Abstract
BACKGROUND
Extensively drug-resistant tuberculosis has been reported in 45 countries, including countries with limited resources and a high burden of tuberculosis. We describe the management of extensively drug-resistant tuberculosis and treatment outcomes among patients who were referred for individualized outpatient therapy in Peru.
METHODS
A total of 810 patients were referred for free individualized therapy, including drug treatment, resective surgery, adverse-event management, and nutritional and psychosocial support. We tested isolates from 651 patients for extensively drug-resistant tuberculosis and developed regimens that included five or more drugs to which the infecting isolate was not resistant.
RESULTS
Of the 651 patients tested, 48 (7.4%) had extensively drug-resistant tuberculosis; the remaining 603 patients had multidrug-resistant tuberculosis. The patients with extensively drug-resistant tuberculosis had undergone more treatment than the other patients (mean [±SD] number of regimens, 4.2±1.9 vs. 3.2±1.6; P<0.001) and had isolates that were resistant to more drugs (number of drugs, 8.4±1.1 vs. 5.3±1.5; P<0.001). None of the patients with extensively drug-resistant tuberculosis were coinfected with the human immunodeficiency virus (HIV). Patients with extensively drug-resistant tuberculosis received daily, supervised therapy with an average of 5.3±1.3 drugs, including cycloserine, an injectable drug, and a fluoroquinolone. Twenty-nine of these patients (60.4%) completed treatment or were cured, as compared with 400 patients (66.3%) with multidrug-resistant tuberculosis (P=0.36).
CONCLUSIONS
Extensively drug-resistant tuberculosis can be cured in HIV-negative patients through outpatient treatment, even in those who have received multiple prior courses of therapy for tuberculosis.
Extensively drug-resistant tuberculosis has been reported in 45 countries1 since it was first described in 2006. This seminal survey found extensively drug-resistant tuberculosis — then defined as Mycobacterium tuberculosis strains with resistance to at least isoniazid, rifampin, and members of three of six classes of second-line drugs — in 10% of multidrug-resistant tuberculosis strains collected on six continents.2 Isoniazid and rifampin are the anchors of standard first-line therapy.3 Resistance to these two drugs, which defines multidrug-resistant tuberculosis, is associated with a decreased probability of cure.4–6 Treatment regimens for multidrug-resistant tuberculosis rely on the most active second-line drug classes — fluoroquinolones and injectable agents (amikacin, capreomycin, and kanamycin).7,8 For patients with infecting isolates that are resistant to drugs in these classes — now defined as extensively drug-resistant tuberculosis9 — the probability of cure is often even lower.10–12
A report from KwaZulu–Natal Province, South Africa, suggested that in patients coinfected with human immunodeficiency virus (HIV), extensively drug-resistant tuberculosis is rapidly fatal if not treated13; other reports have raised the specter of untreatable tuberculosis.10,14,15 The standard of care for patients with resistant tuberculosis consists of hospital-based regimens that are customized according to the treatment history and strain-susceptibility profile. In many resource-poor settings, however, obstacles to appropriate treatment exist, including inadequate infrastructure and insufficient numbers of trained staff.16
Outpatient therapy, provided free of charge to patients through a country’s public health system and delivered by community health workers, overcomes many of these impediments. Such a program has been established in Peru, with supervised outpatient treatment of resistant tuberculosis delivered under the aegis of a model, decentralized national tuberculosis program.17,18 Individualized treatment for multidrug-resistant tuberculosis was introduced in a pilot project in northern Lima in 1996 and subsequently offered nationwide.19,20 Care is provided to patients not cured by first-line tuberculosis regimens. Here, we report the baseline prevalence of extensively drug-resistant tuberculosis and the characteristics of patients receiving individualized tuberculosis treatment. We also describe the strategy used and the outcomes achieved in treating patients with extensively drug-resistant tuberculosis.
METHODS
We conducted a retrospective study of patients referred for individualized tuberculosis treatment in metropolitan Lima, Peru, between February 1, 1999, and July 31, 2002. Comprehensive outpatient treatment, free of charge to patients, was delivered to all eligible patients in the catchment area by a consortium led by the National Tuberculosis Program. The study was approved by the Office for Research Subject Protection at Harvard Medical School and the Ministry of Health in Peru. Written informed consent was obtained from all participants.
STUDY POPULATION
Our study included 810 patients with tuberculosis. Most had been treated unsuccessfully for the disease (defined as treatment failure or relapse); a few had had contact with patients with multidrug-resistant tuberculosis. Eligibility for treatment was determined on the basis of the treatment and contact history, irrespective of the severity of the disease and whether or not the patient had been hospitalized. During the study period, changes in program policy permitted enrollment of patients who had received fewer prior treatment regimens.18,21 Drug-susceptibility profiles were not normally known at the time of referral.
Drug-susceptibility testing was ordered for all referred patients. Inclusion in the study was restricted to patients with baseline drug-susceptibility test results for at least four drugs: isoniazid and rifampin, one fluoroquinolone, and one second-line injectable drug (kanamycin, capreomycin, or amikacin). Seven patients who underwent drug-susceptibility testing were not included because they died before individualized treatment could be initiated.
DRUG-SUSCEPTIBILITY TESTING AND TREATMENT
Drug-susceptibility testing was performed at the Massachusetts State Laboratory Institute.19 At the start of the enrollment period, the standard drug-susceptibility test panel included isoniazid, rifampin, ethambutol, pyrazinamide, streptomycin, kanamycin, cycloserine, capreomycin, ethionamide, and ciprofloxacin. By 2001, tests for aminosalicylic acid (para-aminosalicylic acid, or PAS), amikacin, and levofloxacin were also routinely performed. (Concentrations and methods used for drug-susceptibility testing are detailed in Table 1 in the Supplementary Appendix, available with the full text of this article at www.nejm.org.) Clinicians could request additional drug-susceptibility testing for patients as required to develop a satisfactory regimen.8
Regimens were constructed with a goal of prescribing at least five antituberculosis agents likely to be effective, including a fluoroquinolone and an injectable agent (see Table 2 in the Supplementary Appendix for details on dosing)8; there were no special treatment protocols for patients with extensively drug-resistant tuberculosis. Empirical therapy, based on the treatment and contact history, was started pending the results of the drug-susceptibility tests. Once test results were available, regimens were adjusted as necessary. A drug was considered likely to be effective if all baseline drug-susceptibility tests showed susceptibility to that drug; if no drug-susceptibility test results were available, a drug was considered likely to be effective if the patient had not received that drug for at least 30 days. The duration of treatment was at least 18 months for oral agents and at least 8 months after culture conversion for the injectable drug. If a regimen did not contain five medications categorized as likely to be effective, reinforcing strategies were implemented; these were extending the duration of treatment with the injectable agent or the duration of the whole regimen, adding other drugs, or both. Added drugs included those that the patient had received previously but to which the patient’s isolate had confirmed in vitro susceptibility. Clarithromycin, amoxicillin–clavulanate, clofazimine, and rifabutin, which have questionable activity against multidrug-resistant tuberculosis, could also be added. Patients with localized disease were referred for resective thoracic surgery, according to criteria described previously.22 After surgery, medical therapy was continued, often for more than 18 months.
Comprehensive treatment included other standard elements. Community health workers supervised daily ambulatory treatment.20 Hospitalization was available, if medically indicated. Patients requiring hospitalization were transferred to outpatient care once their condition had stabilized and they had been discharged. Baseline screening and ongoing monitoring are detailed elsewhere.19 Monthly sputum samples were collected for smear microscopy and culture, which was performed at local laboratories.
In patients without conversion of sputum culture after 4 months of treatment, drug-susceptibility testing was performed, with reinforcement of the regimen (defined as the addition or substitution of two agents that were likely to be effective), if possible. This practice was repeated as necessary. Adverse events were managed aggressively with the use of standard algorithms.23,24 Nutritional support, group therapy, and opportunities for income generation were provided, as needed.25,26 After the completion of treatment, patients were screened, clinically and bacteriologically, for recurrent disease. If disease was detected, efforts were made to reduce household transmission and, when possible, to restart individualized regimens.
DATA COLLECTION
Variables collected through standardized chart abstraction included previous treatment exposure, demographic characteristics, presence or absence of cavitary and bilateral disease on chest radiography, presence or absence of coinfection with HIV (HIV testing was routinely offered at baseline), and limited hospitalization data. Data on the frequency of adverse events and related regimen changes were not abstracted.
DEFINITIONS OF TERMS AND OUTCOMES
Patients were classified according to the results of all baseline drug-susceptibility tests, which were those performed on sputum specimens collected before, or up to 31 days after, initiation of the individualized regimen. Patients whose isolates were not resistant to at least isoniazid and rifampin were excluded from analysis. Multidrug-resistant tuberculosis was defined as resistance to both isoniazid and rifampin but not to both an injectable agent and a fluoroquinolone. Extensively drug-resistant tuberculosis was defined as laboratory-confirmed resistance to all of the following, at minimum: isoniazid, rifampin, any fluoroquinolone, and any second-line injectable agent.9 Although subsequent resistance testing was performed in cases of nonresponse to treatment, patients were not reclassified according to these results.
Standard definitions for cure, treatment completion, treatment failure, and death were used.27 Treatment default was a physician-defined end point assigned upon the failure of attempts to return to therapy those patients who had not been adhering to their treatment regimen. Recurrent disease was defined as two or more positive bacteriologic results, or reinitiation of therapy, after treatment completion or cure.
STATISTICAL ANALYSIS
We used SAS software, version 9.12, for data analysis. All P values — calculated with the chi-square test for dichotomous variables, odd ratios, and hazard ratios and with Student’s or Satterthwaite’s t-test for continuous values — were two-sided. The effect of extensively drug-resistant tuberculosis on time to an end point (culture conversion, cure, or death) was estimated with the use of the product-limit method.28 Data were censored when an outcome other than death was recorded. Patient follow-up ended (and data were censored) on September 12, 2007, for patients still receiving treatment.
RESULTS
Of 810 patients receiving individualized regimens, 651 had isolates that were tested for the requisite drugs at baseline. Extensively drug-resistant tuberculosis was identified in 48 of these patients (7.4%); multidrug-resistant tuberculosis was identified in the remaining 603 (92.6%). None of the seven patients who died before treatment were infected with HIV; one of these patients (14.3%) had extensively drug-resistant tuberculosis, and all the others (85.7%) had multidrug-resistant tuberculosis.
Patients with extensively drug-resistant tuberculosis had undergone more treatment regimens before the study than had patients with multidrug-resistant tuberculosis (mean [±SD] number of regimens, 4.2±1.9 vs. 3.2±1.6; P<0.001). The former group also had isolates resistant to more of the 12 agents or classes for which drug-susceptibility testing was routinely performed (8.4±1.1 vs. 5.3±1.5, P<0.001). Coinfection with HIV was rare, occurring in nine patients with multidrug-resistant tuberculosis (1.5%) and none of the patients with extensively drug-resistant tuberculosis (P = 1.00) (Table 1).
Table 1.
Distribution of Baseline Patient Characteristics According to Resistance Profile.*
| Characteristic | XDR Tuberculosis (N = 48) | MDR Tuberculosis (N = 603) | P Value† |
|---|---|---|---|
| Resistance and prior exposure | |||
| No. of previous treatment regimens‡ | 4.2±1.9 | 3.2±1.6 | <0.001 |
| Cumulative months of previous treatment | 34.7±23.7 | 25.1±16.6 | <0.001 |
| No. of agents to which baseline isolate was resistant, of possible 12§ | 8.4±1.1 | 5.3±1.5 | <0.001 |
| Previous treatment (>1 mo) with a fluoroquinolone and an injectable agent (other than streptomycin) — no./total no. (%) | 42/48 (87.5) | 378/600 (63.0) | <0.001 |
| Clinical data | |||
| HIV infection — no./total no. (%) | 0/48 | 9/587 (1.5) | 1.00 |
| Bilateral, cavitary findings — no./total no. (%) | 26/45 (57.8) | 315/573 (55.0) | 0.72 |
| Hospitalized at treatment initiation — no./total no. (%) | 3/48 (6.3) | 26/603 (4.3) | 0.47 |
| Demographic data | |||
| Female sex — no./total no. (%) | 17/48 (35.4) | 241/603 (40.0) | 0.54 |
| Age — yr | 32.0±9.9 | 31.5±12.4 | 0.76 |
Plus–minus values are means ±SD. DST denotes drug-susceptibility test, HIV human immunodeficiency virus, MDR multidrug-resistant tuberculosis, and XDR extensively drug-resistant tuberculosis.
P values were calculated with Student’s or Satterthwaite’s t-test for continuous variables, with Fisher’s exact test for HIV infection and hospitalization at treatment initiation, and with the chi-square test for all other baseline characteristics.
Only two patients, one in each group, had not previously received treatment for tuberculosis.
Resistance to the following 12 drugs or drug classes was tested: capreomycin, cycloserine, ethambutol, ethionamide, isoniazid, kanamycin or amikacin, PAS, pyrazinamide, rifampicin, streptomycin, first-generation fluoroquinolones (ciprofloxacin, ofloxacin), and later-generation fluoroquinolones (gatifloxacin, levofloxacin, moxifloxacin).
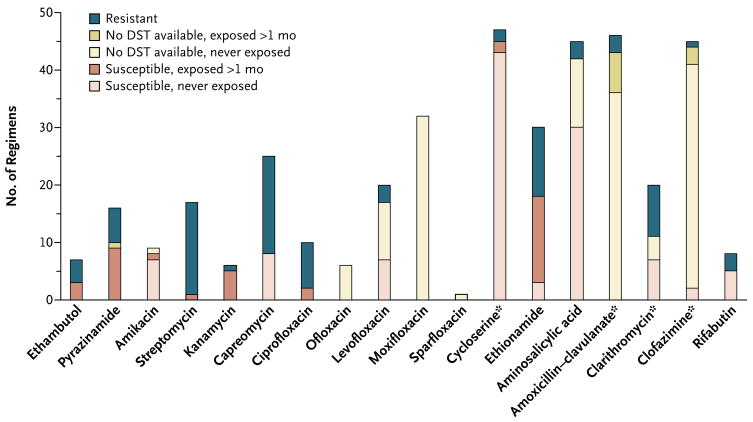
Patients with extensively drug-resistant tuberculosis received an average of 5.3±1.3 antimycobacterial agents for which either susceptibility had been documented or the duration of prior exposure had not exceeded 1 month and susceptibility had not been tested. All patients with extensively drug-resistant tuberculosis received cycloserine, at least one injectable agent, and one fluoroquinolone (Fig. 1). Forty-six patients with extensively drug-resistant tuberculosis (97.9%) received two or more of the following agents: amoxicillin–clavulanate, clarithromycin, clofazimine, and rifabutin (Table 2; see Table 3 in the Supplementary Appendix for details of the regimen for each patient). Additional drugs, either previously administered or with inconsistent results on drug-susceptibility tests, were often included.
Figure 1. Use of Antituberculosis Agents in 48 Individualized Treatment Regimens for MDR Tuberculosis, Drug-Susceptibility Testing, and Prior Exposure to a Particular Agent.
Some susceptibility testing was performed for these agents. Asterisks indicate that some testing was performed for these agents. However, because of the relative infrequency of testing, as well as the lack of standardization or confirmed clinical relevance of tests for these drugs, clinicians relied less on these results than on those for other drugs.
Table 2.
Individualized Regimens Prescribed for 47 Patients with XDR Tuberculosis.*
| Regimen | Value |
|---|---|
| Characteristics | |
| Duration of treatment — mo | |
| Median | 24.9 |
| Interquartile range | 13.3–29.0 |
| Duration of treatment with injectable agents — mo | |
| Median | 15.4 |
| Interquartile range | 11.4–19.7 |
| No. of drugs in regimen (no. without documented resistance or prior exposure for >1 mo) | |
| Among all available agents | 5.3±1.3 |
| Among 12 agents or classes for which routine DST was performed† | 3.2±1.2 |
| Drugs included | |
| First-line oral agents | |
| Ethambutol | |
| No. of patients — % | 7 (14.9) |
| Duration of treatment (mo) — median (interquartile range) | 24.4 (14.0–28.6) |
| Pyrazinamide | |
| No. — % | 16 (34.0) |
| Duration of treatment (mo) — median (interquartile range) | 24.7 (10.0–26.4) |
| First-line injectable agent | |
| Streptomycin | |
| No. of patients — % | 18 (38.3) |
| Duration of treatment (mo) — median (interquartile range) | 19.1 (13.0–25.1) |
| Second-line injectable agents | |
| Amikacin | |
| No. of patients — % | 9 (19.1) |
| Duration of treatment (mo) — median (interquartile range) | 14.5 (8.8–18.1) |
| Capreomycin | |
| No. of patients — % | 25 (53.2) |
| Duration of treatment (mo) — median (interquartile range) | 12.5 (8.8–18.5) |
| Kanamycin | |
| No. of patients — % | 8 (17.0) |
| Duration of treatment (mo) — median (interquartile range) | 9.0 (3.6–13.0) |
| First-generation fluoroquinolones | |
| Ciprofloxacin | |
| No. of patients — % | 10 (21.3) |
| Duration of treatment (mo) — median (interquartile range) | 8.0 (4.2–9.9) |
| Ofloxacin | |
| No. of patients — % | 6 (12.8) |
| Duration of treatment (mo) — median (interquartile range) | 9.9 (6.3–13.7) |
| Later-generation fluoroquinolones | |
| Levofloxacin | |
| No. of patients — % | 20 (42.6) |
| Duration of treatment (mo) — median (interquartile range) | 12.1 (7.4–23.0) |
| Sparfloxacin | |
| No. of patients — % | 1 (2.1) |
| Duration of treatment (mo) | 12.1 |
| Moxifloxacin | |
| No. of patients — % | 34 (72.3) |
| Duration of treatment (mo) — median (interquartile range) | 21.2 (8.8–27.9) |
| Other second-line oral agents | |
| Cycloserine | |
| No. of patients — % | 47 (100) |
| Duration of treatment (mo) — median (interquartile range) | 24.7 (12.7–28.1) |
| Ethionamide | |
| No. of patients — % | 31 (66.0) |
| Duration of treatment (mo) — median (interquartile range) | 24.0 (10.7–27.9) |
| PAS | |
| No. of patients — % | 45 (95.7) |
| Duration of treatment (mo) — median (interquartile range) | 24.9 (12.7–28.3) |
| Other agents | |
| Amoxicillin–clavulanate | |
| No. of patients — % | 47 (100) |
| Duration of treatment (mo) — median (interquartile range) | 24.0 (11.7–28.1) |
| Clarithromycin | |
| No. of patients — % | 21 (44.7) |
| Duration of treatment (mo) — median (interquartile range) | 14.5 (11.6–18.9) |
| Clofazimine | |
| No. of patients — % | 46 (97.9) |
| Duration of treatment (mo) — median (interquartile range) | 24.1 (12.5–27.9) |
| Rifabutin | |
| No. of patients — % | 8 (17.0) |
| Duration of treatment (mo) — median (interquartile range) | 12.8 (8.5–14.7) |
One patient died less than 1 month after starting treatment, before regimen data were recorded. Plus–minus values are means ±SD. DST denotes drug-susceptibility test, and XDR extensively drug-resistant tuberculosis.
Resistance to the following 12 drugs or drug classes was tested: capreomycin, cycloserine, ethambutol, ethionamide, isoniazid, kanamycin or amikacin, PAS, pyrazinamide, rifampicin, streptomycin, first-generation fluoroquinolones (ciprofloxacin, ofloxacin), and later-generation fluoroquinolones (gatifloxacin, levofloxacin, moxifloxacin).
Eleven patients with extensively drug-resistant tuberculosis were hospitalized at one or more points during individualized treatment; the median cumulative duration of hospital care was 14 days (interquartile range, 5 to 54), including hospitalization for surgical resection (Table 3). Surgery, as an adjunctive treatment for tuberculosis, was performed in 7 patients with extensively drug-resistant tuberculosis (14.6%) and in 87 patients with multidrug-resistant tuberculosis (14.4%). Three patients with extensively drug-resistant tuberculosis had positive cultures at the time of surgery. In two of these patients, the sputum culture had remained positive during individualized treatment; both had poor outcomes.
Table 3.
Hospitalization and Resective Thoracic Surgery among Patients with XDR Tuberculosis.*
| Characteristic | Value |
|---|---|
| Patients hospitalized during individualized treatment regimen — no./total no. (%) | 11/48 (22.9) |
| No. of days of hospitalization during individualized treatment regimen — median (interquartile range) | 14 (5–54) |
| Patients undergoing surgical resection during individualized treatment regimen — no./total no. (%) | 7/48 (14.6) |
| Type of surgery — no. (%) | |
| Lobectomy | 5 (10.4) |
| Pneumonectomy | 1 (2.1) |
| Cavitary resection | 1 (2.1) |
| No. of months from treatment initiation to surgery — median (interquartile range) | 11.6 (7.1–24.1) |
| Patients with positive sputum culture at surgery — no. (%) | 3 (42.9) |
| No. of months of treatment for patients undergoing surgery— median (interquartile range) | 31.2 (25.1–57.9) |
| Patients undergoing surgery who subsequently died or whose treatment failed — no. (%) | 2 (28.6) |
XDR denotes extensively drug-resistant.
A median of 99.6% of expected monthly cultures were performed (interquartile range, 90.9 to 100), permitting evaluation of response to therapy and outcomes at the end of treatment.
Among 16 patients (33.3%) with extensively drug-resistant tuberculosis whose sputum cultures remained positive after 4 months of treatment, regimen reinforcement was possible in only 3 (18.8%). Adjustments in the regimen included a change to a later-generation fluoroquinolone, a change in the injectable agent, and the addition of one or more other agents (amoxicillin–clavulanate, clarithromycin, or clofazimine).
The median time to conversion from a positive to a negative culture was longer for patients with extensively drug-resistant tuberculosis than for patients with multidrug-resistant tuberculosis (90 days vs. 61 days; hazard ratio, 0.63; 95% confidence interval [CI], 0.45 to 0.89]) (Table 4). Twenty-nine patients (60.4%) with extensively drug-resistant tuberculosis completed treatment or were cured, as compared with 400 patients (66.3%) with multidrug-resistant tuberculosis (P = 0.36). The hazard ratio for death among patients with extensively drug-resistant tuberculosis, as compared with those with multidrug-resistant tuberculosis, was 1.09 (95% CI, 0.59 to 2.02; P = 0.79). Causes of death and reasons for default were not available.
Table 4.
Response and Time to Response According to Type of Resistance at Beginning of Individualized Treatment Regimen.
| Outcome | XDR Tuberculosis (N = 48) | MDR Tuberculosis(N = 603) | Effect Estimate and P Value* |
|---|---|---|---|
| Response at end of treatment | |||
| Good outcome — no. (%) | 29 (60.4) | 400 (66.3) | |
| Cured | 29 (60.4) | 395 (65.6) | |
| Completed† | 0 (0.0) | 5 (0.8) | |
| Poor outcome — no. (%) | 19 (39.6) | 198 (32.8) | OR, 1.32; 95% CI, 0.72–2.42; P = 0.36 |
| Defaulted‡ | 3 (6.2) | 62 (10.3) | |
| Treatment failed§ | 5 (10.4) | 13 (2.1) | |
| Died | 11 (22.9) | 123 (20.4) | |
| Time to interim response and to response at end of treatment — median (95% CI) | |||
| No. of days to culture conversion | 90 (57–115) | 61 (59–67) | HR, 0.63; 95% CI, 0.45–0.89; P = 0.008 |
| No. of months to cure | 26.0 (24.6–27.8) | 24.8 (24.5–25.2) | HR, 0.83; 95% CI, 0.56–1.21; P = 0.33 |
Effect estimates are for the group of patients with extensively drug-resistant (XDR) tuberculosis as compared with the group that had multidrug-resistant (MDR) tuberculosis. The odds ratio (OR) and the hazard ratios (HR) are unadjusted. Outcomes were not available for five patients, all of whom had MDR tuberculosis; four transferred out of the program and one remained in treatment. P values for the OR and the HRs were calculated with the use of the chi-square test.
Patients who completed treatment are defined as those who finished treatment according to protocol but who did not meet the definition for cure or treatment failure owing to lack of bacteriologic results (i.e., fewer than five cultures were grown in the final 12 months of therapy).
Treatment default was defined as the failure of attempts to return to therapy patients who were not adhering to their treatment regimens.
Treatment was considered to fail for those patients who had two or more positive cultures among the five cultures recorded in the final 12 months of the study or for whom any one of the final three cultures was positive.
Positive bacteriologic results were reported after cure or completion of treatment in 2 patients (6.9%) with extensively drug-resistant tuberculosis and 15 patients (3.8%) with multidrug-resistant tuberculosis (P = 0.40). The median duration of follow up was 19.4 months (interquartile range, 10.7 to 27.0). Data on treatment reinitiation were unavailable as of March 17, 2008.
DISCUSSION
This study shows that an aggressive, comprehensive management program can cure more than 60% of patients with extensively drug-resistant tuberculosis who are not infected with HIV but who have received numerous unsuccessful antituberculosis treatments. Culture conversion was delayed by nearly 1 month in the study patients who had extensively drug-resistant tuberculosis as compared with those who had multidrug-resistant tuberculosis, yet the frequency of cure or relapse and the risk of death did not differ significantly between the two groups of patients. The sample size afforded 80% power to detect differences in risk of more than two. The comparison population — patients with multidrug-resistant tuberculosis — had extensive resistance, prior treatment exposure, and parenchymal damage, which may explain the attenuated difference.
Several principles of management of highly resistant disease were concurrently applied to all patients in this program. First, aggressive regimens — with many drugs, at the highest tolerated doses — were used to maximize the chemotherapeutic benefit. Treatment was protracted, lasting more than 2 years in most patients. The results of drug-susceptibility testing were used to design (and adjust) regimens containing at least five drugs that were likely to be effective whenever possible. Regimens relied heavily on three agents with little prior use in Peru: capreomycin, PAS, and cycloserine.
Since resistance to more than one aminoglycoside was common, capreomycin, a polypeptide, was used in 25 of the patients with extensively drug-resistant tuberculosis (52%). Streptomycin was another alternative; it was prescribed when susceptibility to streptomycin at a concentration of 10 μg per milliliter was documented. In this study the injectable agent was delivered for a median of nearly 15 months as compared with a median of less than 6 months in other studies in which the duration of use of injectables was reported.18,29,30
Moxifloxacin and levofloxacin were commonly included in the individualized regimens — even in patients with isolates that were resistant to ciprofloxacin. This practice reflects the importance of using fluoroquinolones in the treatment of multidrug-resistant tuberculosis7,8,31 and is supported by evidence that the efficacy of later-generation fluoroquinolones may be preserved, despite resistance to ciprofloxacin.32–34
Finally, when necessary, clinicians reinforced regimens with pyrazinamide and ethambutol (despite extensive prior exposure to these drugs) with drugs for which susceptibility test results were inconsistent, and with amoxicillin–clavulanate, clarithromycin, clofazimine, and rifabutin.35–38 Although evidence of the efficacy of these approaches is limited at best, we cannot exclude the possibility that one or more contributed to treatment success, either by increasing the regimen’s activity or by providing protection against the emergence of resistance to more active agents.39 We also cannot exclude the possibility that additional toxic effects induced by this strategy increased the probability of treatment default. With the default from treatment of only three patients with extensively drug-resistant tuberculosis, however, additional toxicity is an unlikely influence.
Second, resective surgery was indicated for patients with high-grade resistance, relatively localized disease, and lack of initial response, even in patients with restricted lung volume.22 Medical treatment was prolonged among patients receiving surgery. Poor outcomes among patients with extensively drug-resistant tuberculosis who had surgery were less frequent (28.6%) than among all patients with extensively drug-resistant tuberculosis (39.6%). Since patients for whom treatment failed or who died were not eligible for surgery, however, we cannot draw a causal inference about its effect. Corticosteroid use, although not recorded, was frequent.24 Other adjunctive therapies were rejected for lack of evidence.40–44
Third, frequent contact with health care workers afforded many benefits. Daily, supervised treatment was delivered in patients’ homes and at health centers. Workers ensured a high level of treatment adherence and promptly identified circumstances requiring additional attention. These included adverse events, which were managed aggressively by nurses and clinicians. Psychosocial needs were also assessed continuously and addressed with a range of interventions.26
Fourth, bacteriologic assessment was integral to the strategy. Monthly monitoring permitted early identification of patients with no response. Individualized regimen design relied on the results of baseline drug-susceptibility testing. In addition, repeated drug-susceptibility tests were performed and regimens adjusted for patients who did not have a response to treatment. The ability to adjust regimens, however, was severely restricted by extensive resistance and prior exposure.
It is noteworthy that the outcomes in our study were better than most outcomes reported from hospitals in Europe, the United States, and Korea, where cure was achieved in fewer than half of patients with extensively drug-resistant tuberculosis.10–12 One exception was a study in Latvia, in which cure was achieved in 61% of such patients. In this study, however, extensively drug-resistant tuberculosis was defined as M. tuberculosis isolates that were resistant to isoniazid, rifampin, and members of three of the six classes of second-line drugs. The hospitalized patients in KwaZulu–Natal13 had advanced acquired immunodeficiency syndrome; more than half had never received treatment for tuberculosis. They were therefore neither expected to have nor treated for resistant disease. The large number of patients who died after a short interval in this study underscores the importance of timely diagnosis of resistant tuberculosis and initiation of effective antituberculosis and antiretroviral therapy in patients who are also coinfected with HIV. The detection of a common tuberculosis strain in a large proportion of the KwaZulu–Natal patients highlights the benefit of avoiding nosocomial transmission by delivering treatment in the community. For severely ill patients, however, hospitalization may be required; in these situations, adequate infection control is essential to protect staff and patients.
Unlike most of the patients in the KwaZulu–Natal study, most of the patients in our study had long-standing tuberculosis. Less than 20% of the study patients had previously been admitted to a hospital. It is therefore improbable that nosocomial transmission of highly resistant strains of tuberculosis accounted for disease in this group. Indeed, in the patients in Peru, extensively drug-resistant tuberculosis probably reflected progressive acquisition of drug-resistance mutations during sequential exposure to inadequate treatment regimens; the patients with extensively drug-resistant tuberculosis had received even more prior therapy than had the patients with multidrug-resistant tuberculosis who had received prior treatment.
Development of extensively drug-resistant tuberculosis is inevitable when injectable agents and fluoroquinolones are used, because of selection for spontaneously occurring resistant mutants.45,46 The speed and extent to which resistance emerges can, however, be mediated through careful use of these agents. This resistance probably reflects the danger of using second-line agents in uncontrolled situations or as part of inadequate regimens.47 In Peru, as in many countries, private practitioners prescribed aminoglycosides and fluoroquinolones for tuberculosis before these agents were formally introduced as part of treatment regimens for multidrug-resistant tuberculosis. In the mid-1990s, the National Tuberculosis Program in Peru included kanamycin as part of a 12-month retreatment regimen. Subsequently, kanamycin and ciprofloxacin were included in the standardized five-drug regimen introduced by the program in October 1997 for the treatment of multidrug-resistant tuberculosis. This regimen also included ethambutol and pyrazinamide, which patients had already received. Of 466 patients treated with that regimen — which consequently contained only two or three new drugs — 48% were cured.18 The use of kanamycin and ciprofloxacin in subcurative regimens probably contributed substantially to the development of extensively drug-resistant tuberculosis; almost 90% of the patients with extensively drug-resistant tuberculosis in our study had prior exposure to these agents. Prescription of a standardized regimen for multidrug-resistant tuberculosis that contained at least four drugs categorized as likely to be effective may have produced better results than the regimen introduced in 1997.48,49
Our study has several limitations. The study design (an observational study involving individualized regimens, without controls) and the small number of patients preclude identification of patient or treatment characteristics that had a causal effect on outcomes. Data on the frequency of adverse events, and their effect on completion of the regimen, were not available; prior work, however, revealed that severe adverse events were uncommon and rarely resulted in discontinuation of the regimen.50,51 Also unavailable were data on other management approaches for extensively drug-resistant tuberculosis in Peru with which our approach could be compared. Future work will estimate the effect of various components of the approach on outcomes.
The patients in our study received therapy for extensively drug-resistant tuberculosis after having received multiple unsuccessful treatments; only one patient with extensively drug-resistant tuberculosis had never received prior treatment for tuberculosis. These patients may therefore represent a survival cohort. It is impossible, however, to assess the effect of a survivor bias on treatment outcomes in the absence of a comparison group — that is, patients with extensively drug-resistant tuberculosis who had not received prior treatment. One relevant comparison can be made between those with more and those with less exposure to prior treatment: more exposure is associated with an increased, not decreased, risk of death. Since it is common under program conditions that therapy for multidrug-resistant tuberculosis or extensively drug-resistant tuberculosis is reserved for patients who have received repeated treatments for tuberculosis, our results can be generalized to many patient populations treated in low- to middle-income countries. Exceptions include patients with HIV coinfection and those who are initially infected with a strain of extensively drug-resistant tuberculosis. As part of future efforts, it will be important to document what will probably be the superior effect of treatment for extensively drug-resistant tuberculosis in patients who have not had prior treatment for tuberculosis.
In conclusion, despite the limited resources in Peru, aggressive regimens, as part of a comprehensive outpatient therapeutic approach, cured more than half of patients with extensively drug-resistant tuberculosis who had previously been treated for tuberculosis. This strategy has now been integrated into the routine approach to treatment in Peru’s National Tuberculosis Program.52 Nevertheless, our study underscores the importance of developing other interventions that will further improve treatment outcomes, as well as stem the development and spread of extensively drug-resistant tuberculosis.53–58
Supplementary Material
Acknowledgments
Supported by grants from the Bill and Melinda Gates Foundation, Thomas J. White, Partners in Health, the Peruvian Ministry of Health, the David Rockefeller Center for Latin American Studies at Harvard University, the Francis Family Foundation, the Pittsfield Antituberculosis Association, the Eli Lilly Foundation, and the Hatch Family Foundation and by career development awards from the National Institute of Allergy and Infectious Diseases (5 K01 A1065836, to Dr. Mitnick) and the National Heart, Lung, and Blood Institute (5 K01 HL080939, to Dr. Becerra).
We thank Drs. Martin Hirsch and Megan Murray for comments on an earlier version of the manuscript, Eva Tomczyk for research assistance, and all the patients, families, and community health care workers who participated in this effort.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Antituberculosis drug resistance in the world: fourth global report. Geneva: World Health Organization; 2008. (Report no. WHO/HTM/TB/2008.394.) [Google Scholar]
- 2.Shah NS, Wright A, Bai GH, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(Suppl 2):S231–S279. [PubMed] [Google Scholar]
- 4.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 5.Coninx R, Mathieu C, Debacker M, et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. Lancet. 1999;353:969–73. doi: 10.1016/s0140-6736(98)08341-x. [DOI] [PubMed] [Google Scholar]
- 6.Lan NTN, Iademarco MF, Binkin NJ, Tung LB, Quy HT, C_ NV. A case series: initial outcome of persons with multidrug-resistant tuberculosis after treatment with the WHO standard retreatment regimen in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2001;5:575–8. [PubMed] [Google Scholar]
- 7.Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA. 1996;276:1229–35. [PubMed] [Google Scholar]
- 8.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–81. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 9.Revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep. 2006;55:1176. [Google Scholar]
- 10.Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs — worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 11.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–6. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 12.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–5. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients coinfected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 14.Masjedi MR, Farnia P, Sorooch S, et al. Extensively drug-resistant tuberculosis: 2 years of surveillance in Iran. Clin Infect Dis. 2006;43:841–7. doi: 10.1086/507542. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan LS. RNTCP 2007: looking ahead to future challenges. J Indian Med Assoc. 2007;105:192, 194, 196. [PubMed] [Google Scholar]
- 16.Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: no time for denial or complacency. PLoS Med. 2007;4(1):e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global tuberculosis control: WHO report 2001. Geneva: World Health Organization; 2001. (Report no. WHO/CDS/TB/2001.287) [Google Scholar]
- 18.Suárez PG, Floyd K, Portocarrero J, et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–9. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]
- 19.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59:1529–39. doi: 10.1016/j.socscimed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Ministerio de Salud. Actualización de la doctrina, normas y procedimientos para el control de la tuberculosis en el Perú. Lima, Perú: Dirección General de Salud de las Personas, Ministerio de Salud; 2001. [Google Scholar]
- 22.Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax. 2007;62:416–21. doi: 10.1136/thx.2005.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partners In Health, Harvard Medical School, Bill & Melinda Gates Foundation. A DOTS-Plus handbook: guide to the community-based treatment of MDR-TB. Boston: Partners In Health; 2002. [Google Scholar]
- 24.Rich ML, editor. The PIH guide to the medical management of multidrug-resistant tuberculosis: international edition. Boston: Partners In Health; 2003. [Google Scholar]
- 25.Vega P, Sweetland A, Acha J, et al. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:749–59. [PubMed] [Google Scholar]
- 26.Chalco K, Wu DY, Mestanza L, et al. Nurses as providers of emotional support to patients with MDR-TB. Int Nurs Rev. 2006;53:253–60. doi: 10.1111/j.1466-7657.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- 27.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–5. [PubMed] [Google Scholar]
- 28.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley; 1980. [Google Scholar]
- 29.Park SK, Kim CT, Song SD. Outcome of chemotherapy in 107 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. Int J Tuberc Lung Dis. 1998;2:877–84. [PubMed] [Google Scholar]
- 30.Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–51. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 31.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–26. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang JY, Lee LN, Lai HC, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J Antimicrob Chemother. 2007;59:860–5. doi: 10.1093/jac/dkm061. [DOI] [PubMed] [Google Scholar]
- 33.Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3:432–42. doi: 10.1016/s1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 34.Matrat S, Veziris N, Mayer C, et al. Functional analysis of DNA gyrase mutant enzymes carrying mutations at position 88 in the A subunit found in clinical strains of Mycobacterium tuberculosis resistant to fluoroquinolones. Antimicrob Agents Chemother. 2006;50:4170–3. doi: 10.1128/AAC.00944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donald PR, Sirgel FA, Venter A, et al. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis. 2001;33:466–9. doi: 10.1080/00365540152029954. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Tomioka H, Akaki T, Kawahara S. Antimicrobial activities of levofloxacin, clarithromycin, and KRM-1648 against Mycobacterium tuberculosis and Mycobacterium avium complex replicating within Mono Mac 6 human macrophage and A-549 type II alveolar cell lines. Int J Antimicrob Agents. 2000;16:25–9. doi: 10.1016/s0924-8579(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 37.Janulionis E, Sofer C, Song HY, Wallis RS. Lack of activity of orally administered clofazimine against intracellular Mycobacterium tuberculosis in whole-blood culture. Antimicrob Agents Chemother. 2004;48:3133–5. doi: 10.1128/AAC.48.8.3133-3135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CN, Lin TP, Chang MF, Jimenez MV, Dolfi L, Olliaro P. Rifabutin as salvage therapy for cases of chronic multidrug-resistant pulmonary tuberculosis in Taiwan. J Chemother. 1996;8:137–43. doi: 10.1179/joc.1996.8.2.137. [DOI] [PubMed] [Google Scholar]
- 39.Mitchison DA. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin Respir Crit Care Med. 2004;25:307–15. doi: 10.1055/s-2004-829503. [DOI] [PubMed] [Google Scholar]
- 40.de Bruyn G, Garner P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev. 2003;1:CD001166. doi: 10.1002/14651858.CD001166. [DOI] [PubMed] [Google Scholar]
- 41.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–5. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 42.Park SK, Cho S, Lee IH, et al. Subcutaneously administered interferon-gamma for the treatment of multidrug-resistant pulmonary tuberculosis. Int J Infect Dis. 2007;11:434–40. doi: 10.1016/j.ijid.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Jouveshomme S, Dautzenberg B, Bakdach H, Derenne JP. Preliminary results of collapse therapy with plombage for pulmonary disease caused by multidrug-resistant mycobacteria. Am J Respir Crit Care Med. 1998;157:1609–15. doi: 10.1164/ajrccm.157.5.9709047. [DOI] [PubMed] [Google Scholar]
- 44.Nitta AT, Iseman MD, Newell JD, Madsen LA, Goble M. Ten-year experience with artificial pneumoperitoneum for end-stage, drug-resistant pulmonary tuberculosis. Clin Infect Dis. 1993;16:219–22. doi: 10.1093/clind/16.2.219. [DOI] [PubMed] [Google Scholar]
- 45.Normark BH, Normark S. Evolution and spread of antibiotic resistance. J Intern Med. 2002;252:91–106. doi: 10.1046/j.1365-2796.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- 46.Iseman MD. Extensively drug-resistant Mycobacterium tuberculosis: Charles Darwin would understand. Clin Infect Dis. 2007;45:1415–6. doi: 10.1086/522988. [DOI] [PubMed] [Google Scholar]
- 47.Pillay M, Sturm AW. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin Infect Dis. 2007;45:1409–14. doi: 10.1086/522987. [DOI] [PubMed] [Google Scholar]
- 48.Rich ML, Socci AR, Mitnick CD, et al. Representative drug susceptibility patterns for guiding design of retreatment regimens for MDR-TB. Int J Tuberc Lung Dis. 2006;10:290–6. [PubMed] [Google Scholar]
- 49.Timperi R, Han LL, Sloutsky A, et al. Drug resistance profiles of Mycobacterium tuberculosis isolates: five years’ experience and insight into treatment strategies for MDR-TB in Lima, Peru. Int J Tuberc Lung Dis. 2005;9:175–80. [PubMed] [Google Scholar]
- 50.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–55. [PubMed] [Google Scholar]
- 51.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–4. [PubMed] [Google Scholar]
- 52.Ministerio de Salud. Norma técnica de salud para el control de la tuberculosis. Lima, Perú: Ministerio de Salud; 2006. [Google Scholar]
- 53.Spigelman M, Gillespie S. Tuberculosis drug development pipeline: progress and hope. Lancet. 2006;367:945–7. doi: 10.1016/S0140-6736(06)68388-8. [DOI] [PubMed] [Google Scholar]
- 54.Raviglione MC, Smith IM. XDR tuberculosis — implications for global public health. N Engl J Med. 2007;356:656–9. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 55.Dukes Hamilton C, Sterling TR, Blumberg HM, et al. Extensively drug-resistant tuberculosis: are we learning from history or repeating it? Clin Infect Dis. 2007;45:338–42. doi: 10.1086/519292. [DOI] [PubMed] [Google Scholar]
- 56.Matteelli A, Migliori GB, Cirillo D, Centis R, Girard E, Raviglione M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev Anti Infect Ther. 2007;5:857–71. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]
- 57.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–7. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farnia P, Masjedi MR, Mohammadi F, et al. Colorimetric detection of multidrug-resistant or extensively drug-resistant tuberculosis by use of malachite green indicator dye. J Clin Microbiol. 2008;46:796–9. doi: 10.1128/JCM.01435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.