Abstract
To gain a better understanding of coordinate regulation of protease gene expression in the mosquito midgut, we undertook a comprehensive molecular study of digestive carboxypeptidases in Aedes aegypti. Through a combination of cDNA cloning using degenerate PCR primers, and database mining of the recently completed Ae. aegypti genome, we cloned and characterized 18 Ae. aegypti carboxypeptidase genes. Bioinformatic analysis revealed that 11 of these genes belong to the carboxypeptidase A family (AaCPA-I through AaCPA-XI), and seven to the carboxypeptidase B gene family (AaCPB-I through AaCPB-VII). Phylogenetic analysis of 32 mosquito carboxypeptidases from five different species indicated that most of the sequence divergence in the carboxypeptidase gene family occurred prior to the separation of Aedes and Anopheles mosquito lineages. Unlike the CPA genes that are scattered throughout the Ae. aegypti genome, six of seven CPB genes were found to be located within a single 120 kb genome contig, suggesting that they most likely arose from multiple gene duplication events. Quantitative expression analysis revealed that 11 of the Ae. aegypti carboxypeptidase genes were induced up to 40-fold in the midgut in response to blood meal feeding, with peak expression times ranging from 3-36 hours post-feeding depending on the gene.
Keywords: phylogenetic analysis, blood meal, gene expression, VectorBase
1. INTRODUCTION
Carboxypeptidases are hydrolases that cleave one amino acid residue at a time from the carboxyl-terminus of proteins. Carboxypeptidases can be categorized as either digestive or regulatory carboxypeptidases. Digestive carboxypeptidases A and B are involved in the degradation of proteins in the digestive tract. Carboxypeptidase A cleaves most amino acid residues efficiently, but is less effective in cleaving C-termini containing aspartic acid, glutamic acid, arginine, lysine, or proline (Vendrell et al., 2000). In contrast, carboxypeptidase B enzymes specifically hydrolyze arginine and lysine residues at C-termini. Digestive carboxypeptidases are metallocarboyxpeptidases that require the presence of zinc as cofactor for enzymatic activity. Regulatory carboxypeptidases play an important physiological role and appear to recognize and hydrolyze specific protein substrates. For example, mosquitoes use a vitellogenic carboxypeptidase to degrade yolk proteins in oocytes (Cho et al., 1991).
The female Ae. aegypti is an anautogenous mosquito requiring blood meal protein for egg development. A major advance towards understanding the molecular events in blood meal digestion has been the molecular cloning and characterization of midgut digestive enzyme genes from mosquitoes. In Ae. aegypti, a number of digestive enzyme genes have been identified, including trypsins (Barillas-Mury et al., 1993), chymotrypsins (Jiang et al., 1997), aminopeptidases (Morlais et al., 2003), and carboxypeptidase A (Edwards et al., 2000). A carboxypeptidase A gene from Anopheles gambiae named AaCPA-1 was first cloned and characterized by Edwards et al., (Edwards et al., 1997), and more recently Lavazec et al. (Lavazec et al., 2005) characterized the expression of 23 carboxypeptidase-related genes in the same species. Although it has been shown that the AaCPA-I gene in Ae. aegypti, and the cpaAg1 (gene AGAP009593) in An. gambiae (Edwards et al., 1997, Edwards et al., 2000), are both up-regulated in the mosquito midgut by blood meal feeding, nothing is known about the expression, genomic organization, or molecular evolution of the other midgut carboxypeptidase genes that also contribute to blood meal metabolism.
2. MATERIALS AND METHODS
The Rockefeller strain of Ae. aegypti mosquito was maintained in a rearing room kept at constant temperature (27°C), relative humidity (80%), and light (16:8 L:D) conditions. Adult mosquitoes were constantly provided with 10% sucrose solution. Five day old female mosquitoes were fed porcine blood supplemented with ATP (5.0 mM final concentration).
Prior to the release of the Ae. aegypti genome database, we employed cDNA cloning with degenerate oligonucleotide primers based on conserved carboxypeptidase amino acid sequences found in the genomes of An. gambiae and Drosophila melanogaster to isolate putative Ae. aegypti carboxypeptidase sequences. Two forward and one reverse degenerate primers were used for these studies and had the sequence: F1-5′-ATHCAYGCNMGNGARTGGAT, F2-5′-GGNATHCAYGCNMGNGARTGG, and R1-5′-CGGAATTCTCTAGACTCGAGNCKNGTYTTNCKCCA. A first strand carboxypeptidase-specific cDNA was synthesized using the R1 primer and total RNA from whole body preparations of Ae. aegypti mosquitoes. Standard polymerase chain reactions (PCR) were performed with either F1 or F2 primers with R2-adapter primer (5′-CGGAATTCTCTAGACTCGAG). The PCR products were gel purified and ligated into the pCR4-TOPO vector (Invitrogen, Carlsbad, CA) according to the manufacture’s instructions. Full-length open reading frames were isolated from phage cDNA and genomic libraries constructed in Lambda Zap Expression Vector (Stratagene, La Jolla, CA) using the cDNA fragments obtained by PCR as hybridization probes. Genomic phage libraries of Ae. polynesiensis, Ochlerotatus triseriatus and Oc. epactius were also screened. In some cases, 5′ and 3′ RACE methods were used to obtain full-length cDNA sequences.
Amino acid sequences were deduced from each carboxypeptidase cDNA sequence and used to infer the location of signal peptides and to perform phylogenetic analysis. Signal peptides were predicted using PSORT II (Horton et al., 1997) and cleavage junctions were assigned based on conserved sequences amongst characterized carboxypeptidases in other organisms. Nucleotide sequences encoding the mature peptide were aligned by the ClustalW. The aligned nucleotide sequences were then onverted into deduced amino acid residues, and the protein sequences were re-aligned by ClustalW using SeaView software (Galtier et al., 1996). Then new nucleotide sequence alignments were obtained based on the amino acid alignments. An unrooted phylogram was constructed on the basis of the multiple sequence alignment for amino acids and nucleotides using the neighbor-joining method, and the robustness of topology nodes was tested by the bootstrap method with 1000 iterations.
In the gene expression study, real-time RT-PCR was performed to quantify differences in midgut carboxypeptidase gene expression after blood meal feeding. To design optimized gene-specific sense and antisense oligonucleotide primers without primer dimer formation and self-priming formation, we used OLIGO software (V.6.0, Molecular Biology Insights, Cascade, CO) for each carboxypeptidase gene (Supplement Table 1). Oligonucleotide primers were obtained from Operon, Inc. (Huntsville, AL). Real-time RT-PCR was carried out in the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) using a 96-well microtiter plate with a 10.0 μl reaction volume containing 5.0 μl SYBR Green PCR Master Mix, 3.0 μl of each primer set (0.5 µM final concentration), and 2.0 μl of a diluted cDNA template. The cDNA was synthesized using 1.0 μg of total midgut RNA isolated from three different cohorts of blood fed mosquitoes as previously described (Scaraffia et al., 2005). The resulting cDNA was contained in a final volume of 20 μl reaction and diluted 1-10 fold for use in the RT-PCR reactions depending on transcript abundance. Replica cDNA samples from each of the three mosquito cohorts were amplified for 40 cycles (15 sec at 95°C and 1 min at 60°C) and the data were analyzed by ABI software (version 1.2). Data are presented as relative transcript levels using the S7 ribosomal protein gene as an internal control. Statistical analysis of the gene expression data was done by ANOVA using GraphPad Prism software (version 4.02; GraphPad Software, Inc.).
3. RESULTS and DISCUSSION
Carboxypeptidases have distinct functional domains including a zinc binding motif and amino acid residues involved in substrate binding and enzymatic catalysis that are evolutionary conserved in a wide range of organisms (Vendrell et al., 2000). Since the Ae. aegypti genome sequence was not completed until 2007 (Nene et al., 2007), we initially used BLAST database searches to identify putative carboxypeptidase genes in the genomes of An. gambiae and D. melanogaster. To clone and sequence the homologs from Ae. aegypti, reverse transcriptase-mediated PCR was performed using total RNA from whole body mosquito preparations with degenerate primers based on highly conserved carboxypeptidase amino acid sequences. After verifying the isolation of unique digestive carboxypeptidases by nucleotide sequencing, gene-specific primers were subsequently designed for each gene in order to obtain full-length clones using a combination of cDNA library screening and 5′ and 3′ RACE. This approach resulted in the isolation of complete coding sequences for seven carboxypeptidase A genes and three carboxypeptidase B genes. Based on sequence analysis, seven carboxypeptidase A genes were isolated and subsequently named AaCPA-I through AaCPA-VII. In addition, three carboxypeptidase B genes were isolated and named AaCPB-I through AaCPB-III.
Once sequencing of the Ae. aegypti genome was completed, we used bioinformatic approaches to mine the Ae. aegypti sequence data contained in the VectorBase online resource (www.vectorbase.org). This search identified an additional eight carboxypeptidase genes using the same sequence alignment criteria applied to the other ten carboxypeptidase genes. Four of these newly identified genes were found to encode carboxypeptidase A proteins (AaCPA-VIII through AaCPA-XI) and four corresponded to carboxypeptidase B genes (AaCPB-IV through AaCPB-VII). All 18 of the Ae. aegypti carboxypeptidase genes listed in table 1 have been deposited in GENBANK.
Table 1.
Characterization of mosquito carboxypeptidases.
| Gene name | GenBank accession | Predicted protein length (aa) | Residue Length S/P/M | Specificity residue | Larval expression | Peak MG expression (hours PBM) | Rel. basal transcript level |
|---|---|---|---|---|---|---|---|
| Ae. aegypti | |||||||
| AaCPA-I | AY590487 | 427 | 21/92/314 | V | NE | 12-24 | 1.00 |
| AaCPA-II | AY590488 | 442 | 19/102/321 | V | Expressed | 12-36 | 0.77 |
| AaCPA-III | AY590489 | 421 | 18/90/313 | P | Expressed | 6-36 | 0.52 |
| AaCPA-IV | AY590490 | 414 | 19/88/307 | N | Expressed | NE | NE |
| AaCPA-V | AY590491 | 415 | 17/88/310 | R | Expressed | 36 | 8.77 |
| AaCPA-VI | AY590492 | 417 | 21/88/308 | I | Expressed | 36 | 9.10 |
| AaCPA-VII | AY590493 | 423 | 21/92/310 | M | NE | 3-24 | 10.63 |
| AaCPA-VIII | EAT39608 | 415 | 17/89/309 | V | Expressed | NE | NE |
| AaCPA-IX | EAT37218 | 422 | 21/92/309 | S | Expressed | NE | NE |
| AaCPA-X | EAT46817 | 414 | 22/84/308 | I | NE | NE | NE |
| AaCPA-XI | EAT44906 | 424 | 14/100/310 | I | Expressed | NE | NE |
| AaCPB-I | AY590494 | 412 | 18/89/305 | D | NE | 24-36 | 2.70 |
| AaCPB-II | AY590495 | 412 | 18/88/306 | D | Expressed | 3-6 | 2.32 |
| AaCPB-III | AY590496 | 425 | 20/93/312 | D | Expressed | 24-36 | 1.36 |
| AaCPB-IV | EF423586 | 437 | 20/93/324 | D | Expressed | 24 | 6.90 |
| AaCPB-V | EF423587 | 417 | 14/102/301 | D | NE | 3-36 | 0.08 |
| AaCPB-VI | EF423588 | 435 | 20/106/310 | D | NE | NE | NE |
| AaCPB-VII | EAT36173 | 457 | 19/129/309 | D | NE | NE | NE |
| Ae. polynesiensis | |||||||
| ApCPB-I | AY593983 | 412 | 18/89/305 | D | ND | ND | ND |
| Oc. triseriatus | |||||||
| OtCPB-I | AY593984 | 411 | 18/89/304 | D | ND | ND | ND |
| Oc. epactius | |||||||
| OeCPB-I | AY593985 | 411 | 18/89/304 | D | ND | ND | ND |
S: signal peptide, P: propeptide, M: mature peptide, NE: not expressed, ND: not determined,MG: midgut. Note that relative basal transcript levels were calculated from normalized levels of CPA-I transcripts in unfed mosquitoes using S7 ribosomal protein transcripts as the internal standard.
Table 1 summarizes the salient features of these 18 carboxypeptidase genes. First, the number of amino acid residues encoded by the open reading frames is very similar amongst the 18 genes. It can be seen that they all contain ~420 amino acids residues which can be broken down into a signal peptide (~20 amino acids), an activation peptide (~90 amino acids), and a mature peptide (~310 amino acids). Second, while the seven AaCPB gene products contain an aspartate residue at the position responsible for recognition of carboxyl termini with an arginine or lysine residue (Titani et al., 1975), the same position in AaCPA gene products is highly variable (see specificity residue in supplemental figure 1). Specifically, six of the predicted AaCPA proteins contain valine (AaCPA-I, AaCPA-II, AaCPA-VIII) or isoleucine (AaCPA-VI, AaCPA-X, AaCPAXI) at this position, whereas, the other five predicted AaCPA proteins contain proline (AaCPA-III), asparagine (AaCPA-IV), arginine (AaCPA-V), methionine (AaCPA-VII), or serine (AaCPA-IX) at this position. This difference in the substrate specificity residue might indicate that the AaCPA proteases are capable of degrading a broad range of proteins present in the blood meal. We also identified a number of conserved amino acid residues in the predicted AaCPA proteins that have been biochemically characterized in bovine carboxypeptidase A (Quiocho et al., 1971). Using the AaCPA-I open reading frame as a reference (signal peptide and propeptide included), these include the canonical zinc metal-binding residues (His-179, Glu-182, His-292), a pair of cysteine residues that form a disulfide bridge (Cys-245, Cys-267), and active site Tyr-356 and Glu-379 residues (supplemental figure 1).
Phylogenetic analysis of the 18 Ae. aegypti carboxypeptidase genes, based on both amino acid and nucleotide sequences, is presented in figure 1. This analysis included a selection of 24 other mosquito carboxypeptidase genes from An. gambiae, Ae. polynesiensis, Oc. triseriatus, and Oc. epactius. The tree topologies are remarkably similar and both indicate that carboxypeptidase gene duplication and divergence most likely occurred before the separation of Ae. aegypti and An. gambiae. This can be seen by the fact that the Ae. aegypti and An. gambiae carboxypeptidase genes share orthologs in all of the main branches of the tree. For example, the AaCPA-1 gene is orthologous to a carboxypeptidase A gene in An. gambiae (AGAP009593), which also shares sequence similarity with two additional Ae. aegypti carboxypeptidase A genes (AaCPA-VII and AaCPA-IX). Similarly, the AaCPB-I gene is orthologous to a carboxypeptidase B gene (AGAP006206) and shares the same branch with AaCPB-V. The three carboxypeptidase B genes we isolated from Ae. polynesiensis, Oc. triseriatus, and Oc. epactius, also share this same branch (ApCPB-I, OtCPB-I, OeCPB-I). Several differences between the two phylogenetic trees can be seen. Most notably, the three gene cluster containing AaCPA-VI, AGAP011435, and AGAP011434 was found in two related, but distinct, branches depending on whether the analysis was performed using amino acid or nucleotide sequence alignments. Other differences included the relative placement of the ancestral sequence in several two gene clusters (AaCPB-V vs. AGAP006206 and AaCPA-IX vs. AGAP009593).
Figure 1.
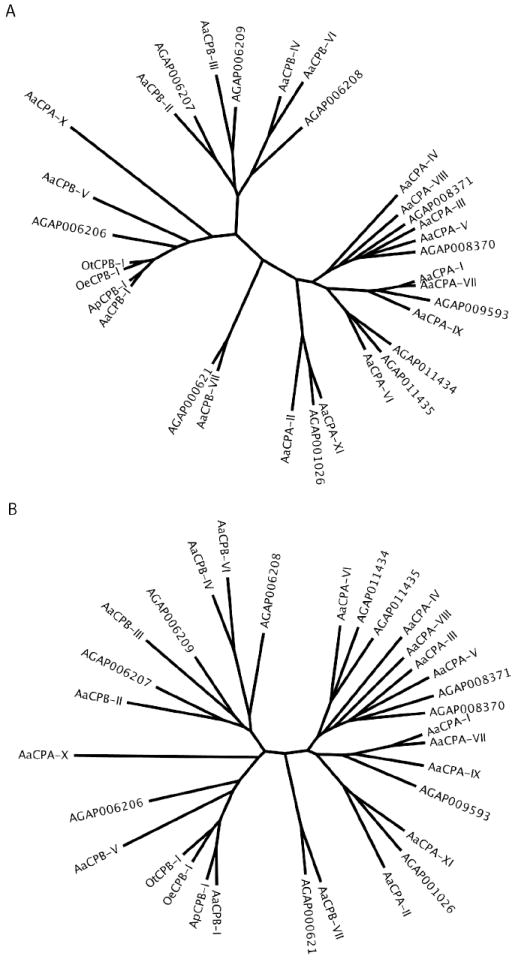
Phylogenetic analysis of amino acid and nucleotide sequences of mosquito carboxypeptidases belonging to the M14 family of zinc-metallopeptidases. A) Unrooted phylogenetic tree of 32 carboxypeptidase genes based on amino acid sequences derived from the mature peptide, omitting the signal and propeptide regions. Multiple sequence alignments were first prepared by ClustalW based on nucleotide sequences encoding the mature peptide. The aligned nucleotide sequences were then converted into deduced amino acid residues, and the protein sequences were re-aligned by ClustalW using SeaView software (see supplemental figure 1). B) Unrooted phylogenetic tree of the same 32 carboxypeptidase genes as in “A,” based on the corresponding nucleotide sequences derived from the mature peptide. The five mosquito species used in these analyses are Ae. aegypti (Aa), An. gambiae (AG), Ae. polynesiensis (Ap), Oc. triseriatus (Ot), and Oc. epactius (Oe).
While the AaCPA and AaCPB genes fall into two major regions of the tree as would be expected, the AaCPA-X gene is more similar to carboxypeptidase B genes than to carboxypeptidase A genes in regions outside of the specificity residue. This residue is an isoleucine in AaCPA-X rather than aspartate as found in all other carboxypeptidase B proteins. Based on the specificity residue, we have named it as AaCPA-X to be consistent with conventional nomenclature and its putative biochemical function, however, structurally, it may be closer to carboxypeptidase B protein (Supplemental Table 1). Based on the fact that AaCPA-X is not expressed in larvae, or in the midgut of adult mosquitoes (table 1), and no corresponding AaCPA-X sequences are present in the Ae. aegypti EST database (data not shown), the AaCPA-X gene may have accumulated mutations in its gene regulatory region or coding sequence, and as such, encodes a nonfunctional protein.
When we examined the genomic organization of the Ae. aegypti carboxypeptidase genes using VectorBase, we found that six of the seven AaCPB genes were localized to a single 120 kb contig in the genome (figure 2). This is in contrast to the 11 AaCPA genes that are located on a number of different large contigs (data not shown). The arrangement of the six AaCPB genes within the contig suggests that these genes arose by more than one gene duplication. The phylogenetic analysis reveals that the AaCPB-II and AaCPB-III genes are closely related, as are the AaCPB-VI and AaCPB-IV genes (see also supplemental figure 1). Moreover, these four genes are more similar to each other than they are to AaCBP-V or AaCBP-I. Since AaCPB-II and AaCBP-VI are on one coding strand, and AaCPB-III and AaCPB-IV are on the opposite coding strand, these genes are related to each perhaps through a secondary duplication event that resulted in an inversion of a two gene cluster. In this scenario, duplication and inversion of AaCBP-II and AaCBP-VI would give rise to AaCPB-III and AaCPB-IV. The relatedness of AaCPB-I and AaCPB-V suggests that they arose from a separate gene duplication event, whereas, AaCPB-VII, which is not found in this contig, is the most divergent of the AaCPB genes (supplemental figure 1).
Figure 2.
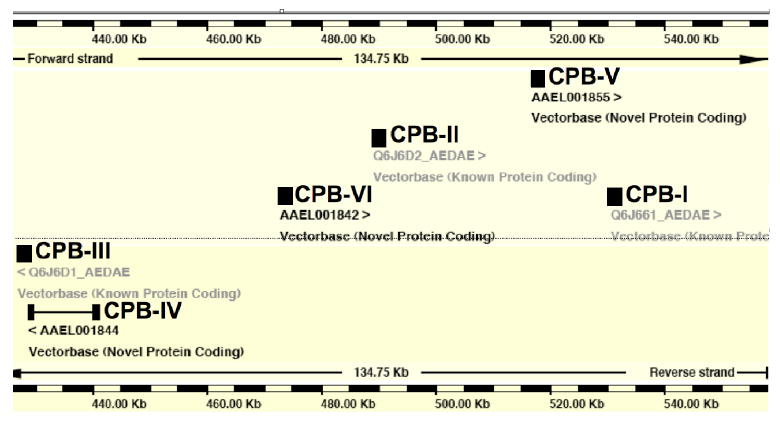
A cluster of carboxypeptidase B genes in the Ae. aegypti genome. Six of the seven carboxypeptidases in Ae. aegypti are contained within supercontig 1.44 retrieved from VectorBase (http://www.vectorbase.org). Four of the genes are encoded on the forward strand (AaCPB-I, AaCPB-II, AaCPB-IV, AaCPB-V), and two are encoded on the reverse strand (AaCPB-III, AaCPB-IV). No other protein-coding genes are predicted to reside within this contig.
In order to determine which of the Ae. aegypti carboxypeptidase genes might be required for blood meal digestion in adults, we examined CPA and CPB gene expression in both larvae and in blood fed mosquitoes using quantitative real-time PCR. As shown in table 1 and figure 3, eleven of the genes were induced in the midgut by blood meal feeding, while four were only expressed in larvae (AaCPA-IV, AaCPA-VIII, AaCPA-IX, AaCPA-XI). Three of the genes we characterized by bioinformatics were not expressed in either larvae or the midgut (AaCPA-X, AaCPB-VI, and AaCPB-VII). A search of the Ae. aegypti EST database identified five EST sequences corresponding to the AaCPB-VII gene (not shown), which indicates that it is expressed in other tissues, or during different times of development. Transcript sequences corresponding to the AaCPB-VI and AaCPA-X genes were not represented in this same database.
Figure 3.
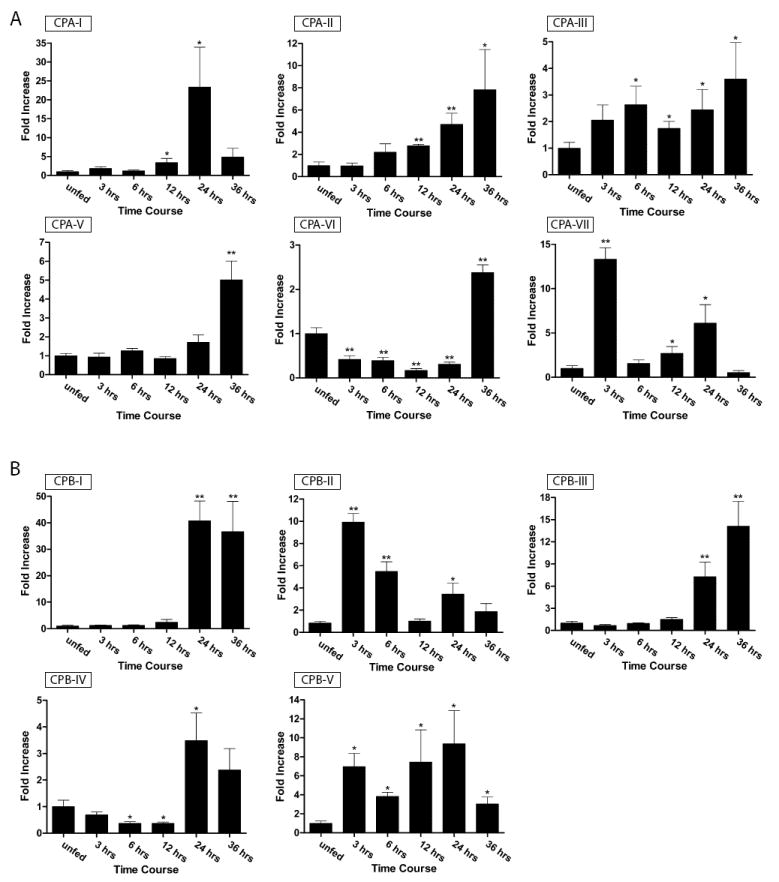
Midgut gene expression pattern of Ae. aegypti carboxypeptidase genes after blood meal feeding. A) Carboxypeptidase A gene family. B) Carboxypeptidase B gene family. RNA expression levels of the indicated genes in the midgut of unfed and fed mosquitoes were quantitated by SYBR Green-based real-time RT–PCR using gene-specific primers. The results were normalized to S7 ribosomal protein gene expression. Data were collected from three separate cohorts of mosquitoes that were blood fed on day five post-eclosion. Statistical analysis was done by ANOVA with p<0.05 (*) and p<001 (**).
The peak time of expression after blood meal feeding for most carboxypeptidase genes was found to be between 24 and 36 hours as can be seen in the AaCPA-I, AaCPA-II, AaCPA-V, AaCPA-VI, AaCPB-I, AaCPB-III, and AaCPB-IV genes (figure 3). However, several genes had peak times of expression that were much earlier (AaCPA-VII and AaCBP-II). The most highly induced genes were AaCPA-I (~25-fold) and AaCPB (~40-fold), with all other genes showing induction profiles in the ~3-15-fold range. Importantly, the relative basal transcript levels of the different carboxypeptidase genes in unfed mosquitoes were quite different as shown in table 1. Using the S7 ribosomal protein transcript level as the internal control, a gene whose transcript levels are unaffected by blood meal feeding (Scaraffia et al., 2008), we found that the AaCPA-II, AaCPA-III, AaCPB-I, AaCPB-II, and AaCPB-III genes had similar basal transcript levels to that of AaCPA-I. In contrast, the basal levels of the AaCPA-V, AaCPA-VI, AaCPA-VII, and AaCPB-IV genes were ~7-10 fold higher than AaCPA-I. The AaCPB-V gene had the lowest transcript levels overall, with a basal transcript level that was ~10 times lower than Aa-CPA-I and only a ~10-fold induced at 24 hours post-feeding.
Taken together, these expression analyses indicate that carboxypeptidase-mediated blood meal digestion involves coordinate expression of multiple genes that are regulated in response to feeding. This is similar to late phase serine protease induction (Noriega et al., 1999), but distinct from aminopeptidases which appear to be expressed at high levels prior to feeding (Noriega et al., 2002). The basal and induced transcript levels of these carboxypeptidase genes, as well as, the peak time of induction post-feeding, could be useful in developing transgenic mosquito lines that complement those generated with the AaCPA-I promoter (Moreira et al., 2000). Moreover, characterization of these 18 carboxypeptidase genes in Ae. aegypti could lead to the development of novel vector control strategies that reduce fecundity through inhibition of blood meal digestion, and thereby, decrease pathogen transmission frequencies.
Supplementary Material
Acknowledgments
We thank Robin Roche and Mary Hernandez for rearing mosquitoes, Roberto Nussenzveig for assisting in the initial phylogenetic tree studies, and the late Michael A. Wells for inspiration during the initial phases of this project. This work was supported by NIH Grant AI31951 to RLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barillas-Mury C, Wells MA. Cloning and sequencing of the blood meal-induced late trypsin gene from the mosquito Aedes aegypti and characterization of the upstream regulatory region. Insect Mol Biol. 1993;2:7–12. doi: 10.1111/j.1365-2583.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Cho WL, Deitsch KW, Raikhel AS. An extraovarian protein accumulated in mosquito oocytes is a carboxypeptidase activated in embryos. Proc Natl Acad Sci U S A. 1991;88:10821–4. doi: 10.1073/pnas.88.23.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Lemos FJ, Donnelly-Doman M, Jacobs-Lorena M. Rapid induction by a blood meal of a carboxypeptidase gene in the gut of the mosquito Anopheles gambiae. Insect Biochem Mol Biol. 1997;27:1063–72. doi: 10.1016/s0965-1748(97)00093-3. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Moskalyk LA, Donnelly-Doman M, Vlaskova M, Noriega FG, Walker VK, Jacobs-Lorena M. Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol Biol. 2000;9:33–8. doi: 10.1046/j.1365-2583.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12:543–8. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Horton P, Nakai K. Better prediction of protein cellular localization sites with the k nearest neighbors classifier. Proc Int Conf Intell Syst Mol Biol. 1997;5:147–52. [PubMed] [Google Scholar]
- Jiang Q, Hall M, Noriega FG, Wells M. cDNA cloning and pattern of expression of an adult, female-specific chymotrypsin from Aedes aegypti midgut. Insect Biochem Mol Biol. 1997;27:283–9. doi: 10.1016/s0965-1748(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Lavazec C, Bonnet S, Thiery I, Boisson B, Bourgouin C. cpbAg1 encodes an active carboxypeptidase B expressed in the midgut of Anopheles gambiae. Insect Mol Biol. 2005;14:163–74. doi: 10.1111/j.1365-2583.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, Jacobs-Lorena M. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2000;97:10895–8. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlais I, Mori A, Schneider JR, Severson DW. A targeted approach to the identification of candidate genes determining susceptibility to Plasmodium gallinaceum in Aedes aegypti. Mol Genet Genomics. 2003;269:753–64. doi: 10.1007/s00438-003-0882-7. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. J Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Edgar KA, Bechet R, Wells MA. Midgut exopeptidase activities in Aedes aegypti are induced by blood feeding. J Insect Physiol. 2002;48:205–212. doi: 10.1016/s0022-1910(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Quiocho FA, Lipscomb WN. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Scaraffia PY, Isoe J, Murillo A, Wells MA. Ammonia metabolism in Aedes aegypti. Insect Biochem Mol Biol. 2005;35:491–503. doi: 10.1016/j.ibmb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Scaraffia PY, Tan G, Isoe J, Wysocki VH, Wells MA, Miesfeld RL. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2008;105:518–23. doi: 10.1073/pnas.0708098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K, Ericsson LH, Walsh KA, Neurath H. Amino-acid sequence of bovine carboxypeptidase B. Proc Natl Acad Sci U S A. 1975;72:1666–70. doi: 10.1073/pnas.72.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell J, Querol E, Aviles FX. Metallocarboxypeptidases and their protein inhibitors. Structure, function and biomedical properties. Biochim Biophys Acta. 2000;1477:284–98. doi: 10.1016/s0167-4838(99)00280-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


