Abstract
Homeostatic signaling systems are thought to interface with the mechanisms of neural plasticity to achieve stable yet flexible neural circuitry. However, the time course, molecular design and implementation of homeostatic signaling remain poorly defined. Here we demonstrate that a homeostatic increase in presynaptic neurotransmitter release can be induced within minutes following postsynaptic glutamate receptor blockade. The rapid induction of synaptic homeostasis is independent of new protein synthesis and does not require evoked neurotransmission, indicating that a change in the efficacy of spontaneous quantal release events is sufficient to trigger the induction of synaptic homeostasis. Finally, both the rapid induction and the sustained expression of synaptic homeostasis are blocked by mutations that disrupt the pore-forming subunit of the presynaptic CaV2.1 calcium channel encoded by cacophony. These data confirm the presynaptic expression of synaptic homeostasis and implicate presynaptic CaV2.1 in a homeostatic retrograde signaling system.
Keywords: Drosophila, plasticity, glutamate receptor, CaV2.1, calcium channel, excitability, migraine, ataxia, learning, memory, development, neuromuscular junction, synapse
Introduction
Homeostasis is an essential type of feedback regulation that enables a biological system to maintain a constant functionality within a changing environment. In the nervous system, homeostatic signaling systems have been implicated in the control of both nerve and muscle excitability (Davis, 2006; Davis and Bezprozvanny, 2001; Marder and Prinz, 2002; Perez-Otano and Ehlers, 2005; Turrigiano and Nelson, 2004). In the central nervous system homeostatic signaling systems have been shown to counteract chronic perturbations of neuronal excitation though modulation of ion channel density and neurotransmitter receptor abundance (Beattie et al., 2002; Murthy et al., 2001; Thiagarajan et al., 2005; Turrigiano et al., 1994; Turrigiano et al., 1998). In the peripheral nervous system, homeostatic control of synaptic efficacy has been documented at the neuromuscular junction (NMJ) of organisms including Drosophila, rodent and human (Davis and Bezprozvanny, 2001; Davis et al., 1998; Petersen et al., 1997; Plomp et al., 1994). At the NMJ, mutations that decrease the sensitivity of postsynaptic neurotransmitter receptors lead to a compensatory, homeostatic increase in synaptic efficacy. The increase in synaptic efficacy precisely offsets the perturbation of postsynaptic receptor function and leads to normal muscle excitation (Davis et al., 1998; DiAntonio et al., 1999; Petersen et al., 1997; Plomp et al., 1992).
It is generally believed that homeostatic signaling at the neuromuscular junction is slow, being induced and expressed over a period of several hours to days (Davis and Bezprozvanny, 2001; Davis and Goodman, 1998; Petersen et al., 1997; Plomp et al., 1992). This model is thought to reflect the time over which muscle activity is integrated by the homeostatic signaling system (Davis and Bezprozvanny, 2001; Turrigiano and Nelson, 2004). Consistently, it is believed that the mechanisms of homeostatic compensation may be directly linked to the slow, developmental growth of the NMJ (Davis and Bezprozvanny, 2001; Davis and Goodman, 1998; Turrigiano and Nelson, 2004). However, since prior experiments have exclusively utilized manipulations that persist for several days of development, it has not been possible to test the time course or molecular mechanisms that are responsible for the induction of synaptic homeostasis.
Many additional questions remain. For example, homeostatic signaling implies the existence of mechanisms that monitor nerve or muscle activity and integrate this information over time (Davis and Bezprozvanny, 2001; Davis 2006; Marder and Prinz, 2002). The molecular identity of an activity “monitor” and the time over which neural activity is integrated to achieve homeostatic compensation remain unknown both centrally and peripherally, though postsynaptic calcium and CamKII signaling are thought to be involved (Haghighi et al., 2003; Thiagarajan et al., 2002; Turrigiano et al., 1994). Homeostatic modulation of synaptic efficacy at the NMJ, and possibly central synapses, could involve the modulation of presynaptic neurotransmitter release (Murthy et al., 2001; Piedras-Renteria et al., 2004). This implies the existence of a retrograde signaling system. The identity of this retrograde signal, and the identity of the presynaptic effector proteins that mediate altered neurotransmission also remain generally unknown.
Here we demonstrate that homeostatic signaling at the Drosophila NMJ can potentiate presynaptic transmitter release within 5–10 minutes following pharmacological blockade of postsynaptic neurotransmitter receptors. Therefore, changes in muscle activity must be integrated by the homeostatic system over a period of seconds to minutes, a process that is far more rapid than previously demonstrated at the NMJ (Plomp et al., 1992) or central synapses (Murthy et al., 2001; Sutton et al., 2006; Sutton and Schuman, 2005; Thiagarajan et al., 2005; Turrigiano and Nelson, 2004). We then demonstrate that the rapid induction of synaptic homeostasis occurs in the absence of motoneuron activity and, therefore, does not require evoked neurotransmission. These data suggest that the altered efficacy of spontaneous miniature release events that persist in the absence of motoneuron activity is sufficient to induce rapid homeostatic signaling at the NMJ. Finally, we demonstrate that mutations in the pore forming subunit of an essential presynaptic calcium channel (CaV2.1), encoded by the cacophony gene, block both the rapid induction and the sustained expression of synaptic homeostasis. These data identify a presynaptic protein necessary for the homeostatic modulation of synaptic efficacy following postsynaptic neurotransmitter receptor blockade. We present a model for the role of presynaptic calcium channels during the retrograde, homeostatic regulation of transmitter release at the NMJ.
Results
We have conducted a series of experiments of increasing temporal resolution to define the time course of synaptic homeostasis at the Drosophila NMJ. We previously demonstrated that muscle-specific expression of the Kir2.1 potassium channel (UAS-Kir2.1-GFP) throughout the four days of synapse development at the Drosophila larval NMJ specifically inhibits muscle excitability and initiates a homeostatic, compensatory increase in quantal content (Paradis et al., 2001). In the first set of experiments, we took advantage of a temperature sensitive GAL80 transgene driven by the tubulin promoter (TubGAL80ts) (McGuire et al., 2003) to temporally restrict muscle-specific, GAL4-dependent expression of UAS-Kir2.1-GFP (Supplemental Figure 1). Using these reagents we restricted robust, muscle-specific expression of UAS-Kir2.1-GFP to the final 24 hr of larval development (Supplemental Figures 1 and 2). Importantly, we observed a homeostatic increase in quantal content that is quantitatively identical to the homeostatic increase in quantal content observed following four days of UAS-Kir2.1-GFP muscle expression (Supplemental Figure 2). There is no change in bouton number (data not shown; Paradis et al., 2001) or active zone density following 24 hr of UAS-Kir2.1-GFP muscle expression, suggesting that homeostatic compensation occurs through modulation of existing synaptic machinery (Supplemental Figure 2D–E). Together, these data demonstrate that synaptic homeostasis can be induced at the mature NMJ within an intact, behaving animal.
Rapid induction of synaptic homeostasis following postsynaptic receptor blockade
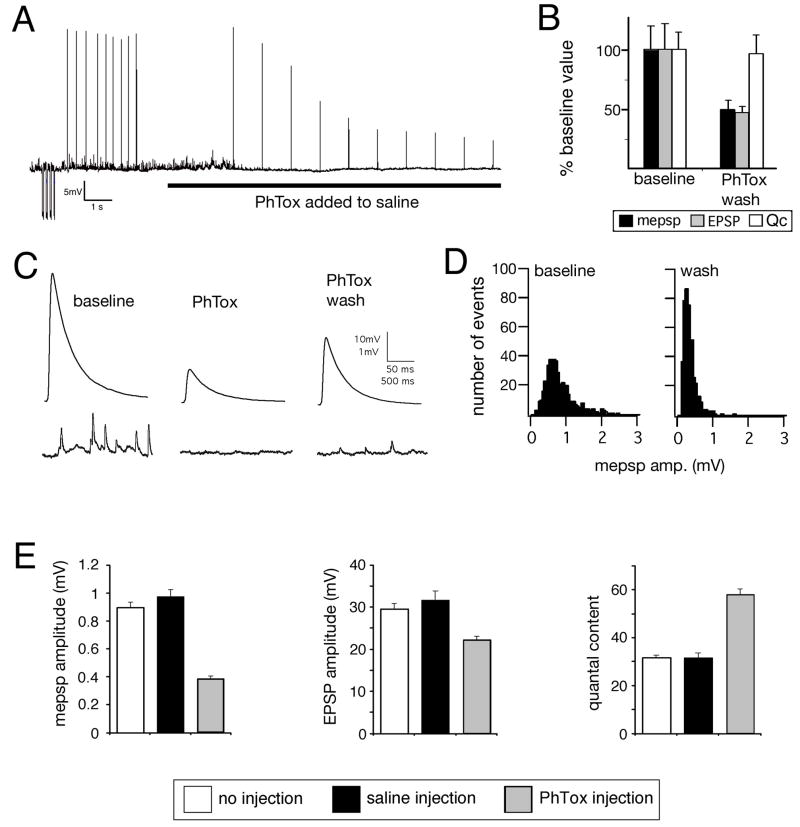
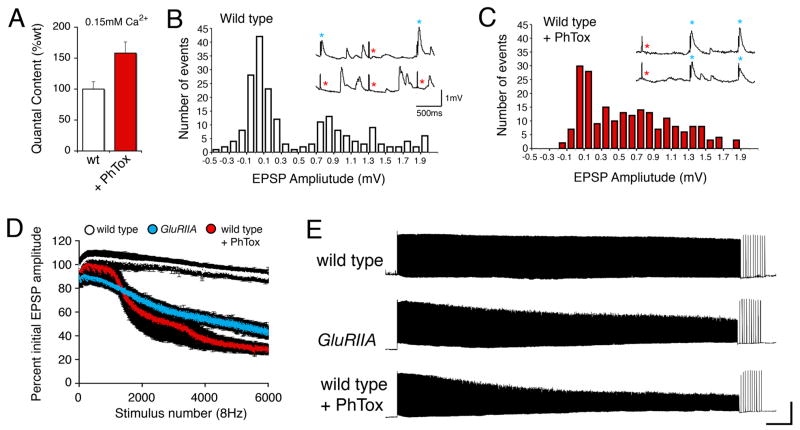
We next sought to refine the time necessary to induce synaptic homeostasis. It was previously shown that null mutations in a postsynaptic AMPA/Kainate-type glutamate receptor subunit (GluRIIA) lead to a decrease in spontaneous miniature excitatory postsynaptic potential (mepsp) amplitude and a compensatory increase in quantal content (Petersen et al., 1997). To refine the time course of this homeostatic compensation we turned to pharmacological manipulation of postsynaptic glutamate receptor function. We first characterized the properties of Philanthotoxin-433 (PhTox), an insect glutamate receptor antagonist originally purified from wasp venom (Eldefrawi et al., 1988; Karst and Piek, 1991). We demonstrate that PhTox is a persistent, use-dependent inhibitor of postsynaptic glutamate receptors at the Drosophila NMJ (Figure 1A–D). PhTox bath application (4μM) rapidly inhibits both excitatory postsynaptic potential (EPSP) amplitudes and mepsp amplitudes without an immediate effect on quantal content (estimated by average EPSP/average mepsp amplitude) (Figure 1A–D). PhTox washout after a 2 min incubation results in only a partial recovery of EPSP and mepsp amplitudes, demonstrating a persistent component of PhTox receptor blockade (Figure 1C–D). The persistent effects of PhTox cannot be accounted for by glutamate receptor internalization. This possibility was assessed by surface labeling epitope tagged-GluRIIA receptors prior to and following PhTox application (Stephanie Albin and G.W.D unpublished observations).
Figure 1. Rapid induction of synaptic homeostasis following injection of a glutamate receptor antagonist.
A) Sample recording from the NMJ showing a stimulus-dependent decline in mepsp and EPSP amplitudes following PhTox bath application (horizontal line). B) Average mepsp, EPSP amplitudes and quantal content (Qc) prior to PhTox application (baseline; n=12) and following a 2 min PhTox incubation with 30 sec washout (PhTox-wash; n=13). Recording saline lacks PhTox. C) Sample traces for the data presented in (B). D) Histograms reveal that the mepsp amplitude distribution is shifted toward smaller values following PhTox application and washout (right graph; wash). Samples sizes are equivalent. E) Quantification of average mepsp amplitude (graph at left), average EPSP amplitude (middle graph) and quantal content (graph at right). Quantification was performed for three conditions including: 30 min after 200μM PhTox injection (PhTox injection; n=30), 30 min after saline injection (saline injection; n=17) versus uninjected controls (no injection, n=10). There is a significant, homeostatic increase in quantal content in the PhTox-injected animals compared to controls (p<0.001).
Using PhTox we were able to narrow the time necessary for the induction of synaptic homeostasis in an intact animal to 30 min. We injected PhTox (200μM) into third instar larvae, achieving a sub-blocking final toxin concentration that significantly decreased mepsp amplitudes (Figure 1E). Following PhTox injection, animals were initially paralyzed (within ~30s) though the heart muscles continued to beat. Larvae remained paralyzed for ~30 min before recovering the ability to move. By comparison, animals injected with carrier saline moved normally within 1 min of injection. We next measured synaptic function 30 min following PhTox injection. The average mepsp amplitude was decreased 30 min following PhTox injection and we observed a homeostatic increase in quantal content that restored EPSP amplitudes toward wild-type values (Figure 1E). These data demonstrate that a homeostatic increase in quantal content can be induced within 30 min at the mature NMJ in an intact animal.
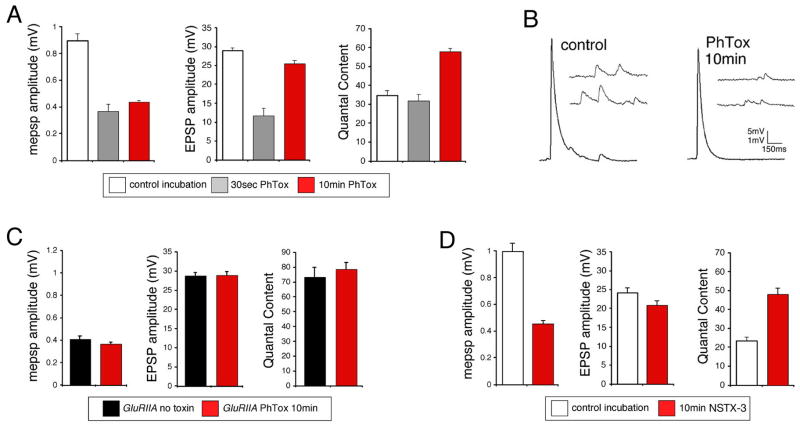
In order to precisely define the time necessary to induce synaptic homeostasis we developed a semi-intact larval neuromuscular preparation (see methods). Using a semi-intact preparation we demonstrate that a 30-second incubation in PhTox decreased mepsp amplitudes without a change in quantal content. However, a 10-minute incubation in PhTox caused a significant increase in quantal content that restored EPSP amplitudes toward wild-type levels (Figure 2A, B). These data demonstrate that synaptic homeostasis can be induced following 10 minutes of impaired postsynaptic receptor function in a semi-intact preparation.
Figure 2. Rapid induction synaptic homeostasis in a semi-intact preparation.
A) Quantification of average mepsp amplitude (left), average EPSP amplitude (middle) and quantal content (right) for the three conditions listed below the graphs; 10min saline incubation (control incubation), 30 second incubation in PhTox (30sec PhTox) and a 10 minute incubation in PhTox (10min PhTox). There is a statistically significant increase in quantal content after a 10 min PhTox incubation (n=48; p<0.01) compared to a 30 sec PhTox incubation (n=10) or saline incubation (control incubation; n=42). B) Sample traces prior to and following PhTox incubation for 10min. Scale bar 150ms; 5mV for EPSPs and 150ms; 1mV for mepsps. C) Quantification as in (A). Incubating GluRIIA mutants for 10 min in PhTox (n=12) does not reduce average mepsp amplitude beyond that observed in GluRIIA mutants without toxin (p>0.2; n=12) and does not increase quantal content beyond that observed in GluRIIA mutants without toxin (p>0.5). D) Data are shown for wild-type synapses incubated in control saline (10 min) versus wild-type synapses incubated in NSTX-3 (NSTX-3; 10μM, 15 min). Prior to recording, synapses were washed in normal saline without NSTX-3. A persistent inhibition of postsynaptic receptors following the wash is shown by the significant decrease in mepsp amplitudes compared to mock treated wild-type controls (p<0.01). After a 15 min incubation in NSTX-3, EPSP amplitudes are near wild-type values (p>0.1) and there is a significant increase in quantal content (p<0.01).
A number of control experiments were performed to ensure that the observed change in quantal content is consistent with the induction of synaptic homeostasis. First, rapid homeostatic compensation occurs in the continued presence of PhTox demonstrating that these effects are not a consequence of toxin withdrawal (see Figure 4 below). Second, we demonstrate that two additional pharmacological inhibitors of postsynaptic receptors (10μM NSTX-3 and 4μM PhTox-343) are also capable of inducing synaptic homeostasis in 10–15 minutes (Figure 2D and data not shown). Finally, we performed experiments to control for the possibility that PhTox directly modulates transmitter release by acting upon a presynaptic target. In these experiments, GluRIIA null mutant animals were incubated in PhTox for 10 min. GluRIIA mutants have decreased quantal size and a homeostatic increase in presynaptic release (Petersen et al., 1997). PhTox treatment did not alter the average mepsp amplitude compared to mock-treated GluRIIA animals suggesting that PhTox binds with higher affinity to GluRIIA-containing receptor complexes. (Figure 2C). Importantly, quantal content remains unaffected compared to mock treated GluRIIA controls suggesting that PhTox does not directly influence presynaptic release by acting upon an unidentified presynaptic target (Figure 2C). An alternate interpretation could be that the synapse is already maximally potentiated in the GluRIIA mutant. However, we find that PhTox treatment can induce a compensatory increase in presynaptic release that is significantly beyond that observed, on average, in the GluRIIA mutant animals (compare Figures 2C and 6A). From these data we conclude that PhTox acts primarily to inhibit postsynaptic glutamate receptors and thereby induces a homeostatic increase in quantal content at the Drosophila NMJ.
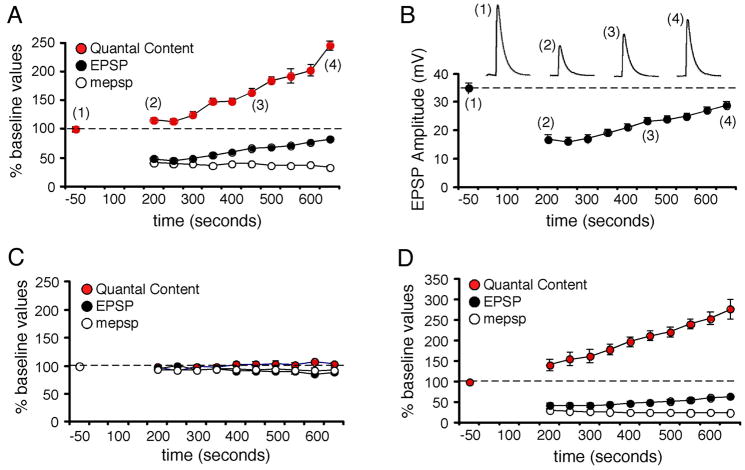
Figure 4. Induction of synaptic homeostasis in real time.
A) mepsp, EPSP and quantal content are plotted over time for a single recording (normalized to baseline values prior to toxin application). Time zero is the time of toxin application. EPSPs delivered at 0.2Hz are averaged every 50 s. mepsps are averaged over identical 50 s intervals. Quantal content is calculated based on these average measurements for each 50 s interval. Baseline stimulation is followed by a perfusion with PhTox (4μM) that remains present in the bath solution throughout the remainder of the recording. 10 stimuli are delivered immediately following PhTox application to maximally suppress mepsp amplitudes (amplitudes not shown on graph). 0.2Hz stimulation is then resumed for the duration of the experiment in the continued presence of PhTox. B) EPSP amplitudes for the experiment in (A). C) Average values for experiments performed as in (A) without the addition of PhTox (n=10). D) Average values for all experiments that included PhTox as in (A) (n=12).
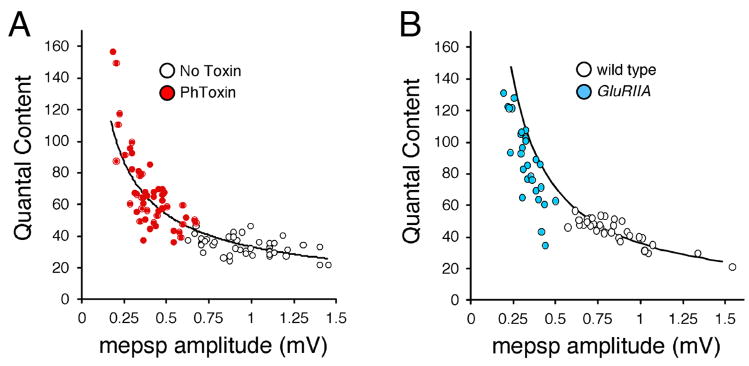
Figure 6. Presynaptic release precisely offsets decreased quantal size.
Data for individual recordings are shown, plotting quantal content versus mepsp amplitude. Each point is a separate experiment. (A) Wild type synapse (white) and synapses incubated in PhTox for 10 min (red) are shown. Quantal content scales continuously with decreased mepsp amplitude. The line represents the function describing perfect compensation (quantal content/mepsp = constant EPSP). The constant EPSP value is taken as the average wild-type amplitude (no drug). B) Data plotted as in (A) for wild-type (white) versus GluRIIA mutant animals.
Evidence that the rapid induction of synaptic homeostasis is achieved by a change in presynaptic release probability
It is thought that the homeostatic increase in synaptic efficacy observed in the GluRIIA mutant is due to a change in the probability of presynaptic neurotransmitter release (Petersen et al., 1997). Therefore, we examined whether the rapid induction of homeostatic signaling is also due to a change in the probability of presynaptic release. First, we induced synaptic homeostasis with a 10 min PhTox incubation in a semi-intact preparation and calculated quantal content using the method of failures (0.15mM Ca2+, 10mM Mg2+ saline). We find a significant 155% increase in quantal content at PhTox incubated synapses compared to mock-incubated synapses (Figure 3A–C; p<0.001). These data are consistent with a homeostatic increase in presynaptic release probability following a 10 min incubation in PhTox.
Figure 3. Evidence for increased release probability during the rapid induction of synaptic homeostasis.
A) Quantal content, estimated by the method of failures in 0.15mM Ca2+ is significantly increased following application of PhTox (+PhTox; 10min semi-intact preparation) compared to wild type in the absence of PhTox (p<0.01). Quantal content values are: wild type (0.43 ± 0.04, N=14) and PhTox (0.68 ± 0.05, N=15). B) Sample histogram of EPSP amplitudes measured in wild type. Inset shows sample traces from this recording. Three stimuli are shown for each trace (vertical transients are stimulus artifacts). Blue stars indicate stimulus-locked release events and red stars indicated putative failures following the stimulus artifact. C) Sample histogram of EPSP amplitudes measured at a PhTox treated synapse with inset as in (B). D) Graph examining vesicle depletion during a stimulus train (6000 stimuli at 8Hz) comparing wild type (white symbols; N=6) to GluRIIA mutant (blue symbols; N=6) and PhTox treated (10 min semi-intact; N=8) animals. The average value (± SEM) is shown for each stimulus to generate the curves. There is significantly greater EPSP rundown comparing GluRIIA and PhTox treated animals with wild type (p<0.01). E) Sample traces for data shown in (D). There is significantly greater EPSP rundown in GluRIIA and PhTox treated synapses. Rapid recovery to initial EPSP amplitudes is demonstrated by low frequency stimulation (0.2Hz) following the cessation of the stimulus train.
An increase in presynaptic release probability should also result in faster depletion of the vesicle pool during prolonged nerve stimulation. We examined vesicle depletion during prolonged nerve stimulation under conditions of high vesicular release (6000 stimuli at 8Hz, 2mM extracellular Ca2+). We compared wild type synapses with PhTox treated synapses (10 min PhTox incubation) and GluRIIA mutant synapses (Figure 3D,E). Wild type synapses sustain synaptic transmission without significant depression under these conditions. By contrast, synapses treated with PhTox and GluRIIA mutant synapses both show a gradual decline in EPSP amplitudes resulting in significant synaptic depression compared to wild type (p<0.001). Together with the failure analysis, these data are consistent with a rapid, homeostatic increase in the probability of release following PhTox application. It should be noted that there is not an increase in spontaneous miniature release frequency that parallels the increase in quantal content following PhTox application (data not shown). However, there is also no change in mepsp frequency in GluRIIA mutants that express a homeostatic increase in presynaptic release (Petersen et al., 1997). It is possible that a change in mini frequency is not indicative of altered presynaptic release probability at this synapse, or that a population of mepsp events remains below detection levels in our recordings (Petersen et al., 1997).
Rapid homeostatic signaling is achieved by a low-gain homeostatic signaling system
We next sought to characterize the rate of homeostasis at the NMJ in order to address two questions. First, does homeostatic signaling potentiate synaptic efficacy following a delay after toxin application, or is homeostatic signaling initiated immediately? A delay would be indicative of the time over which muscle depolarization is monitored prior to the homeostatic modulation of synaptic efficacy. Second, does the homeostatic increase in synaptic efficacy asymptotically retarget baseline values, implying a low-gain homeostatic system, or is compensation rapid and prone to over-shoot baseline values, implying a high-gain homeostatic system (Stelling et al., 2004)? To characterize these properties of synaptic homeostasis we used chronic recordings to follow the induction of homeostatic compensation at single synapses in real time. Initially, following PhTox application (4μM), both mepsp and EPSP amplitudes decrease in parallel and no immediate change in quantal content is observed (Figure 4A). A sample recording made in the continued presence of PhTox shows that mepsp amplitudes remain at a constant depressed amplitude while EPSP amplitudes gradually increase in size, eventually approaching baseline values (Figure 4A–B). Increased EPSP amplitude is associated with a gradual, homeostatic potentiation of quantal content in the continued presence of PhTox (Figure 4A). In some recordings mepsp amplitudes continued to decline over the course of the recording while EPSP amplitudes and input resistance remained constant. Again, a gradual increase in quantal content was calculated. When we average our data across all experiments we reveal a gradual monotonic increase in quantal content following a delay of 3–4 minutes after PhTox application (Figure 4D). Control recordings in the absence of PhTox reveal no change in mepsp amplitude, EPSP amplitude or quantal content over the same time interval (Figure 4C). The gradual increase in release is consistent with a low-gain homeostatic system that requires only a short period (2–5 min) during which muscle depolarization is integrated. Finally, consistent with this rapid timecourse, we present evidence that new protein synthesis is not required for the induction of synaptic homeostasis at the NMJ (Supplemental Figure 3).
Motoneuron activity and evoked muscle depolarization are not necessary for the induction of synaptic homeostasis
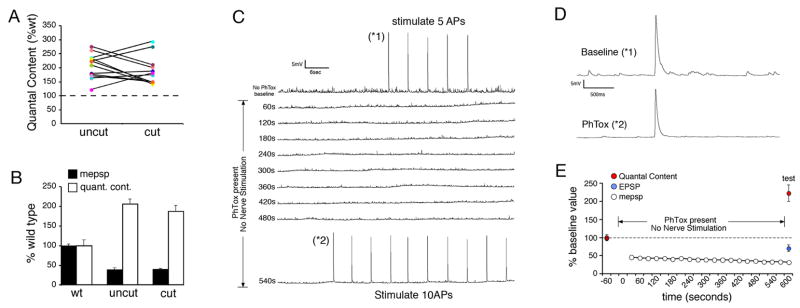
To test the role of neural activity during the induction of synaptic homeostasis we severed the motor axons on one side of the CNS prior to the application of PhTox to a semi-intact preparation. Axons that remained intact on the other side of the CNS served as an internal control. Drosophila motor axons rarely, if ever, fire spontaneous action potentials when the segmental nerve is cut (GWD personal observations). Consistently, muscle contraction was not observed on the half of the animal where the motor axons were severed and spontaneous action potentials were never observed during chronic recordings in the presence of a severed motor axon (see below).
We examined the PhTox-dependent induction of synaptic homeostasis by comparing recordings from muscles with cut motor axons on one side of the animal to recordings from muscles in the same abdominal segment on the other side of the animal innervated by intact motor axons (Figure 5A,B). We find that homeostatic compensation is always present at synapses with a cut motor axon, indicating that motoneuron action potentials and evoked neurotransmitter release are not required for the induction of synaptic homeostasis (Figure 5A,B). As a control, we have recorded, chronically, from NMJs innervated by cut motor axons for 10–15 minutes and demonstrate that cutting the motor axon alone is not sufficient to potentiate neurotransmitter release at the NMJ (Figure 4C). In addition, we have recorded from semi-intact preparations in which the nerve was cut and the preparations experience no activity or nerve stimulation for 10–15 minutes. There was no change in quantal content comparing animals with a cut nerve (quantal content = 33.3 ± 4.0) to wild-type controls recorded immediately following dissection (quantal content = 35.3 ± 1.6; p>0.5). Thus, the absence of action potentials in the motor nerve does not induce a change in presynaptic release. Finally, we have performed chronic recordings to follow the induction of synaptic homeostasis in the absence nerve stimulation and thereby document the absence of evoked neurotransmission during the induction of synaptic homeostasis. Prior to PhTox application the nerve was stimulated briefly to quantify baseline presynaptic release. PhTox was then added and the recording was continued in the absence of stimulation for 9 min before we again tested evoked synaptic transmission (Figure 5C–E). The muscle recordings document a complete absence of evoked synaptic transmission (Figure 5C). We observe a significant, homeostatic increase in presynaptic release in the absence of neural activity (Figure 5D,E) that is quantitatively similar to chronic recordings where nerve stimulation was present (Figure 4D). Taken together, these data suggest that the altered efficacy of spontaneous miniature release events that persist in the absence of evoked neurotransmission can initiate a homeostatic change in presynaptic release.
Figure 5. Motoneuron activity is not required for the rapid induction of synaptic homeostasis.
A) Quantal content is shown for individual recordings and presented as a percent relative to the average quantal content observed in wild type animals recorded in normal saline (dotted line at 100%). In each preparation recordings were made from muscle 6 in segment A3 on both sides of the animal (coded with the same color symbol and connected by a line). In one hemi-segment the motor axons were severed prior to application of PhTox (cut) and in the other hemi-segment the motor axons remained intact (uncut). Every recording showed a homeostatic increase in quantal content. There is no obvious trend in the degree of compensation comparing cut versus uncut axons in a single animal. B) Average values for the data presented in (A). The cut axons show a statistically significant increase in quantal content compared to controls (p<0.01). The quantal content recorded in uncut axons is not significantly greater than that observed in cut axons (p>0.1). C) A sample 10 minute recording showing the induction of synaptic homeostasis in the absence of evoked transmission. The recording is initiated in the absence of PhTox and 5 stimuli are delivered to quantify basal synaptic efficacy (*1). PhTox is then applied without further nerve stimulation. The absence of evoked neurotransmission is evident until test stimuli are delivered at the end of the recording (10 action potentials; *2). D) Sample EPSPs from the recording in (C) from the indicated time points. At time point (*2) the EPSP amplitude is close to baseline while mepsps are significantly smaller than baseline. (E) Average data for experiments as in (C) calculating quantal content (red), EPSP amplitude (blue) and mepsp amplitude (white). The average mepsp amplitude is calculated at 30s intervals. No evoked release was observed during PhTox incubation until test stimuli are delivered at the end of the recording. In the absence of evoked neurotransmission PhTox application (over time indicated) induces a significant, homeostatic increase in quantal content compared to baseline values prior to PhTox application (p<0.01).
If synaptic homeostasis does not require evoked neurotransmission, then synaptic homeostasis should also be independent of the muscle safety factor. Neuromuscular synapses, including those in Drosophila, release 50–100% more neurotransmitter than is necessary to depolarize the muscle to contract (Marrus and DiAntonio, 2005). This excess release is classically referred to as a “safety factor”. If impaired muscle contraction initiates homeostatic signaling at the NMJ, then a homeostatic change in presynaptic release should not be induced until muscle excitation (assessed by mepsp amplitude) falls below some threshold value related to the safety factor and, at that point, we should observe a significant increase in presynaptic release. We plotted average mepsp amplitudes versus quantal content for individual recordings from wild-type NMJs with or without PhTox treatment. There is no evidence for a threshold induction of synaptic homeostasis. Instead, quantal content scales precisely with the change in mepsp amplitude (Figure 6A). A similar relationship is observed when we examine recordings from GluRIIA null mutant and wild-type animals on a similar plot (Figure 6B). Although PhTox-treated preparations appear to demonstrate perfect compensation, it should be noted that our recordings were made in the absence of PhTox, which should increase the measured mepsp amplitudes by approximately 25% (Figure 1C). Taking this into account the GluRIIA and PhTox-treated preparations should appear similar, falling just to the left of the line indicating perfect homeostatic compensation (Figure 6A, B). Two conclusions can be made from these data. First, these data argue that synaptic homeostasis at the NMJ is independent of the muscle safety factor and support the observation that evoked muscle depolarization is not required for the rapid induction of synaptic homeostasis in PhTox treated animals and, perhaps, the GluRIIA mutants as well. Second, these data demonstrate that homeostatic modulation of presynaptic transmitter release is precisely controlled by the homeostatic signaling system such that it is able to precisely offset even small changes in postsynaptic quantal size.
Synaptic homeostasis is disrupted by mutations that disrupt the pore forming subunit of CaV2.1 encoded by cacophony
To identify the molecular mechanisms responsible for synaptic homeostasis we initiated a candidate screen for mutations that prevent the rapid induction of synaptic homeostasis at the Drosophila NMJ. Altered calcium channel function could, in principle, mediate a rapid homeostatic modulation of presynaptic release probability. Therefore, we genetically tested the involvement of presynaptic calcium channels in the mechanisms of synaptic homeostasis. In Drosophila, the cacophony (cac) gene encodes the α-1 subunit of the CaV2.1 channel. This channel is exclusively expressed in the nervous system (Tomancak et al., 2002; see also Berkeley Drosophila Genome Project), the protein is restricted to the presynaptic terminal, localizes to the presynaptic zone and is absolutely required for stimulus-evoked neurotransmitter release at the NMJ (Brooks et al., 2003; Smith et al., 1996). In our experiments we took advantage of hypomorphic mutations in the cac gene that impair, but do not eliminate, calcium channel function (Brooks et al., 2003; Dellinger et al., 2000; Kawasaki et al., 2000). The cacTS2 and cacS mutations are hypomorphic alleles that, when raised at room temperature, cause a deficit in basal synaptic transmission when recordings are made in 0.5mM extracellular calcium (cacTS2 = 47% decrease in EPSP amplitude; cacS = 71% decrease in EPSP amplitude; absolute amplitudes are presented in Table 1) (Dellinger et al., 2000; Kawasaki et al., 2000). The cacTS2 mutation is a missense mutation (P1385S) in the cytoplasmic C-terminal domain of the channel adjacent to a putative EF-hand motif and near a putative IQ domain (Brooks et al., 2003; Kawasaki et al., 2002). The intragenic suppressor of cacTS2, [su(TS2)2], is a missense mutation (F916L) in the S5 transmembrane domain in repeat three (see summary in Brooks et al., 2003). The residues that are altered in both mutations are conserved in mouse and worm. The cacS mutation is a missense mutation (F1029I) in the S6 transmembrane domain in repeat three (Smith et al., 1998).
Table 1.
Physiological data demonstrating a role for cacophony in synaptic homeostasis.
| Condition | Figure | Genotype | mepsp | EPSP | quantal content | N |
|---|---|---|---|---|---|---|
| 0.5mM Ca2+ Reared at 22°C | 8A, 9A | wt | 0.85 (0.03) | 33.9 (0.7) | 41.8 (1.5) | 33 |
| 8A, 9A | GluRIIA | 0.32 (0.01) | 24.1 (0.9) | 77.0 (4.0)** | 31 | |
| 8B | cacS/+ | 0.75 (0.03) | 29.2 (1.7) | 39.5 (1.8) | 21 | |
| 8B | cacS/+; GluRIIA | 0.32 (0.02) | 17.0 (0.8) | 55.5 (4.2)** a | 16 | |
| 8C | cacS | 0.82 (0.07) | 10.3 (2.0) | 13.9 (3.0) | 9 | |
| 8C | cacS; GluRIIA | 0.39 (0.02) | 7.2 (1.1) | 18.2 (2.6) ns^ | 8 | |
| 1.5mM Ca2+ Reared at 22°C | 8D, 9D | wt | 0.62 (0.04) | 36.7 (2.2) | 118.3 (10.2) | 17 |
| 8D, 9D | GluRIIA | 0.40 (0.03) | 40.8 (2.1) | 212.9 (25.6)** | 7 | |
| 8E | cacS | 0.67 (0.06) | 32.5 (2.1) | 85.5 (8.4) | 7 | |
| 8E | cacS; GluRIIA | 0.39 (0.02) | 20.7 (1.3) | 73.8 (4.4) ns^ | 6 | |
| 0.5mM Ca2+ Reared at 22°C | 9A | cacTS2 | 0.93 (0.04) | 18.1 (2.1) | 19.6 (2.3) | 11 |
| 9A | cacTS2; GluRIIA | 0.35 (0.01) | 13.3 (2.0) | 38.3 (8.5)** | 13 | |
| 0.5mM Ca2+ Reared at 29°C | 9B | wt | 0.68 (0.03) | 28.7 (2.6) | 42.7 (2.0) | 7 |
| 9B | GluRIIA | 0.34 (0.03) | 27.6 (2.9) | 80.1 (5.3)** | 6 | |
| 9B | cacTS2 | 0.66 (0.02) | 14.0 (1.5) | 22.2 (1.9) | 11 | |
| 9B | cacTS2; GluRIIA | 0.35 (0.01) | 9.8 (1.4) | 27.7 (3.5) ns^ | 13 | |
| 9C | cacTS2,cacsu(TS2)2 | 0.78 (0.07) | 29.1 (3.6) | 37.5 (3.4) | 8 | |
| 9C | cacTS2,cacsu(TS2)2; GluRIIA | 0.27 (0.01) | 14.7 (0.8) | 55.1 (4.7)** b | 6 | |
| 1.5mM Ca2+ Reared at 29°C | 9D | cacTS2 | 0.71 (0.07) | 36.8 (1.9) | 100.1 (10.6) | 6 |
| 9D | cacTS2; GluRIIA | 0.37 (0.02) | 27.6 (3.7) | 125.2 (27.7) ns^ | 6 | |
| 0.5mM Ca2+ 29°C for 24hr | 9E | cacTS2 | 1.07 (0.07) | 26.6 (1.4) | 25.7 (2.1) | 10 |
| 9E | cacTS2; GluRIIA | 0.40 (0.02) | 13.2 (1.3) | 33.0 (3.1)* | 11 |
Gray shading identifies recordings made under identical conditions.
Note: The column titled “Figure” indicates the location of these data within Figures 8 and 9. Data recorded at 1.5mM extracellular calcium are corrected for non-linear summation.
indicates significance at p=0.03 compared to cacTS2 reared under identical conditions
indicates significance at p≤0.01 compared to the genotype within the same box (ns) indicates p>0.25 compared to the genotype within the same box.
Quantal content in cacS/+; GluRIIA is significantly less than GluRIIA alone shown in Figure 8A, p<0.01.
Quantal content in cacTS2, cacsu(TS2)2; GluRIIA is significantly smaller than GluRIIA in Figure 9B p<0.01 and significantly greater than wild type in Figure 9B, p<0.05.
Indicates that quantal content is different than GluRIIA alone under identical conditions (p<0.01).
For the analysis of the cacophony mutations, we present data both as raw amplitudes (Table 1) and as normalized to the control genotype (Figures 7–9). In the figures, data are normalized to the appropriate cacophony mutant background. This helps to account for changes in baseline neurotransmission and highlight the effects of the GluRIIA mutant within a given cac mutant background. For example, if we observe that decreased mepsp amplitude, caused by the presence of the GluRIIA mutation, correlates with increased quantal content compared to the appropriate genetic control, then we conclude that homeostatic compensation has occurred, even if absolute synaptic strength remains below that observed in wild type.
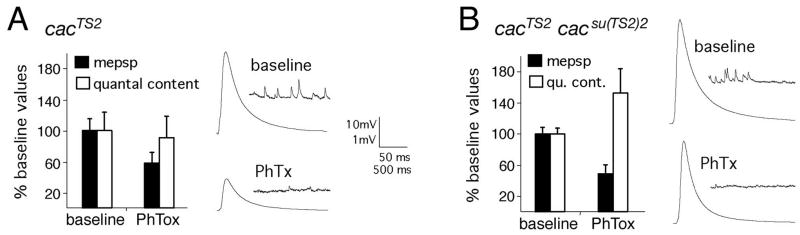
Figure 7. The induction of synaptic homeostasis is suppressed by mutations in the CaV2.1 alpha-1 subunit.
A) Average mepsp and quantal content, normalized to baseline, for cacTS2 animals with and without PhTox incubation (10 min). Sample traces are at right. No homeostatic increase in quantal content is observed following PhTox incubation (p>0.4; n=13). B) Quantification as in (A) for the cacTS2cacsu(TS2)2 intragenic suppressor double mutation. Sample traces at right, scale as in (A). A homeostatic increase in quantal content is restored in the cacTS2cacsu(TS2)2 double mutant (p<0.02; n=13).
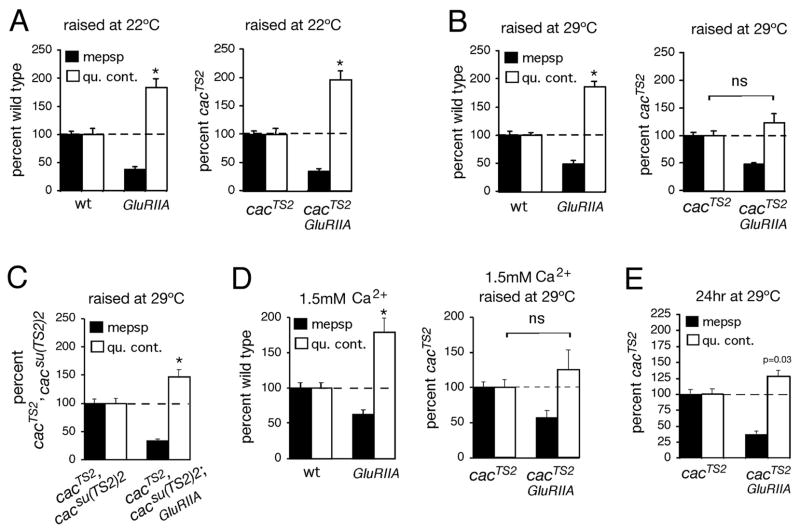
Figure 9. Conditional disruption of synaptic homeostasis using temperature sensitive mutations in the CaV2.1 alpha-1 subunit.
A) Quantification of average mepsp amplitude (filled bars) and quantal content (open bars). Values are normalized to wild-type control (dotted line) recorded in 0.5mM extracellular calcium. (Left) GluRIIA mutants have decreased mepsp amplitudes and a homeostatic increase in quantal content (p<0.001 for both). Data are re-plotted from Figure 8A for figure clarity. (Right) There remains a significant homeostatic increase in quantal content in the cacTS2; GluRIIA double mutant compared to cacTS2 alone (p<0.01). B) Quantification as in (A) for wild type (wt), GluRIIA, cacTS2 and cacTS2;GluRIIA raised at 29°C (assayed at room temperature, 0.5mM Ca2+). (Left) GluRIIA animals show a compensatory increase in quantal content compared to wild type (p<0.01). At right, the cacTS2;GluRIIA double mutants fail to show a homeostatic increase in quantal content when compared to cacTS2 alone (p>0.25). C) Quantification as in (A) for the double mutant cacTS2, cacsu(TS2)2 and the triple mutant cacTS2, cacsu(TS2)2;GluRIIA raised at 29°C (assayed at room temperature, 0.5mM Ca2+). Values are normalized to cacTS2, cacsu(TS2)2. A homeostatic increase in quantal content is observed in triple mutant animals (p<0.01) indicating partial restoration of homeostatic compensation by the intragenic suppressor mutation. D) Quantification as in (A) for wild type (wt), GluRIIA, cacTS2 and cacTS2;GluRIIA for the indicated calcium and rearing conditions. Data for wt and GluRIIA are repeated from figure 8D for visual clarity. At right, the cacTS2;GluRIIA double mutants fail to show a significant increase in quantal content compared to cacTS2 alone (p>0.25). Quantal contents were corrected for non-linear summation prior to normalization. E) cacTS2 and cacTS2;GluRIIA animals were raised at room temperature for three days of larval development before being shifted to 29°C for the final 24 hours of larval development. A homeostatic increase in quantal content is observed (p=0.03). However, the homeostatic increase in quantal content is significantly suppressed (p<0.01) in the cacTS2; GluRIIA double mutant animals compared to that observed when cacTS2;GluRIIA animals are raised at 22°C throughout development (A – right graph). Furthermore, the increase in quantal content is not different from that observed when cacTS2;GluRIIA animals are raised at 29°C throughout development (B – right graph). Throughout, the notation ‘ns’ indicates that quantal content is not significantly different (p>0.25). Sample size for each genotype and non-normalized values (including EPSP amplitudes) are presented in Table 1.
We first demonstrate that PhTox application the cacTS2 mutant decreases mepsp amplitudes without causing a significant homeostatic increase in presynaptic quantal content indicating strong suppression of synaptic homeostasis (Figure 7A). As a control, we took advantage of an intragenic suppressor mutation isolated within the cacTS2 mutant gene, cacTS2cacsu(TS2)2 (Brooks et al., 2003). Channel function is significantly restored in the cacTS2cacsu(TS2)2 double mutant, as demonstrated by the restoration of EPSP amplitudes toward wild type values (Table 1; wild type EPSP = 33.9 ±0.7mV and cacTS2cacsu(TS2)2 = 26.5 ± 2.0mV – recorded at room temperature) (Brooks et al., 2003). When PhTox is applied to the cacTS2cacsu(TS2)2 animals, acute synaptic homeostasis is significantly restored (Figure 7B). It should be noted that synaptic homeostasis does not reach the levels observed in wild-type animals. This is consistent with the observation that the intragenic suppressor mutation improves channel function, but does not recapitulate a wild-type channel (Brooks et al., 2003). It remains unknown how the intragenic suppressor mutation alters channel function. However, at a minimum, these data support the conclusion that a mutation in cacophony is the cause of altered homeostasis in the cacTS2 animal and that presynaptic Cacophony (alpha subunit of presynaptic CaV2.1) is required for the rapid, homeostatic modulation of transmitter release.
We next tested whether cac mutations block synaptic homeostasis when placed in the GluRIIA mutant background. These experiments test whether the induction of synaptic homeostasis is achieved by similar mechanisms in GluRIIA mutants and PhTox-treated animals, and tests whether the expression of synaptic homeostasis over four days of development also requires Cacophony. We generated double mutant animals, combining a null allele of GluRIIA with either cacS or cacTS2 and generated a triple mutant by combining GluRIIA with cacTS2cacsu(TS2)2. First, we demonstrate that synaptic homeostasis is suppressed in the cacS;GluRIIA double mutant animals (compare Figure 8A and 8C). These data indicate that synaptic homeostasis in the GluRIIA mutants also requires normal presynaptic Cacophony function.
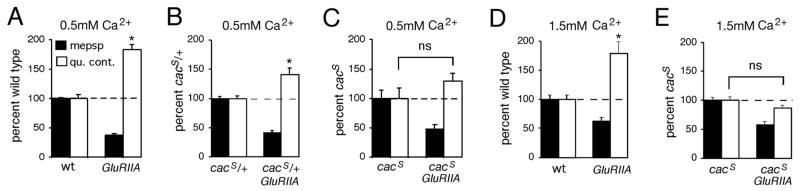
Figure 8. The expression of synaptic homeostasis is suppressed by mutations in the CaV2.1 alpha-1 subunit.
A) Quantification of average mepsp amplitude (filled bars) and quantal content (open bars). Values are normalized to wild-type control (dotted line). GluRIIA mutants have decreased mepsp amplitudes and a homeostatic increase in quantal content (p<0.001 for both). B) Values as in (A) are normalized to those observed in the cacS/+ heterozygous condition. A homeostatic increase in quantal content is observed in the cacS/+; GluRIIA animals (p<0.01). However, the magnitude of the increase in quantal content is significantly less than that observed in GluRIIA mutants shown in (A) (p<0.01). C) Quantification as in (A) for control and cacS;GluRIIA double mutants. Data are normalized to values observed in cacS alone. A homeostatic increase in quantal content is suppressed in the cacS;GluRIIA double mutant compared to cacS alone (p>0.2). D) Quantification as in (A) for control and GluRIIA mutants recorded in 1.5mM extracellular calcium. GluRIIA mutants show a compensatory increase in quantal content at 1.5mM extracellular calcium compared to wild type (p<0.01). Quantal contents were corrected for non-linear summation prior to normalization. E) The homeostatic increase in quantal content is suppressed in the cacS;GluRIIA double mutant compared to cacS mutants alone, recorded in 1.5mM extracellular calcium (p>0.5).
Since baseline neurotransmission is compromised in the cacS mutant, it remains possible that decreased calcium influx through the mutant cacS channel cannot support increased vesicle release of any kind. To address this issue we performed two control experiments. First, we asked whether heterozygous (cacS/+) mutants suppress synaptic homeostasis in the GluRIIA mutant background. The baseline EPSP amplitudes in cacS/+ animals are nearly wild type indicating that these synapses can support near normal transmitter release (wild type EPSP = 33.9±0.7mV and cacS/+ = 29.2mV±1.7, p<0.01). A significant increase in quantal content is observed in the cacS/+; GluRIIA double mutant compared to cacS/+ alone indicating significant homeostatic compensation (Figure 8B). However, the magnitude of this homeostatic compensation is less than that observed in GluRIIA mutants alone indicating that the cacS/+ heterozygous condition suppresses homeostatic compensation without substantially altering baseline neurotransmission (compare Figure 8A and 8B; p<0.01). As a further control, we asked whether the expression of synaptic homeostasis could be restored in the cacS; GluRIIA double mutant animals by recording in elevated extracellular calcium. At 1.5mM extracellular Ca2+, EPSP amplitudes are restored to wild type levels in the cacS animals (wild type EPSP amplitude = 36.7±2.2mV while cacS = 32.5±2.1mV; p>0.3, Table 1). However, synaptic homeostasis remains blocked in the cacS;GluRIIA double mutants (Figure 9D and E). Since 1.5mM extracellular calcium supports near normal vesicular release in the cacS background, limited calcium influx is unlikely to simply mask the expression of synaptic homeostasis in the cacS;GluRIIA double mutants.
Finally, to confirm that Cacophony functions presynaptically during synaptic homeostasis (as predicted by expression analysis) we rescued a cacophony null mutation by presynaptic expression of a UAS-cacophony-GFP transgene (Kawasaki et al., 2002; 2004) and did so in the presence of the GluRIIA mutation. We observe a robust, homeostatic increase in quantal content in these rescued animals (p<0.01 compared to wild type) that is similar to that seen in GluRIIA mutants alone (p=0.15 compared to GluRIIA) demonstrating that presynaptic Cacophony is sufficient to mediate synaptic homeostasis (values for elaV-GAL4, cacnull/Y; UAS-cac-GFP, GluRIIA/GluRIIA recordings: quantal content = 66.4 ±5.7, EPSP amplitude = 27.4mV ±2.5, quantal size = 0.41mV ±0.02). Since presynaptic Cacophony supports normal homeostatic compensation, these data provide important molecular confirmation that a retrograde signal mediates synaptic homeostasis at this synapse.
A consistent, but more complex set of data were obtained for the cacTS2;GluRIIA double mutants. When the cacTS2;GluRIIA double mutants are raised at room temperature (22°C) there is no deficit in synaptic homeostasis (Figure 9A). This is in contrast to the observation that the cacTS2 mutation, when raised at room temperature, blocks PhTox-induced synaptic homeostasis (Figure 8). This discrepancy could be explained if the cacTS2 mutations delay the induction of synaptic homeostasis without fully blocking the process. This is consistent with observations in other homeostatic signaling systems where mutations can slow the process of homeostatic compensation without blocking the mechanism (Davis, 2006). Thus, normal synaptic homeostasis could be achieved over the four days of larval development in the cacTS2;GluRIIA double mutants. To test this idea, we raised cacTS2;GluRIIA double mutants at an intermediate, non-permissive temperature (29°C), thereby making the cacTS2 mutation more severe during development. Baseline quantal content, assayed at room temperature, is unchanged by raising cacTS2 at 29°C compared to cacTS2 raised at room temperature (Table 1). This indicates that functional synapse development has not been impaired by rearing cacTS2 animals at 29°C. However, synaptic homeostasis is now fully suppressed in the cacTS2;GluRIIA double mutant (Figure 9B). Again, as a control, we demonstrate that synaptic homeostasis is significantly restored in the cacTS2cacsu(TS2)2; GluRIIA triple mutant animals raised in parallel at 29°C and assayed at room temperature (Figure 9C). Quantal content in the cacTS2cacsu(TS2)2; GluRIIA triple mutant is not only significantly elevated beyond the appropriate genetic control (cacTS2cacsu(TS2)2), but it is increased compared to wild-type animals (p<0.05) supporting the conclusion that some homeostatic signaling has been restored. However, as observed in PhTox experiments, the presence of the intragenic suppressor mutation does not restore synaptic homeostasis to levels observed in the GluRIIA mutant alone, consistent with cacTS2cacsu(TS2)2 lacking full functionality. As a further control we assayed synaptic homeostasis at elevated calcium (1.5mM Ca2+) for the cacTS2;GluRIIA animals raised at 29°C. As observed for the cacS animals, baseline transmission is restored toward wild type levels in the cacTS2 controls assayed at elevated calcium (wild type = 36.7±2.2mV and cacTS2 = 36.8±1.9mV in 1.5mM Ca2+, Table 1), but synaptic homeostasis remains severely impaired (Figure 9D).
We also controlled for possible developmental abnormalities caused by the cacophony mutations. It has been previously reported that mutations in the cacophony gene result in decreased synaptic growth (Reikhof et al., 2003; Xing et al., 2005). However, these prior studies utilized mutations and experimental conditions that are different, and possibly more severe, than those utilized here. In order to determine whether altered synaptic homeostasis in the cacophony mutations is a secondary consequence of impaired synaptic growth we controlled for possible anatomical developmental abnormalities associated with raising cacTS2 mutants at 29°C or with the cacS animals raised at room temperature (conditions that block homeostasis). There is no change in synaptic bouton number in animals harboring either the cacTS2 or cacS homozygous mutations raised under conditions that block synaptic homeostasis (Supplemental Figure 4). Thus, impaired synaptic homeostasis in these animals is not a secondary consequence of altered synapse development. Together, these data support the conclusion that presynaptic cacophony is necessary for the induction and expression of synaptic homeostasis at the Drosophila NMJ.
Cacophony is necessary for the sustained expression of synaptic homeostasis
It remains unknown whether synaptic homeostasis at the NMJ is reversible. Homeostatic quantal scaling in the vertebrate CNS decays over a period of days following the removal of chronic activity manipulations (Thiagarajan et al., 2005). If homeostatic compensation is labile, then it may be necessary for the muscle to continually invoke homeostatic signaling to counteract developmental perturbations such as the GluRIIA mutation. Thus, a remaining question is whether the Cacophony channel is also necessary for the sustained expression of homeostatic compensation. We addressed this question by performing a temperature shift experiment on cacTS2;GluRIIA double mutant animals. The cacTS2;GluRIIA double mutants show normal homeostasis when raised at room temperature, but severely impaired homeostasis when raised at 29°C. Therefore, we raised cacTS2;GluRIIA animals at room temperature and asked whether a shift to 29°C for the final 24 hours of larval development was sufficient to block synaptic homeostasis (Figure 9E). We find that homeostatic compensation is strongly suppressed following a 24hr shift to 29°C in the third larval instar (Figure 9E). Although a statistically significant increase in quantal content above baseline is observed, the magnitude of this change is not statistically different from the cacTS2; GluRIIA double mutants that are raised continually at 29°C (p>0.4, compare Figure 9E and 9B). As a control, we demonstrate that synaptic homeostasis is unaltered in GluRIIA mutant animals that are raised at 29°C throughout the four days of larval development (Figure 9B). These data suggest that the normal functionality of presynaptic Cacophony is necessary for the sustained expression of synaptic homeostasis.
DISCUSSION
Rapid induction of homeostatic signaling at the Drosophila NMJ
Here we demonstrate that robust homeostatic modulation of presynaptic release can be observed in as little as 2–3 minutes and that the full expression of homeostatic compensation can be achieved in 10 minutes (Figure 4). By comparison, homeostatic compensation at the vertebrate NMJ following pharmacological AChR blockade requires several days to be expressed (Plomp et al., 1992; Plomp et al., 1994). Similarly, in the central nervous system, homeostatic modulation of synaptic efficacy requires several hours (Sutton et al., 2006) and often several days before detectable changes are observed (Desai et al., 2002; Thiagarajan et al., 2005; Turrigiano et al., 1998). Although the speed with which synaptic efficacy is modulated at the Drosophila NMJ is surprising, prior phenomenological studies using PhTox in other insects agree well with our data (Eldefrawi et al., 1988; Karst and Piek, 1991). Importantly, the time between postsynaptic receptor blockade and the expression of altered presynaptic release should reflect the time over which postsynaptic excitation is integrated by the homeostatic “sensors” in the muscle. Since altered presynaptic release can be detected within 2–3 minutes (Figure 4), our data suggest that muscle excitation is being integrated over a time frame of seconds to minutes, far more rapidly than previously thought to occur at the Drosophila NMJ, or at any other synapse (Davis, 2006).
Spontaneous miniature release events can initiate rapid and precise homeostatic signaling
It has been suspected that synaptic homeostasis is an activity-dependent phenomenon at the NMJ (Petersen et al., 1997; Davis et al., 1998; Davis and Goodman, 1998; Paradis et al., 2001; Davis and Bezprozvanny, 2001). Here we provide evidence that neither activity in the motoneuron, nor evoked neurotransmission is required for the induction of synaptic homeostasis (Figures 4, 5). These data suggest that the muscle is able to detect a change in postsynaptic receptor function through the action of spontaneous miniature release events. Our data do not rule out the possibility that evoked neurotransmission might normally participate in the induction of synaptic homeostasis. However, a change in the efficacy of spontaneous miniature release events is able to convey sufficient information to induce homeostatic compensation. Interestingly, the speed of homeostatic signaling does not appear to be modulated by the presence or absence of motoneuron activity. Regardless of whether the motor axons remain intact or are severed, homeostatic signaling requires at least 2–3 minutes before it is observable as a change in presynaptic release and requires 10 minutes to be fully expressed (Figures 2A, 4, 5).
If spontaneous miniature release events are sufficient to induce homeostatic signaling, two models could be considered. First, homeostatic signaling could be induced at individual active zones. The rate of spontaneous release at this synapse is approximately 4 events per second. If one assumes that there are approximately 300 active zones at the NMJ on muscle 6 (Atwood et al., 1993) then, during the 10 min induction of synaptic homeostasis (assuming an equal probability of spontaneous events across all active zones) each active zone should experience approximately 8 vesicles. This suggests that remarkably few vesicles could provide the information at each active zone to achieve precise homeostatic compensation. In support of this possibility, data from the vertebrate central nervous system indicate that mepsps can act locally to achieve significant effects on synapse stability (McKinney et al., 1999) and synaptic function (Sutton et al., 2006). However, another model is equally plausible at the NMJ. It is not necessary that precise homeostatic compensation be induced at the level of individual active zones. If the muscle can integrate some aspect of mepsp efficacy across multiple active zones over time then several hundred individual quantal release events could be utilized to provide the information necessary for the induction of synaptic homeostasis.
What signaling mechanisms are sensitive enough to detect changes in the efficacy or signaling capacity of quantal release events? An attractive possibility is that calcium signaling at the postsynaptic density is involved. Drosophila glutamate receptors are calcium permeable. Several studies, including work at the Drosophila NMJ, have implicated calcium or calcium sensitive signaling molecules such as CamKII in the mechanisms of synaptic homeostasis (Haghighi et al., 2003; Marder and Prinz, 2002; Thiagarajan et al., 2002; Turrigiano et al., 1994). Given that active zones throughout the nervous system release and respond to one vesicle (or at most a few vesicles) at any given time, it is plausible that postsynaptic calcium signaling has the sensitivity to detect changes in the efficacy of single vesicles. Consistently, there is evidence that spontaneous miniature release events can influence postsynaptic signaling including CamKII activity (Murphy et al., 1994a; Murphy et al., 1994b; Sutton et al., 2006; Sutton and Schuman, 2005; Sutton et al., 2004). An appealing feature of this model is that the postsynaptic active zone could be an isolated signaling compartment. At the NMJ this would seem to be necessary in order to isolate calcium signaling at the postsynaptic density from the calcium waves that propagate throughout the t-tubule network during muscle contraction. In support of this idea, the postsynaptic density is enveloped by an elaborate membrane network termed the subsynaptic reticulum that could function to isolate the postsynaptic density from the calcium transients experienced during muscle contraction.
In this study we also demonstrate that disruption of muscle membrane excitation via muscle-specific expression of the Kir2.1 potassium channel can initiate synaptic homeostasis within 24 hours at a late stage of synapse development (Supplemental Figures 1 and 2). These data suggest that a disruption of muscle excitability is sufficient to induce homeostatic compensation (Paradis et al., 2001). It is important to emphasize that these data do not rule out the possibility that altered current flow through GluRs could also be sufficient to induce homeostatic compensation (Paradis et al., 2001). It also remains possible that multiple mechanisms exist to induce homeostatic compensation at the NMJ (Davis, 2006; Davis and Bezprozvanny, 2001). At present, the time necessary for the induction of Kir2.1 expression in muscle prevents us from directly testing whether homeostatic compensation under these conditions requires neural activity.
Homeostatic control of presynaptic release is achieved via presynaptic CaV2.1 calcium channels
Although homeostatic signaling is a robust phenomenon in both the central and peripheral nervous systems, very little is known about the underlying molecular mechanisms (Davis, 2006 for recent review). Here we demonstrate that two independent point mutations in the cacophony gene block both the rapid induction of synaptic homeostasis in response to application of PhTox and the sustained expression of synaptic homeostasis in glutamate receptor mutants (GluRIIA). These data not only identify an important molecular component of homeostatic signaling but also provide important molecular confirmation that synaptic homeostasis is mediated by a retrograde signal from muscle to nerve at this synapse.
In considering the role of the Cacophony channel in synaptic homeostasis, it is important to assess whether the cacophony mutations primarily alter synapse development and only secondarily impair homeostatic compensation. At vertebrate central and peripheral synapses, for example, a null mutation in the pore forming subunit of CaV2.1 causes diverse developmental defects including altered expression of other calcium channel subunits and changes in synapse morphology and stability (Cao and Tsien, 2005; Piedras-Renteria et al., 2004). Several observations argue against this possibility in our present work. First, because we analyze hypomorphic and temperature sensitive point mutations, the mutant CaV2.1 channels remain at the NMJ and should preclude other channels from occupying their preferred position at the active zone (Cao et al., 2004). This idea is supported by the demonstration that temperature sensitive Cacophony channels can support nearly normal transmitter release at room temperature (1.5mM Ca2+), but show a pronounced temperature-sensitive defect in neurotransmission at elevated temperature (Brooks et al., 2003). We also do not observe any defect in synaptic growth or synapse morphology in the cacophony mutations under the conditions utilized in this study (Supplemental Figure 4). Although prior work from other laboratories have documented synaptic growth defects associated with cacophony mutations, the mutations and conditions used in these prior studies were different, and possibly more severe, than those used here (Rieckhof et al., 2003; Xing et al., 2005).
Another concern is that the deficit in neurotransmission observed in cacophony mutants simply masks our ability to detect an otherwise normal homeostatic change in presynaptic release. However, if this were the case, then the deficit in release that we observe when we record from the synapse should correlate with the degree to which homeostasis is suppressed. Several observations are inconsistent with this expectation. First, the cacS/+ heterozygous animals have normal baseline transmission but show a significant impairment in homeostatic compensation. Second, the degree to which the cacTS2 mutation disrupts homeostasis correlates with the time spent at 29°C but not with a change in baseline transmission assayed at 22°C. These data dissociate the effects of this channel during the induction of homeostasis from its role during basal presynaptic release. Thus, it appears that Cacophony is directly involved in the presynaptic mechanisms that generate a homeostatic change in presynaptic transmitter release.
There are two general mechanisms by which cacophony mutations could block homeostatic compensation. The Cacophony channel could be a direct target of the homeostatic, retrograde signal and thereby modulate presynaptic release probability. Alternatively, calcium influx via Cacophony could be a permissive signal for additional signaling within the presynaptic nerve terminal. Such a mechanism would be analogous to the proposed function of presynaptic R-type calcium channels in mossy fiber LTP (Nicoll and Schmitz, 2005). In either case, the involvement of Cacophony in synaptic homeostasis is intriguing given the numerous mechanisms by which the conductance of CaV2.1 channels can be modulated. For example, both PKC and G-protein signaling have been shown to influence CaV2.1 channel function (Evans and Zamponi, 2006). Thus, any retrograde signal that impinges on these signaling mechanisms could alter the activity of the CaV2.1 channel and modulate presynaptic release during homeostatic signaling. Recent data indicate that the cacTS2 mutation alters channel inactivation in heterologous cells, suggesting that modulation of calcium channel inactivation could be involved in the homeostatic modulation of synaptic function (Macleod et al., 2006).
A revised view of synaptic homeostasis at the Drosophila NMJ
The data presented here demonstrate that homeostatic signaling can be independent of synaptic growth and development at the NMJ. Rather, homeostatic signaling at the NMJ appears to be a rapid form of plasticity capable of tuning presynaptic transmitter release to offset even small changes in postsynaptic excitability. Furthermore, since synaptic homeostasis can be rapidly induced at a late stage of synapse development, it suggests that the mechanisms of synaptic homeostasis are persistently active at the NMJ. Based upon these new data we propose that synaptic homeostasis represents a mechanism to constrain the variability associated with robust, but ultimately imperfect mechanisms of neural development. This property of homeostatic signaling could be particularly important during the development of complex neural circuitry in the CNS because errors that occur early in the development could be compounded as additional layers of neural circuitry are established. We further hypothesize that the capacity for homeostatic signaling will be retained throughout the life of an organism to counteract stress-related, disease-related or injury-related perturbations that alter neural function. In this regard it is interesting to note that mutations in the pore forming subunit of CaV2.1 have been linked to migraine and ataxia in humans (Plomp et al., 2001). Altered channel activity and synaptic function have been probed as an underlying cause of these conditions (Barrett et al., 2005; Cao and Tsien, 2005). Together these data suggest a possible link between impaired homeostatic signaling and neurological disease.
Materials and Methods
Physiology
Recordings were made from muscle 6 in abdominal segment 3 of third instar larvae as previously described (Davis et al., 1998). Recordings are made in HL3 saline at specified calcium concentrations (see text). Care was taken in all recordings to ensure reliable recruitment of both motoneurons innervating muscle 6 (Davis et al., 1998; Albin and Davis, 2004; Paradis et al,. 2001). Semi-intact preparations were achieved by pinning the anterior and posterior extremities of a third instar larva and then making a dorsal incision. It was essential that the animal not be stretched in the longitudinal or lateral directions since stretching the muscles consistently disrupted the induction of homeostatic compensation (GWD unpublished observations). The CNS, fat, and gut were left intact. PhTox (4μM final concentration) was perfused into the animal through this incision. Robust body wall contractions were initially present demonstrating continued presence of motoneuron activity. Following a defined period of incubation (see text) the dissection was completed including the removal of the CNS as previously described (Davis et al., 1998). During chronic recordings, the larvae were dissected without stretching and the CNS removed. The cut motor axon was stimulated as described previously (Davis et al., 1998). In all chronic recordings, muscle input resistance (Rin) was monitored at the beginning and end of the recording. Recordings were excluded if Rin or Vm changed by more than 20%. In no instance did Rin increase during chronic recordings (data not shown). Temperature was controlled during animal rearing in Forma environment chambers with defined temperature and humidity (Forma Scientific). Control animals were reared in parallel and treated identically in all experiments. Philanthatoxin-343, -433 and NSTX-3 (Sigma) were prepared as stock solutions (PhTox, 4mM in DMSO; NSTX-3, 10mM in H2O) and diluted in HL3 saline to the indicated concentration (see text). Larval injections were achieved using glass microelectrodes filled with HL3 saline/PhTox and pressure injection under visual control using an inverted microscope (Zeiss). Following injection, animals were placed at a food source on moist apple juice plates and allowed to recover. In all cases, continued heartbeat was assessed visually with a dissection microscope. Quantal content was calculated for each individual recording by calculating the average EPSP/average mepsp (Davis et al., 1998; Davis and Goodman, 1998; Paradis et al., 2001; Albin and Davis, 2004). Quantal content was also calculated by the method of failures according to ln N/n0 where N=trials and n0 is the number of failures (Petersen et al., 1997; Davis and Goodman, 1998). Quantal contents calculated for each recording were then averaged across animals for a given genotype.
Anatomical analysis
Third instar larval fillets were fixed (Bouins) and stained with anti-HRP (DHSB, Iowa) and either mAb nc82 [gift of Eric Buchner; see (Wagh et al., 2006) or anti-GluRIIA (DHSB, Iowa)]. Anti-HRP staining allowed quantification of bouton number as previously described (Albin and Davis, 2004). Active zones visualized by nc82 or anti-GluRIIA were quantified according to previously published methods (Albin and Davis, 2004).
Genetics
Drosophila mutations were utilized as described in the text. GluRIIA null mutations are GluRIIASp16 (Petersen et al., 1997). Conditional Kir2.1-GFP expressing animals were generated by crossing MHC-Gal4, UAS-Kir2.1-GFP/TM6b females to males carrying the Tub-Gal80ts transgene (McGuire et al., 2003; Paradis et al., 2001). One-hour egg lays were performed on apple juice plates. Animals were grown at 18°C through embryogenesis and early larval development. For the ‘0 Hours of Kir2.1 Expression’ condition, animals were raised at 18°C for 8 days. For the ’24 Hours of Kir2.1 Expression’ condition, animals were raised at 18°C for 7 days and then 30°C for 24 hours. The hypomorphic cacophony mutations utilized are cacS (Smith et al., 1998), cacTS2 (Dellinger et al., 2000), and cacTS2, cacsu(TS2)2 (Brooks et al., 2003). The lethal cacophony mutation is l(1)L13HC129 (Kawasaki et al,. 2002). UAS-cacophony-GFP flies were obtained from Richard Ordway (Kawasaki et al., 2004). Animals were raised and assayed by electrophysiology as described in the text. The w1118 strain was used as a control genotype for the effects of cacophony mutations on synapse morphology.
Supplementary Material
Acknowledgments
We thank Eric Buchner for the nc82 antibody. We thank Richard Ordway for providing the cacophony alleles used in this study. We thank Roger Nicoll, Dion Dickman, Jan Pielage, Bruno Marie and Kira Poskanzer for comments on an earlier version of this manuscript. This work was supported by NIH grant number NS39313 to GWD, an NIH NRSA to CAF NS049694 and predoctoral fellowships from NSF to CPG and KWM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Albin SD, Davis GW. Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci. 2004;24:6871–6879. doi: 10.1523/JNEUROSCI.1538-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Cao YQ, Tsien RW. Gating deficiency in a familial hemiplegic migraine type 1 mutant P/Q-type calcium channel. J Biol Chem. 2005;280:24064–24071. doi: 10.1074/jbc.M502223200. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Brooks IM, Felling R, Kawasaki F, Ordway RW. Genetic analysis of a synaptic calcium channel in Drosophila: intragenic modifiers of a temperature-sensitive paralytic mutant of cacophony. Genetics. 2003;164:163–171. doi: 10.1093/genetics/164.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YQ, Tsien RW. Effects of familial hemiplegic migraine type 1 mutations on neuronal P/Q-type Ca2+ channel activity and inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:2590–2595. doi: 10.1073/pnas.0409896102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43:387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Dellinger B, Felling R, Ordway RW. Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel alpha1-subunit gene, cacophony. Genetics. 2000;155:203–211. doi: 10.1093/genetics/155.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi AT, Eldefrawi ME, Konno K, Mansour NA, Nakanishi K, Oltz E, Usherwood PN. Structure and synthesis of a potent glutamate receptor antagonist in wasp venom. Proc Natl Acad Sci U S A. 1988;85:4910–4913. doi: 10.1073/pnas.85.13.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Zamponi GW. Presynaptic Ca(2+) channels - integration centers for neuronal signaling pathways. Trends Neurosci. 2006 Aug 28; doi: 10.1016/j.tins.2006.08.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Karst H, Piek T. Structure-activity relationship of philanthotoxins--II. Effects on the glutamate gated ion channels of the locust muscle fibre membrane. Comp Biochem Physiol C. 1991;98:479–489. doi: 10.1016/0742-8413(91)90237-n. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active Zone localization of Presynaptic Calcium Channels Encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- Marrus SB, DiAntonio A. Investigating the safety factor at an invertebrate neuromuscular junction. J Neurobiol. 2005;63:62–69. doi: 10.1002/neu.20120. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Macleod GT, Chen L, Karunanithi S, Peloquin JB, Atwood HL, McRory JE, Zamponi GW, Charlton MP. The Drosophila cacts2 mutation reduces presynaptic calcium entry and defines an important element for CaV2.1 channel inactivation. Eur J Neurosci. 2006;23:3230–44. doi: 10.1111/j.1460-9568.2006.04873.x. [DOI] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Baraban JM, Wier WG, Blatter LA. Visualization of quantal synaptic transmission by dendritic calcium imaging. Science. 1994a;263:529–532. doi: 10.1126/science.7904774. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Bhat RV, Fiore RS, Wier WG, Baraban JM. Differential regulation of calcium/calmodulin-dependent protein kinase II and p42 MAP kinase activity by synaptic transmission. J Neurosci. 1994b;14:1320–1331. doi: 10.1523/JNEUROSCI.14-03-01320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Pyle JL, Diehn M, Glickfeld LL, Harata NC, Cao Y, Kavalali ET, Brown PO, Tsien RW. Presynaptic homeostasis at CNS nerve terminals compensates for lack of a key Ca2+ entry pathway. Proc Natl Acad Sci U S A. 2004;101:3609–3614. doi: 10.1073/pnas.0308188100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, van den Maagdenberg AM, Molenaar PC, Frants RR, Ferrari MD. Mutant P/Q-type calcium channel electrophysiology and migraine. Curr Opin Investig Drugs. 2001;2:1250–1260. [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. The upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats: its dependency on calcium. J Physiol . 1994;478(Pt 1):125–136. doi: 10.1113/jphysiol.1994.sp020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel alpha1 subunit in Drosophila. Genetics. 1998;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle FJ, 3rd, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature Neurotransmission Stabilizes Synaptic Function via Tonic Suppression of Local Dendritic Protein Synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol. 2005;64:116–131. doi: 10.1002/neu.20152. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tomancak P, Beaton A, Weiszmann R, Kwan E, Shu S, Lewis SE, Richards S, Ashburner M, Hartenstein V, Celniker SE, Rubin GM. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2002;3(12) doi: 10.1186/gb-2002-3-12-research0088. RESEARCH0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Xing B, Ashleigh Long A, Harrison DA, Cooper RL. Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function. Synapse. 2005;57:132–147. doi: 10.1002/syn.20165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.