A key attribute of drug delivery systems (DDSs) is their ability to regulate drug release, minimizing side effects and improving therapeutic efficacy of conventional pharmaceuticals.1 Two approaches can be used to regulate the release of the therapeutic payload from the carrier: endogenous and exogenous activation. Endogenous activation strategies2 exploit specific physiochemical characteristics of the pathological microenvironment, providing biologically-controlled release. Exogenous activation3 provides a complementary approach, employing orthogonal external stimuli to effect drug release.
Light provides a highly orthogonal external stimulus, allowing spatiotemporal control of payload release. In a recent applications of this strategy, drug encapsulated carriers of 100-500nm size (i.e. mesoporous silica, self-assembled molecular aggregates) containing a photo switch for cargo release has been developed.4 In an alternative approach, caged drugs have been developed where the activity of the drug is suppressed by attaching it to a blocking element through a photoremovable protecting group.5
Monolayer protected gold nanoparticls (AuNPs) provide an appealing synthetic scaffold for the creation of DDSs due to their functional versatility, better biocompatibility, and low toxicity.6 Moreover, through appropriate choice of particle size (2-10 nm), enhanced biodistribution (i.e. passive targeting) can be obtained through the enhanced permeation and retention (EPR) effect.7 The EPR effect arises from the increased permeability of the tumor tissue vasculature, which allows nanocarriers to extravasate into the interstitial space,1, 7 resulting in an enrichment of the carriers within the tumor tissue. We describe here the use of AuNPs for photocontrolled release of a caged anticancer drug (5-fluorouracil, 5-FU) by conjugating the drug to the particle surface through a photoresponsive o-nitrobenzyl (ONB) linkage. In this approach, the particle serves both to cage and transport the therapeutic.
The fluorouracil conjugated gold nanoparticles (Au_PCFU) synthesized for this study possess a gold core diameter of ~2-nm and feature a surface functionality comprising of a mixed self-assembled monolayer of photocleavable and zwitterionic thiol ligands. The two ligands feature a common basic structure, where an alkyl segment is used to confer stability on the particle, while the tetra(ethylene glycol) component provides water solubility and superior biocompatibility.8, 6d The zwitterionic ligand serves to enhance solubility and prevent cellular uptake,9 while the photocleavable ligand integrates fluorouracil (5-FU) moieties to the nanoparticle surface through a terminally anchored orthonitrobenzyl (ONB) group. The ONB group has long term stability under ambient light in biological environments. However, it undergoes photolytic cleavage at 365 nm when exposed to UV radiation, thus allowing controlled uncaging of the covalently attached 5-FU moieties.5b,10
The time course of the photolytic release was first monitored by means of UV-Vis spectroscopy. We characterized the uncaging behavior of free photocleavable thiol ligand and Au_PCFU by irradiating solutions using 365 nm UV-A radiation. As the photolytic reaction proceeds, a decrease of absorbance was observed at 200, 230, 280 nm, along with a noticeable increase at 215, 250 and 375 nm (see Supporting Information Figure S8). These results confirm that the photolytic reactions of Au_PCFU proceeds in an analogous fashion as that of free photocleavable thiol ligand.
The identity and amount of photo released product (5-FU) of Au_PCFU were verified by irradiation followed by spin filteration through a 50,000 MW cutoff filter to remove the nanoparticle from solution. As shown in Figure 1b the absorption spectra of solution clearly indicate the presence of free 5-FU. This release is dependent on irradiation time, with maximum release observed at ~10 min (inset, Figure 1b). Based on the absorption value at 265 nm, the maximum number of 5-FU molecule released from a single nanoparticle surface was estimated to be 14, 82% of the 17 photocleavable ligands on Au_PCFU as determined by UV-Vis spectroscopy.11 The release of 5-FU was also monitored after exposing the nanoparticle solution to alternating periods of light and dark (see Supporting Information Figure S9). The step profile of the product formation reveals no release is observed in the dark, and that that uncaging is spatio-temporally restricted to the illuminated region.
Figure 1.
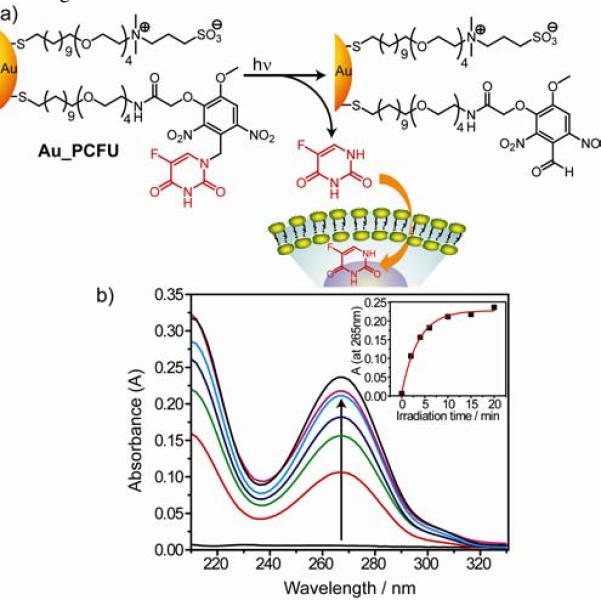
a) Photochemical reaction of Au_PCFU and delivery of payload to cell. b) Overlaid UV-Vis spectral changes showing light dose dependent increase of 5-FU concentration. Inset: The plot of absorbance at 265 nm against irradiation time.
We next evaluated the use of Au_PCFU as a photocontrolled DDS through MCF-7 cell culture studies. Au_PCFU dispersed in cell culture media was added to the cells, which were then irradiated at 365 nm for 20 min. After 96 h further incubation, optical micrographs were taken to visualize the change in cell morphology, and the live cells were stained with calcien AM (Figure 2a-d). Cell viability was quantified by Alamar blue assay. An IC50 value of 0.7 μM was observed upon irradiation for Au_PCFU on a per particle basis, corresponding to 11.9 μM on a per drug basis, In contrast, when the cells were first exposed to light and afterward incubated with the particle there were no signs of toxicity (Figure 3a). Likewise, no significant cell death was observed in cells treated with only light or only Au_PCFU (Figure 3b), demonstrating lack of toxicity from either light or particle. Taken together, these observations demonstrate that Au_PCFU serves as a drug carrier as well as a caging group for 5-FU function.12
Figure 2.
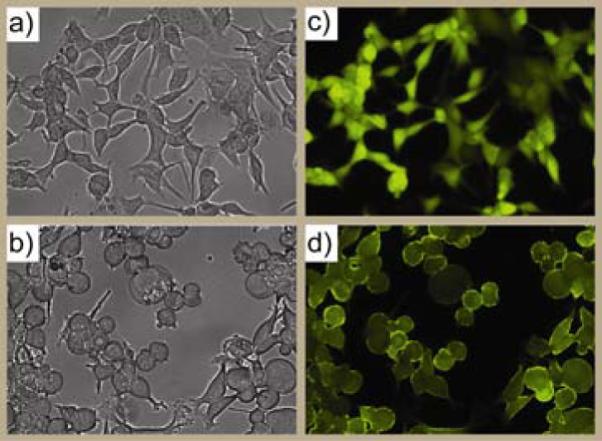
The bright-field and fluorescence-microscopy images of the cells irradiating before treated with Au_PCFU (a and c) and irradiating after treated with Au_PCFU (b and d) with light.
Figure 3.
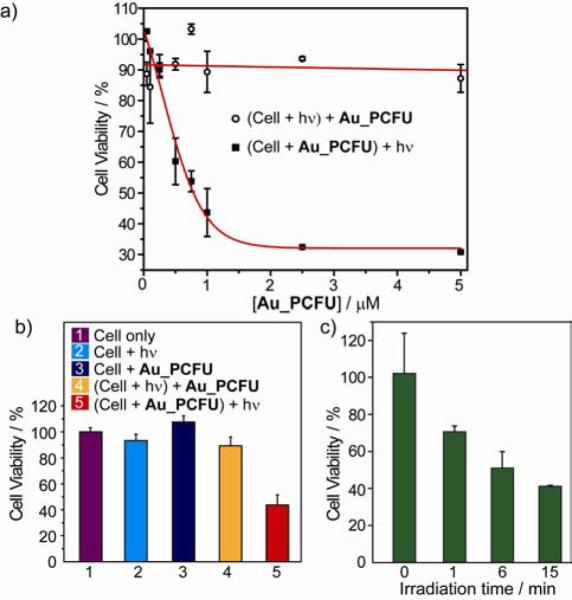
a) The cytotoxicity of different concentration of Au_PCFU under caging and control condition. The IC50 value was 0.7 μM per particle, 11.9 μM per drug. b) The effect of different condition on the cell viability of MCF-7 cell line. The concentration of Au_PCFU used is 1 μM and the light exposure time is 20 min. c) Cell viability with different durations of light exposure.
An important benefit of exogenous control is the ability to externally regulate drug dosing. To demonstrate this capability MCF-7 cells were first treated with 1 μM solution of Au_PCFU and then exposed to the 365 nm UV light for 0, 1, 6 and 15 min. The cytotoxicity studies after 96 h of incubation show that the cell viability decreases with increasing duration of the applied light (Figure 3c). The light dependent change in cell viability thus effectively correlates the dose of the liberated drug with duration of the exposure to light.
In summary, we have demonstrated the light-controlled release of a therapeutic from a nanoscale gold nanoparticle carrier. In this system, the particle served as both cage and carrier for the drug, providing a non-toxic conjugate that effectively released payload upon long wavelength UV irradiation. The small size (hydrodynamic diameter ~10 nm, as measured by DLS) of the carrier coupled with the engineered monolayer of this system should provide long circulation time and preferential accumulation into the tumor tissues via EPR effect.7 We envision that using photolabile linkers responsive to two photon excitation will enhance tissue penetration and reduce phototoxicity for in vivo application, which is an area under investigation.
Supplementary Material
Acknowledgments
This research is supported by the NIH (GM077173).
Footnotes
Supporting Information Available: Synthesis of ligands, preparation of the nanoparticle, protocols for in vitro and cellular studies, and other experimental procedures. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- (2).(a) Rooseboom M, Commandeur JNM, Vermeulen NPE. Pharmacol. Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]; (b) Ulbrich K, Subr V. Adv. Drug Delivery Rev. 2004;56:1023–1050. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]; (c) Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Angew. Chem., Int. Ed. 2005;44:5038–5044. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]; (d) Kam NWS, Liu Z, Dai H. J. Am. Chem. Soc. 2005;127:12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- (3).(a) Kim HJ, Matsuda H, Zhou HS, Honma I. Adv. Mater. 2006;18:3083–3088. [Google Scholar]; (b) Kwon IC, Bae YH, Kim SW. Nature. 1991;354:291–293. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]; (c) Babincova M, Sourivong P, Chorvat D, Babinec P. J. Magn. Magn. Mater. 1999;194:163–6. [Google Scholar]; (d) Wu CL, Chen C, Lai JP, Chen JB, Mu X, Zheng JS, Zhao YB. Chem. Commun. 2008:2662–2664. doi: 10.1039/b804886j. [DOI] [PubMed] [Google Scholar]; (e) Park H, Yang J, Seo S, Kim K, Suh J, Kim D, Haam S, Yoo KH. Small. 2008;4:192–196. doi: 10.1002/smll.200700807. [DOI] [PubMed] [Google Scholar]; (f) Skirtach AG, Javier AM, Kreft O, Kohler K, Alberola AP, Mohwald H, Parak WJ, Sukhorukov GB. Angew. Chem., Int. Ed. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]; (g) Park C, Lee K, Kim C. Angew. Chem., Int. Ed. 2009;48:1275–1278. doi: 10.1002/anie.200803880. [DOI] [PubMed] [Google Scholar]
- (4).(a) Mal NK, Fujiwara M, Tanaka Y. Nature. 2003;421:350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]; (b) Zhu YC, Fujiwara M. Angew. Chem., Int. Ed. 2007;46:2241–2244. doi: 10.1002/anie.200604850. [DOI] [PubMed] [Google Scholar]; (c) Liu NG, Dunphy DR, Atanassov P, Bunge SD, Chen Z, Lopez GP, Boyle TJ, Brinker CJ. Nano Lett. 2004;4:551–554. [Google Scholar]; (d) Park C, Lim J, Yun M, Kim C. Angew. Chem., Int. Ed. 2008;47:2959–2963. doi: 10.1002/anie.200705271. [DOI] [PubMed] [Google Scholar]
- (5).(a) McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. J. Am. Chem. Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]; (b) Zhang Z, Hatta H, Ito T, Nishimoto S. Org. Biomol. Chem. 2005;3:592–596. doi: 10.1039/b417734g. [DOI] [PubMed] [Google Scholar]; (c) Mayer G, Heckel A. Angew. Chem., Int. Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- (6).(a) Gibson JD, Khanal BP, Zubarev ER. J. Am. Chem. Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]; (b) Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. J. Am. Chem. Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]; (c) Han G, You CC, Kim BJ, Turingan RS, Forbes NS, Martin CT, Rotello VM. Angew. Chem., Int. Ed. 2006;45:3165–3169. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]; (d) Tshikhudo TR, Wang Z, Brust M. Mater. Sci. Technol. 2004;20:980–984. [Google Scholar]; (e) Bhattacharya R, Mukherjee P. Adv. Drug Deliver. Rev. 2008;60:1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- (7).(a) Brigger I, Dubernet C, Couvreur P. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]; (b) Baban DF, Seymour LW. Adv. Drug Deliv. Rev. 1998;34:109–119. doi: 10.1016/s0169-409x(98)00003-9. [DOI] [PubMed] [Google Scholar]; (c) D'Emanuele A, Attwood D. Adv. Drug Deliv. Rev. 2005;57:2147–2162. doi: 10.1016/j.addr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- (8).Hong R, Fischer NO, Verma A, Goodman C, Emrick T, Rotello VM. J. Am. Chem. Soc. 2004;126:739–743. doi: 10.1021/ja037470o. [DOI] [PubMed] [Google Scholar]
- (9).(a) Kim CK, Ghosh P, Pagliuca C, Zhu ZJ, Menichetti S, Rotello VM. J. Am. Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rouhana LL, Jaber JA, Schlenoff JB. Langmuir. 2007;23:12799–12801. doi: 10.1021/la702151q. [DOI] [PubMed] [Google Scholar]; (c) Jin Q, Xu JP, Ji J, Shen JC. Chem. Commun. 2008:3058–3060. doi: 10.1039/b801959b. [DOI] [PubMed] [Google Scholar]
- (10).(a) Kostiainen MA, Smith DK, Ikkala O. Angew. Chem., Int. Ed. 2007;46:7600–7604. doi: 10.1002/anie.200701200. [DOI] [PubMed] [Google Scholar]; (b) Ohmuro-Matsuyama Y, Tatsu Y. Angew. Chem., Int. Edit. 2008;47:7527–7529. doi: 10.1002/anie.200802731. [DOI] [PubMed] [Google Scholar]
- (11).See Supporting Information for detail analysis and characterization.
- (12).Cytotoxicity experiment with free 5-FU under similar condition showed an IC50 value of 4.5 μM (see Supporting Information Figure S10), roughly 50% of the per drug IC50 of Au_PCFU (9.7 μM) after factoring in loading and release efficiency.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


