Abstract
Expression of oncogenes, such as the human papillomavirus type 16 (HPV16) E7 oncoprotein, promotes aberrant cell proliferation. In the absence of concurrent mitogenic stimuli, this triggers a cell-intrinsic defense mechanism, the “trophic sentinel response”, which eliminates such aberrant cells. The molecular pathways that elicit this response, however, remain obscure. We set up an experimental system to investigate the trophic sentinel pathway triggered by HPV16 E7 expression in normal human keratinocytes, the natural host cells of HPVs. Keratinocytes expressing HPV16 E7 cultured in E-medium undergo cell death and show increased sub-G1 DNA content when grown to confluence or under conditions of serum deprivation. Moreover, HPV16 E7 expressing human keratinocytes express higher levels of the autophagy marker, LC3-II, which can be abrogated by 3-methylaldenine, an autophagy inhibitor. These findings indicate that even under normal culture conditions, HPV16 E7 expression triggers metabolic stress that may result in autophagy, a pathway implicated in carcinogenesis.
Keywords: human papillomavirus, keratinocytes, autophagy, cell death, E7 oncoprotein, trophic sentinel signaling, metabolic stress
INTRODUCTION
Normal cells undergo growth arrest when they reach confluence and/or are deprived of growth factors. Cells that have suffered a single oncogenic hit, however, can encounter a situation of conflicting growth signals when the oncogenic proliferative signal is inconsistent with environmental antiproliferative cues. As a consequence, such cells undergo an abortive process, the “trophic sentinel response”, which can result in cell death, differentiation or senescence (reviewed in Evan et al., 2001). This phenomenon was initially discovered in cells expressing the adenovirus (Ad) E1A oncogene (Debbas et al., 1993; Putzer et al., 2000; Rao et al., 1992; Teodoro et al., 1995; White et al., 1991), or c-myc (Evan et al., 1992). Co-expression of Ad E1B or Bcl-2 in Ad E1A or c-myc expressing cells, respectively, mutes this response and results in cellular transformation (Debbas et al., 1993; Pelengaris et al., 2002; White et al., 1991). Hence, the trophic sentinel response is thought to represent a cellular tumor suppressive process that eliminates aberrantly proliferating cells from an organism (reviewed in Evan et al., 2001).
High-risk human papillomaviruses (HPVs) are etiological agents of cervical carcinoma and are also associated with other anogenital tract malignancies as well as head and neck cancers (reviewed in Schiffman et al., 2007). Due to frequent viral genome integration during malignant progression, high-risk HPV associated cancers consistently express the two viral oncoproteins, E6 and E7. The best known cellular targets of high-risk HPV E6 and E7 oncoproteins are the p53 and retinoblastoma (pRB) tumor suppressor proteins, respectively (reviewed in Munger et al., 2001).
Our group showed that IMR90 normal human diploid fibroblasts that express the high-risk HPV type 16 (HPV16) E7 oncoprotein are predisposed to undergo cell death once the cells become confluent and/or are serum starved (Jones et al., 1997b). Induction of the trophic sentinel response by HPV16 E7 correlated with its ability to destabilize the retinoblastoma tumor suppressor pRB and to stabilize the p53 tumor suppressor. This effect was abrogated when the HPV16 E6 oncoprotein, which targets p53 for degradation (Scheffner et al., 1993; Scheffner et al., 1990), or a dominant negative p53 mutant was co-expressed (Eichten et al., 2004). Although caspase 3 is activated and DNA fragmentation occurs in serum starved HPV16 E7 expressing IMR90 cells, treatment with a pan-caspase inhibitor did not block cell death in HPV16 E7 expressing IMR-90 cells. Hence, the trophic sentinel response in HPV16 E7 expressing normal human fibroblasts depends on p53, but does not involve prototypical apoptosis (Eichten et al., 2004) and requires additional mechanistic investigation.
To analyze the trophic sentinel response in a biologically relevant cell type, normal human keratinocytes, we developed an assay system where normal human keratinocytes are grown in E medium supplied with 5% fetal bovine serum (FBS). We show that under these conditions, normal human epithelial cells undergo growth arrest upon serum deprivation or when the cells reach confluence. In contrast, HPV16 E7 expressing keratinocytes undergo cell death as evidenced by an increase in the sub-G1 population. Furthermore, we detected evidence of autophagy in HPV16 E7 expressing keratinocytes, even when the cells were grown under normal tissue culture conditions. These findings will allow a detailed mechanistic investigation of the trophic sentinel response triggered by HPV16 E7 expression in keratinocytes and suggest that E7 expression results in metabolic stress even under normal tissue culture conditions.
RESULTS
HPV16 E7 expressing primary human foreskin keratinocytes are prone to cell death upon serum deprivation in E medium
Our previously published experiments on the HPV16 E7 induced trophic sentinel pathway were performed with normal human fibroblasts rather than epithelial cells, the normal host cell type for HPVs. In order to investigate the mechanistic details of the trophic sentinel pathway in normal human keratinocytes, we first needed to develop a workable experimental system. Normal human keratinocytes are most conveniently cultured in serum free growth media such as Keratinocyte Serum Free Medium (K-SFM) (Pirisi et al., 1987), which is supplemented with pituitary extract and recombinant EGF. Upon depletion of the pituitary extract from K-SFM, normal human keratinocytes failed to undergo a cell cycle phase specific growth arrest (J. Hasskarl and K. Münger, unpublished) whereas EGF depletion inhibited cell migration but increased their proliferative capacity (Hasskarl et al., 2006). Based on these results, we explored alternative growth conditions for human keratinocytes. E medium, based on Dulbecco’s modified Eagle medium (DMEM) supplemented with F-12 nutrient mixture and a variety of other components such as insulin, transferrin, adenine, triiodothyronine and hydrocortisone, as well as fetal bovine serum (FBS) supports the growth of normal human keratinocytes (Rheinwald, 1980; Rheinwald et al., 1980). While the original formulation also requires the presence of mitomycin C treated Swiss 3T3 J2 murine fibroblasts or their conditioned medium, we did not include these components for our studies.
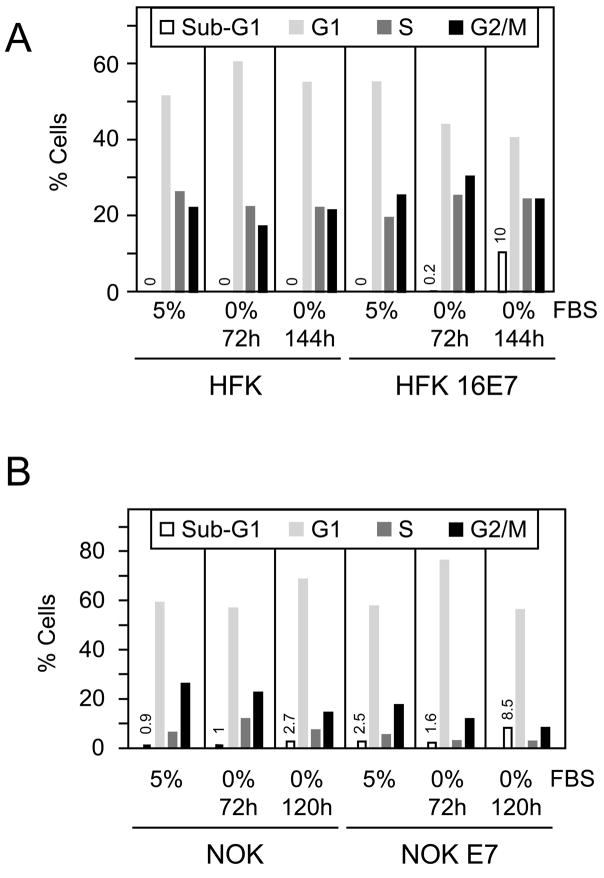
Primary human foreskin keratinocytes (HFKs) cultured in E medium showed decreased cell growth in response to serum deprivation as evidenced by lower cell density after 48 hours of treatment (data not shown). Cell cycle analysis by FACS revealed a modest increase in the G1/S ratio, from 1.9 to 2.7, representing cell growth arrest, but no apoptotic cells with sub-G1 DNA content were detected at 72 or 144 hours after serum withdrawal (Figure 1A, left panels). In order to determine whether these culture conditions may be used to analyze trophic sentinel signaling, we subjected a passage and donor matched population of HPV16 E7 expressing HFKs to the same treatment. There was no marked decrease in cell density after 48 hours of serum deprivation in E-medium as compared to cells grown in E-medium supplemented with 5% fetal bovine serum (FBS) (data not shown). FACS analysis revealed an increase of cells in S and G2/M phases with a concomitant decrease in G1 phase cells. Most importantly, there was a dramatic increase of cells with sub-G1 DNA content from 0.2% at 72 hours to 10% at 144 hours after serum deprivation (Figure 1A, right panels). Similar results were obtained in three independent experiments. Hence, HPV16 E7 predisposes human foreskin keratinocytes to cell death in response to growth factor withdrawal, similar to what we previously observed with normal human diploid fibroblasts (Jones et al., 1997b).
Figure 1.
HPV16 E7 expressing normal human keratinocytes cultured in E medium are prone to cell death upon serum deprivation. (A) Representative flow cytometric cell cycle analysis of control human foreskin keratinocytes (HFK) and HPV16 E7 expressing populations (HFK 16E7) grown in E-medium with 5% FBS or in serum free E medium for 72 and 144 hours. Similar results were obtained in three independent experiments. (B). Representative flow cytometric cell cycle analysis of telomerase immortalized normal human oral keratinocytes (NOK) and a donor matched HPV16 E7 expressing line (NOK E7) (Piboonniyom et al., 2003) grown in complete E-medium or in serum free medium E medium for 72 and 120 hours. Similar results were obtained in three independent experiments.
HPV16 E7 expressing hTERT immortalized human oral keratinocytes are predisposed to cell death upon serum deprivation
Given that HFKs have a limited lifespan and their growth characteristics change upon repeated passaging, they need to be re-derived regularly. Due to genetic differences between individual donors, there can be significant differences between cell populations. To minimize these effects and to further confirm that the trophic sentinel pathway is functional in normal human keratinocytes, we investigated a set of matched hTERT immortalized normal oral human keratinocyte lines (NOK) with and without HPV16 E7 expression (Piboonniyom et al., 2003). As with the primary HFKs, these cells were adapted to grow to E-medium containing 5% FBS. After serum withdrawal for 24 and 48 hours, we observed increased cell shrinkage and rounding in NOK E7 cells (data not shown). To quantify this phenotype, we performed FACS analysis after serum withdrawal for 72 hours and 120 hours (Figure 1B). In NOK cells we observed an ~10% increase in the G1 population, with concomitant decreases in the S and G2/M populations. There was only a minor increase in the population of cells with a sub-G1 DNA content from 0.9% to 2.7%. In NOK E7 cells, however, we observed an increase in the sub-G1 population from 1.6% to 8.5% at 120 hours after serum deprivation (Figure 1B). Similar results were obtained in three independent experiments.
Increased incidence of cell death in HPV16 E7 expressing human keratinocytes upon cell-cell contact
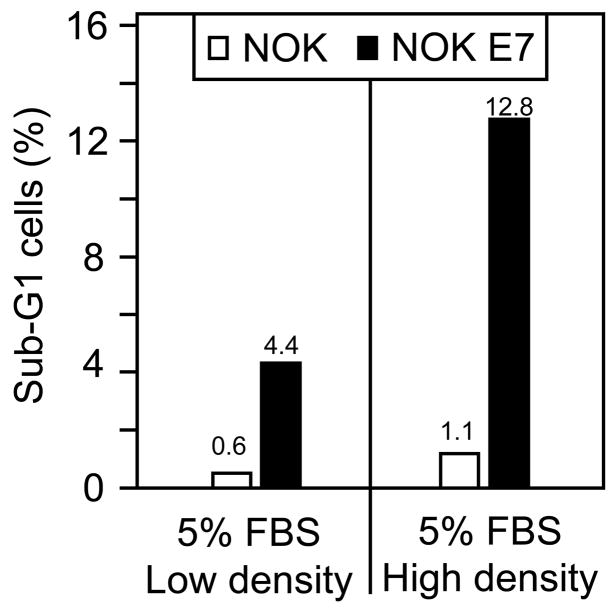
In the course of these experiments, we observed that the overall incidence of cell death upon serum deprivation of NOK E7 cells was dependent on the saturation density that the cells reached. We consistently observed a higher number of floating cells in dense cultures of NOK E7 cells than in NOK control cells, even when the cells were maintained in E medium supplied with 5% FBS. Hence, we investigated whether E7 expression can trigger trophic sentinel signaling in confluent cells. To address this question, we seeded NOK and NOK E7 cells at low and high density and cultured them in E medium with 5% FBS. Under high-density conditions, the cells immediately reached confluence and were maintained at confluence for three days before being harvested for FACS analysis (Figure 2). NOK E7 cells exhibited a 2.9 fold increase of cells with a sub-G1 DNA content after they reached confluence (12.8% versus 4.4%), whereas the NOK cells showed a 1.8 fold increase (1.1% versus 0.6%). The fact that NOK E7 cells show a higher incidence of sub-G1 cells (4.4%) than NOK cells (0.6%), even when seeded at low density, may be related to the fact that NOK cells grow in dense clusters even in a sparsely seeded plate, which appears sufficient to cause cell death with NOK E7 cells. Similar results were obtained in two independent experiments.
Figure 2.
NOK E7 cells are prone to cell death upon cell-to-cell contact. The percentage of NOK and NOK E7 cells with sub-G1 DNA content as determined by flow cytometry is graphed for subconfluent and confluent NOK and NOK E7 cells.
Hence, consistent with our earlier studies in human diploid fibroblasts, the trophic sentinel response in E7 expressing keratinocytes is also triggered by a growth inhibitory signal in response to cell-to-cell contact.
HPV16 E7 expression in human keratinocytes induces an autophagy-like process
Autophagy is a highly conserved cellular pathway that serves to recycle damaged cellular structures (Mizushima et al., 2008). Under conditions of nutrient limitation, this pathway is triggered as a survival mechanism to mobilize necessary nutrients. During this process, autophagosomes are formed which subsequently fuse to lysosomes to form autolysosomes where their contents are digested and recycled. When autophagy is triggered over extended periods of time in the absence of sufficient exogenous energy sources, cells eventually undergo death by self-digestion. A commonly used molecular marker to assess autophagy is microtubule-associated protein 1 light chain 3 (LC3). LC3 occurs in two forms in cells; cytoplasmic LC3 I and lipidated LC3 II, which is associated with autophagosome membranes and forms cytoplasmic speckles (“puncta”) (Mizushima, 2004).
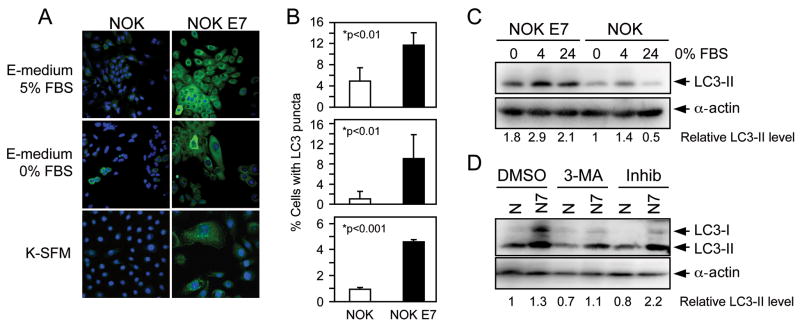
Given the enhanced sensitivity of HPV16 E7 expressing cells to growth factor withdrawal, we set out to determine whether HPV16 E7 expressing normal human keratinocytes show evidence for autophagy-related processes. NOK and NOK E7 cells grown in complete E medium or after 24 hours of serum withdrawal in E-medium were stained with LC3 antibody. We detected stronger LC3 staining in NOK E7 cells as compared to NOK control cells, both under normal tissue culture conditions and after serum withdrawal. In addition, there was increased incidence of cells with LC3 puncta in the NOK E7 cells as compared to NOK cells, indicating increased incidence of autophagosomes in NOK E7 cells (Figure 3A). To test whether HPV16 E7 expression activates an autophagy related process regardless of culture conditions, we also stained NOK and NOK E7 cells grown in K-SFM with LC3 antibody. Similar to what we observed when the cells were grown in E-medium, the NOK-E7 population showed an increased number of cells with LC3 puncta. A quantification of these results corresponding to an assessment of >600 cells in four (E medium) and three (K-SFM) repeats is shown in Figure 3B. NOK and NOK E7 cells grown in E medium showed stronger LC3 staining than cells grown in K-SFM. This may be due to one of the components of E medium, hydrocortisone, which can activate autophagy (Kalamidas, 2000). Although the percentage of cells with LC3 puncta is different under the various conditions tested, there was a consistent 2 to 5 fold increase of cells with LC3 puncta with NOK E7 cells as compared to NOK cells.
Figure 3.
Evidence for an autophagy-related process in NOK E7 cells. (A) Representative confocal immunofluorescence images of the LC3 staining patterns in NOK (left panels) and NOK E7 (right panels) cells cultured in complete E-medium (top panels), serum free E-medium for 24 hours (middle panels) or in K-SFM (bottom panels). Cell nuclei were counterstained with TO-PRO-3 (indicated by the pseudo blue coloration). (B). Quantification of cells with punctate LC3 staining (“LC3 puncta”) indicative of autophagy in NOK and NOK E7 cells under the different growth conditions. Bar graphs represent means and standard deviations of 4 (E-medium) or 3 (K-SFM) independent experimental repeats. More than 600 cells were counted in each group. p-Values as determined by unpaired t test are indicated. (C) Expression of autophagosome associated LC3 II in NOK and NOK E7 cells. NOK and NOK E7 cells were cultured in complete E medium before starvation. Serum was withdrawn for 4 hours and 24 hours. α-actin was used as loading control to quantify the relative amount of LC3-II in the different cell populations. (D) Expression level of LC3-II in NOK (N) and NOK E7 (N7) cells. Cells cultured in keratinocyte serum free medium (K-SFM) were treated with autophagy inhibitor 3-MA (5mM) or the lysosomal protease inhibitors (Inhib) E64d (10 μg/ml) and pepstatin A (10 μg/ml) for 4 hours and subjected to immunoblot analysis using LC3 antibody. α-actin was used as loading control to quantify the relative amount of LC3.
In these experiments, NOK and NOK E7 cells showed somewhat fewer LC3 puncta after 24 hours of serum deprivation. While these differences were not statistically significant, we reasoned that this might due to fusion of autophagosomes with lysosomes. In order to determine whether there was evidence of increased autophagy in response to serum withdrawal, we analyzed expression of LC3 II at 4 hours after serum deprivation by Western blotting. LC3 II levels increased in both NOK and NOK E7 cells, with NOK E7 cells exhibiting overall stronger LC3 staining than NOKs (Figure 3C). Consistent with the immunofluorescence experiments (Figure 3A, B), the levels of LC3 II decreased at 24 hours after serum withdrawal (Figure 3C).
In order to confirm that the observed increase of LC3 puncta (Figure 3A, B) and LC3 II expression (Figure 3C) in NOK E7 cells is caused by activation of autophagy, we treated NOK and NOK E7 cells with 3-methyladenine (3-MA), a compound that inhibits autophagy at an early stage (Seglen, 1982 #89). Consistent with this notion, 3-MA treatment decreased LC3 II expression in both NOK and NOK E7 cells (Figure 3D). Furthermore, when cells were treated with the lysosomal protease inhibitors pepstatin A and E-64d, which inhibit LC3 II degradation once autophagosomes have fused with lysosomes, we detected increased amounts of LC3 II in NOK E7 cells (Figure 3D). This indicates that NOK E7 cells not only have more autophagosomes, but also have an active autophagic flux (Mizushima, 2007 #90).
DISCUSSION
The productive phase of the HPV life cycle in an infected epithelium is confined to terminally differentiated layers. Squamous epithelial cells normally undergo irreversible growth arrest upon differentiation. Since HPV genome replication critically depends on the availability of host replication enzymes, these viruses encode proteins that establish and/or maintain a replication competent cellular milieu in differentiated epithelial cells. HPV16 E7 has been shown to uncouple cellular growth arrest from differentiation and induces aberrant S-phase entry/competence through a number of mechanisms including degradation of the pRB tumor suppressor and inactivation of the cyclin dependent kinase inhibitors p21CIP1 and p27KIP1 (Funk et al., 1997; Jones et al., 1997a; Zerfass-Thome et al., 1996).
Such induction of aberrant cellular and/or viral DNA synthesis in the absence of concurrent environmental mitogen stimulation, however, can give rise to a situation of conflicting growth signals. In otherwise normal cells, this triggers a cellular defense mechanism, the “trophic sentinel response” that eliminates such deviant cells from the proliferative pool through cell-type specific abortive processes such as cell death, differentiation or senescence (reviewed in Evan et al., 2001). To avoid elimination, high-risk HPVs encode a second oncoprotein, E6, which targets the p53 tumor suppressor protein for ubiquitin-mediated, proteasomal proteolysis. Indeed, the HPV16 E7-triggered trophic sentinel response in normal human diploid fibroblasts is abrogated by co-expression of HPV16 E6 or a dominant negative p53 mutant (Eichten et al., 2004).
Our previous studies with HPV16 E7 expressing normal human diploid fibroblast have yielded interesting and somewhat unexpected results. While E7 expression causes p53 stabilization similar as is observed upon p53 activation through a DNA damage signal (Jones et al., 1999), p53 remains transcriptionally inert even when the cells are subjected to serum deprivation. In contrast to serum starved control cells, we detected reduced phosphorylation of AKT in HPV-16 E7 expressing IMR90 fibroblasts, indicating a potential role of this survival kinase in HPV-16 E7 induced trophic sentinel signaling in fibroblasts. Moreover, we detected a marked induction of NF-kB activity and transcriptional upregulation of two IGF-binding proteins, IGFBP2 and IGFBP5, when serum was withdrawn and addition of IGF-I could partially rescue the serum deprivation induced cell death response (Eichten et al., 2004). Another surprising aspect of these studies was that a pan-caspase inhibitor, zVAD, did not inhibit the cell death response despite of inhibition of DNA degradation. Hence the mechanism of HPV16 E7 induced cell death in response to growth factor deprivation is distinct from standard apoptosis and its mechanism remains enigmatic.
In order to warrant further mechanistic study, we first needed to establish a workable assay system of HPV16 induced trophic sentinel signaling in the normal host cell type of these viruses, primary human epithelial cells. Here we report that HPV16 E7 expression in two human epithelial cell types, primary human foreskin keratinocytes (HFKs) and hTERT immortalized normal oral keratinocytes (NOK) reproducibly causes cell death when grown in E-medium and subjected to serum deprivation. While normal cells stopped proliferating, we only detected minimal cell death under these conditions (Figure 1). Similar to what he had previously reported for HPV16 E7 expressing normal human diploid fibroblasts, we also observed cell death in HPV16 E7 expressing keratinocytes when they were maintained at high cell density in complete, serum containing medium (Figure 2). Hence, these results suggest that similar to what we observed in the fibroblasts, HPV16 E7 expressing normal human keratinocytes undergo cell death when they experience conflicting growth signals.
While we have not yet performed detailed mechanistic studies on trophic sentinel signaling triggered by HPV16 E7 expression in normal human keratinocytes and do not know whether it is similar to what we observed in fibroblasts, we discovered evidence for activation of an autophagy-related process in E7-expressing keratinocytes. Autophagy is a type II form of cell death and represents an ancient, genetically well-defined signaling pathway that serves to maintain cell homeostasis by recycling misfolded proteins as well as defective cellular organelles. Under conditions of metabolic stress, such as growth factor deprivation, autophagy is activated to provide cells with alternative sources of energy for survival. Prolonged activation of autophagy, however, can eventually lead to cell death by self-digestion (Mizushima et al., 2008).
Given that HPV16 E7 expressing fibroblast can undergo caspase independent cell death, we decided to assess HPV16 E7 expressing cells for autophagy using LC3 staining as a marker (Klionsky et al., 2007; Tasdemir et al., 2008). In general, HPV16 E7 expressing keratinocytes contained higher levels of diffuse cytoplasmic LC3 staining. This is consistent with a recent report that some autophagy genes, including LC3, are transcriptionally regulated by E2F1 (Polager et al., 2008), which is activated through pRB degradation in HPV16 E7 expressing keratinocytes. Indeed, we detected decreased LC3 expression in NIH 3T3 cells expressing the pRb/p107/130 defective HPV16 E7 DLYC mutant as compared to wild type HPV16 E7 expressing NIH3T3 cells (data not shown). More importantly, we also observed an increased number of cells where LC3 staining was confined to discrete cytoplasmic puncta, even when the cells were grown under nutrient-rich conditions such as serum containing E-medium or standard K-SFM (Figure 3A). We also detected increased amounts of autophagosome-associated LC3, LC3-II, in NOK E7 cells by Western blot (Figure 3C, 3D). Moreover, when cells were treated with lysosomal protease inhibitors, LC3-II levels further increased in NOK E7 cells, indicating active autophagic flux in NOK E7 cells. These findings suggest that HPV16 E7 expression in keratinocytes activates an autophagy-like process. One possibility is that aberrant cell proliferation induced by E7 expression increases energy requirements. Moreover, E7 expression has been shown to cause a metabolic switch from normal aerobic respiration to anaerobic metabolism (Zwerschke et al., 1999) that generates considerably less ATP. Hence, HPV16 E7 expressing cells may be experiencing metabolic stress even under normal growth conditions, which may induce an autophagy-like process.
The ability of HPV16 E7 to induce an autophagy-related process is interesting, given that the process of autophagy has been evaluated as a target for anticancer therapy. Increased LC3 staining has been observed in many tumor types including pancreatic (Fujii et al., 2008), gastrointestinal (Yoshioka et al., 2008) and colon cancers (Sato et al., 2007) and stronger LC3 staining correlated with particularly poor prognosis in pancreatic cancer patients (Fujii et al., 2008). While inhibiting autophagy decreased tumor cell survival both in vitro and in vivo (Degenhardt et al., 2006) and increased the sensitivity to chemotherapy and radiotherapy in multiple carcinoma cell lines (Apel et al., 2008), cells with defects in the autophagy pathway also showed increased chromosome instability, signs of persistent DNA damage and aneuploidy, which may promote carcinogenesis (Mathew et al., 2007). Conversely, upregulation of autophagy has also been shown to induce growth inhibition in glioma (Aoki et al., 2007) and cholangiocellular carcinoma cells (Enomoto et al., 2007), and enhanced response to radiotherapy of lung cancer in a mouse model (Kim et al., 2008). Therefore, one might envision strategies that inhibit or enhance autophagy to be tested for clinical efficacy (Carew et al., 2007; Karantza-Wadsworth et al., 2007). It will be important to determine whether there is increased autophagy in cervical cancers and how these tumors respond to inhibition or activation of autophagy.
In summary, we have established a model system to investigate the mechanistic details of the trophic sentinel response triggered by HPV16 E7 expression in normal human keratinocytes. Moreover, we provide evidence that HPV16 E7 expression in keratinocytes activates an autophagy-related process, presumably as a result of metabolic stress due to sustained proliferative activity and/or reprogramming of the cellular metabolism to a less efficient anaerobic mechanism (Zwerschke et al., 1999). It will be important to determine whether and how trophic sentinel signaling is connected to the induction of this autophagy-related process.
MATERIALS AND METHODS
Cells and culture conditions
Primary human foreskin keratinocytes (HFKs) derived from neonatal foreskins were isolated as described previously (Jones et al., 1997a). HFK populations with stable expression of HPV16 E7 were prepared by transfecting primary HFKs with p1435, a human β-actin HPV16 E7 expression plasmid, or p1318, the parental vector (Munger et al., 1989) as a control, using the Amaxa Human Keratinocyte Nucleofector Kit (Amaxa Biosystems) according to the manufacturer’s instructions. Transfections included pcDNA3.1 (Invitrogen) (1:10 ratio) to allow selection of transfected cells in 0.2 mg/ml G418. The hTERT immortalized NOK and NOK E7 cells have been previously described (Piboonniyom et al., 2003).
HFKs and NOKs were cultured either in Keratinocyte Serum Free Medium (K-SFM, Invitrogen) supplemented with human recombinant epidermal growth factor 1–53 (EGF 1–53), bovine pituitary extract (BPE), penicillin (100 U/ml), streptomycin (100μg/ml), gentamicin (10 μg/ml), and amphotericin B (0.5 μg/ml) or in E medium (Rheinwald, 1980; Rheinwald et al., 1980). E-medium was prepared by dissolving powdered Dulbecco’s modified Eagle Medium (DMEM; Invitrogen) and Ham’s formulation of F-12 Nutrient Mixture (Invitrogen) in distilled water. The solution was supplemented with sodium bicarbonate (3.07 g/l), adenine (180 μM), insulin (5 μg/ml), transferrin (5 μg/ml), triiodothyronine T3 (20 pM), hydrocortisone (0.4 μg/ml), cholera enterotoxin (10 ng/ml), penicillin (100 U/ml), streptomycin (100 μg/ml), nystatin (100 U/ml) and 5% Fetal Bovine Serum (FBS).
For serum deprivation experiments, cells were seeded into six-well plates at a density of 4 to 5 × 104 cells/well in complete E medium. Unattached cells were removed by replacing the medium the day after seeding. Cells were starved in serum-free E-medium or grown in complete E-medium for the indicated times before analysis by FACS, immunofluorescence or Western blotting, as indicated.
For confluence experiments, cells were seeded into six-well plates at 6 × 105 cells/well in complete E-medium. Unattached cells were removed by replacing the medium the day after seeding and the cells were kept in complete E-medium for 72 hours.
For some experiments NOK and NOK E7 cells were treated with the autophagy inhibitor 3-methyladenine (#M9281, Sigma) at 5 mM or the lysosomal protease inhibitors E-64d (#330005, Calbiochem) at 10 μg/ml and pepstatin A (#516481, Calbiochem) at 10 μg/ml for 4 hours.
Fluorescence-activated cell sorting analysis (FACS)
Cells were washed with phosphate buffered saline (PBS) and fixed in 75% ethanol at room temperature for 10 minutes. After treatment with RNAse A (20 μg/ml), DNA was stained with propidium iodide (50 μg/ml). Samples were analyzed with a FACSCalibur system and CellQuest software (Becton Dickinson).
Immunological Methods
For immunofluorescence experiments, cells were plated onto coverslips and fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.5% Triton X-100. Cells were blocked with 10% goat serum in PBS at room temperature for one hour and incubated with a rabbit polyclonal LC3B antibody (#2775, Cell Signaling) at a 1:200 dilution in PBS for 12 to 18 hours at 4°C followed by an Alexa Fluor 488-conjugated goat anti rabbit antibody (1:1000 in PBS; Invitrogen). Nuclei were counterstained with Hoechst 33258 (Sigma) and TO-PRO-3 (Invitrogen) dyes. Images were taken with laser scanning Zeiss Axioskop PCM2000 confocal fluorescence microscope.
For detection of LC3-II by Western blotting, cell lysates were prepared in 0.5% Nonidet P-40 (NP40), 150 mM NaCl, 50 mM Tris-HCl (pH7.5) and protease inhibitor cocktail (Roche). Extracts were cleared by centrifugation at 4°C at 16,000 g for 5 min and protein concentrations were determined by the Bradford method (Bio-Rad). 200 μg aliquots were immediately analyzed by SDS-PAGE and transferred to PVDF membranes (Immobilon-P; Millipore). The membranes were blocked at room temperature for one hour in 5% nonfat dry milk in TNET buffer (200 mM Tris-HCl, 1 M NaCl, 50 mM EDTA, 0.1% Tween 20 [pH7.5]) and then probed with rabbit LC3B polyclonal antibody (#2331, Novus biologicals; 1: 500) for one hour at room temperature. After three times of washing with TNET buffer, secondary antibody was added to the membrane for 45 minutes at room temperature. Immunodetection was performed using the enhanced chemiluminescence system (PerkinElmer Life Sciences, Inc.), followed by digital acquisition and quantification on a Kodak 4000R Image Station (Kodak) using Kodak Imaging Software (version 4.0). LC3-II signals were normalized to α-actin (#MAB1501, Chemicon; 1:1000).
Acknowledgments
Supported by PHS grant R01 CA081135 (KM). We thank Dr. Amy Baldwin for help in preparing for HFKs and Dr. Margaret McLaughlin-Drubin for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72(1):29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68(5):1485–94. doi: 10.1158/0008-5472.CAN-07-0562. [DOI] [PubMed] [Google Scholar]
- Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007;3(5):464–7. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7(4):546–54. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichten A, Rud DS, Grace M, Piboonniyom SO, Zacny V, Munger K. Molecular pathways executing the “trophic sentinel” response in HPV-16 E7-expressing normal human diploid fibroblasts upon growth factor deprivation. Virology. 2004;319(1):81–93. doi: 10.1016/j.virol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Tsuchida A, Miyazawa K, Yokoyama T, Kawakita H, Tokita H, Naito M, Itoh M, Ohyashiki K, Aoki T. Vitamin K2-induced cell growth inhibition via autophagy formation in cholangiocellular carcinoma cell lines. Int J Mol Med. 2007;20(6):801–8. [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69(1):119–28. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fujii S, Mitsunaga S, Yamazaki M, Hasebe T, Ishii G, Kojima M, Kinoshita T, Ueno T, Esumi H, Ochiai A. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11(16):2090–100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasskarl J, Velupillai P, Munger K. Increased in vitro lifespan of primary human keratinocytes correlates with decreased migration. J Invest Dermatol. 2006;126(5):1179–81. doi: 10.1038/sj.jid.5700205. [DOI] [PubMed] [Google Scholar]
- Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997a;11(16):2101–11. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Munger K. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997b;239(1):97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Suh-Burgmann E, Grace M, Munger K. Expression of the HPV E7 oncoprotein mimics but does not evoke a p53-dependent cellular DNA damage response pathway. Virology. 1999;258(2):406–14. doi: 10.1006/viro.1999.9733. [DOI] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Autophagy. 2007;3(6):610–3. doi: 10.4161/auto.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha YI, Lu B. Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer. Autophagy. 2008;4(5):659–68. doi: 10.4161/auto.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3(3):181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–81. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36(12):2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20(54):7888–98. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109(3):321–34. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Piboonniyom SO, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Munger K. Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res. 2003;63(2):476–83. [PubMed] [Google Scholar]
- Pirisi L, Yasumoto S, Feller M, Doniger J, DiPaolo JA. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987;61(4):1061–6. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27(35):4860–4. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- Putzer BM, Stiewe T, Parssanedjad K, Rega S, Esche H. E1A is sufficient by itself to induce apoptosis independent of p53 and other adenoviral gene products. Cell Death Differ. 2000;7(2):177–88. doi: 10.1038/sj.cdd.4400618. [DOI] [PubMed] [Google Scholar]
- Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992;89(16):7742–6. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 1980;21A:229–54. doi: 10.1016/s0091-679x(08)60769-4. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980;22(2 Pt 2):629–32. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, Ueno T, Ochiai A, Esumi H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007;67(20):9677–84. doi: 10.1158/0008-5472.CAN-07-1462. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol. 2008;445:29–76. doi: 10.1007/978-1-59745-157-4_3. [DOI] [PubMed] [Google Scholar]
- Teodoro JG, Shore GC, Branton PE. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11(3):467–74. [PubMed] [Google Scholar]
- White E, Cipriani R, Sabbatini P, Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991;65(6):2968–78. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka A, Miyata H, Doki Y, Yamasaki M, Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y, Monden M. LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int J Oncol. 2008;33(3):461–8. [PubMed] [Google Scholar]
- Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13(11):2323–30. [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96(4):1291–6. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]