SUMMARY
Many bacterial pathogens and symbionts utilize type III secretion systems to deliver bacterial effector proteins into host cells. These effector proteins have the capacity to modulate a large variety of cellular functions in a highly regulated manner. Here we report that the phosphoinositide phosphatase SopB, a Salmonella Typhimurium type III secreted effector protein, diversifies its function by localizing to different cellular compartments in a ubiquitin-dependent manner. We show that SopB utilizes the same enzymatic activity to modulate actin-mediated bacterial internalization and Akt activation at the plasma membrane, and vesicular traffic and intracellular bacterial replication at the phagosome. Thus by exploiting the host cellular machinery, Salmonella Typhimurium has evolved the capacity to broaden the functional repertoire of a virulence factor to maximize its ability to modulate cellular functions.
Keywords: bacterial pathogenesis, phosphoinositide phosphatase, type III secretion, vesicular trafficking, actin remodeling
INTRODUCTION
Bacterial pathogens that have co-existed with their hosts for extended periods of time have evolved complex functional interfaces involving specific bacterial adaptations to modulate cellular functions and secure the pathogen’s survival and replication. One of these adaptations is the type III secretion system (TTSS), a multiprotein nano-machine that mediates the delivery of bacterial effector proteins into target host cells (Galan and Wolf-Watz, 2006). Salmonella enterica serovar Typhimurium (S. Typhimurium) encodes two of these machines within its pathogenicity islands 1 and 2 (SPI-1 and SPI-2), which at different times during infection, deliver more than 60 proteins into host cells (Galán, 2001; Waterman and Holden, 2003). These effector proteins have the capacity to modulate a variety of cellular processes, including actin dynamics, vesicular trafficking, and transcriptional responses (Galán, 2001; Waterman and Holden, 2003), and many do so by mimicking the activities of host cell proteins (Stebbins and Galán, 2001). It is thought that the activity of type III secreted effector proteins within target cells must be regulated so that their function is exerted at the appropriate time and subcellular space. However, little is known about mechanisms that control these critical aspects of bacterial effector protein function. One of the effector proteins delivered by the S. Typhimurium SPI-1 TTSS is the phosphoinositide phosphatase SopB (Galyov et al., 1997; Hong and Miller, 1998; Norris et al., 1998). This effector protein mediates a diverse set of responses at different times during infection. Through the activation of SGEF, an exchange factor for the Rho-family GTPase RhoG, SopB mediates actin-dependent bacterial internalization (Patel and Galan, 2006; Zhou et al., 2001). In addition, SopB modulates vesicular trafficking by altering the metabolism of phosphoinositides at the Salmonella phagosomal membrane (Hernandez et al., 2004; Mallo et al., 2008). Furthermore, SopB activates the serine protein kinase Akt (Steele-Mortimer et al., 2000) and stimulates the production of nitric oxide (Drecktrah et al., 2005) by poorly understood mechanisms. Remarkably, all of these activities are strictly dependent on the phosphatase activity of SopB. The mechanism by which this effector modulates such a diverse set of functions with the same enzymatic activity is not understood. We show here that SopB diversifies its function by localizing to different cellular compartments at different times during infection. Early in infection, SopB localizes to the plasma membrane to mediate bacterial entry and Akt activation. Later in infection, SopB localizes to the Salmonella containing vacuole where it is required for bacterial replication. We also show that the translocation of SopB from the plasma membrane to the Salmonella-containing vacuole requires its ubiquitination. Therefore by co-opting the host-cell ubiquitination machinery, SopB can modulate distinct functions during the infection process utilizing the same enzymatic activity.
RESULTS
Ubiquitinated SopB persists during S. Typhimurium infection of cultured epithelial cells
The observation that SopB modulates different cellular processes at different times during infection prompted us to examine the levels of translocated SopB throughout the infection process. We used a S. Typhimurium strain expressing carboxyl-terminus FLAG-tagged SopB, which had been introduced in its chromosome by allelic exchange (see Materials and Methods). Epitope-tagged SopB was found to behave in a manner that was indistinguishable from wild type SopB (Fig. S1). SopB could be detected within infected cells from as early as a few minutes to up to several hours after infection (Fig. 1A), which is consistent with previous results (Drecktrah et al., 2005). Although a proportion of SopB detected late in infection represented newly synthesized protein, we found that a significant fraction represented protein delivered early in infection since it was detected even in the presence of a bacterial protein synthesis inhibitor (Fig. 1B). Shortly after infection, a significant proportion of translocated SopB was rapidly modified resulting in a change of its mobility in SDS PAGE (Fig. 1A and B), an observation previously attributed to its ubiquitination (Marcus et al., 2002; Rogers et al., 2008). To confirm that the change in SopB mobility was due to its ubiquitination, COS-2 cells transiently expressing HA-tagged ubiquitin were infected with S. Typhimurium, and 60 min after infection, SopB was immunoprecipitated with a specific monoclonal antibody. Immunoprecipitated proteins were separated by SDS-PAGE and the presence of ubiquitin conjugated to SopB was probed by Western immunoblotting using a specific antibody to the HA epitope tag. As shown in Fig. 1C, ubiquitinated SopB was readily detected in infected cells confirming that the observed change in its mobility upon translocation is due to its ubiquitination.
Figure 1. SopB is ubiquitinated upon bacterial delivery and persists during infection.
A. SopB persists during infection. Henle-407 cells were infected with a S. Typhimurium strain expressing FLAG epitope-tagged SopB for 1 hr and its presence in the protein translocated fraction was examined by western immunoblot. B. Effect of bacterial protein synthesis inhibitors on the levels of translocated SopB at different times after infection. Henle-407 cells were infected with a S. Typhimurium strain expressing FLAG epitope-tagged SopB and at the indicated times after infection, chloramphenicol was added. Infected cells were lysed 6 hours after infection and the presence of SopB in the translocated fraction was examined by western immunoblot. C. SopB is ubiquitinated upon translocation into epithelial cells. COS-2 cells transfected with a plasmid encoding HA epitope tagged ubiquitin, were infected with a S. Typhimurium strain expressing FLAG-epitope-tagged SopB for 1 hr. The presence of ubiquitinated SopB in infected cells was analyzed by immunoprecipitation and western blot analysis. D. SopB ubiquitination does not require the bacterially-encoded E3 ubiquitin ligases. Henle-407 cells were infected for 1 hr with S. Typhimurium strains expressing FLAG epitope-tagged SopB and lacking the bacterially-encoded E3 ubiquitin ligases SlrP, SspH2, or SopA, as indicated. The presence of SopB in the protein translocated fraction was examined by western immunoblot. E. SopB is conjugated to lysineless and K63 ubiquitin. Cultured cells transfected with a plasmid encoding HA-tagged lysineless (K0) or K63 ubiquitin were infected with a S. Typhimurium strain expressing FLAG-epitope-tagged SopB. The presence of ubiquitinated SopB in infected cells was analyzed by immunoprecipitation and western blot analysis. In all panels the star denotes the predicted mobility of unmodified SopB.
S. Typhimurium encodes three E3 ubiquitin ligases, the Hect-like protein SopA (Zhang et al., 2006), as well as three other proteins, SspH1 (absent from some strains including the one used in this study), SlrP, and SspH2, which belong to a novel family of E3 ligases (Quezada et al., 2008; Rohde et al., 2007). These effector proteins are also delivered by the SPI-1 and SPI-2-encoded type III secretion systems (Miao et al., 1999). We investigated the potential involvement of threes E3 ligases and found that the pattern of SopB ubiquitination after its delivery by the S. Typhimurium ΔsspH2 ΔslrP ΔsopA triple mutant was indistinguishable from that observed after its delivery by wild type S. Typhimurium (Fig. 1D). These results indicate that SopB ubiquitination does not require any of the bacterially-encoded E3 ubiquitin ligases.
We have previously shown that two other effectors delivered by the SPI-1 TTSS, SopE and SptP, are also ubiquitinated upon translocation (Kubori and Galan, 2003). In this case, ubiquitination serves as a degradation signal since these effectors are degraded upon translocation. In contrast, ubiquitinated SopB persisted within cells for extended periods of time even in the presence of a bacterial protein synthesis inhibitor (Fig. 1B). Furthermore, ubiquitinated SopB could be readily detected in the absence of proteasome inhibitors (Fig. 1A and B), and addition of a proteasome inhibitor did not significantly alter the levels of ubiquitinated SopB (Fig. S2). These results suggest that, in the case of SopB, ubiquitination may play a role other than that of a degradation signal. Consistent with this hypothesis, the mobility shift observed in SopB subsequent to its translocation into mammalian cells indicates that monoubiquitination is the most common modification observed in this effector (Fig. 1A-D). In fact, SopB may undergo multi-monoubiquitination since in immunoprecipitation experiments conducted in cells over-expressing lysine-less ubiquitin, which cannot form ubiquitin chains although it can be conjugated to substrates, we still observed laddering in the SopB mobility (Fig. 1E). We also observed the presence of ubiquitinated SopB in infected cells overexpressing a ubiquitin mutant in which its lysine 63 (K63-ubiquitin) was the only lysine available for conjugation (Fig. 1E). Like monoubiquitination, K63-linked ubiquitin is not usually associated with degradation (Urbe, 2005). However, these results do not rule out that the laddering observed under these conditions may also be the result of multi-monoubiquitination by K63 ubiquitin. Together, these results further support the hypothesis that ubiquitination of SopB plays a role other than that of a degradation signal.
Identification of the SopB ubiquitination sites
To evaluate the role of ubiquitination in SopB function we mapped its ubiquitination sites. We isolated translocated SopB from infected cells by affinity purification and subjected it to mass spectrometric analysis to identify ubiquitinated peptides. This analysis identified ubiquitinated SopB fragments consisting of amino acids 14-23, and 24-41 (Fig. S3). These results, in conjunction with the ubiquitin laddering observed in infected cells expressing lysine-less ubiquitin (see above), suggested that several lysine residues located at the amino terminus of SopB could serve as potential ubiquitination sites. Consequently, we focused our analysis on the amino terminus of SopB, which comprises 9 lysine residues (Fig. 2A). We generated a series of FLAG-tagged point mutants, replacing each of these lysine residues, either individually or in combination, in the context of full-length SopB. The different SopB mutants were expressed in S. Typhimurium, tested for their secretion from the bacteria, and assayed for ubiquitination upon TTSS-mediated translocation into cultured epithelial cells. All constructs were expressed, secreted, and translocated into cultured cells at levels indistinguishable from those of wild type SopB (Fig. 2B). Surprisingly, individual substitution of each one of all the lysine residues located within the first 120 amino acids of SopB did not prevent its ubiquitination (Fig. 2B). Furthermore, even pair-wise substitution of lysine residues within this region had a negligible effect on SopB ubiquitination (Fig. 2B). Only when all the lysine residues contained within this region were substituted was SopB ubiquitination abrogated, resulting in its migration as a predominantly single species at the predicted molecular weight of non-ubiquitinated SopB (Fig. 2B). Moreover, additional mutation of a lysine residue located at the carboxy terminus (K541), which has previously been shown to be ubiquitinated upon transient over expression of SopB in cultured cells (Rogers et al., 2008), had no effect on the ubiquitination profile of SopB (Fig. S4). In addition, the mutant in which all the amino terminal lysines had been substituted did not co-immunoprecipitate with epitope tagged ubiquitin (data not shown). These results revealed a high degree of complexity in the pattern of SopB ubiquitination, with different lysines potentially serving as targets for ubiquitination. Similar observations have been made for the ubiquitination sites of the epidermal growth factor receptor (Haglund et al., 2003a; Haglund et al., 2003b; Huang et al., 2006).
Figure 2. Identification of the SopB ubiquitination sites.
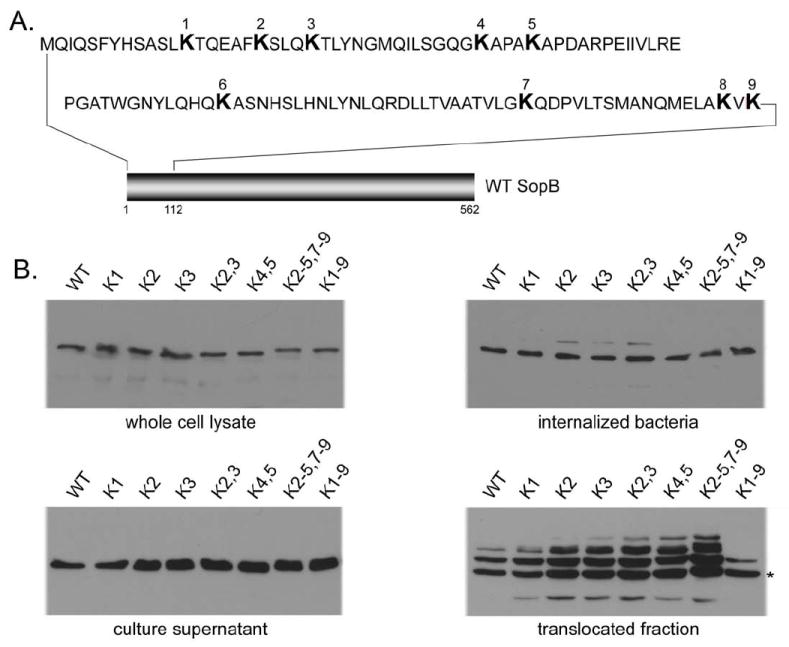
A. Diagram indicating the location of lysine residues within the amino terminus of SopB. B. Effect of mutations in SopB lysine residues on its secretion, translocation, and ubiquitination. Bacterial strains were examined for their ability to express and secrete the different mutant constructs (left panels). In addition, mutants were examined for their ubiquitination upon translocation into cultured cells (right panels). Numbers correspond to the lysine residues indicated in the diagram shown in A. The star denotes the predicted mobility of unmodified SopB.
The non-ubiquitinated SopB mutant accumulates at the plasma membrane of infected cells following its TTSS-mediated delivery
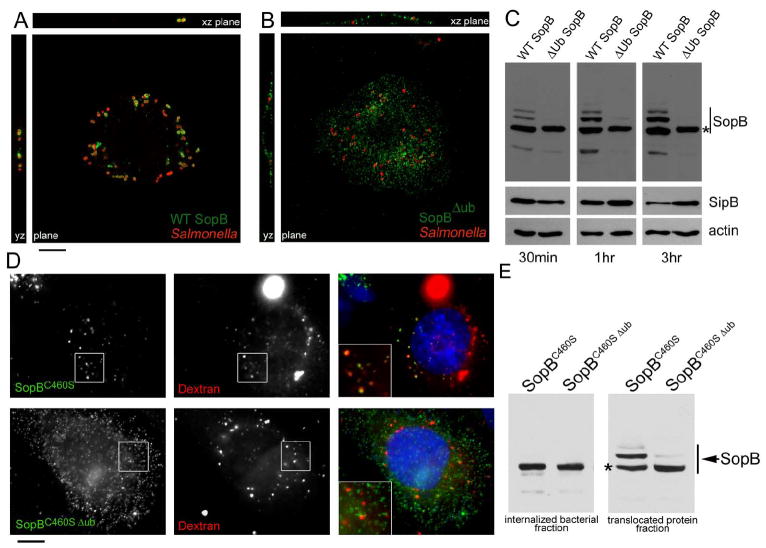
To investigate the role of SopB and its ubiquitination during infection we compared the localization of the non-ubiquitinated (K1-9 substituted, see Fig. 2) SopB mutant (from here forth referred to as SopBΔub) with that of wild type SopB. Cultured epithelial cells were infected with S. Typhimurium strains expressing a chromosomally encoded FLAG tagged wild-type SopB or its SopBΔub mutant derivative constructed by allelic exchange, and the localization of these effector proteins was examined by confocal microscopy. Ten minutes after infection, wild-type SopB was observed localized at the plasma membrane, tightly around entering bacteria (Fig. S5). Following internalization, wild-type SopB was seen decorating the Salmonella containing vacuoles, and its association with this compartment was maintained for up to 3 hrs (Fig. 3A, supplementary Video S1, and data not shown). In contrast, the SopBΔub mutant revealed a strikingly different localization at the plasma membrane in the form of surface puncta radiating from the site of infection (Fig. 3B, Supplementary Video S2, and data not shown). Within 30 minutes of infection, diffuse SopBΔub staining over the entire plasma membrane was readily evident in x-z and y-z projections following reconstruction of confocal z-stacks (Supplementary Video S2). Despite the significant differences in localization, biochemical fractionation of lysates of host cells infected with bacteria expressing the different constructs indicated that the levels of translocated SopBΔub mutant protein were equivalent to those of wild type SopB (Fig. 3C). The half-life within host cells of the non-ubiquinated SopB mutant was equivalent to wild type SopB (data not shown), further demonstrating that, in the case of this effector, ubiquitination does not serve as a degradation signal. Consistent with the role of ubiquitination in SopB localization, mutants in which lysine substitutions (individual or in combination) did not result in changes in its ubiquitination pattern (Fig. 2B) localized in a manner indistinguishable to wild type SopB (Fig. S6).
Figure 3. Subcellular localization of wild-type SopB and its ubiquitination-deficient mutant.
A and B. Henle-407 cells were infected with a S. Typhimurium strain expressing wild type epitope-tagged SopB (A) or its ubiquination-deficient SopBΔub mutant (B) and examined by fluorescence confocal microscopy after staining with antibodies directed to the epitope tag (green) or to S. Typhumrium (red) Bar = 10 μM. C. Levels of SopB or SopBΔub in infected cells. Henle-407 cells were infected with S. Typhimurium strains expressing FLAG epitope-tagged wild type SopB or the SopBΔub mutant. At the indicated times after infection, cells were probed for the presence of SopB in the translocated fraction by western immunoblot. Blots were reprobed with antibodies directed to SipB (another SPI-1 TTSS effector protein) or actin to control for loading. The star denotes the predicted mobility of unmodified SopB. D. Localization of wild-type SopB or its ubiquitination deficient mutant derivative after their delivery by a non-invasive S. Typhimurium mutant. Cultured epithelial cells pre-loaded with Alexa 594-dextran to label endosomal compartments were infected for 1 hr with the invasion-defective ΔsopE ΔsopE2 ΔsopB S. Typhimurium mutant strain expressing FLAG epitope-tagged catalytic-inactive SopBC460S or its ubiquitination defective derivative SopBC460S Δub. Cells were fixed, stained for SopB (green) and DAPI (blue) and examined by fluorescence microscopy. Insets show enlarged detail area. Bar = 10 μM. E. Levels of SopBC460S or its derivative SopBC460S Δub after their TTS-mediated delivery by the non-invasive S. Typhimurium mutant strain. Henle-407 cells were infected with ΔsopE ΔsopE2 ΔsopB S. Typhimurium mutant strains expressing FLAG epitope-tagged wild type SopB or its not ubiquitinitable mutant (SopBΔub) and 1 hr after infection, cells were probed for the presence of SopB in the translocated fraction by western immunoblot. The star denotes the predicted mobility of unmodified SopB.
To further explore the role of ubiquitination in the localization of SopB, we examined the distribution of the SopBΔub mutant upon delivery by bacteria competent for TTSS-mediated protein translocation but defective for internalization. We reasoned that this would allow us to examine the distribution of this effector protein without the potential influence of actin remodeling and bacterial entry. To render the bacteria unable to induce its own internalization, we constructed catalytic mutant derivatives of the wild type (SopBC460S) and its non-ubiquitinated mutant (SopBΔub C460S), and expressed them in a strain lacking the effectors SopE and SopE2, which are functionally redundant with SopB in mediating bacterial entry. When delivered in the context of this non-invasive strain, SopBC460S was seen in small intracellular vesicle-like compartments that could be labeled with endocytic tracers (Fig. 3D). In addition, mixed infection experiments showed that SopB delivered by non-invasive bacteria could be observed surrounding phagosomes of invasive bacteria, demonstrating that this delivery pathway can result in the transfer of translocated SopB to Salmonella-containing phagosomes (supplementary video S3). SopBC460S was readily ubiquitinated upon delivery into cells by non-invasive bacteria, indicating that its phosphatase activity is dispensable for this modification (Fig. 3E). In contrast, SopBΔubC460S delivered by non-invasive bacteria was seen localized exclusively at the plasma membrane, did not co-localize with endocytic tracers (Fig. 3D) or bacterial-containing phagosomes after mixed infections, and as expected, was not ubiquitinated upon its delivery to infected cells (Fig. 3E). These results indicate that the localization of SopB to an intracellular vesicular compartment such as the Salmonella-containing vacuole is not the consequence of its passive trapping in the phagosomal membrane during bacterial uptake. Rather, these results indicate that ubiquitination may serve as a signal necessary for the removal of SopB from the host cell plasma membrane and its delivery to intracellular vesicular compartments such as the Salmonella-containing vacuole. This mechanism is reminiscent of the ubiquitination–dependent internalization mechanisms of growth factors receptors after stimulation by their ligands (Dikic, 2003). However, our results cannot rule out that during bacterial internalization, ubiquitination may also serve as a signal to localize SopB to the nascent phagosome and contribute to its targeting to the bacterial phagosome.
Identification of a domain required for SopB association with the host cell membrane
We found that following translocation into host cells SopB partitioned primarily to a membrane fraction, and could not be extracted by high salt concentrations (Fig. 4A). Similar observations have been previously made following transient over-expression of SopB in epithelial cells (Marcus et al., 2002). SopBΔub also partitioned to the membrane fraction and was equally resistant to extraction by salt, which indicates that ubiquitination is not required for SopB to associate with host-cell membranes after TTSS-mediated translocation (Fig. 4A). The association of SopB with the host-cell membrane in a manner resistant to extraction with high salt treatment suggests a strong interaction equivalent to that of a hydrophobic domain that may be intimately associated with the membrane. We examined the SopB sequence for the presence of such a domain and identified a region between amino acids 288 and 309 whose features would be consistent with those of a transmembrane domain as indicated by the TMpred prediction algorithm (data not shown). However, this domain does not appear to be a bona fide transmembrane domain since other prediction algorithms did not detect it (data not shown). To investigate the potential contribution of this domain to the membrane localization of SopB, we constructed a S. Typhimurium strain expressing a SopB mutant lacking this hydrophobic region (SopBΔ288-309) and examined its phenotype. SopBΔ288-309 was secreted from the bacteria and delivered into host cells by the SPI-1 TTSS, albeit at slightly lower levels than those of the wild-type protein (Fig. 4B). Importantly, translocated SopBΔ288-309 migrated as a single species on SDS-PAGE with the predicted molecular weight of the unmodified protein, which indicates that, unlike wild-type SopB, this mutant form cannot be ubiquitinated (Fig. 4B). Furthermore, translocated SopBΔ288-309 exhibited a cytoplasmic localization (Fig. 4C), indicating that the hydrophobic domain is essential for the localization of SopB to host cell membranes. A S. Typhimurium ΔsopE ΔsopE2 mutant strain expressing SopBΔ288-309 was defective for entry (Fig. 4D) and Akt activation (Fig. 4E), indicating that SopB must be able to associate with host cell membranes to exert these functions. The phenotype of the SopBΔ288-309 mutant is unlikely to be due to gross conformational changes caused by the deletion of the hydrophobic domain since purified SopBΔ288-309 exhibited almost wild-type phosphatase activity in vitro (Fig. 4F). However, this experiment cannot rule out the possibility that loss of function of this SopB mutant may be due to localized conformational changes induced by introduction of the small deletion. Taken together, however, these results indicate that the association of SopB with host cell membranes requires a discrete hydrophobic domain and is critical for its ubiquitination and function.
Figure 4. Identification of a SopB domain required for its membrane localization.
A. Membrane association of SopB after type III secretion mediated translocation. Henle-407 cells were infected with a S. Typhimurium strain expressing FLAG epitope-tagged wild-type SopB (upper panel) or its mutant SopBΔub (lower panel) and their presence in the membrane insoluble (P) or soluble (S) fractions of infected cells before and after extraction with NaCl (1 M), Na2C03 (pH11), or Triton X100 was examined by western immunoblot. The star denotes the predicted mobility of unmodified SopB. B. TTSS-mediated secretion, and translocation into host cells of the SopBΔ288-309 mutant. Whole cell bacterial lysates, bacterial culture supernatants, or TTS-protein translocated fraction of cultured epithelial cells infected with the indicated strains of S. Typhimurium expressing FLAG epitope-tagged SopBΔ288-309 mutant or wild type SopB (as control) were analyzed by western immunoblot. The star denotes the predicted mobility of unmodified SopB. C. Subcellular localization of the SopBΔ288-309 mutant. Henle-407 cells, were infected with a S. Typhimurium strain expressing an epitope-tagged SopBΔ288-309 mutant and examined by immunofluorescence confocal microscopy with an antibody directed to the epitope tag (green) or actin (red). Bar = 10 μM. D. Ability of wild type SopB and the SopB288-309 mutant to mediate bacterial entry into cultured epithelial cells. Henle-407 cells were infected with a S. Typhimurium ΔsopE ΔsopE2 ΔsopB mutant strain (as control indicated “ΔsopE” in the panel) or a S. Typhimurium ΔsopE ΔsopE2 mutant expressing wild type SopB, or SopBΔ288-309 (as indicated), and the levels of internalized bacteria were determined as indicated in the Material and Methods. Values are the mean ± standard deviation of three independent experiments and represent the percentage of the original bacterial inoculum that survived the antibiotic treatment due to internalization. E. Ability of wild type SopB and the SopB288-309 mutant to activate Akt. Henle-407 cells were infected with a S. Typhimurium expressing FLAG epitope tagged wild-type SopB, or the SopBΔ288-309 mutant, and the levels of phosphorylated (activated) or total Akt 1 hr after infection were examined by western immunoblot analysis. The levels of translocated epitope-tagged SopB, or SopBΔ288-309 were determined by western immunoblot analysis of the fraction of infected cells containing the translocated protein (upper panel). F. Comparison of the in-vitro phosphoinositide phosphatase activity of purified wild type SopB, the SopBΔ288-309 mutant, and the phosphatase-inactive SopBC460S mutant. The phosphatase activity was calculated based on the standard curve (shown in inset) for inorganic phosphate standards using a malachite green chromogenic assay.
S. Typhimurium expressing SopBΔub promotes increased actin remodeling, macropinocytosis, and Akt activation
To investigate the functional significance of SopB ubiquitination we examined the SopB-dependent phenotypes of a S. Typhimurium strain expressing the ubiquitination-deficient SopBΔub mutant. Since SopB is functionally redundant with the Rho-family GTPase exchange factors SopE and SopE2 in mediating bacterial internalization (Zhou et al., 2001) (Patel and Galan, 2006), we expressed the SopBΔub mutant in a strain lacking these exchange factors so that signaling for entry was exclusively delivered through SopBΔub. We found that a S. Typhimurium strain expressing SopBΔub was able to enter cells in a manner indistinguishable from that of a strain expressing wild type SopB (Fig. 5A). Interestingly, the S. Typhimurium sopBΔub mutant strain induced more profuse actin cytoskeletal rearrangements than the strain expressing wild type SopB. While the strain expressing wild-type SopB induced localized actin remodeling, the strain expressing SopBΔub induced diffuse membrane ruffling along the cell periphery that coincided with a loss in cellular stress fibers (Fig. 5B and 5C). Moreover, in contrast with the transient actin remodeling induced by wild-type SopB, the actin rearrangements stimulated by the S. Typhimurium sopBΔub mutant strain persisted for up to 3 hours after infection (data not shown). These results suggest that SopB triggers signaling for entry when it is localized at the plasma membrane and that its persistence at this location results in increased signaling to the actin cytoskeleton. These results also suggest that the removal of SopB from the plasma membrane by an ubiquitination-mediated mechanism may contribute to the downregulation of this signaling event after bacterial entry.
Figure 5. The ubiquitination-deficient SopB mutant induces efficient entry and distinct actin reorganization.
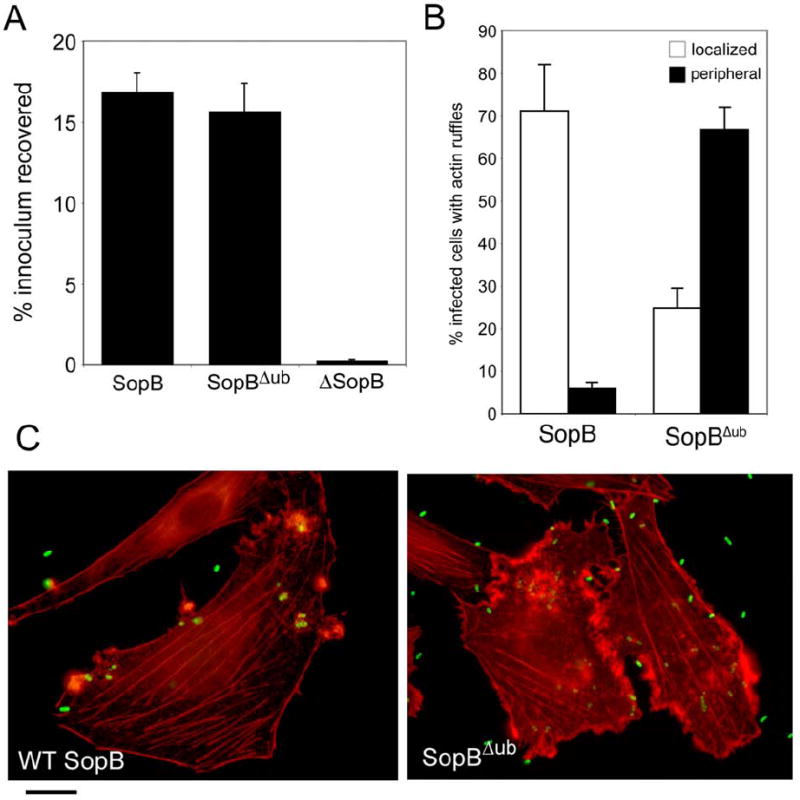
A. Henle-407 cells were infected with a S. Typhimurium ΔsopE ΔsopE2 mutant strain expressing wild type SopB, or the SopBΔub mutant (as indicated), and the levels of internalized bacteria were determined by the gentamicin resistance assay. Values are the mean ± standard deviation of three independent experiments and represent the percentage of the original bacterial inoculum that survived the antibiotic treatment due to internalization. B and C. Ref-52 cells were infected with a ΔsopE ΔsopE2 S. Typhimurium strain expressing either wild-type SopB or its ubiquitination deficient mutant derivative (SopBΔub). Cells were stained with rhodamine-phalloidin to visualize the actin cytoskeleton, with a FITC-labeled antibody to visualize S. Typhimurium, and examined by fluorescence microscopy. Cells exhibiting either localized or peripheral actin cytoskeletal changes were quantified. Values (shown in B) represent the percentage of infected cells showing the indicated changes and are the mean ± standard deviation of 3 experiments in which a minimum of 200 cells were examined for each category. The values obtained in cells infected with the SopBΔub mutant were statistically significant (p < 0.02) when compared to those of wild type with the student t test.
We investigated whether ubiquitination of SopB influences the magnitude and temporal kinetics of macropinocytosis, a phenotype also dependent on SopB (Hernandez et al., 2004; Mallo et al., 2008). Henle-407 cells infected with S. Typhimurium expressing wild-type SopB or the SopBΔub mutant were analyzed by live microscopy for their ability to induce macropinocytosis. In comparison to a wild type strain, cells infected with the S. Typhimurium sopBΔub mutant exhibited increased and more prolonged macropinocytic activity (Fig. 6A and 6B). These results suggest that the persistence of SopBΔub at the plasma membrane enhances its ability to stimulate macropinocytosis.
Figure 6. The ubiquitination-deficient SopB mutant induces prolonged macropinocytosis and Akt activation.

A and B. Henle-407 cells were infected with a S. Typhimurium strain expressing either wild-type SopB or its ubiquitination deficient mutant derivative (SopBΔub) and visualized by live phase contrast microscopy (A). Cells exhibiting macropinosomes were quantified at the indicated times (B). Values represent the percentage of infected cells showing macropinosomes and are the mean ± standard deviation of 3 experiments in which a minimum of 200 cells were examined for each category and time point. * indicates statistically significant (p < 0.05) differences when compared to those of wild type with the student t test. C. Henle-407 cells were infected with a S. Typhimurium expressing either wild-type SopB or its ubiquitination deficient mutant derivative (SopBΔub) and the levels of phosphorylated (activated) Akt and total Akt at different times (as indicated) after infection were examined by western immunoblot analysis.
We also compared the activation of Akt over time in cells infected with S. Typhimurium expressing wild type SopB or the SopBΔub mutant. Although at 30 minutes after infection the levels of Akt activation were equivalent in cells infected with either bacterial strain, cells infected with the S. Typhimurium sopBΔub mutant showed more sustained kinase activation (Fig. 6C). Three hours after infection the levels of phosphorylated (activated) Akt were higher in cells infected with the S. Typhimurium sopBΔub mutant (Fig. 6C), which correlates with the prolonged presence of SopBΔub at the plasma membrane. These results suggest that SopB activates Akt at the plasma membrane.
In summary, persistence of SopB at the plasma membranes resulted in enhanced ability to stimulate actin remodeling, macropinocytosis, and Akt activation, suggesting that these activities require the presence of SopB at this location.
S. Typhimurium expressing SopBΔub is defective for intracellular replication
It has been previously shown that SopB is required for the efficient intracellular replication of S. Typhimurium by modulating the vesicular trafficking of the Salmonella-containing vacuole (SCV) (Hernandez et al., 2004). It has also been recently shown that recruitment of Rab5 to the SCV is dependent on the presence of SopB, and it has been postulated that this recruitment is necessary for efficient S. Typhimurium intracellular replication (Mallo et al., 2008). We reasoned that these phenotypes must depend on the presence of SopB at the SCV, in which case a S. Typhimurium expressing SopBΔub, which is retained at the plasma membrane, may exhibit a defect in these phenotypes. To test this hypothesis, we first compared the ability of S. Typhimurium expressing either wild-type SopB or the SopBΔub mutant with a ΔsopB strain for their ability to recruit Rab5 to the SCV. Both the ΔsopB and the strain expressing the SopBΔub mutant were equally defective in their ability to recruit Rab5 to the SCV (Fig. 7A and Supplementary Fig. S7). To further evaluate the importance of ubiquitin-mediated SopB localization to the SCV, we compared the ability of a S. Typhimurium ΔsopE ΔsopE2 mutant strain expressing either wild-type SopB or the SopBΔub mutant to grow within epithelial cells. Although both strains were internalized at equivalent levels, 24 hs after infection a significantly lower number of colony forming units were recovered from cells infected with the strain expressing the SopBΔub mutant (Fig. 7B). We also compared the S. typhimurium ΔsopB mutant with wild type S. Typhimurium or an isogenic mutant strain expressing SopBΔub for their ability to survive within primary bone-marrow-derived macrophages. As shown in Fig. 7C, when compared to wild type S. Typhimurium, the ΔsopB and the strain expressing SopBΔub were equally defective in their ability to survive within macrophages. Introduction of a plasmid encoding wild type sopB readily complemented the S. Typhimurium strain expressing the SopBΔub mutant for its ability to survive within macrophages. Combined, these results are consistent with the hypothesis that SopB modulation of bacterial intracellular replication requires the localization of SopB at the SCV. In addition, together with the results presented above, these findings indicate that the differential localization of SopB at different sites allows it to modulate different cellular processes and hence diversify its function.
Figure 7. The ubiquitination-deficient SopB mutant is defective for Rab5 recruitment and intracellular growth.
A. Rab5 recruitment by different strains of S. Typhimurium. Henle-407 cells that had been transfected with a plasmid expressing Rab5-GFP were infected with a ΔsopB S. Typhimurium mutant strain (ΔsopB) or a strain expressing either wild-type SopB (WT SopB) or its ubiquitination deficient mutant derivative (SopBΔub). At the indicated times the percentages of the different S. Typhimurium strains showing co-localization with Rab5-GFP were determined. Values are mean ± standard deviation of three independent determinations in which the indicated number of bacteria (n) were examined. * indicates statistically significant (p < 0.05) differences when compared to the values of cells infected with wild type bacteria using the student t test. B. S. Typhimurium expressing SopBΔub is defective for intracellular growth in cultured epithelial cells. Henle-407 cells were infected with a ΔsopE ΔsopE2 S. Typhimurium strain expressing either wild type SopB or expressing SopBΔub. At the indicated times after infection, the levels of intracellular c. f. u. of the different strains were determined. Values represent the mean ± standard deviation of three independent experiments. * indicates statistically significant (p < 0.05) differences when compared to the values of cells infected with wild type bacteria using the student t test. C. S. Typhimurium expressing sopBΔub is defective for survival within primary macrophages. Primary bone marrow-derived macrophages were infected with wild type S. Typhimurium (WT), a ΔsopB mutant (ΔsopB), a strain expressing expressing sopBΔub (sopBΔub) or a derivative of the same strain complemented with a plasmid expressing wild type sopB (sopBΔub + WT sopB). At the indicated times after infection, the levels of intracellular c. f. u. of the different strains were determined. Values are normalized to those of wild type and represent the mean ± standard deviation of three independent experiments. * indicates statistically significant (p < 0.05) differences when compared to the values of cells infected with wild type bacteria using the student t test. D. Model for ubiquitin-mediated diversification of SopB function. Dx Upon delivery by the S. Typhimurium TTSS, SopB localizes to the plasma membrane through is membrane localization domain (I). While at the plasma membrane SopB mediates entry and macropinocytosis by activating SGEF, a RhoG exchange factor, and Akt activation by unknown mechanisms (I). Membrane-localized SopB is ubiquitinated (mono ubiquitinated or linked via K63), which results in its trapping in the incoming phagosome or its removal from the plasma membrane and its subsequent translocation to the Salmonella- containing vacuole, where it modulates vesicle trafficking by altering phosphoinositide metabolism at that site thus avoiding the delivery of S. Typhimurium to lysosomes (II). See text for further details.
DISCUSSION
Type III secretion systems have the capacity to mediate a very diverse array of cellular functions. They do so by delivering a variety of effector proteins, which in many cases function as “mimics” of eukaryotic cell proteins (Galan, 2007; Stebbins and Galán, 2001). In order to exert their function, the activity of these effector proteins must be precisely coordinated and regulated in time and space. For example, in S. Typhimurium, two effector proteins, SopE and SptP, modulate Rho-family GTPases through opposing biochemical activities. SopE activates Rho-family members functioning as a guanine nucleotide exchange factor (GEF) for these GTPases (Hardt et al., 1998) while SptP down-modulates the activity of the same GTPases by functioning as a GTPase-activating protein (GAP) (Fu and Galán, 1999). These effectors ensure activation of the GTPases to allow bacterial internalization while preserving cellular homeostasis, which is transiently altered during bacterial entry. The opposing activities of these two effectors are coordinately regulated by their ubiquitin-dependent differential degradation (Kubori and Galan, 2003). We have shown here another example of ubiquitin-mediated regulation of effector function. Rather than degradation, the mechanism described here implicates ubiquitination in the differential localization of the effector protein SopB during the infection cycle, resulting in the diversification of its function.
Upon translocation from the bacteria, SopB localizes to the plasma membrane of infected cells from where it mediates actin cytoskeleton reorganization, macropinocytosis, bacterial entry, and Akt activation (Fig. 7D). We found that this localization depends on a hydrophobic region of SopB, which tightly tethers this effector protein to the host-cell plasma membrane. However, membrane localization may involve additional determinants since previous transient transfection studies have identified an additional region of SopB capable of mediating its putative membrane association or affecting its solubility under transient over expression conditions (Marcus et al., 2002). Shortly after its translocation, SopB is multi-monoubiquitinated. This modification acts as a signal required for its removal from the host-cell plasma membrane and its subsequent delivery to internal vesicular compartments and/or for its “trapping” on the nascent bacterial phagosome. At this location, SopB modulates vesicle traffic and facilitates intracellular bacterial replication. Consistent with this model, an ubiquitination-defective SopB mutant remained localized to the plasma membrane throughout bacterial infection, and a S. Typhimurium strain expressing this mutant exhibited an increased and prolonged ability to stimulate actin cytoskeleton rearrangements and Akt activation. In contrast, this strain was defective for intracellular survival, which is dependent on the appropriate vesicle trafficking of the SCV. Therefore, ubiquitination is central not only for the down modulation of the early activities of SopB but also for the diversification of its function. In this context, the parallels with the role of ubiquitination in the modulation and diversification of growth factor receptor function are striking (Dikic, 2003; Haglund et al., 2003a). Upon stimulation by its ligand, the EGFR is monoubiquitinated and removed from the plasma membrane to be delivered to an endocytic compartment where it can further stimulate downstream signaling, or it can be sorted for recycling or degradation. Like SopB, in the absence of ubiquitination, EGFR remains at the plasma membrane resulting in constitutive signaling. Furthermore, the similarities extend to the highly redundant nature of the potential sites for ubiquitination. We found that several lysines within the amino terminus of SopB can be targets for monoubiquitination. Likewise, several lysines clustered around the kinase domain of EGFR can be alternatively monoubiquitinated (Haglund et al., 2003a; Haglund et al., 2003b; Huang et al., 2006). However, despite these striking similarities, there are significant differences in the cellular machinery utilized for the removal and transport of SopB and EGFR from the plasma membrane to endocytic compartments. The ubiquitination-dependent removal of the EGFR from the plasma membrane is mediated by a complex mechanism that involves proteins belonging to the endosomal sorting complexes required for transport (ESCRT) (Saksena et al., 2007), as well as dynamin and Epsin (Bache et al., 2006; Chen et al., 1998; Damke et al., 1994; Dikic, 2003; Malerød et al., 2007; Raiborg et al., 2008). In contrast, depletion of or interference with dynamin, clathrin, Epsin, or members of the ESCRT complex did not affect the location of SopB (Supplementary Fig. S8) implying that an as yet unidentified but potentially different cellular machinery is involved in SopB’s removal from the plasma membrane and translocation to endocytic compartments.
In summary, we have described here a remarkable mechanism by which, through differential localization, a virulence factor diversifies its function to modulate rather distinct cellular processes with the same biochemical activity. This is another example of the extraordinary complexity of the pathogen/host cell functional interface sculpted by evolutionary forces directed at securing pathogen and host survival.
EXPERIMENTAL PROCEDURES
Plasmids, Bacterial Strains and Cell Lines
Full-length wild-type SopB and its derivatives (including truncations and deletions), tagged at the C-terminus by a FLAG epitope were cloned into the arabinose inducible vector pBAD24 (Guzman et al., 1995) by standard recombinant DNA procedures. SopB mutants harboring single or combinatorial Lys (K) to Glu (Q) substitutions at positions 13, 19, 23, 37, 41, 67, 93, 109 and 111 were generated by site-directed mutagenesis and verified by DNA sequencing. Eukaryotic expression vectors encoding Epsin UIM and K44A Dynamin were provided by Pietro de Camilli (Yale University), YFP tagged Vps4a derivatives (pEYFP-Vps4a and pEYFP-E228QVps4a) were provided by W. Sandquist (University of Utah), and HA tagged wild-type, K63, and K0 ubiquitin were provided by Yihong Ye (NIH). siGENOME SMARTpool (M-016835-00, Dharmacon) at a final concentration of 50nM was used to knock down expression of endogenous HRS. RNAi efficacy was verified by qRT-PCR, using HRS primers as previously described (Patel and Galan, 2006). Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturers instructions.
The wild-type strain of S. Typhimurium SL1344 (Hoiseth and Stocker, 1981) and its isogenic derivatives used in this study, ΔsopB (SB1120) (Hernandez et al., 2004), ΔsopE ΔsopE2 (SB1301), and ΔsopE ΔsopE2 ΔsopB (SB1302) (Zhou et al., 2001), have been previously described. Strains expressing chromosomally encoded FLAG tagged wild-type SopB and its derivatives were constructed by standard recombinant DNA and allelic exchange procedures as previously described (Kaniga et al., 1994). All bacterial strains were cultured under conditions that stimulate the expression of the Salmonella Pathogenicity Island-1-encoded type III secretion system (Galán and Curtiss III, 1990). Where appropriate, 0.05% l-arabinose was added to cultures at the early logarithmic phase of growth (OD600nm of 0.4) to induce expression of genes under the control of the para BAD promoter.
Type III protein secretion and translocation assay
Bacterial protein secretion and translocation into host cells were examined by biochemical fractionation and western immunoblot as previously described (Collazo and Galán, 1997; Kubori and Galan, 2003). Where appropriate, the proteasome inhibitor MG132 (5μM, Calbiochem) or its vehicle solvent DMSO, was added to cells 30 min prior to infection and maintained throughout the infection and lysis procedure. In bacterial protein synthesis inhibition experiments, chloramphenicol was added at a final concentration of 100μg/ml at different time points during infection.
SopB immunoprecipitation
COS-2 cells seeded in 10 cm dishes and transfected with a plasmid encoding HA-tagged ubiquitin were infected with S. Typhimurium expressing chromosomally encoded SopB FLAG (m.o.i. 30) at 37°C, 5% CO2. At different time points following infection, cells were harvested on ice by scraping into 1ml modified RIPA lysis buffer (50mM Tris pH7.6, 150mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 20mM NEM, 1x protease cocktail (Roche), 1mM PMSF) per well. Lysates were centrifuged at 20,000 × g at 4°C for 20 min to remove insoluble material. An aliquot of the supernatant was saved (~ 100μl) to monitor levels of SopB FLAG translocation and HA-ubiquitin expression whilst the remainder (~900μl) was incubated with 15μl pre-washed anti-FLAG M2 agarose (50% slurry, Sigma), rotating for 1 hr at 4°C to precipitate translocated SopB. Immune complexes were collected by centrifugation at 1,000 × g for 5 min at 4°C, washed twice with 1ml cold lysis buffer and boiled in SDS loading buffer to dissociate proteins from beads. Samples were analyzed by SDS PAGE (8% gel) and western blotting using antibodies directed against HA and FLAG.
Subcellular fractionation and extraction
Henle-407 cells (10 cm plate) were infected (m.o.i. 60) for 1 hr as described above, washed and harvested by scraping gently into homogenization buffer (20mM Hepes, pH 7.2, 200mM sucrose, 0.5mM EGTA, 1mM PMSF, 1x protease inhibitor cocktail). Cells were disrupted by mechanical lysis using a dounce homogeniser (25x passage) and lysates were centrifuged twice at 20,000g, 4°C for 30 min to remove bacteria and debris. The resulting supernatant was centrifuged at 100,000g 4°C to separate the membrane (pellet) from the cytoplasmic (supernatant) fractions. Extraction of SopB from the pellet fraction was performed by resuspending in buffer containing 1M NaCl, 100mM sodium bicarbonate (pH11) or 1% Triton X-100 and incubating on ice for 30 min. Samples were centrifuged at 100,000g to separate membrane and cytoplasmic fractions. The presence of SopB in the supernatant fraction was determined by TCA precipitation and western blot analysis.
S. Typhimurium induced ruffling, macropinocytosis assays, and Rab5 recruitment
S. Typhimurium-induced ruffling and macropinocytosis assays were performed in Ref-52 and Henle-407 cells as previously described (Chen et al., 1996; Patel and Galán, 2008; Patel and Galan, 2006). Rab5 recruitment was examined in Henle-407 cells following transfection of GFP-Rab5 as previously described (Mallo et al., 2008).
Extracellular delivery of SopB
To monitor the fate of SopB delivered into cells from extracellular bacteria we infected Henle-407 cells with a DsopE ΔsopE2 ΔsopB S. Typhimurium mutant strain expressing a catalytically inactive FLAG-epitope tagged SopB mutant (SopBC460S) at an m.o.i. of 25. This strain is non-invasive but it is fully competent for type III secretion and protein delivery. Prior to infection, cultured cells were loaded with alexa 594 dextran (100,00MW, 1mg/ml) to label the endocytic pathway. One hour after infection, cells were washed three times with cold HBSS, fixed and processed for immunofluorescence. To determine whether SopB delivered from extracellular bacteria could associate with phagosomes derived from invasive S. Typhimurium we performed mixed infection experiments. Henle-407 cells were co-infected with wild type S. Typhimurium expressing DsRED as a marker and a non-invasive a DsopE ΔsopE2 ΔsopB S. Typhimurium mutant strain expressing a catalytically inactive FLAG-epitope tagged SopB mutant (SopBC460S) at a ratio of 1:5. Cells were infected with a combined m.o.i of 40 for 1 hr before fixing and processing for immunofluoresence as described above.
S. Typhimurium-induced Akt activation
S. Typhimurium induced Akt activation after infection was monitored in soluble cell lysates by probing with antibodies directed to phospho-specific Ser473Akt and Akt (Cell Signalling. Cells were lysed in 50mM Tris pH7.6, 150mM NaCl, 10% glycerol, 1% Triton X100, 1mM EDTA, 2mM MgCl2, 100mM NaF, 200μM NaVO4, 1mM PMSF, 1x protease inhibitor cocktail.
S. Typhimurium replication assay
S. Typhimurium replication assays were preformed in Henle-407 cells and bone marrow-derived macrophages from caspase-1-deficient mice (which are resistant to to rapid S. Typhimurium-induced killing) as previously described (Hernandez et al., 2004).
SopB in vitro phosphatase assay
GST tagged derivatives of SopB and SopB288-309 were expressed and purified by standard affinity purification procedures using glutathione-coupled Sepharose 4B beads (Pharmacia). SopB protein phosphatase activity was measured using a malachite green chromogenic assay, as previously described (Lee et al., 2004).
Acknowledgments
We thank Walther Mothes for providing reagents, Christopher Case and Catarina Nogueira for caspase-1 deficient bone marrow derived macrophages, and members of the Galán laboratory for critical review of this manuscript. K. H. was supported by a fellowship from the Damon Runyon Cancer Research Foundation. This work was supported by Public Health Research Grant AI055472 to J. E. G.
Footnotes
SUPPLEMENTAL DATA Supplemental data for this article include 7 figures and 3 videos
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Lukacs GL, Brech A, Stenmark H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol Biol Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fre S, Slepnev V, Capua M, Takei K, Butler M, Di Fiore P, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Chen LM, Hobbie S, Galan JE. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- Collazo C, Galán JE. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock D, Schmid S. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- Drecktrah D, Knodler L, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 2005:105–113. doi: 10.1111/j.1462-5822.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Galán JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- Galan JE. SnapShot: effector proteins of type III secretion systems. Cell. 2007;130:192. doi: 10.1016/j.cell.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Galán JE. Salmonella interaction with host cells: Type III Secretion at Work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- Galán JE, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Galyov EE, Wood MW, Rosqvist R, Mullan PB, Watson PR, Hedges S, Wallis TS. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:1903–1912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Di Fiore P, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003a;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore P, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003b;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Hardt W-D, Chen L-M, Schuebel KE, Bustelo XR, Galán JE. Salmonella typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Hernandez LD, Hueffer K, Wenk MR, Galán JE. A Salmonella protein modulates vesicular trafficking by altering phosphoinositide metabolism. Science. 2004;18:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hong KH, Miller VL. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J Bacteriol. 1998;180:1793–1802. doi: 10.1128/jb.180.7.1793-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Kaniga K, Bossio JC, Galán JE. The Salmonella typhimurium invasion genes invF and invG encode homologues to the PulD and AraC family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Kubori T, Galan JE. Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell. 2003;115:333–342. doi: 10.1016/s0092-8674(03)00849-3. [DOI] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerød L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Mallo G, Espina M, Smith A, Terebiznik M, Alemán A, Finlay B, Rameh L, Grinstein S, Brumell J. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S, Knodler L, Finlay B. Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell Microbiol. 2002;4:435–446. doi: 10.1046/j.1462-5822.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- Miao EA, Scherer CA, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ, Miller SI. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol Microbiol. 1999;34:850–864. doi: 10.1046/j.1365-2958.1999.01651.x. [DOI] [PubMed] [Google Scholar]
- Norris FA, Wilson MP, Wallis TS, Galyov EE, Majerus PW. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sc U S A. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J, Galán J. Investigating the function of Rho family GTPases during Salmonella/host cell interactions. Methods Enzymol. 2008;439:145–158. doi: 10.1016/S0076-6879(07)00411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Galan JE. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J Cell Biol. 2006;175:453–463. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada C, Hicks S, Galán J, Stebbins E. A Salmonella Virulence Factor is a Novel E3 Ligase Mimic. 2008 submitted for publication. [Google Scholar]
- Raiborg C, Malerod L, Pedersen NM, Stenmark H. Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking. Exp Cell Res. 2008;314:801–813. doi: 10.1016/j.yexcr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Rogers L, Kristensen A, Boyle E, Robinson D, Ly R, Finlay B, Foster L. Identification of cognate host targets and specific ubiquitylation sites on the Salmonella SPI-1 effector SopB/SigD. J Proteomics. 2008;71:97–108. doi: 10.1016/j.jprot.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Rohde J, Breitkreutz A, Chenal A, Sansonetti P, Parsot C. Type III Secretion Effectors of the IpaH Family Are E3 Ubiquitin Ligases. Cell Host Microbe. 2007;1:77–83. doi: 10.1016/j.chom.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr S. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Galán JE. Structural mimicry in bacterial virulence. Nature. 2001;412:701–705. doi: 10.1038/35089000. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O, Knodler L, Marcus S, Scheid M, Goh B, Pfeifer C, Duronio V, Finlay B. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J Biol Chem. 2000;275:37718–37724. doi: 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- Urbe S. Ubiquitin and endocytic protein sorting. Essays Biochem. 2005;41:81–98. doi: 10.1042/EB0410081. [DOI] [PubMed] [Google Scholar]
- Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5:501–511. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Higashide W, McCormick B, Chen J, Zhou D. The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol. 2006;62:786–793. doi: 10.1111/j.1365-2958.2006.05407.x. [DOI] [PubMed] [Google Scholar]
- Zhou D, Chen LM, Hernandez L, Shears SB, Galán JE. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39:248–259. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]