate the inhibition of adaptive immunity. Our studies are in agreement with the Heikkinen et al results and add the information that isolated, purified decidual macrophages produce IL-10 but not a second inhibitory cytokine, TGF-β1. Further experiments documented that TGF-β1 is present in cell cultures that contained both decidual macrophages and other types of decidual cells, the latter of which is known to produce this cytokine,40 and that levels increase as cultures continue over time. Also of interest was evidence showing that levels of HLA-DR and CD86 in the mixed decidual cell cultures are double that of purified decidual macrophages, which suggests 2 possibilities: (1) the presence of other decidual cells allows for even greater expression of these activation markers by decidual macrophages or (2) other cell types, such as fibroblasts, vascular cells, and other leukocytes including dendritic cells in term decidual tissue, contribute to the HLA-DR and CD86 signals. However, while nonmacrophage cells from first-trimester decidua have been documented to express these markers,41,42 nonmacrophage cells in term decidua are negative for HLA-DR and CD86.43 With regard to IL-15, production of IL-15 in nonmacrophage cells has been reported in early deciduas,44 but this has not been reported in term decidual cells. In this study, the finding that purified term decidual macrophage levels of IL-15 were similar to those of decidual cell mixtures suggests that macrophages are the major contributors of this cytokine at term.
Both IL-10 and TGF-β1 are of critical biological importance during pregnancy. IL-10 counteracts the effects of proinflammatory cytokines, and alterations in IL-10 levels have been correlated with pathologic pregnancy.45,46 TGF-β1 may also contribute to immunosuppression as it modulates production of tumor necrosis factor–α, a major inflammatory cytokine. TGF-β1 production is often associated with an anti-inflammatory profile, as shown recently by Arnold et al,47 and it may promote differentiation of NK cells into their characteristic decidual phenotype.40 It was therefore not surprising to find that these 2 anti-inflammatory cytokines are produced by cells in the uterine decidua and, as with other cytokines, differ in their conditions for production.
The decidual macrophage phenotype we report here confirms Gordon's1 statement that tissue macrophages can only rarely be strictly categorized as either classically or alternatively activated or as portraying either the M1 or M2 phenotype. In contrast to the findings of Cupurdija et al,48 a report on first-trimester decidual macrophages, our results show that term decidual macrophages display some of the characteristics of several categories, including classically IFN-γ–activated, alternatively activated, and deactivated/immunosuppressive macrophage types. These differences may reflect important functional requirements of macrophages in early versus late gestation. It will be of considerable interest to determine whether the cells tested here are exhibiting a single, homogenous phenotype or, as is more likely, the results represent the average of values for a heterogeneous mixture of macrophage phenotypes residing in the decidua. In other contexts, macrophage environmental programming is impermanent. Arnold et al47 have shown that as needs change, the original phenotype is revised; in skeletal muscle healing following an injury, resident macrophages that originally exhibit a proinflammatory profile characterized by phagocytic activity reprogram into an anti-inflammatory phenotype releasing TGF-β1. Thus, environmental reprogramming of macrophages may be a constant feature of the decidua through the course of pregnancy.
Acknowledgments
This study was supported by National Institutes of Health grants to JSH (HD24212; HD39878 Project III), and RHM was supported by the University of Kansas Medical Center Biomedical Training Grant.
The authors appreciate the gifts of mouse monoclonal anti-LILRB1 (M401) and anti-LILRB2 (M422) antibodies from Amgen, Inc. The assistance of S. Fernald, University of Kansas School of Medicine Image Analysis Center, with preparation of the figures is greatly appreciated.
References
- 1.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–618. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 3.Vince GS, Johnson PM. Immunobiology of human uteroplacental macrophages—friend and foe? Placenta. 1996;17:191–199. doi: 10.1016/s0143-4004(96)90038-7. [DOI] [PubMed] [Google Scholar]
- 4.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003;1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt JS, Pollard JW. Macrophages in the uterus and placenta. Curr Top Microbiol Immunol. 1992;181:39–63. doi: 10.1007/978-3-642-77377-8_2. [DOI] [PubMed] [Google Scholar]
- 6.Nehemiah JL, Schnitzer JA, Schulman H, Novikoff AB. Human chorionic trophoblasts, decidual cells, and macrophages: a histochemical and electron microscopic study. Am J Obstet Gynecol. 1981;140:261–268. doi: 10.1016/0002-9378(81)90271-4. [DOI] [PubMed] [Google Scholar]
- 7.Bulmer JN, Smith J, Morrison L, Wells M. Maternal and fetal cellular relationships in the human placental basal plate. Placenta. 1988;9:237–246. doi: 10.1016/0143-4004(88)90031-8. [DOI] [PubMed] [Google Scholar]
- 8.Reister F, Frank HG, Kingdom JC, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- 9.Dorman PJ, Searle RF. Alloantigen presenting capacity of human decidual tissue. J Reprod Immunol. 1988;13:101–112. doi: 10.1016/0165-0378(88)90054-x. [DOI] [PubMed] [Google Scholar]
- 10.Mizuno M, Aoki K, Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol. 1994;31:180–188. doi: 10.1111/j.1600-0897.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 12.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol. 2003;131:498–505. doi: 10.1046/j.1365-2249.2003.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narahara H, Johnston JM. Effects of endotoxins and cytokines on the secretion of platelet-activating factor-acetylhydrolase by human decidual macrophages. Am J Obstet Gynecol. 1993;169:531–537. doi: 10.1016/0002-9378(93)90614-o. [DOI] [PubMed] [Google Scholar]
- 14.Arcuri F, Ricci C, Ietta F, et al. Macrophage migration inhibitory factor in the human endometrium: expression and localization during the menstrual cycle and early pregnancy. Biol Reprod. 2001;64:1200–1205. doi: 10.1095/biolreprod64.4.1200. [DOI] [PubMed] [Google Scholar]
- 15.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 16.Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood CJ, Matta P, Krikun G, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcuri F, Buchwalder L, Toti P, et al. Differential regulation of colony stimulating factor 1 and macrophage migration inhibitory factor expression by inflammatory cytokines in term human decidua: implications for macrophage trafficking at the fetal-maternal interface. Biol Reprod. 2007;76:433–439. doi: 10.1095/biolreprod.106.054189. [DOI] [PubMed] [Google Scholar]
- 19.Veith GL, Rice GE. Interferon gamma expression during human pregnancy and in association with labour. Gynecol Obstet Invest. 1999;48:163–167. doi: 10.1159/000010165. [DOI] [PubMed] [Google Scholar]
- 20.Platt JS, Hunt JS. Interferon-gamma gene expression in cycling and pregnant mouse uterus: temporal aspects and cellular localization. J Leukoc Biol. 1998;64:393–400. doi: 10.1002/jlb.64.3.393. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, He H, Colonna M, Seya T, Takai T, Croy BA. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod. 2005;73:510–518. doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- 22.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- 23.Lessin DL, Hunt JS, King CR, Wood GW. Antigen expression by cells near the maternal-fetal interface. Am J Reprod Immunol Microbiol. 1988;16:1–7. doi: 10.1111/j.1600-0897.1988.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 24.Pollard JW. Role of colony-stimulating factor-1 in reproduction and development. Mol Reprod Dev. 1997;46:54–60. doi: 10.1002/(SICI)1098-2795(199701)46:1<54::AID-MRD9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Daiter E, Pampfer S, Yeung YG, Barad D, Stanley ER, Pollard JW. Expression of colony-stimulating factor-1 in the human uterus and placenta. J Clin Endocrinol Metab. 1992;74:850–858. doi: 10.1210/jcem.74.4.1548350. [DOI] [PubMed] [Google Scholar]
- 26.Pampfer S, Daiter E, Barad D, Pollard JW. Expression of the colony-stimulating factor-1 receptor (c-fms proto-oncogene product) in the human uterus and placenta. Biol Reprod. 1992;46:48–57. doi: 10.1095/biolreprod46.1.48. [DOI] [PubMed] [Google Scholar]
- 27.McIntire RH, Petroff MG, Phillips TA, Hunt JS. In vitro models for studying human uterine and placental macrophages. Methods Mol Med. 2006;122:123–148. doi: 10.1385/1-59259-989-3:123. [DOI] [PubMed] [Google Scholar]
- 28.Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods. 1990;132:181–189. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 29.Roby KF, Hamlin GP, Soares MJ, Hunt JS. Differential responses of phenotypically distinct rat trophoblast cell lines to MHC class I antigen-inducing cytokines. Placenta. 1994;15:577–590. doi: 10.1016/s0143-4004(05)80405-9. [DOI] [PubMed] [Google Scholar]
- 30.Lobo SC, Huang ST, Germeyer A, et al. The immune environment in human endometrium during the window of implantation. Am J Reprod Immunol. 2004;52:244–251. doi: 10.1111/j.1600-0897.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 31.Okada S, Okada H, Sanezumi M, Nakajima T, Yasuda K, Kanzaki H. Expression of interleukin-15 in human endometrium and decidua. Mol Hum Reprod. 2000;6:75–80. doi: 10.1093/molehr/6.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Musso T, Calosso L, Zucca M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 33.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 34.Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J Reprod Immunol. 2002;56:3–17. doi: 10.1016/s0165-0378(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 36.McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-beta1. J Leukoc Biol. 2004;76:1220–1228. doi: 10.1189/jlb.0604337. [DOI] [PubMed] [Google Scholar]
- 37.Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991;148:273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- 38.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003;50:444–452. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 39.Hunt JS, Manning LS, Wood GW. Macrophages in murine uterus are immunosuppressive. Cell Immunol. 1984;85:499–510. doi: 10.1016/0008-8749(84)90262-4. [DOI] [PubMed] [Google Scholar]
- 40.Keskin DB, Allan DS, Rybalov B, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16-NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montes MJ, Aleman P, Garcia-Tortosa C, Borja C, Ruiz C, Garcia-Olivares E. Cultured human decidual stromal cells express antigens associated with hematopoietic cells. J Reprod Immunol. 1996;30:53–66. doi: 10.1016/0165-0378(96)00954-0. [DOI] [PubMed] [Google Scholar]
- 42.Olivares EG, Montes MJ, Oliver C, Galindo JA, Ruiz C. Cultured human decidual stromal cells express B7-1 (CD80) and B7-2 (CD86) and stimulate allogeneic T cells. Biol Reprod. 1997;57:609–615. doi: 10.1095/biolreprod57.3.609. [DOI] [PubMed] [Google Scholar]
- 43.Oliver C, Cowdrey N, Abadia-Molina AC, Olivares EG. Antigen phenotype of cultured decidual stromal cells of human term decidua. J Reprod Immunol. 1999;45:19–30. doi: 10.1016/s0165-0378(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 44.Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2000;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 46.Wilczynski JR, Tchorzewski H, Banasik M, et al. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;109:8–15. doi: 10.1016/s0301-2115(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 47.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cupurdija K, Azzola D, Hainz U, et al. Macrophages of human first trimester decidua express markers associated to alternative activation. Am J Reprod Immunol. 2004;51:117–122. doi: 10.1046/j.8755-8920.2003.00128.x. [DOI] [PubMed] [Google Scholar]


