Abstract
Regulatory cells play a crucial role in the induction and maintenance of tolerance by controlling T cell as well as B and natural killer (NK) cell-mediated immunity. In transplantation, CD4+CD25+forkhead box P3+ T regulatory cells are instrumental in the maintenance of immunological tolerance, as are several other T cell subsets such as NK T cells, double negative CD3+ T cells, γδ T cells, interleukin-10-producing regulatory type 1 cells, transforming growth factor-β-producing T helper type 3 cells and CD8+CD28− cells. However, not only T cells have immunosuppressive properties, as it is becoming increasingly clear that both T and non-T regulatory cells co-operate and form a network of cellular interactions controlling immune responses. Non-T regulatory cells include tolerogenic dendritic cells, plasmacytoid dendritic cells, mesenchymal stem cells, different types of stem cells, various types of alternatively activated macrophages and myeloid-derived suppressor cells. Here, we review the mechanism of action of these non-lymphoid regulatory cells as they relate to the induction or maintenance of tolerance in organ transplantation.
Keywords: alloreactivity, regulatory cells, transplantation
Myeloid dendritic cells (DCs) (tolerogenic DC, regulatory DC) (Fig. 1a)
Fig. 1.
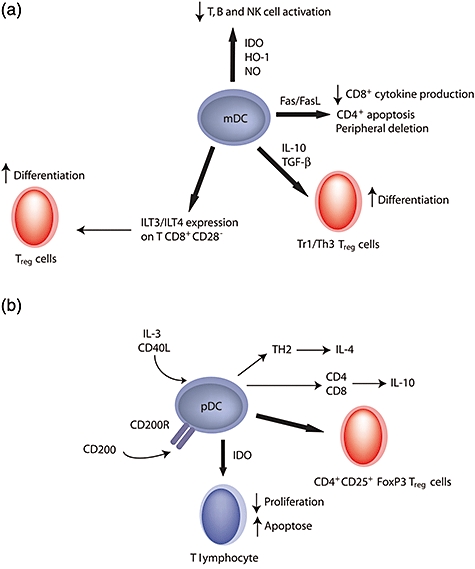
Suppressive function of myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC). (a) mDCs induce peripheral tolerance via different mechanisms: peripheral deletion, CD4+ T cell apoptosis or decrease of CD8+ T cell cytokine production through Fas/Fas ligand (FasL) interaction. The synthesis of nitric oxide (NO), haem oxygenase (HO)-1 or indoleamine 2,3-dioxygenase (IDO) inhibits T, B and natural killer (NK) cell activation. After contact with CD8+CD28− cells or through the synthesis of interleukin (IL)-10 and transforming growth factor (TGF)-β, mDC also induce regulatory T cell (Treg) differentiation. (b) In the presence of IL-3 and CD40L, pDC activate IL-4 secretion by T helper type 2 (Th2) cells and IL-10 production by CD4 and CD8 T cells. They induce Treg generation. Stimulation of pDC with CD200-immunoglobulin (Ig) induces IDO.
Thymic DCs mediate the negative selection of T lymphocytes in vivo and play a role in the peripheral tolerance of self-reactive T lymphocytes [1]. Upon maturation, DCs increase their expression of major histocompatibility complex (MHC), adhesion and co-stimulatory molecules and secrete cytokines necessary to enhance T lymphocyte activation and to generate immune responses [2]. Therefore, this raises the question of whether preventing DC maturation would prevent T lymphocyte activation and promote tolerance in transplantation. This does indeed seem to be the case, as the injection of immature DCs has been shown to prolong cardiac allograft survival in mice [3] as well as rats [4,5]. Several mechanisms may be responsible for the peripheral tolerance induced by immature DCs. Some of these include T lymphocyte anergy in the absence of co-stimulatory signals, peripheral deletion of reactive T cells through Fas/Fas ligand (FasL) interactions and the capture of apoptotic cells by DCs [6], which then present antigens in a context where proinflammatory cytokine production is inhibited [7]. Other mechanisms include T cell starvation through tryptophan metabolism via indoleamine 2,3-dioxygenase (IDO) [8], T cell inhibition through the action of haem oxygenase-1 (HO-1) [9], induction of regulatory T cells (Treg) (reviewed in [8]) or release of nitric oxide (NO) [5]. Certain cytokines, such as interleukin (IL)-10 [10], transforming growth factor (TGF)-β, hepatocyte growth factor (HGF) and granulocyte colony-stimulating factor, can induce immature DCs to become tolerogenic. Immature DCs induce the differentiation of T regulatory type 1 or T helper type 3 (Th3) regulatory cells following IL-10 or TGF-β production [11]. Paradoxically, mature DCs cannot induce differentiation of Treg but are nevertheless required to promote their survival and function [12]. Several types of membrane molecules have been identified as markers of tolerogenic DCs, such as signalling lymphocyte activation molecule (SLAM), programmed death ligand 1 (PD-L1), DC receptor for endocytosis (DEC)-205 (CD205) and the inhibitory receptors of the immunoglobulin (Ig)-like transcript family (ILT3/4). Immature DCs exposed to CD8+CD28− Treg increase their expression of the immunoreceptor tyrosine-based inhibitory motif-containing ILT3 and ILT4 receptors, which prevents the overexpression of co-stimulatory molecules and thereby prevents the activation of CD4+ T cells [13] and promotes the differentiation of Treg[14]. DEC-205 appears to be a good marker for tolerogenic DCs without, however, transmitting any signal to T cells [15]. CD8+ DEC-205+ DCs in mice [15] block CD4+ T cell responses totally by inducing T cell apoptosis through Fas/FasL interactions [16]. They also block cytokine production by CD8+ T cells without inducing their apoptosis [17]. SLAM and PD-L1, although expressed on activated DCs, generate two negative signals that cause immunosuppression. SLAM–SLAM homotypic interactions inhibit the production of IL-6, tumour necrosis factor (TNF)-α and IL-12 by CD40-stimulated DC [18], whereas DC expression of PD-L1, which shares the same B7-1 receptor as cytotoxic T lymphocyte antigen (CTLA)-4, suppresses the function of activated T cells [19]. In transplantation, interactions of B7 molecules with CTLA-4, the latter being expressed by activated T cells and Treg cells, plays a critical role in peripheral tolerance by inhibiting T cell activation [20]. This effect has been attributed to signalling via signal transducer and activator of transcription 1 (STAT1), p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB, leading to the synthesis of IFN-γ which, in turn, in the absence of IL-6, leads to the induction of IDO [21,22]. IDO is responsible for down-regulating T [23–25], B and natural killer (NK) cell [26] activation and proliferation through depletion of tryptophan from the microenvironment [21] and through the immunosuppressive action of tryptophan catabolites (Kynurenin, quinolinic acid and 3-hydroxyanthranilic acid) [27]. DCs themselves escape from being destroyed via the action of IDO by a concomitant overexpression of tryptophanyl (Trp)–tRNA synthetase. The Trp–tRNA complex provides a reservoir of the amino acid in a form that is protected from IDO-mediated degradation and is directly available for protein synthesis [28].
Plasmacytoid DCs (Fig. 1b)
Plasmacytoid DCs (pDCs) are found mainly in the peripheral blood and bone marrow, and in the T cell areas of secondary lymphoid organs. pDCs are a specialized cell population that exhibit a plasma-cell like morphology and produce large amounts of type I IFN in response to viruses. These cells play a crucial role in the induction and maintenance of tolerance, properties that depend upon expression of the type I IFN receptor [8]. pDCs are considered as an immature DC subtype able to differentiate in vitro into mature DCs, in response to different stimuli [29]. In mice, pDCs express B220, Ly6C or CD11c but no CD123 (IL-3Rα) [30,31]. In humans, pDCs express CD4, CD123, HLA-DR, CD68, ILT-3 and CD45RA but lack CD11c, ILT-1, CD3, CD14, CD16, CD19, CD20 and CD56 [32,33]. Blood DC antigen (BDCA)-2 (CD303) and BDCA-4 (identical to neuropilin-1) are also human pDC-specific markers in the blood [34]. BDCA-2 is a type II lectin C [34] that plays a role in antigen internalization and presentation as well as in the synthesis of IL-12. In the presence of IL-3, CD40L or viruses, pDC can differentiate into mature DCs. This process involves up-regulation of MHC class II, co-stimulatory molecules and the chemokine receptor CCR7, as well as the production of IL-12, and leads in turn to an enhancement of T lymphocyte activation [29,30,35]. Activated pDCs are able to produce a large variety of proimmune cytokines including IFN-α/β, granulocyte–macrophage colony-stimulating factor, TNF-α, IL-6 and IL-8 [36]. In addition, human as well as murine mature pDCs affect T cells functions, leading to earlier activation, prolonged survival, IFN-γ production and Th1 differentiation, via different molecular mechanisms [37]. In contrast, in humans and mice, freshly isolated immature pDCs express low levels of MHC class II and co-stimulatory molecules and are consequently very limited in their capacity to present antigen to T lymphocytes and to induce cytokine polarization [29,38]. In vivo, the tolerogenic potential of pDCs was demonstrated initially following the administration of murine liver pDCs in a cardiac allograft model [39]. The latter cells were shown to acquire alloantigens in the allograft and then home, via the blood, to peripheral lymph nodes where they induced the generation of CD4+CD25+forkhead box P3 (FoxP3)+ Treg expressing chemokine (C-C motif) receptor 4 (CCR4) [40]. Stimulation of pDCs with CD200-Ig [41], CTLA-4-Ig or glucocorticoid-induced TNF receptor family-related gene-Ig-induced IDO production and contributed to the tolerogenic state of these cells [42]. Furthermore, pDCs from tumour-draining lymph nodes express IDO constitutively, suggesting that they help to maintain the state of immunosuppression within the tumour [43].
CD19+ DCs also have a plasma cell-like morphology, but unlike pDC they are found in the red pulp of the mouse spleen. These cells are normally capable of stimulating T cells. However, they can synthesize large amounts of IDO after ligation of CD80/86 by CTLA-4 bound on the surface of Treg[44] or by ligation of Toll-like receptor 9 (TLR-9) [45], and thereby become immunosuppressive. As for pDC, the suppressive action of CD19+ DCs is under the control of type I IFN.
Mesenchymal stem cells (Fig. 2)
Fig. 2.
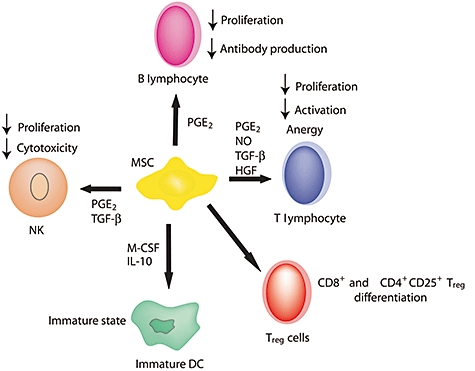
Immunomodulatory properties of mesenchymal stem cells (MSC). MSC induce regulatory T cell differentiation and amplification. Prostaglandin E2 (PGE2), nitric oxide (NO), transforming growth factor (TGF)-β and hepatocyte growth factor produced by MSC are involved in the suppression of T cell proliferation and activation and induce anergy. PGE2 and TGF-β block natural killer (NK) cell proliferation and cytotoxicity, whereas PGE2 suppresses B cell proliferation and differentiation into plasmocytes. Secretion of macrophage colony-stimulating factor and interleukin (IL)-10 by MSC prevents dendritic cell maturation.
Mesenchymal stem cells (MSC) are multi-potent non-haematopoietic cells located in the bone marrow, and to some extent in fat tissue [46], placenta, amniotic fluid [47] and umbilical cord blood [48]. MSC can differentiate into multiple mesenchymal lineages such as bone, fat and cartilage [49], endothelial cells, neural cells or endodermic cells [50,51]. MSC are characterized by the expression of single-strand conformation analysis (SSCA)-1 [52] and SSCA-4 [53] in mice, or ganglioside GD-2 [54] in humans, but lack expression of CD11b, CD14, CD31 or CD45 haematopoietic and endothelial markers [49]. MSC possess immunomodulatory properties thought to play a role in the maintenance of peripheral tolerance, in the control of autoimmunity [55] and in fetal–maternal tolerance [56]. In vitro, MSC are able to suppress T lymphocyte activation and proliferation induced by mitogens and polyclonal activators as well as by cognate antigens [57]. MSC induce a cell cycle arrest at the G0/G1 phase, rendering T cells anergic [58]. TGF-β and HGF [57] are involved in the suppression of T cell proliferation by MSC. Prostaglandin E2 (PGE2) [59] or NO [60], both secreted constitutively by MSC, may also play a role in their suppressive activity. So far it is not entirely clear whether IDO is [61] or is not [59] involved in the immune inhibition by MSC. Recently, a pivotal role was reported for HO-1 in the immunosuppressive properties of rat and human MCS [62]. In vivo, MSC can also modulate immune responses by inducing CD8+ Treg cell generation [63] and CD4+CD25+ Treg amplification [59]. Also, MSC modulate DC differentiation and maintain them in an immature state (inhibition of co-stimulatory molecule and MHC class II overexpression). This modulation takes place through the action of macrophage colony-stimulating factor [64] and IL-10 [65]. In addition to T cell suppression, the immunosuppressive action of MSC extends to B cells: their proliferation, differentiation and antibody production is prevented without modification of co-stimulatory molecule expression or cytokine production through the synthesis of PGE2[66]. NK cells also stop proliferating and secreting cytokines upon contact with MSC or in the presence of TGF-β or PGE2 secreted by MSC [67]. In vivo, MSC delayed the T cell-mediated rejection of skin allografts in primates [68] and decreased rejection of allogeneic BM transplantation in mice [69]. MSC were also found to be protective in a rat model of kidney ischaemia/reperfusion injury [70] as well as in a model of heart transplantation [71].
Other stem cells
The immune-privileged properties of embryonic stem cells (ESC) [72] also makes these cells candidates as a source of regulatory cells for cell therapy. ESC express low levels of MHC class I and class II molecules [73], albeit sufficient in quantity to elicit their rejection by cytotoxic T cells [74]. The reason for their immunomodulatory properties resides in their synthesis of HO-1, which reduces proliferative responses in vitro[75]. Neural stem cell (NSC) transplantation or transplantation of ESC-derived neural precursors has also been proposed as a means of cell replacement therapy. It was shown initially that intraventricular transplantation of NSC attenuated brain inflammation in acute and chronic experimental autoimmune encephalomyelitis (EAE) and reduced demyelination and axonal pathology [76,77]. However, their intravenous injection also inhibited EAE and reduced central nervous system (CNS) inflammation and tissue injury, although they did not enter the CNS [78]. In these experiments, NSC were found to have migrated to lymph nodes and spleen where they inhibited the activation and proliferation of T cells and reduced markedly their encephalitogenicity. Although the mechanism of action involved has not yet been elucidated, it seems that NSC inhibit the activation of T cells without inducing their apoptosis [79].
Alternatively activated macrophages (Fig. 3a)
Fig. 3.
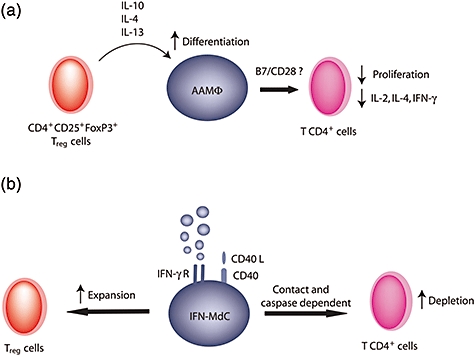
Suppressive function of alternatively activated macrophages (AAMΦ) and interferon-γ-stimulated monocyte-derived cells (IFN-γ-MdC). (a) Cytokines secreted by regulatory cells induce AAMΦ differentiation. Interaction with CD4+ T cells induces suppression of proliferation and inhibition of cytokine production. (b) IFN-γ-MdC induce depletion of activated CD4+ T cells through contact and caspase-dependent mechanisms. On the other hand, IFN-γ-MdC induce regulatory T cell expansion.
Upon activation, macrophages present a heterogeneous phenotype that divides them into two categories: ‘classically’ activated macrophages (CAMΦ or M1) and ‘alternatively’ activated macrophages (AAMΦ or M2) [80]. Such distinct differentiation depends on the presence of specific cytokines in the microenvironment. Inflammatory responses are induced by CAMΦ under the influence of microbial agent or type 1 cytokines such as IFN-γ or IL-12. This classical activation is associated with a large production of NO and proinflammatory cytokines such as IL-6 or TNF-α. In contrast, an anti-inflammatory environment is generated by the action of AAMΦ induced by anti-inflammatory agents such as IL-4, IL-10, IL-13, TGF-β, granulocyte–macrophage colony-stimulating factor or glucocorticoids [80,81]. Following administration of IL-4 or IL-13, murine AAMΦ overexpress mannose receptors (CD206) [82] as well as scavenger receptors (CD163) [83] and MHC class II molecules [84] that stimulate endocytosis and antigenic presentation. IL-4 and IL-13 also induce the expression of the co-stimulatory molecule PD-L2 [85], the surface markers CD23 [86], CD163 [87] and CD14 [88]), the chemokines CCL2 (MCP1) [89], CCL22 (MDC) [90], CCL17 (TARC) [91] and CCL18 (AMAC1) [92], the cytokines IL-1 [93], IL-10 [94] and TGF-β[95] and intracellular enzymes such as arginase I [81]. The expression and secretion of galectin-3 is also a major characteristic of AAMΦ[96]. Functionally, AAMΦ are immunosuppressive and inhibit the proliferation of activated CD4+ T cells and the secretion of IL-2, IFN-γ and IL-4 in allogeneic mixed leucocyte reaction [97]. The neutralization of IL-10 with antibodies, or the blockade of NO release, fails to reverse the inhibition of AAMΦ, indicating that these factors are not essential for their suppressive activity. The suppression is also independent of co-stimulatory molecules (in contrast with CAMΦ) [98]. However, AAMΦ can be induced secondarily after B7/CD28 T cell co-stimulation blockade, suggesting that they might play a role in tolerance induction in transplantation [99]. Because they are immunosuppressive, AAMΦ are involved in a large variety of pathologies including allergy as well as cellular and humoral responses against parasites and extracellular pathogens. They are found in the placenta and in the lung, where they protect against unwanted immune reactivity and down-regulate inflammatory reactions [100,101]. Recently, a novel function was reported for CD4+CD25+FoxP3+ Treg cells in the induction of AAMφ; Treg cells produced high levels of IL-10, IL-4 or IL-13, which in turn enhanced AAMΦ differentiation [102]. Tumour-associated macrophages are a type of AAMΦ that are the most abundant immunosuppressive cells within the tumour microenvironment. They exhibit the IL-10highIL-12low AAMΦ profile and proliferate in response to the cytokines leukemia inhibitory factor and IL-6 present in the tumour microenvironment [103].
The IFN-γ-stimulated monocyte-derived cells (IFN-γ-MdC; Fig. 3b)
The IFN-γ-MdC are a macrophage subset arising when the latter are cultured in the presence of CD40L-expressing CD4+ T cells, macrophage colony-stimulating factor and IFN-γ. IFN-γ-MdC express F4/80, CD11b/c, CD86 and CD274, but lack CD4, CD8, Gr1, CD19, CD80 and CD207. Functionally, IFN-γ-MdC induce a cell contact and caspase-dependent depletion of activated T cells and an expansion of CD4+CD25+FoxP3+ regulatory cells. Although their mechanism of action has not yet been identified, the expression of IFN-γ receptor and CD40 has been shown to be necessary for their functional activity. Contrary to expectation, IDO is not thought to be involved in the immunosuppressive activities of IFN-γ-MdC [104]. In mice, IFN-γ-MdC delivered intravenously can migrate to gut-associated peripheral lymphoid tissues and promote the clinical and histological resolution of chronic colitis and dampen acute rejection of allogenic heart transplants [104].
Myeloid-derived suppressor cells (Fig. 4)
Fig. 4.
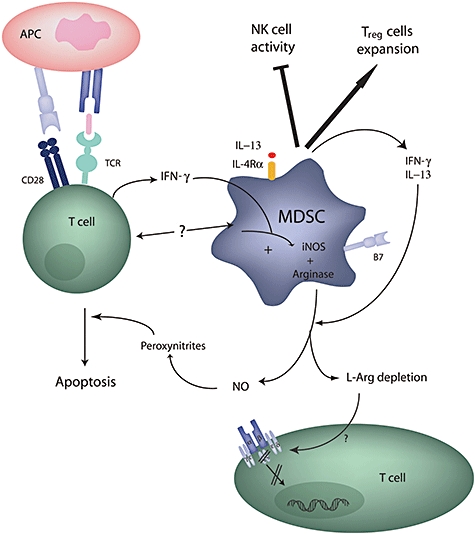
Immunoregulatory properties of myeloid-derived suppressor cells (MDSC). The release of interferon (IFN)-γ by T lymphocytes and the stimulation through CD40 trigger IFN-γ and interleukin (IL)-13 production by MDSC, which leads secondarily to the production of both inducible nitric oxide synthase (iNOS) and arginase I. These enzymes are able to suppress T cell receptor signalling and induce T cell apoptosis. In addition, MDSC are also able to enhance regulatory T cell expansion and suppress natural killer (NK) cell activity.
In the 1980s, cells named natural suppressor cells, distinct from T and NK cells, were described in mice bearing transplantable tumours [105]. These cells, originating from the bone marrow, derive from a heterogeneous mixture of myeloid cells at different stages of differentiation. They were defined initially as immature myeloid cells or myeloid suppressor cells (MSC) because of their capacity to suppress immune responses [106]. To minimize confusion with MSC, Gabrilovitch proposed to name these cells ‘myeloid-derived suppressor cells’ (MDSC) [107]. These cells accumulate in mice in various lymphatic organs [108] in pathological conditions, including bacterial infections [109], chronic inflammation, tumour progression, graft-versus-host disease [110] or immune stress following activation by superantigens [111]. They are characterized by the co-expression of Gr-1 (Ly-6G) and CD11b [106] together with the immature cell marker CD31 [112]. Other markers that could be correlated with the suppressive function of these cells include CD80 [113], F4/80, CD115 (macrophage colony-stimulating factor receptor) [114] and CD16 [115]. MDSC also express major histocompatibility class I but not class II molecules [116]. In humans, MDSC are defined by the expression of immature markers such as CD34+, CD33+, CD15+, CD14− and CD13+ and are increased in the peripheral blood of cancer patients [117]. MDSC are able to suppress T and B cell proliferation and cytokine production by blocking entry into the cell cycle, without killing target cells [118], in a contact-dependent manner [116]. They are also able to suppress NK cell activity [119]. Several studies showed that the interaction itself between MDSC and T cells, as well as IFN-γ secretion by T lymphocytes, are instrumental in the induction of MDSC suppressive activity [118]. Indeed, in tumour-bearing mice, both release of IFN-γ by T lymphocytes and stimulation through CD40 [118] induce MDSC to produce IFN-γ and IL-13, which are utilized in an autocrine manner to enhance their suppressive properties [120]. To control T lymphocyte responses, activated MDSC enhance the production and activity of two enzymes involved in the metabolism of L-arginine, a non-essential amino acid that plays a role in immune responses and regulation of T lymphocyte function [121]. These enzymes are inducible NO synthase (iNOS), which induces NO production [122], and arginase-1 (ARG-1), which depletes arginine from the microenvironment [123]. ARG-1 converts L-arginine into urea and L-ornithine whereas iNOS oxidizes L-arginine into L-citrulline and NO [124]. The activation of ARG-1 and iNOS suppresses T cell proliferation by interfering with intracellular signals involved in the transduction pathways leading to T cell apoptosis [125]. Indeed, reversal of the inhibitory effect on T cell proliferation was achieved following addition of N(G)-mono-methyl-L-arginine, an inhibitor of iNOS [122]. Although low concentrations of NO act in synergy with the T cell receptor (TCR) to stabilize p53 and allow for IL-2 synthesis by effector T cells [126], higher doses inhibit phosphorylation and activation of signalling molecules such as Janus kinases, STAT5, extracellular-regulated kinase and protein kinase B (Akt) [118], and therefore block the IL-2 and MAPK signalling pathways. The use of phosphodiesterase-5 inhibitors, which down-regulate ARG 1 and iNOS expression, was shown to reduce tumour recruited-MDSC expansion and indirectly enhance T cell proliferation [127]. Inhibition of T cell proliferation by MDSC is also characterized by the loss of the TCR ζ chain [108] in a L-arginine-dependent manner [128].
In addition to their direct activity on T cells, MDSC are able to down-regulate T cell proliferation indirectly by enhancing the development of CD4+CD25+FoxP3+ Treg[114]. Tumour growth in mice was shown to be delayed by the action of anti-CTLA-4 antibodies that block the CTLA-4/CD80 interactions occurring between Treg and MDSC [129]. Also, the cross-talk between MDSC and macrophages of tumour-bearing mice results in a reduction in IL-12 release by macrophages and an increase in IL-10 production by MDSC, reorientating the response towards a type 2 response that favours tumour progression [130].
In transplantation, a role for MDSC was described recently for the first time in a rat model of kidney allograft tolerance induced by anti-CD28 antibodies [131]. In this model, MDSC were found to accumulate in the graft and blood of recipient animals and the production of NO was found to be responsible for the maintenance of tolerance.
Clinical potential of regulatory ‘non-T’ cells
Regulatory ‘non-T’ cells basically present non-antigen-specific immunosuppressive properties. They usually do not induce dominant tolerance [132]. However, they co-operate with Treg cells and reinforce an antigen-specific and dominant immunosuppression driven by these Treg cells. The therapeutic potential of regulatory ‘non-T’ cells is best exemplified in animal models where transplant tolerance or the improvement of immunoinflammatory diseases could be obtained after adoptive transfer of MDC [4], pDC [39], NSC [78] or IFN-γ DC [104]. In man, the immunoregulatory potential of regulatory ‘non-T’ cells is currently being tested. Transplant acceptance-inducing cells (TAIC) are immunoregulatory macrophages with the capacity to specifically dampen allogeneic rejection [104]. In a safety clinical trial study, the pretransplant infusion of these donor-derived regulatory cells in kidney allograft recipients did not provide conclusive evidence of a beneficial effect. However an alloantigen-specific unresponsiveness was observed which suggested that TAIC injection was not immunogenic but immunoregulatory, and could allow minimization of pharmacological immunosuppression. The transfer of autologous ex vivo expanded MSCs (NCT00752479 phase I/II study; available at: http://clinicaltrials.gov/) to kidney allograft recipients under a standard immunosuppressive regimen is also tested to suppress immune rejection further and improve donor kidney survival. In type I diabetes patients, autologous monocyte-derived DC are also used in a phase I clinical trial (NCT00445913). The cells are first treated ex vivo with anti-sense phosphorothioate-modified oligonucleotides targeting the primary transcripts of the CD40, CD80 and CD86 co-stimulatory molecules to produce iDC and infused to assess their diabetes-suppressive potential.
Concluding remarks
In addition to Treg, a series of other cell types have been described that share the common feature of being able to modulate the reactivity of T and other immune cells. The most common mechanism of action of these cells is a direct induction of target T cell death or growth arrest, mediated by immunosuppressive enzymes, cytokines or cytotoxic mechanisms. In addition, a consistent finding has been the non-T cell-mediated induction of Treg cells, which secondarily mediate the immune suppression. Inversely, Treg cells can also trigger immunoregulation by non-T cells (as is the case for AAMΦ; Table 1). Therefore, immune regulation should not be seen as a consequence of the action of one regulatory cell type but rather of the synergistic interactions of T and regulatory ‘non-T’ cells.
Table 1.
Non-lymphoid cells with immunosuppressive activity.
| Cell type | Mechanism of action | Transplant or inflammation model |
|---|---|---|
| Myeloid DC | Fas/FasL [16], TGF-β, IL-10, induction of T cell anergy, induction of Treg[6] | Cardiac allograft survival in mice [3] and rats [4,5] |
| IDO [25], HO-1 [9], NO [5] | ||
| Plasmacytoid DC | Controls T cell polarization [38], IDO [133], induction of Treg[40] | Cardiac allograft survival in mice [39] |
| MSC | PGE2[59], TGF-β[57], NO[60], IDO [61], HO-1 [62]Induction of T cell anergy [58], induction of Treg[59]. | Heart transplantation in mice [71] |
| ESC | Low MHC expression [73]Production of HO-1 [75] | Transplantation of ESC for tissue repair [134] |
| NSC | n.d. | Intracerebral injection of neuronal precursors in EAE [78] |
| AAMΦ | TGF-β[80] | Suppression of T cell proliferation [99] |
| Arginase I [80] | ||
| IFN-γ–MdC | Induction of Treg, CD40 and IFN-γ-dependent [104] | Heart transplantation in mice Mouse DSS-induced colitis [104] |
| MDSC | Release of nitric oxide [122]Action of arginase I [123] | Kidney transplantation in rats [131] |
AAMΦ, alternatively activated macrophages; DC, dendritic cell; DSS, dextran sulphate sodium; EAE, experimental autoimmune encephalomyelitis; ESC, embryonic stem cells; IFN-γ–MdC, interferon (IFN)-γ-stimulated monocyte-derived cells; MDSC, myeloid-derived suppressor cells; MSC, mesenchymal stem cells; n.d., not defined; NSC, neural stem cells; IDO, indoleamine 2,3-dioxygenase; HO-1, haem oxygenase; PGE2, prostaglandin E2; IL, interleukin; IFN, interferon; TGF, transforming growth factor; Treg, T regulatory cells.
References
- 1.Moser M. Dendritic cells in immunity and tolerance – do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86–) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DePaz HA, Oluwole OO, Adeyeri AO, et al. Immature rat myeloid dendritic cells generated in low-dose granulocyte macrophage-colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation. 2003;75:521–8. doi: 10.1097/01.TP.0000048380.84355.4A. [DOI] [PubMed] [Google Scholar]
- 5.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–10. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 7.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 9.Chauveau C, Remy S, Royer PJ, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 10.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 11.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 14.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 15.Kronin V, Wu L, Gong S, Nussenzweig MC, Shortman K. DEC-205 as a marker of dendritic cells with regulatory effects on CD8 T cell responses. Int Immunol. 2000;12:731–5. doi: 10.1093/intimm/12.5.731. [DOI] [PubMed] [Google Scholar]
- 16.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–96. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronin V, Winkel K, Suss G, et al. A subclass of dendritic cells regulates the response of naive CD8 T cells by limiting their IL-2 production. J Immunol. 1996;157:3819–27. [PubMed] [Google Scholar]
- 18.Rethi B, Gogolak P, Szatmari I, et al. SLAM/SLAM interactions inhibit CD40-induced production of inflammatory cytokines in monocyte-derived dendritic cells. Blood. 2006;107:2821–9. doi: 10.1182/blood-2005-06-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HK, Guan H, Zu G, et al. High-level expression of B7-H1 molecules by dendritic cells suppresses the function of activated T cells and desensitizes allergen-primed animals. J Leukoc Biol. 2006;79:686–95. doi: 10.1189/jlb.0805436. [DOI] [PubMed] [Google Scholar]
- 20.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–17. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Vacca C, Orabona C, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–8. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Terness P, Bauer TM, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 26.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Diff. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 28.Murray MF. Tryptophan depletion and HIV infection: a metabolic link to pathogenesis. Lancet Infect Dis. 2003;3:644–52. doi: 10.1016/s1473-3099(03)00773-4. [DOI] [PubMed] [Google Scholar]
- 29.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–50. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 31.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 34.Dzionek A, Inagaki Y, Okawa K, et al. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–48. doi: 10.1016/s0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- 35.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 36.Bauer M, Redecke V, Ellwart JW, et al. DNA triggers activation and maturation of human CD11c–, CD123+ dendritic cells. J Immunol. 2001;166:5000–7. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- 37.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 38.Boonstra A, Asselin-Paturel C, Gilliet M, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J Exp Med. 2003;197:101–9. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Bonham CA, Liang X, et al. Liver-derived DEC205+B220+CD19– dendritic cells regulate T cell responses. J Immunol. 2001;166:7042–52. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- 40.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 41.Fallarino F, Asselin-Paturel C, Vacca C, et al. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–54. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 42.Puccetti P, Fallarino F. Generation of T cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol Dis. 2008;40:101–5. doi: 10.1016/j.bcmd.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 44.Mellor AL, Chandler P, Baban B, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 45.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 47.In’t Anker PS, Scherjon SA, Kleijburg C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 48.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 49.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 50.Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells – a critical review. APMIS. 2005;113:831–44. doi: 10.1111/j.1600-0463.2005.apm_3061.x. [DOI] [PubMed] [Google Scholar]
- 51.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 52.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 53.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 54.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–8. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–79. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 57.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 58.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 61.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 62.Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 63.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 64.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 65.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–19. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 66.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 67.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 68.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 69.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 71.Fandrich F, Lin X, Chai GX, et al. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–8. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 73.Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drukker M, Katchman H, Katz G, et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–9. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 75.Trigona WL, Porter CM, Horvath-Arcidiacono JA, Majumdar AS, Bloom ET. Could heme-oxygenase-1 have a role in modulating the recipient immune response to embryonic stem cells? Antioxid Redox Signal. 2007;9:751–6. doi: 10.1089/ars.2007.1602. [DOI] [PubMed] [Google Scholar]
- 76.Ben-Hur T, Einstein O, Mizrachi-Kol R, et al. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia. 2003;41:73–80. doi: 10.1002/glia.10159. [DOI] [PubMed] [Google Scholar]
- 77.Einstein O, Karussis D, Grigoriadis N, et al. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–82. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Einstein O, Fainstein N, Vaknin I, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61:209–18. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–4. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 81.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–7. [PubMed] [Google Scholar]
- 82.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1883–90. [PubMed] [Google Scholar]
- 84.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 85.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–41. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becker S, Daniel EG. Antagonistic and additive effects of IL-4 and interferon-gamma on human monocytes and macrophages: effects on Fc receptors, HLA-D antigens, and superoxide production. Cell Immunol. 1990;129:351–62. doi: 10.1016/0008-8749(90)90211-9. [DOI] [PubMed] [Google Scholar]
- 87.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 88.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 89.Gao JL, Wynn TA, Chang Y, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–68. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonecchi R, Sozzani S, Stine JT, et al. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood. 1998;92:2668–71. [PubMed] [Google Scholar]
- 91.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–8. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 92.Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J Immunol. 1998;160:1411–18. [PubMed] [Google Scholar]
- 93.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 94.Kambayashi T, Jacob CO, Strassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell Immunol. 1996;171:153–8. doi: 10.1006/cimm.1996.0186. [DOI] [PubMed] [Google Scholar]
- 95.Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mackinnon AC, Farnworth SL, Hodkinson PS, et al. Regulation of alternative macrophage activation by Galectin-3. J Immunol. 2008;180:2650–8. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 97.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 98.Schebesch C, Kodelja V, Muller C, et al. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology. 1997;92:478–86. doi: 10.1046/j.1365-2567.1997.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2002;99:1465–73. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- 100.Chang MD, Pollard JW, Khalili H, Goyert SM, Diamond B. Mouse placental macrophages have a decreased ability to present antigen. Proc Natl Acad Sci USA. 1993;90:462–6. doi: 10.1073/pnas.90.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holt PG, Schon-Hegrad MA, Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988;167:262–74. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 104.Brem-Exner BG, Sattler C, Hutchinson JA, et al. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008;180:335–49. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 105.Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–37. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- 106.Bronte V, Wang M, Overwijk WW, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–20. [PMC free article] [PubMed] [Google Scholar]
- 107.Gabrilovich DI. Molecular mechanisms and therapeutic reversal of immune suppression in cancer. Curr Cancer Drug Targets. 2007;7:1. [PubMed] [Google Scholar]
- 108.Ezernitchi AV, Vaknin I, Cohen-Daniel L, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–72. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 109.Goni O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int Immunol. 2002;14:1125–34. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- 110.Bobe P, Benihoud K, Grandjon D, Opolon P, Pritchard LL, Huchet R. Nitric oxide mediation of active immunosuppression associated with graft-versus-host reaction. Blood. 1999;94:1028–37. [PubMed] [Google Scholar]
- 111.Cauley LS, Miller EE, Yen M, Swain SL. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-gamma. J Immunol. 2000;165:6056–66. doi: 10.4049/jimmunol.165.11.6056. [DOI] [PubMed] [Google Scholar]
- 112.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 113.Mencacci A, Montagnoli C, Bacci A, et al. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunol. 2002;169:3180–90. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- 114.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 115.Marshall MA, Jankovic D, Maher VE, Sher A, Berzofsky JA. Mice infected with Schistosoma mansoni develop a novel non-T-lymphocyte suppressor population which inhibits virus-specific CTL induction via a soluble factor. Microbes Infect. 2001;3:1051–61. doi: 10.1016/s1286-4579(01)01499-x. [DOI] [PubMed] [Google Scholar]
- 116.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 117.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 118.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 119.Brooks JC, Hoskin DW. The inhibitory effect of cyclophosphamide-induced MAC-1+ natural suppressor cells on IL-2 and IL-4 utilization in MLR. Transplantation. 1994;58:1096–103. [PubMed] [Google Scholar]
- 120.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–68. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 123.Mills CD, Shearer J, Evans R, Caldwell MD. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992;149:2709–14. [PubMed] [Google Scholar]
- 124.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–6. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 125.Brito C, Naviliat M, Tiscornia AC, et al. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–66. [PubMed] [Google Scholar]
- 126.Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl)(3):iii37–40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–15. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 130.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 131.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 132.Degauque N, Lair D, Dupont A, et al. Dominant tolerance to kidney allografts induced by anti-donor MHC class II antibodies: cooperation between T and non-T CD103+ cells. J Immunol. 2006;176:3915–22. doi: 10.4049/jimmunol.176.7.3915. [DOI] [PubMed] [Google Scholar]
- 133.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–41. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]


