In previous reports (1, 2), we have provided a detailed technical description of right hepatic trisegmentectomy and discussed the relation of this procedure to other more commonly used resections of the liver. At that time, the four standard kinds of anatomic liver resection were right lobectomy; right trisegmentectomy, extended right lobectomy; left lobectomy, and left lateral segmentectomy. We report herein upon a fifth procedure, left hepatic trisegmentectomy, or an extended left lobectomy, with which the true left lobe of the liver and most, or all, of the anterior segment of the right lobe have been removed in continuity.
METHODS
Patient material
The four patients in this study ranged in age from nine to 61 years (Table I). The hepatic lesions were diagnosed as hepatoma, squamous cell carcinoma which probably originated in an epithelial liver cyst, cavernous hemangioma associated with arteriovenous malformation of the liver and metastatic carcinoma from a previously resected carcinoma of the colon and rectum. The lesions were predominantly in the left lobe of the liver, but the area of involvement crossed the interlobar plane into the anterior segment of the right lobe (Fig. 1). The diagnosis of a malignant condition justified an aggressive attempt at excision in three of the four patients. In the fourth patient, an extensive arteriovenous malformation had ruptured into the biliary tract with the resulting deposition of an inspissated blood clot throughout all of the intrahepatic and extrahepatic biliary tree. This patient had disabling pain in the upper abdominal quadrant, a weight loss of 50 pounds and remittent jaundice.
TABLE I. CLINICAL FEATURES.
| Patient No. | Age, yrs. Sex | Diagnosis | Left caudate fragment removed | Blood transfusions, L | Complications | Outcome |
|---|---|---|---|---|---|---|
| 1 | 9, M | Hepatoma | No | 3.0 | None | Tumor-free, 20 mos. |
| 2 | 61, M | Squamous cell carcinoma from from? liver cyst | Yes | 2.7 | Hepatic duct anastomotic stricture, dilated; bile fistula drained | Died 8 mos., carcinomatosis |
| 3 | 37, M | Cavernous hemangioma with arteriovenous malformation | Yes | 20.0 | Intraoperative cardiac arrest; hepatic duct stricture, dilated; bile fistula, drained | Well, 9 mos. |
| 4* | 50, M | Metastatic carcinoma from colon, primary resected two years previously | No | 8.5 | Bile fistula, drained | Tumor-free, 9 mos. |
Only one-half of the anterior segment was removed. Chorioembryonic antigen fell from 2,500 to <3 ngm./100 ml.
Fig. 1.
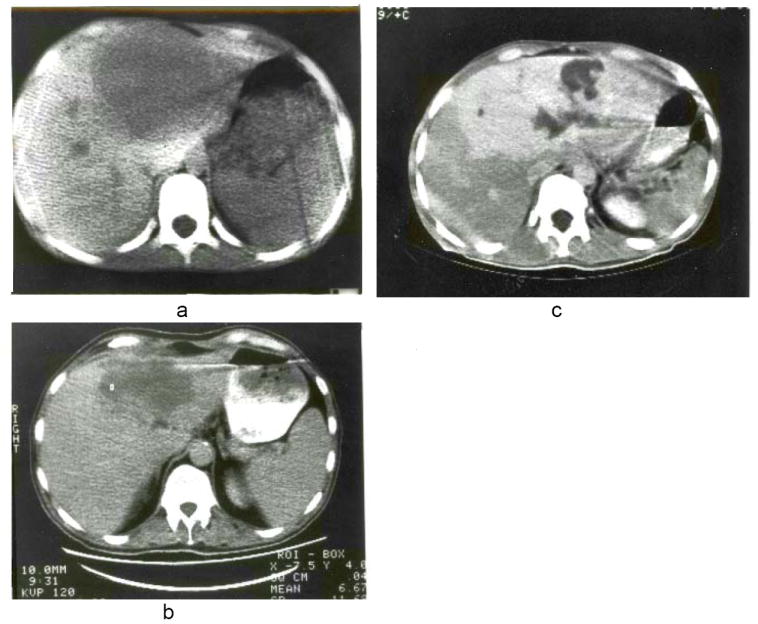
a, b and c, Scans of liver in Patients 1, 2 and 3 obtained with computerized axial tomography. Note predominant involvement of the left lobes but with extension into the anterior segments of the right lobes. c, The white appearance of the lesion was due to the prior injection of contrast medium intravenously.
Surgical technique
Although we have previously recommended routine angiography preoperatively (1), we no longer do this procedure in most instances. Broad spectrum antibiotics are begun preoperatively.
A bilateral subcostal incision is used with a superior midline extension to above the xiphoid process. The xiphoid process is excised to facilitate exposure of the entrance of the hepatic veins into the suprahepatic inferior vena cava.
The hilus of the liver is examined (Fig. 2). The cystic duct and cystic artery are ligated and divided to facilitate subsequent hilar dissection. The ligamenta teres hepatis is ligated and divided, and the falciform ligament is incised superiorly to beyond where its leaves diverge to ensheath the suprahepatic bare area in which lie the main hepatic veins and suprahepatic inferior vena cava. The left triangular ligament is incised, fully exposing the anterior surface of the left hepatic vein and the entry into it of the left phrenic vein.
Fig. 2.
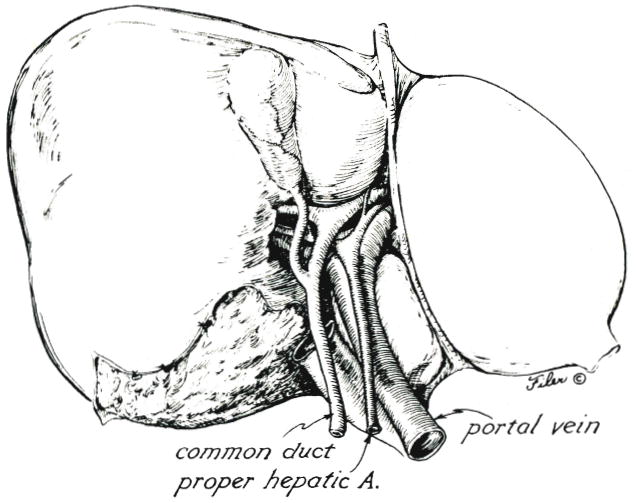
Structures of the hilus of the liver.
The lateral segment of the left lobe is lifted anteriorly and retracted toward the right. With this maneuver (Fig. 3), the principal left lobar branches of the portal triad structures can be safely approached from their posterolateral aspect. The posteriorly located left portal vein is encircled first, ligated and divided. The more anteriorly positioned left hepatic artery and left hepatic duct can then be more easily seen, dissected and divided.
Fig. 3.
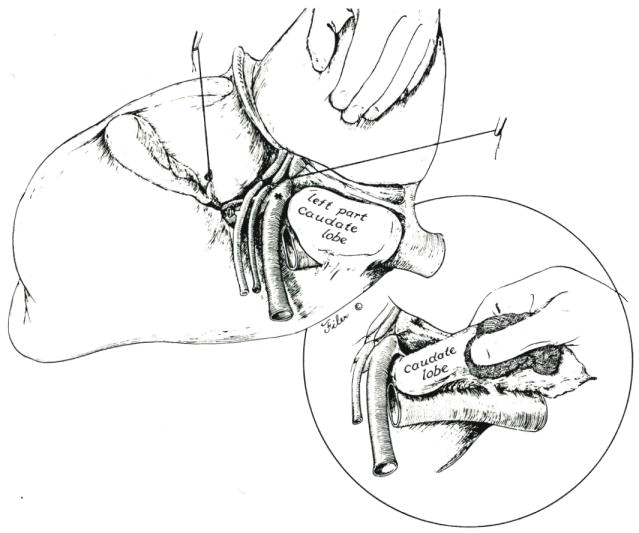
Exposure of the left hilar structures is accomplished by retracting the lateral segment of the left lobe anteriorly and to the right. Branches to the left portion of the caudate lobe are preserved if the latter liver fragment is to be retained. Otherwise, the left structures are ligated at their origin, *. Inset, If the left portion of the caudate lobe is removed, it must be dissected from the retrohepatic inferior vena cava, and several small hepatic veins must be ligated.
If the caudate lobe to the left of the inferior vena cava is to be removed, the left hilar structures should be ligated flush with their origin (Fig. 3, at asterisk). If this left portion of the caudate lobe is to be spared, the ligatures usually should be distal to the posteriorly directed first branches (Fig. 3). When these maneuvers are complete, the true left lobe becomes cyanotic, with the line of color demarcation being in the center of the gallbladder bed (Fig. 4).
Fig. 4.
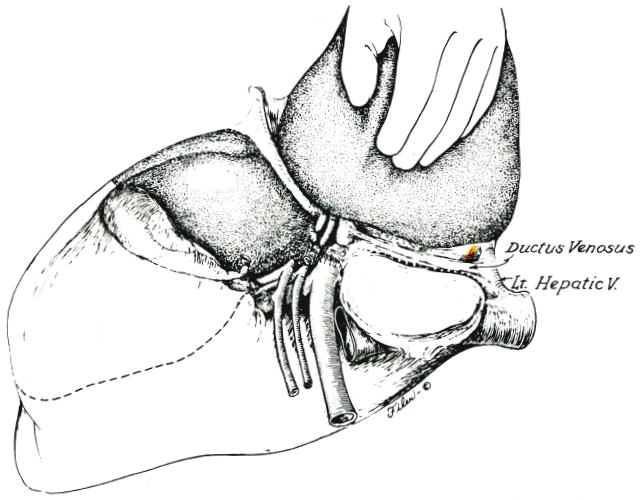
Line of incision into the capsule of the liver along obliterated ductus venosus which is used if the left portion of the caudate lobe is retained. Alternatively, the line of posterior incision is along the vena cava (Fig. 3, inset) if the caudate fragment is removed. Eventual plane between anterior and posterior segments of the right lobe is shown.
With continued traction of the lateral segment of the left lobe anteriorly and to the right, the posterior incision of the eventual specimen is developed through the liver capsule. If a decision has been made to preserve the first left lobar branches and the left part of the caudate lobe, the parenchyma of the liver is entered along the natural line of the obliterated ductus venosus (Fig. 4). Alternatively, the caudate remnant can be removed (Fig. 3, inset) with consequent visualization of the anterior and left lateral surface of the retrohepatic vena cava. The caudate extension was removed in two of the four patients in our study.
The elected posterior line of transection is continued superiorly (Fig. 4) until the left hepatic vein can be encircled, transected and either ligated or closed with a vascular suture. If it is readily accessible, the middle hepatic vein can be similarly treated at the same time. Earlier attempts to encircle the left hepatic vein may be dangerous because of posteriorly located tributaries which can be injured by premature efforts at encirclement of the left hepatic vein.
Until now, the steps of the technique have been those followed for a standard left hepatic lobectomy. A left lobectomy would be completed by entering the lobar plane defined by the color demarcation (Fig. 4) and by splitting the liver from the hilus to the diaphragm. The additional requirement of a left hepatic trisegmentectomy is to scalp off the anterior segment of the right lobe of the liver. The main difficulty is to identify correctly the plane between the anterior and posterior right lobar segments.
In most instances, the search for this plane is best begun superiorly. With blunt dissectiom, the superior end of the previously defined line of posterior incision is deepened near the diaphragm, using the point of transection of the left hepatic vein as the starting point. The dissecting finger is first brought forward, then turned at right angles so that it can be swept transversely in the coronal plane (Fig. 5). The finger tip can be felt emerging just anterior to the right hepatic vein (Fig. 5, inset). The middle hepatic vein will be encountered and must be transected and ligated or sutured, if this has not already been accomplished (Fig. 5, inset). Intraparenchymal tributaries to the right hepatic vein are ligated as encountered.
Fig. 5.
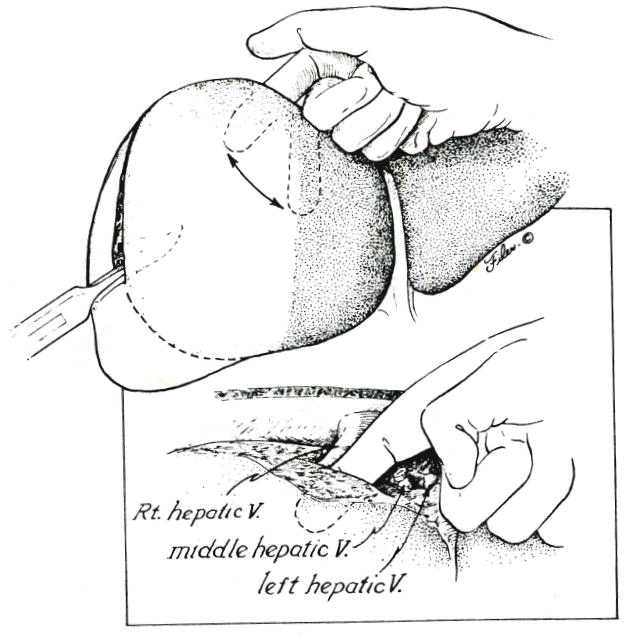
Superior to inferior scalping of the anterior segment of the right lobe. Inset, Note that the dissecting finger is kept anterior to the right hepatic vein, the left and middle hepatic vein having been ligated or sutured.
The superior to inferior scalping maneuver is continued (Fig. 6), now aided by downward traction of the specimen. A resistance-free plane is sought, and all strands encountered are clamped or ligated. Inferiorly, the dissecting finger should emerge near the base of, and at right angles to, the gallbladder bed (Fig. 6). The scalping must be done expeditiously, since the anterior segment retains its portal venous and hepatic arterial inflow almost until the specimen is out. If the blood loss becomes excessive during the deepening of the intersegmental plane, the portal triad can be temporarily cross clamped, the Pringle maneuver. When the anterior segmental blood supply is finally interrupted, the previously obvious cyanosis of the true left lobe extends to include the anterior segment, as illustrated in Figure 6.
Fig. 6.
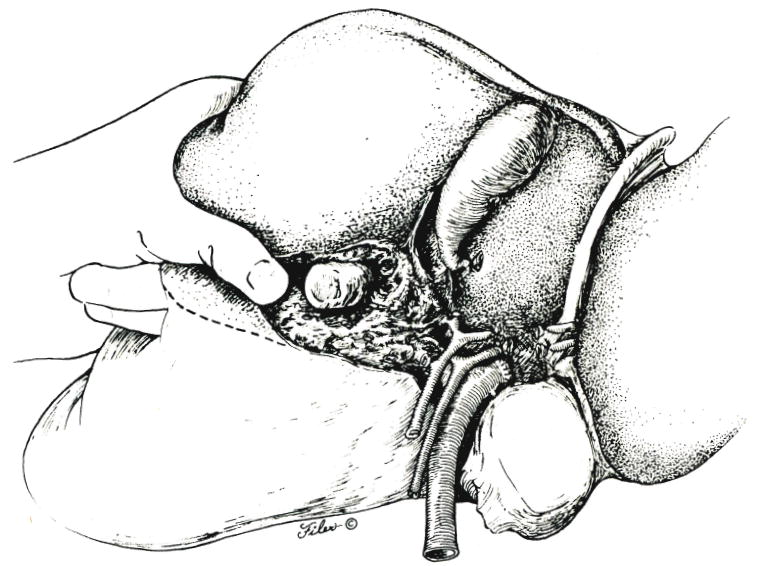
Further development of plane between the anterior and posterior segments of the right lobe of the liver.
Some livers have a natural transverse groove near the base of the gallbladder which delineates the plane between the anterior and posterior segments at the hilus. With the advantage of this anatomic landmark, it was possible in one of the patients in our study to start the scalping process at the hilus (Fig. 7), ligating the ductal, arterial and portal venous strands that ran anteriorly, while protecting the vital residual posterior structures from injury.
Fig. 7.
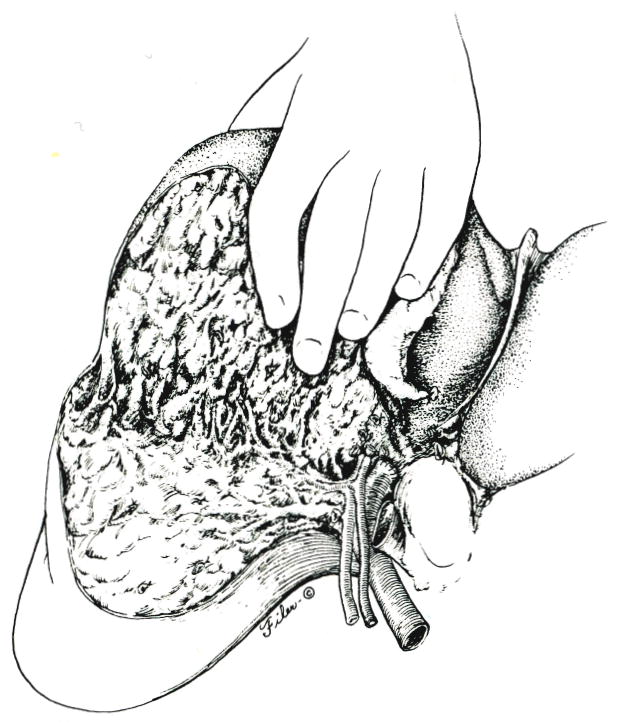
Alternative approach, with which the anterior segment is scalped from the hilus of the liver to the diaphragm.
The orientation of the frontally presenting raw surface of the posterior segment after removal of the specimen presents an unfamiliar sight (Fig. 8) but one which permits precise visualization of residual bleeding points. The extent to which the remaining ducts are exposed is so much greater than with other kinds of anatomic resections that we have considered the duct arborization to be the determining factor for the inferior part of the intersegmental plane. Meticulous attention is paid to closure with fine sutures of minute bile leaks from the exposed ducts. If the residual posterior duct is distorted or kinked, it is stented with a straight tube which is brought to the outside through the cystic or common duct, transhepatically, or through both routes as a U. After obtaining perfect hemostasis, the wound is drained, either by leaving open the upper midline component of the incision or with multiple closed drainage catheters.
Fig. 8.
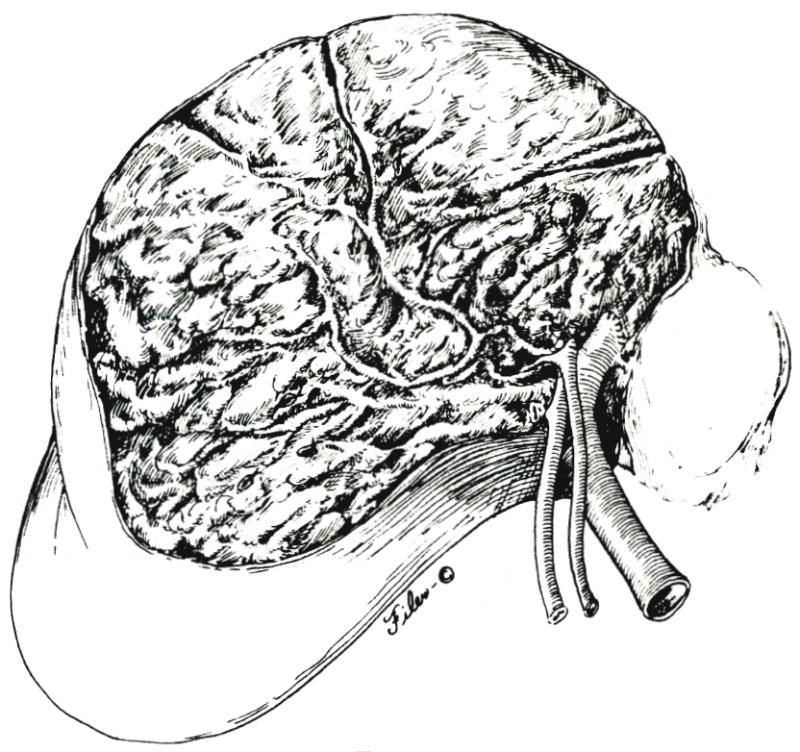
Operative field after removal of specimen.
Postoperative management
Ventilatory support is continued overnight or longer, if necessary. Antibiotics are continued for several days. Exceptional steps to treat hepatic insufficiency, coagulation defects or hematologic alterations are not necessary. If there is concern about kinking or injury of the duct, drainage of the remaining posterior segment, cholangiographic and other studies and roentgenographically directed instrumentation of the ducts are considered or begun as soon as the patient has recovered from the acute effects of the operation.
RESULTS
Mortality
All of the patients survived the operation, and three are well after a period of nine to 20 months (Table I). The fourth patient died of disseminated squamous cell carcinoma eight months after resection. The patients with hepatocellular carcinoma and localized metastases from carcinoma of the colon and rectum have no evidence of a recurrence of carcinoma.
Complications
The procedures were all technically difficult, requiring six to ten hours. One of the four patients had only partial removal of the anterior segment. In two patients, 2 and 3, blood loss during the scalping of the anterior segment of the right lobe was so severe that a Pringle maneuver was necessary for approximately 30 minutes. Total blood loss ranged from 2.7 to 20.0 liters (Table I).
The most sever hemorrhage was in the patient who had a hemangioma with associated arteriovenous malformation. This patient had an intraoperative cardiac arrest for two minutes. It was never possible to obtain complete hemostasis of the raw surface, and consequently, the wound was tightly packed at operation with a gauze roll that was placed through the open midline component of the incision and which was removed on the ward 48 hours later. The frontal position of the raw surface made the packing unusually effective. The residual ducts of this patient were left crammed with old blood which was irrigated later under roentgenographic control, using the entry catheter that had been left in the common duct at operation. By using the same access catheter, a delayed stricture of the right hepatic duct was later dilated under roentgenographic control.
Patient 2 also required dilation of the hepatic duct postoperatively. At operation, a portion of the residual right hepatic duct was involved with tumor and was excised. Anastomosis of the upper common duct to the posterior segmental duct was performed with four 6-0 catgut sutures. A small feeding tube was passed through the anastomosis. One end of this stent was brought externally through the liver and, the other end, through the cystic duct and to the outside. Postoperatively, the anastomosis first leaked, and later a stricture developed (Fig. 9) which was effectively treated by dilation under roentgenographic control.
Fig. 9.
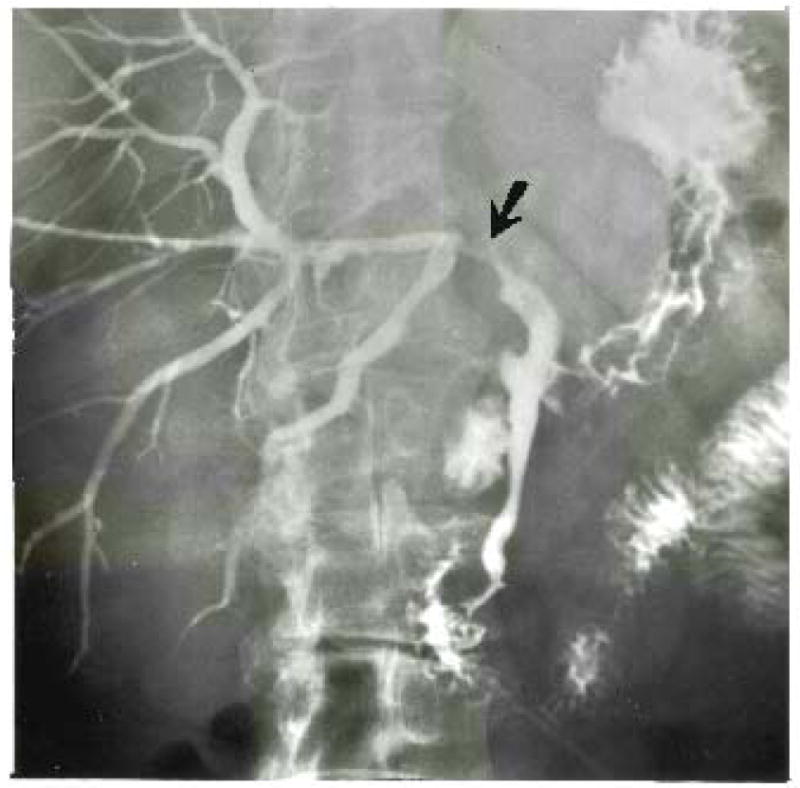
Patient 2. Stricture of right hepatic duct at its termination in the posterior segmental duct. The exposed and denuded duct is susceptible to injury at this location.
Patient 4 had a bile lake develop postoperatively. Closed drainage was converted to open drainage.
Three of the four patients had transient jaundice postoperatively, but in two of these patients, there had been a duct complication. There was no evidence of hepatic insufficiency. The length of hospitalization was for six, 17, 28 and 49 days.
Discussion
The surgical-anatomic units that could theoretically be removed from the liver were slowly appreciated over a 25 year period, largely because of the anatomic descriptions of McIndoe and Counseller (3), Hjortsjö (4), Healey (5), Healey and Schroy (6), Couinaud (7) and Goldsmith and Woodburne (8). In his classical treatise on the liver, Couinaud (7) speculated about and described the anatomic basis for a variant of left trisegmentectomy in which the left part of the caudate lobe is retained. The operation which he proposed was almost identical to the limited procedure which we eventually performed upon Patient 4.
In spite of the predictions of Couinaud (7), the procedure has never previously been performed to our knowledge. Balasegarum and Joishy (9) listed eight examples of extended left hemihepatectomies among 288 liver resections. However, they stated that removal of the anterior segment of the right lobe was impractical. The limited scope of their extensions beyond a left lobectomy was evident in the technical descriptions and accompanying illustrations published by Joishy and Balasegarum (10). The line of hepatic transection was just to the right of the interlobar plane and often did not necessitate removal of the gallbladder.
The frequency with which left hepatic trisegmentectomy will be used is not yet defined. The four examples described herein were from a series of 147 liver resections. However, in the past, we have declared lesions of the left lobe inoperable, because of involvement of the anterior segment of the right lobe. Some of these masses could have been removed with a left trisegmentectomy.
The difficulty of performing a left hepatic trisegmentectomy could be a limiting factor in its general acceptance. Blood loss was always severe, leading to an intraoperative cardiac arrest in one instance. The technical problems were due, in part, to our unfamiliarity with that part of the procedure in which the plane was developed between the anterior and posterior segments of the right lobe in the presence of an intact blood supply to both segments. The Pringle maneuver of cross clamping the portal triad, which was eventually required in two patients, could be applied from the outset of this phase, if deep hilar dissection and initial control of the anterior segmental blood supply are not possible. Huguet and co-workers (11) reported that normothermic ischemia of the human liver usually can be tolerated for upward of an hour, far longer than the previously accepted 15 or 20 minute time limit.
Next to hemorrhage, the greatest risk was damage to the residual structures passing to the posterior segment. The right hepatic duct was unusually exposed far into the plane of intersegmental transection, and its terminal segmental branch was turned posterior, at what seemed a dangerous angle without support of the adjacent parenchyma. Later, dilation under roentgenographic control was required in one patient in whom the duct had been partially removed and anastomosed because of tumor involvement and in another in whom the residual duct was left intact.
In future instances, it will be important to determine if it is worthwhile to retain the left portion of the caudate lobe, as was done in two of the four patients. Caudate removal in continuity with the specimen was not difficult, but it was not known if the hepatic function of this small fragment would be an important survival factor. It has become obvious that the posterior segment of the right lobe alone is enough to support life postoperatively. None of the four patients had serious hepatic insufficiency develop, although three of the four temporarily had jaundice.
SUMMARY
Left hepatic trisegmentectomy was successfully performed upon four patients in whom the true left lobe of the liver and all, or part, of the anterior segment of the right lobe of the liver were removed in continuity. Three of the patients had carcinomas of the liver, and the fourth patient had a hemangioma and arteriovenous malformation. This procedure, which has not been described before, should allow subtotal hepatic resection to be performed upon some patients who have lesions that have been classified as inoperable, in the past.
Acknowledgments
Supported by grants from the Veterans Administration, and Grant Nos. AM-17260 and AM-07772 from the National Institutes of Health.
References
- 1.Starzl TE, Bell RH, Beart RW, Putnam CW. Hepatic trisegmentectomy or extended right lobectomy; relation to other liver resections. Surg Gynecol Obstet. 1975;141:429–438. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Koep LJ, Weil R, III, et al. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–214. [PMC free article] [PubMed] [Google Scholar]
- 3.Mclndoe AH, Counseller VS. The bilaterality of the liver. Arch Surg. 1927;15:589–612. [Google Scholar]
- 4.Hjortsjo CH. The topography of the intrahepatic duct systems. Acta Anat. 1950;11:599–615. [PubMed] [Google Scholar]
- 5.Healey JE., Jr Clinical anatomic aspects of radical hepatic surgery. J Int Coll Surg. 1954;22:542–550. [PubMed] [Google Scholar]
- 6.Healey JE, Jr, Schroy PC. Anatomy of the biliary ducts within the human liver. Arch Surg. 1953;66:599–616. doi: 10.1001/archsurg.1953.01260030616008. [DOI] [PubMed] [Google Scholar]
- 7.Couinaud C. Etudes Anatomiques et Chirurgicales. Paris: Masson & Cie; 1957. Le Foie. Les hépatectomies élargies; pp. 400–409. [Google Scholar]
- 8.Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957;105:310–318. [PubMed] [Google Scholar]
- 9.Balasegaram M, Joishy SK. Hepatic resection; pillars of success built on a foundation of 15 years of experience. Am J Surg. 1981;141:360–365. doi: 10.1016/0002-9610(81)90197-5. [DOI] [PubMed] [Google Scholar]
- 10.Joishy SK, Balasegaram M. Hepatic resections for malignant tumors of the liver; essentials for a unified surgical approach. Am J Surg. 1980;139:360–369. doi: 10.1016/0002-9610(80)90294-9. [DOI] [PubMed] [Google Scholar]
- 11.Huguet C, Nordlinger B, Galopin JJ, et al. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689–693. [PubMed] [Google Scholar]


