Abstract
Background
Animal studies have shown that prenatal stress has persisting effects on several aspects of offspring development; more recent studies show that this effect may be eliminated by positive postnatal rearing. Human studies of prenatal anxiety/stress are now also beginning to document links between antenatal stress/anxiety and behavioural and cognitive development of the child; however, there is no human evidence as to whether the early caregiving environment moderates the effect of antenatal anxiety/stress on child outcomes.
Methods
Antenatal and postnatal measures of stress were collected on 123 women who were recruited from an antenatal clinic. Laboratory-based assessment of the children's cognitive development and fearfulness were assessed when the children were aged 17 months. In addition, child-parent attachment quality was assessed using the Strange Situation.
Results
Attachment classification moderated the link between antenatal stress and observed fearfulness. The effect of antenatal stress on fearfulness was most accentuated in children with an Insecure/Resistant attachment classification; the significant antenatal stress X attachment classification interaction held after controlling for postnatal stress and obstetric, social and demographic factors. Attachment did not moderate the effects of antenatal anxiety on cognitive development.
Discussion
These findings provide the first human evidence that postnatal parenting may moderate the adverse effects of antenatal stress. These results raise developmental questions about the timing and effect of interventions to reduce the adverse effects of antenatal stress exposure.
Keywords: antenatal stress, attachment, fearfulness, cognitive development
There is considerable evidence from experimental animal research that maternal stress during pregnancy predicts a range of adverse neurodevelopmental outcomes in the offspring. Studies from several countries and dating back decades link antenatal stress with impaired behavioural, neurological, immunological, and physiological outcomes (Barbazangas et al., 1996; Coe et al., 1996; Schneider, 1992). Collectively, animal studies point to three important findings: a) the effects of antenatal stress on offspring outcome persist into adulthood, suggesting a programming mechanism and a model for environmentally-mediated intergenerational transmission of risk; b) the hypothalamic-pituitary-adrenal (HPA) axis is key mechanism serving the range of long-term effects on offspring; c) the adverse effects of antenatal stress exposure on the developing offspring are remediable with a positive postnatal rearing environment. The animal data are remarkably persuasive, but the relevance to human development and clinical conditions is far from clear.
Antenatal anxiety/stress and child development
Translating these findings to humans is well underway. We focus in the current paper on the third observation from animal studies noted above, namely, the role of the postnatal parenting environment as a moderator of the effects of antenatal stress. First, however, we briefly consider the human evidence linking antenatal stress to child development.
Naturalistic investigations in humans provide robust evidence of an association between antenatal anxiety/stress and child development. That is so whether the measure of antenatal risk is anxiety symptoms (Brouwers et al., 2001; Davis et al., 2007; O'Connor et al., 2002; van den Bergh et al., 2004), worries particular to pregnancy (Huizink et al. 2003), stressful life events (Bergman et al., 2007; Gutteling et al., 2006), or acute major stresses (Laplante et al., 2004). Further support for a robust link between antenatal anxiety/stress is that, as in the case of the animal work, a wide range of outcomes has been identified. Most studies focus on behavioural problems - most typically attention and anxiety — and cognitive development, with follow-up periods ranging from infancy to adolescence. But there are additional replicated findings, including atypical laterality indexed by mixed handedness (Glover et al., 2004; Obel et al., 2003).
Does postnatal rearing moderate the effects of antenatal anxiety/stress on the child?
Experiences in the early postnatal environment in animal models have persisting effects consistent with a programming effect. Specifically, rat pups whose mothers engage in high levels of optimal parenting, operationalized as licking/grooming and arch-back nursing, show reduced behavioural effects of stress, persisting physiological changes in the stress response system, and changes in expression in the brain of genes involved in the stress system (Caldji et al., 1998; Weaver et al., 2004). Somewhat comparable effects have been noted from postnatal handling (Meaney, 2001; O'Donnell et al., 1994). Interestingly, in animal studies, the beneficial effects of positive postnatal experiences, from naturally occurring individual differences or experimental manipulation, are as robust as the adverse prenatal stress effects; moreover, positive postnatal rearing and prenatal stress seem to have opposite effects on the HPA axis (Vallee et al., 1999). Perhaps as a consequence of this, there is considerable evidence that the adverse effects of prenatal stress on the offspring are attenuated by positive postnatal rearing (Barros et al., 2004; Maccari et al., 1995; Smythe, McCormick, & Meaney, 1996; Wakshlak & Weinstock, 1990).
Whether postnatal rearing experiences also moderate the effects of antenatal stress in humans is a matter of considerable conceptual and practical significance; however, no studies have yet tested this hypothesis directly. In the current study, we adopted the most widely-researched model of caregiving, attachment theory, to test the hypothesis that postnatal rearing would moderate the effects of antenatal stress. Quality of child-parent attachment was indexed by Ainsworth's Strange Situation (Ainsworth, Blehar, Waters, & Wall, 1978), the “gold standard” index of attachment in infancy. The choice of attachment theory follows from the substantial evidence linking individual differences in mother-infant attachment with current and subsequent functioning (Sroufe, 2005). Furthermore, extensive animal and modest human evidence suggests that individual differences in attachment experiences have biological consequences, including on the HPA axis (Nachmias, Gunnar, Mangeelsdorf, Parritz, & Buss, 1996), the leading candidate mechanism in the antenatal stress research.
Several alternative predictions can be made about how and if early attachment may moderate the effects of prenatal stress. One form, explicit in the resilience literature, is that secure attachment would buffer children from the adverse effects of stress exposure. This is the form of moderation in the animal studies of antenatal stress. Human evidence of a stress-buffering effect of early attachment experiences is mixed, however, and most of these studies consider psychosocial rather than biological risk exposures. For example, although some studies suggest that infant attachment may protect children from subsequence adverse psychosocial exposure (Sroufe et al., 1990), other studies suggest no clear stress `inoculation' (Belsky & Fearon, 2002).
An alternative moderation effect is exacerbation. That is, early stress exposure would have greater impact among those children who experienced subsequent poor caregiving. This model has received little attention in the animal literature of antenatal stress. In human studies, this model, like the buffering model, has received mixed support. Some studies suggest that prior risk exposure increases vulnerability to subsequent stress, as in the case of premature birth and low SES (Brooks-Gunn et al., 1998). Other studies fail to find that there is a carrying forward of vulnerability that increases the likelihood of disturbance following subsequent stress exposure (e.g., Kurstjens & Wolke, 2001).
Set against these moderation models is a third possibility, a non-moderation or cumulative risk model. According to this approach, there is not an interaction between early and subsequent risk exposure; rather, risk exposure at the two time points accumulates and leads, in an additive manner, to worse outcomes. This is the more common model in human developmental studies of psychosocial risk and their effects (Burchinal et al., 2000; Sameroff et al., 1993). Explicit in this model is the hypothesis that antenatal stress exposure would have lasting adverse effects, on average, and that this link would operate at least somewhat independently of early attachment experiences.
Which of the above models would be most likely fit data from the current study is difficult to predict a priori. Although the animal findings (cited above) confirm a stress-buffering model, there are obvious concerns about extending this animal finding to humans. For example, rats are born at a comparatively earlier point in ontogeny than are humans; thus, the early postnatal manipulations that offset antenatal stress in rats would still be an antenatal effect in humans (i.e., in ontogenetic terms). Furthermore, in most of the above studies showing the postnatal rearing modification, the pregnant dams who are exposed to stress are not the one whose parenting eliminates the antenatal stress effect. That is, the postnatal rearing depends on cross-fostering design, which has uncertain application to humans. Accordingly, it is far from clear that human mothers who were anxious/stressed antenatally would be able to provide a parenting environment that would eliminate the adverse effect of antenatal stress exposure.
In this study we have tested these alternative models connecting antenatal exposure to stress and early attachment for two of the most commonly reported outcomes of antenatal stress or anxiety, fearfulness and cognitive ability. The availability of attachment data provided an important opportunity to expand prior work (Bergman et al. 2007) in an important new direction. Assessing two distinct outcomes provides some leverage in testing the robustness of a moderation effect and provides an index of whether risk mechanisms operate similarly for these developmental outcomes.
METHOD
Participants
Women were recruited sequentially from an amniocentesis clinic in a large London UK maternity hospital between December, 2001 and January, 2005. The majority of referrals were for karyotyping for Down's syndrome. Written informed consent was obtained. The study was approved by the institutional research ethics committee. All English-speaking mothers with full-term (≥37 weeks), healthy and singleton infants, whose birth outcomes were known, were invited to return to the pediatric clinic in the hospital when the child was between 14 and 19 months old. As detailed elsewhere (Bergman et al., 2007), 365 women were recruited at amniocentesis, of whom 109 were excluded because of known abnormalities, incomplete data on birth outcome or the procedure was for non-routine amniocentesis. Of the 256 remaining mothers, we were unable to locate 71 and a further 60 did not wish to participate or could not attend the clinic (e.g., because of moving away from London). In addition, two mothers were subsequently excluded because of insufficient English. Therefore, complete data on obstetric outcomes, antenatal and postnatal life events stress, and cognitive outcomes were available on 123 children; data on temperament were available on 106 children (for 17 children it was not possible to complete the temperament assessment, which occurred later in the visit, primarily because of fatigue or time constraints). Sample sizes in multivariate analyses may differ slightly because of missing data.
Measures
Maternal stress
Mothers completed a 26-item Stressful Life Events Questionnaire (SLEQ; adapted from Barnett, Hanna, & Parker, 1983), at the postnatal visit, and reported if the event occurred and whether the event “affected me a little” or “affected me a lot.” Mothers reported if the event occurred antenatally or postnatally (birth to follow-up) or both. The SLEQ is similar to measures of stressful life events used in studies of non-pregnant adults; there are two scales: the number of events experienced and the perceived impact of the events. We focus on the number of events because it is a more objective assessment of stress exposure, but we note in the results section that the findings are substantially identical for the frequency of events and perceived impact of events. Details of the items can be found in Bergman et al. (2007).
Mother-infant attachment
Ainsworth's Strange Situation (Ainsworth, Blehar, Waters, & Wall, 1978) was administered to determine the quality of mother-infant attachment. This is an extensively researched 7-episode laboratory assessment that capitalizes on the separation-reunion paradigm to assess the extent to which the child uses the parent as a secure base for exploration. The procedure was videotaped and later rated by a researcher who was blind to antenatal data. Coding were made using established procedures for classifying dyads as Insecure-Avoidant, Secure, Insecure-Ambivalent/Resistant, and Insecure-disorganized (Ainsworth, Blehar, Waters, & Wall, 1978; Main & Solomon, 1990). The primary coder underwent extensive training with L.A. Sroufe and E. Carlson at the Institute of Child Development, University of Minnesota, and achieved at least 80% agreement on a reliability test of 35 cases.
Infant cognitive development
A developmental researcher who was blind to antenatal data administered the Bayley Scales of Infant Development - Second Edition (BSID-II) (Bayley, 1993), a widely-used standardized assessment of infant mental development (Mental Developmental Index, MDI) and physical development (Physical Developmental Index, PDI). The researcher underwent extensive Bayley training and achieved inter-rater reliability was 90% for MDI and 97% for PDI.
Infant fearfulness
Part of the Laboratory Temperament Assessment Battery (Lab-TAB) - Locomotor Version (Goldsmith & Rothbart, 1999) was used to assess infant temperament. The Lab-TAB is a leading observational measure of childhood temperament, with considerable support for its validity and clinical value (e.g. Askan & Kochanska, 2004). We used the unpredictable mechanical toy paradigm from the fear subscale. The child sat at a table and a robotic dog was presented when the child was calm and alert. Each trial lasted about 20 seconds and consisted of the dog barking walking towards the child as its eyes, mouth and head moved. Three trials were presented unless the child became too distressed and subsequent trials could not be administered; in that case, scores from the first trial are carried through to the subsequent trials, as per coding guidelines. We report findings from the first trial because it meant that results were based on the most complete raw observed data (but we note that the findings for the composite across trials - which carried forward scores for those unable to complete latter trials - were substantively identical). The episode was videotaped and later rated by a researcher blind to maternal data using standard scoring procedures. Indicators of fear were assessed from facial expression, body posture, vocalizations, and escape behavior. Inter-rater reliability was calculated on 22 randomly selected tapes with a rater who was blind to all child and parent data. Intraclass correlations were 0.80 for facial expression, 0.70 for body posture, 0.92 for vocalizations, and 0.89 for escape behavior (all p's <0.001); a composite score derived from the four indicators had an intraclass correlation of 0.93.
Psychosocial and obstetric covariates
Information on maternal age, parity, ethnicity (categorized according to UK National Health Service ethnic codes), smoking (cigarettes per day), alcohol (units per week) and prescription drug use during pregnancy (prescription drug categories: Selective serotonin reuptake inhibitors; anti-hypertensive; anti-asthmatic; anti-epileptic; steroids; and other) was collected at recruitment. Information regarding birth outcomes (birth weight, gestational age at birth, method of delivery) and child sex was collected from the child's hospital notes. Standard deviation score of birth weight adjusted for gestational age and sex was calculated using software based upon 1990 British Growth Reference data.
We included detailed measures of maternal mental health in the postnatal period as covariates to provide a rigorous test of the antenatal stress effect. The Spielberger Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1983) was included as a measure of habitual (trait) anxiety and as a possible index of heritable anxiety-like traits in the child. The STAI is a widely used index of anxiety symptoms and has considerable validity, reliability, and clinical utility. The Edinburgh Postnatal Depression Scale (EPDS), a widely used index with considerable validity (Cox, Holden, & Sagovsky, 1987), was used to measure maternal depressive symptoms, a reliable predictor of child outcomes.
Statistical analyses
A P-P plot was used to check the normality of distributions; non-normally distributed variables (antenatal and postnatal stressful life events, and smoking and alcohol intake during pregnancy) were log transformed and both non-parametric (Spearman rs) and parametric statistics were used to confirm the findings. The findings were substantively identical with and without transformed scores and using parametric and nonparametric statistics. We report findings on the raw data for descriptive analyses and regression analyses because these values are more readily interpretable than estimates of transformed variables. Importantly, both outcome measures were normally distributed. Maternal age, smoking and alcohol in pregnancy, birth weight corrected for gestational age, and maternal education were included as covariates because they can affect the outcomes (e.g., Li & Poirier, 2003). We also included as covariates postnatal life events and postnatal maternal symptoms of anxiety and depression to provide a strong test of the hypothesis that the effects of antenatal stress on child outcomes are not explained by concurrent maternal mental state.
Attachment moderation hypotheses were initially tested by assessing correlations between antenatal stress and developmental outcomes separately by attachment classification; formal testing of the moderation was conducted by hierarchical regression analyses in which attachment X antenatal stress variables were entered into the model after including main effects and covariates.
RESULTS
Descriptive statistics and preliminary analyses
Sample characteristics are provided in Table 1. There were no significant differences between the sample on whom fearfulness data were collected (n=106) and the small group of individuals (n=17) on whom this measure was not administered (not shown). Demographic data suggest that this is a normal risk group indexed by such factors as levels of symptoms (the means were comparable to other normal risk samples); there was the predictable range of risk with the possible exception of smoking and alcohol intake, which were low. Maternal age was older than an average antenatal sample, but ranged widely. The distribution of attachment classifications was also generally consistent with what is found in normal risk samples.
Table 1.
Sample characteristics for mothers and infants
| Means±(SD) and No.(%) (N=123) | |
|---|---|
| Maternal education | |
| No exam passed | 4(3.3) |
| GCSE or equivalent | 14(11.4) |
| A levels or equivalent | 18(14.6) |
| Diploma or equivalent | 23(18.7) |
| University degree | 41(33.3) |
| Postgraduate degree | 23(18.7) |
| Parity | 0.89±0.88 |
| Nulliparous | 49(39.8) |
| 1 previous child | 45(36.6) |
| 2 previous children | 23(18.7) |
| 3 previous children | 6(4.9) |
| Racial background | |
| White Caucasian | 102(82.9) |
| Asian - Indian/Sub-continent | 8(6.5) |
| Black | 10(8.1) |
| Middle-eastern | 3(2.4) |
| Smoking during pregnancy | 0.29±1.84 |
| 0 cigarettes/day | 110(89.4) |
| 1-2 cigarettes/day | 12(8.8) |
| >2 cigarettes/day | 1(0.8) |
| Alcohol during pregnancy | 0.54±1.54 |
| 0 units/week | 85(69.1) |
| 1-2 units/week | 33(26.8) |
| 2 units/week | 5(4.1) |
| Prescription drugs in pregnancy | |
| None | 106(86.2) |
| SSRIs | 1(0.8) |
| Anti-hypertensive | 2(1.6) |
| Anti-asthmatic | 8(6.5) |
| Steroids | 1(0.8) |
| Other | 3(2.4) |
| Unknown | 2(1.6) |
| Maternal age | 36.55±4.12 |
| range: 25.00-45.00 | |
| Maternal STAI trait (postnatal) | 37.90±10.15 |
| Postnatal depression (postnatal) | 8.33±4.61 |
| Method of delivery | |
| Normal vaginal delivery | 62(50.4) |
| Assisted vaginal delivery | 18(14.6) |
| Elective caesarean | 19(15.4) |
| Emergency caesarean | 15(12.2) |
| Unrecorded | 9(7.3) |
| Child age (months) | 16.76±1.41 |
| Gestational age at birth (weeks) | 39.47±1.17 |
| Birth weight (grams) | 3490±480 |
| Child sex | |
| Female | 63(51.2) |
| Male | 60(48.8) |
| Attachment pattern | |
| A (Avoidant) | 17(13.8) |
| B (Secure) | 69(56.1) |
| C (Resistant) | 16(13.0) |
| D (Disorganized) | 20(16.3) |
Associations between attachment and predictor and outcomes variables
Antenatal stress was significantly associated with attachment classification (F(3,118)=2.99, p<.05). Post hoc analyses showed that children with Insecure-disorganized (mean 2.00 [SD 2.00]) and Insecure-avoidant (2.00[1.58]) attachments had mothers who reported higher levels of antenatal stress than children with Secure (1.32[1.39]) and Insecure-Ambivalent (.75[1.07]) attachments. No significant differences were observed for postnatal life events, although the overall pattern in means was similar: Insecure-disorganized (3.05[2.89]), Insecure-Avoidant (2.24[2.11]), Insecure-Ambivalent (2.19[2.61]), Secure (1.83[1.77]).
There was not a significant association between observed fearfulness and attachment using the 4-way attachment classifications (F(3,101)=.43). The correlation between observed fearfulness and antenatal stressful life events was r(106)=.38,p<.01); in addition, there were significant bivariate associations between observed fearfulness and maternal trait anxiety (r(106)=.24,p<.05) and EPDS (r(105)=.25,p<.01).
There was a main effect of attachment on the mental development index from the Bayley scale (F(3,118)=6.28, p<.001). Follow-up contrasts indicated that children with Insecure-Disorganized attachment (91.05[8.30]) and Insecure-Avoidant children (92.53[9.89]) had significantly lower scores than children with Secure (99.43[9.30]) and Insecure-Ambivalent children (101.00[11.28]). Bivariate analyses also indicated that infant cognitive development was also significantly associated with antenatal stress (r(123)=-.43,p<.001), female gender (r(123)= .19,p<.05), age at infant assessment (r(123)= -.21,p<.05).
Does child-parent attachment moderate the effects of maternal antenatal stress on fearfulness?
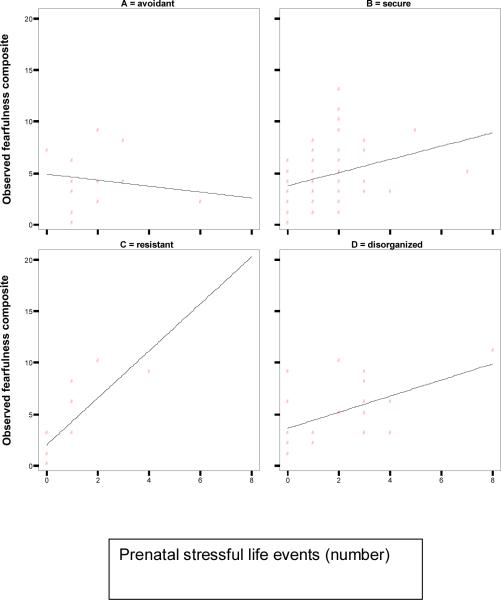
The correlation between antenatal stress and observed fearfulness in the lab-TAB assessment varied widely according to attachment classification. As Figure 1 shows, the association (both Pearson and Spearman correlations are provided given the small numbers in some classifications) was large in the Insecure-ambivalent group (r(12)=0.78, p<0.001; rs=.84), moderate in the Secure group (r(62)=0.34, p<0.01; rs =.35) and Insecure-disorganized (r(18)=0.53, p<0.05; rs =.47) groups, and minimal in the Avoidant group (r(13)= -0.17;ns; rs =-.06).
Figure 1.
Spearman correlation between number of antenatal stressful life events and fearfulness scores from Trial 1 (compositing facial, body, vocal, and escape behaviors) according to attachment classification (*p<0.05; **p<0.01)
Hierarchical regression analyses was used to test the difference in the magnitude of effect across attachment classifications, controlling for main effects and covariates. Results are given in Table 2. In this model, Insecure-Ambivalent is the control condition for the 4-way attachment classifications (this was determined after assessing the correlations by attachment classification). Model 1 includes the main effects and covariates; Model 2 includes the 3 2-way interactions between attachment classification and antenatal stress. Results indicate significant interactions for all three attachment X antenatal stress interactions, indicating that the prediction from antenatal stress to fearfulness is significantly greater in the Insecure-Ambivalent group compared the other three attachment classifications (i.e., the interaction with Insecure-Avoidant, Secure, and Insecure-Disorganized are all negative). Antenatal stress is also a significant main effect in the model. The final model predicted 32% of the variance; F(17,79)=2.21,p<.01). These interaction effects were essentially identical when the impact of events was used as a main effect and moderator of attachment classification (not shown).
Table 2.
Stepwise linear regression model predicting infant Lab-TAB fearfulness.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| B(SE) | beta | B(SE) | beta | |
| Antenatal stress | .68(.20) | .39*** | 2.40(.70) | 1.38*** |
| Postnatal stress | -.18(.15) | -.14 | -.25(.15) | -.20 |
| Maternal education | .25(.20) | .12 | .13(.20) | .07 |
| Maternal age | .05(.08) | .06 | .06(.07) | .08 |
| Child sex (female=1,male=2) | -.16(.57) | -.03 | .10(.56) | .02 |
| Child age (months) | .10(.20) | .05 | .04(.20) | .02 |
| Smoking in pregnancy | -.24(.15) | -.16 | -.24(.14) | -.16 |
| Alcohol in pregnancy | .07(.18) | .04 | .07(.18) | .04 |
| Birthweight/Gestational age | -.17(.27) | -.07 | -.11(.26) | -.05 |
| Trait anxiety (postnatal) | .02(.04) | .06 | .01(.04) | .03 |
| EPDS (postnatal) | .09(.09) | .14 | .06(.08) | .10 |
| Insecure-Avoidant | -1.00(1.07) | -.13 | 2.09(1.46) | .27 |
| Secure | -.39(.90) | -.07 | 1.44(1.19) | .26 |
| Insecure-Disorganized | -.55(1.09) | -.08 | 1.32(1.43) | .18 |
| Antenatal stress X Attachment | ||||
| Insecure-Avoidant X Antenatal stress | -2.44(.80) | -.73** | ||
| Secure X Antenatal stress | -1.65(.73) | -.68* | ||
| Insecure-Disorganized X Antenatal stress | -1.63(.71) | -.79* | ||
Note: Insecure-Ambivalent is the control condition for the attachment classification analyses. n=97;
p<0.05
p<0.01
p<001.
Does child-parent attachment moderate the effects of maternal antenatal stress on infant cognitive outcome?
The correlation between antenatal stress life events and infant BSID-II MDI scores showed some variability across the four attachment classifications, ranging from rs -.02 in the Insecure-ambivalent group to rs -.62 in the Insecure-disorganized group; the association in the Secure and Insecure-Avoidant groups were rs -.33 and rs -.47, respectively. We formally tested the antenatal stress X attachment interaction in predicting MDI scores using the hierarchical regression model; results are displayed in Table 3. After controlling for the main effects and covariates, there was not evidence that attachment significantly moderated the effects of antenatal stress on cognitive development. It is somewhat arbitrary which classification is considered as the control condition in analyses; we report the final model using Secure classification as the control condition (i.e., attachment classification effects are assessed relative to Secure). Results from the final model show that antenatal stress, maternal education, and Insecure-disorganized attachment were significant predictors, collectively accounting for 39% of the variance (F(14,99)=4.38,p<.001). The results were essentially identical when the impact of events was used rather than the number of events (not shown).
Table 3.
Stepwise linear regression model predicting infant BSID-II MDI.
| Model 1 | ||
|---|---|---|
| B(SE) | beta | |
| Antenatal stress | -2.70(.60) | -.42*** |
| Postnatal stress | .63(.44) | .14 |
| Maternal education | 1.82(.59) | .25** |
| Maternal age | -.07(.21) | -.03 |
| Child sex (female=1,male=2) | -2.61(1.66) | -.13 |
| Child age (months) | -1.45(.61) | -.20* |
| Smoking in pregnancy | .24(.43) | .05 |
| Alcohol in pregnancy | .04(.57) | .01 |
| Birthweight/Gestational age | -.79(.78) | -.09 |
| Trait anxiety (postnatal) | .02(.12) | .02 |
| EPDS (postnatal) | .02(.26) | .01 |
| Insecure-Avoidant | -3.34(2.31) | -.12 |
| Insecure-Ambivalent | 1.14(2.59) | .04 |
| Insecure-Disorganized | -6.75(2.34) | -.26* |
Note: Secure is the control condition for the attachment classification analyses. n=114;
p<0.05
p<0.01
p<001.
Discussion
This is the first human study to consider caregiving as a moderator of the effects of antenatal stress on the offspring. Results indicated that an early Insecure-Ambivalent attachment accentuated the link between exposure to antenatal stress and infant fearfulness assessed at approximately 17 months, as observed as part of a standardized laboratory assessment. There was no evidence of early caregiving moderating the impact of antenatal stress on cognitive development. These findings build on and extend the research on antenatal stress in humans and carry both conceptual and clinical applications. We consider the study strengths and limitations before addressing the implications of the findings.
There are several limitations to the existing study. One of these is the moderate sample size, which was comparatively large relative to studies collecting detailed observational data, but more modest to detect interactions. Second, we obtained life events stress about pregnancy in the postnatal period. That meant that we were able to cover the antenatal period (which would be difficult if we had assessed life events during pregnancy), but it does raise methodological concerns about recall bias. Countering these concerns are data suggesting that the short time-frame and objective nature of events recalled are less likely to lead to bias (Henry et al., 1994); more fundamentally, the likelihood of a simple recall bias is incompatible with the finding that we included multiple measures of stress and symptoms in the postnatal period as covariates. There are alternative explanations to be considered, including genetics, and the non-experimental nature of the design (i.e., women were not assigned to stress and non-stressed conditions) which means that there could be selection biases that confound causal inferences. The inclusion of multiple postnatal measures of stress and symptoms in the postnatal period means that simple genetic transmission account of the data seems unlikely (i.e., if that were so, than the antenatal effect would likely be eliminated by postnatal stress, anxiety or depression); the inclusion of postnatal data are consistent with the hypothesis that there is something particular to stress in the antenatal period that predicts child outcomes. Finally, because of the broader study focus on fetal hormone exposure, all women were recruited from an amniocentesis clinic. They were a generally normal risk group according to maternal symptom and child outcome data, but there was a tendency to include predominantly, white, older, and well-educated mothers. These limitations are offset by several strengths of the study, including standard observational measures of attachment, cognitive ability and temperamental fearfulness.
Antenatal exposure X caregiving environment interactions
Human research has reliably replicated the basic association between antenatal stress and offspring outcomes that has been so extensively reported in experimental animal work. Accordingly, human research is moving on to consider other aspects of this phenomena that derive from animal findings such as the mechanisms involved and the role of postnatal rearing or other particularly human concerns, such as the generalizability of this link across economic and socio-demographic groups. What seems clear at this point is that antenatal stress may be one of the most promising scientific proving grounds for translating, and back-translating, animal and human research findings.
The observation in the animal studies that maternal care, postnatal handling, and environmental enrichment can eliminate the effects of prenatal adversity (Meaney, 2001; Morley-Fletcher, Rea, Maccari, & Laviola, 2003; Smythe, McCormick, & Meaney, 1996; Wakshlak & Weinstock, 1990) is significant for suggesting that, even if antenatal stress may have programming effects on the fetal brain, the `programming window' extends to the early postnatal period. Protective or otherwise counter-acting influences in the early postnatal period may be evident because the `programmability' of the stress-response (or connected) system is not yet closed. Animal work in this area has been centrally concerned with early postnatal experience (but see Barbaganzes et al., 1996); therefore, the developmental limits of the postnatal period for eliminating the antenatal stress exposure effect are not yet clear - and the comparatively brief period of maternal care in animals make it practically challenging to interpret caregiving effects at different points in offspring development.
For observed fearfulness, we found no compelling evidence that early caregiving, indexed by attachment, eliminated the effect of antenatal stress. Thus, we failed to replicate the animal finding. However, early caregiving, specifically, Insecure-Ambivalent attachment, accentuated the link between antenatal stress and fearfulness. This exacerbation effect is not a non-replication of the animal data because the focus in virtually all of the animal experiments is reducing the adverse effects of antenatal stress - not exacerbating them. Why would an Insecure-Ambivalent attachment accentuate the effects of previous stress exposure on fearfulness? Several groups have reported that Insecure-Ambivalent attachment, characterized as inconsistent availability of the caregiver (Isabella & Belsky, 1991), is linked with inhibited temperament in infancy (Calkins & Fox, 1992) and anxiety and anxious behavior (Warren et al., 1997; Cassidy & Berlin, 1994). The observation that Insecure-Ambivalent caregiving accentuates fearfulness among those children exposed to antenatal stress - and who are already vulnerable to anxious/fearful behavior - therefore fits well with prior work on this attachment pattern. Why we did not find, as others have, a main effect of attachment on fearfulness is not clear, but might be explicable in terms of the age of assessment or the small number of children in this category.
What mechanisms might account for the caregiving effect - either as a main effect or moderator -is not yet clear, but there is considerable interest in the HPA axis as a candidate (Nachmias et al., 1996) - the same mechanism that has attracted the most attention in the work on antenatal stress and child outcomes. It may be that the increased exposure of the fetal brain to cortisol, a product of elevated maternal antenatal stress, makes children more vulnerable to a particular form of insecure caregiving that seems also to trigger HPA-related physiological changes associated with anxiety/fearfulness. That hypothesis needs direct testing.
The near absence of a link between antenatal stress and fearfulness in Insecure-Avoidant infants is intriguing; whether that implies that there is something about the avoidant caregiving that is “protective” against fearfulness in prenatally stress children requires further consideration.
A caregiving moderation effect was not found for cognitive ability. Whether that is a non-replication of the animal work is not yet clear because most of the reported moderation effects are from studies assessing fearfulness-related behavioral or physiological phenotypes. Although there was not a significant interaction, we did find a main effect of attachment insecurity (especially Disorganized attachment according to the multivariate models). Much of the research on Insecure-Disorganized attachment focuses on behavioral/emotional problems and social competence; nonetheless, the finding of lower cognitive ability is consistent with past studies (van IJZendoorn et al., 1999). The novel finding in this report is that the effect adds to the antenatal stress effect. Taken together, the findings support a cumulative risk model that is familiar in developmental studies.
Finally, the current findings also underscore the need for further clinical research into the biobehavioral nature of psychological interventions (Cicchetti & Blender, 2006). There are now many efforts underway to test the hypothesis that psychological interventions may alter both behavioral and neuroendocrine processes. That is a significant line of investigation because it seeks to translate some of the more impressive animal findings on the role of early caregiving and because it would help to provide a broader public health context to psychosocial interventions. Multiple candidate mechanisms may be regulated by stress exposure such as the HPA axis (Gunnar & Quevedo, 2007) or the immune system (Caserta et al., in press); these are natural targets for further developmental studies of intervention effects.
Acknowledgements
We wish to thank Diana Adams for help with patient recruitment and the scoring of the questionnaires. The study was supported by a grant from the March of Dimes. Support was also provided by NIH grant MH073019 and MH073842.
References
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of Attachment: A Psychological Study of the Strange Situation. Lawrence Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- Askan N, Kochanska G. Links between systems of inhibition from infancy to preschool years. Child Development. 2004;75:1477–1490. doi: 10.1111/j.1467-8624.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le MM, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J.Neuroscience. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett BE, Hanna B, Parker G. Life event scales for obstetric groups. Journal of Psychosomatic Research. 1983;27:313–320. doi: 10.1016/0022-3999(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Barros VG, Berger MA, Martijena ID, Sarchi MI, Perez AA, Molina VA, Tarazi FI, Antonelli MC. Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Journal of Neuroscience Research. 2004;76:488–496. doi: 10.1002/jnr.20119. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2nd ed. The Psychological Corporation; New York: 1993. [Google Scholar]
- Belsky J, Fearon RM. Early attachment security, subsequent maternal sensitivity, and later child development: does continuity in development depend upon continuity of caregiving? Attachment and Human Development. 2002;4:361–387. doi: 10.1080/14616730210167267. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O'Connor T,G, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1097/chi.0b013e31814a62f6. (in press) [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, McCormick MC, Klebanov PK, McCarton C. Health care use of 3-year-old low birth weight premature children: effects of family and neighborhood poverty. Journal of Pediatrics. 1998;132:971–975. doi: 10.1016/s0022-3476(98)70393-2. [DOI] [PubMed] [Google Scholar]
- Burchinal MR, Roberts JE, Hooper S, Zeisel SA. Cumulative risk and early cognitive development: A comparison of statistical risk models. Developmental Psychology. 2000;36:793–807. doi: 10.1037//0012-1649.36.6.793. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, O'Connor TG, Wyman PA, Wang H, Moynihan J, Cross W, Tu X, Jin X. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain, Behavior and Immunity. doi: 10.1016/j.bbi.2008.01.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy J, Berlin LJ. The insecure/ambivalent pattern of attachment: theory and research. Child Development. 1994;65:971–991. [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Annals of the NY Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Karaszewski JW, Ershler WB. Prenatal endocrine activation alters postnatal cellular immunity in infant monkeys. Brain Behavior and Immunity. 1996;10:221–234. doi: 10.1006/brbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Development of the Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:737–46. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Glover V, O'Connor TG, Heron J, Golding J. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Human Development. 2004;79:107–118. doi: 10.1016/j.earlhumdev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The Laboratory Temperament Assessment Battery Locomotor Version 3.1 Description of Procedures. University of Wisconsin; Madison, Wisconsin: 1999. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de WC, Zandbelt N, Mulder EJ, Visser GH, Buitelaar JK. Does maternal prenatal stress adversely affect the child's learning and memory at age six? Journal of Abnormal Child Psychology. 2006;34:789–798. doi: 10.1007/s10802-006-9054-7. [DOI] [PubMed] [Google Scholar]
- Henry B, Moffitt TE, Caspi A, Langley JD, Silva PA. On the “Remembrance of Things Past”. A longitudinal evaluation of the retrospective method. Psychological Assessment. 1994;6:92–101. [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology & Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Isabella RA, Belsky J. Interactional synchrony and the origins of infantmother attachment: a replication study. Child Development. 1991;62:373–384. [PubMed] [Google Scholar]
- Kurstjens S, Wolke D. Effects of maternal depression on cognitive development of children over the first 7 years of life. Journal of Child Psychology and Psychiatry. 2001;42:623–636. [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du FG, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Li K, Poirier DJ. The roles of birth inputs and outputs in predicting health, behaviour and test scores in early childhood. Statistics in Medicine. 2003;22:3489–3514. doi: 10.1002/sim.1577. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal MM. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. Journal of Neuroscience. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main M, Solomon J. Procedures for identifying infants as disorganized/disoriented during the Strange Situation. In: Greensberg M, Cicchetti D, Cummings EM, editors. Attachment in the Preschool Years: Theory, Research and Intervention. Chicago University of Chicago Press; 1990. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18:3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangeelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment insecurity. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J. Psychological factors in pregnancy and mixed-handedness in the offspring. Developmental Medicine and Child Neurology. 2003;45:557–561. doi: 10.1017/s0012162203001014. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O'Donnell D, Larocque S, Seckl JR, Meaney MJ. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Brain Research Molecular Brain Research. 1994;26:242–248. doi: 10.1016/0169-328x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: the influence of social and family risk factors. Child Development. 1993;64:80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML. Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Developmental Psychobiology. 1992;25:529–540. doi: 10.1002/dev.420250706. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch I, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Sroufe LA. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attachment and Human Development. 2005;7:349–367. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Kreutzer T. The fate of early experience following developmental change: longitudinal approaches to individual adaptation in childhood. Child Development. 1990;61:1363–1373. doi: 10.1111/j.1467-8624.1990.tb02867.x. [DOI] [PubMed] [Google Scholar]
- Vallee M, Maccari S, Dellu F, Simon H, Le MM, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. European Journal of Neuroscience. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Development and Psychopathology. 1999;11:225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- Warren SL, Huston L, Egeland B, Sroufe LA. Child and adolescent anxiety disorders and early attachment. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:637–644. doi: 10.1097/00004583-199705000-00014. [DOI] [PubMed] [Google Scholar]
- Wakshlak A, Weinstock M. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiology and Behavior. 1990;48:289–292. doi: 10.1016/0031-9384(90)90315-u. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]