Abstract
Severe thermal injury induces immunosuppression, involving all parts of the immune system, especially when large fractions of the total body surface area are affected. An animal model was established to characterize the burn-induced immunosuppression. In our novel mouse model a 6% third-degree burn injury was induced in mice with a hot-air blower. The third-degree burn was confirmed histologically. The mice were allocated into five groups: control, shave, burn, infection and burn infection group. At 48 h, a decline in the concentration of peripheral blood leucocytes was observed in the group of mice with burn wound. The reduction was ascribed to the decline in concentration of polymorphonuclear neutrophil leucocytes and monocytes. When infecting the skin with Pseudomonas aeruginosa, a dissemination of bacteria was observed only in the burn wound group. Histological characterization of the skin showed a more polymorphonuclear neutrophil granulocytes (PMNs)-dominated inflammation in the group of mice with infected burn wound compared with the with burn wound group. In contrast, a higher degree of inflammation was observed in the burn wound group compared with the group of mice with infected burn wound. Furthermore, the oxidative burst and the phagocytic capacity of the PMNs were reduced in the group of mice with burn wound. Using this novel mouse model of thermal injury a decline of peripheral leucocytes was observed, whereas the increased local inflammatory response at the site of infection showed reduced capacity to contain and eliminate the infection.
Keywords: animal model, oxidative burst, phagocytosis, PMNs, Pseudomonas aeruginosa, thermal injury
Introduction
Several thousand people die each year because of complications from burn wounds [1,2]. Although some burn patients die of burn shock during the first hours the major course of death is infections, and it is estimated that 75% of all deaths following burns are related to infection [3].
Burn patients are especially susceptible to infections compared with other trauma patients, because of loss of the skin barrier. The burn wound can be regarded as a culture medium, and avascularity of the burn wound prevents the action of the blood-borne immune system. Furthermore, the use of invasive procedures bypassing the remaining mechanical and biological barriers increases the risk of influx of microorganisms. In addition, prolonged hospital stay and translocation of bacteria from the gastrointestinal organs also contribute to the increased risk for contracting infections, complicated further by the increased length of stay at the hospital and the use of more central venous catheters in burn patients [2].
Regarding the immune system, all parts have been reported to be affected. Both the innate and the adaptive, especially the cellular immune system, is influenced by the thermal injury [4]. Furthermore, the size of the burn appears to matter, as burns of greater extent than 30% of the total body surface area (TBSA) particularly affect the immune system [4].
Pseudomonas aeruginosa is known to cause serious infections in immunocompromised hosts, such as patients with neutropenia and burn patients. Because of the armamentarium of exotoxins and cell-associated virulence factors, P. aeruginosa can disseminate within the burn wound. In this respect, P. aeruginosa infection is a complication, as 10% of the burn victims who become infected with P. aeruginosa have a mortality rate of 80% [5]. Furthermore, P. aeruginosa can cause skin grafts to fail or delay healing of the burn wound. Despite improvement in the general treatment and outcome for burn patients, those who become infected with P. aeruginosa have not benefited from this progress, and the mortality rate has been unchanged in the last 25 years.
Given the high morbidity and mortality of P. aeruginosa-infected burn victims in combination with the emergence of resistant P. aeruginosa, it is important to understand the innate immune response in order to be able to improve therapy, in addition to traditional antibiotic treatment. Hence, we present a novel model inducing third-degree burn injury covering 6% of the TBSA of the mouse. Using this burn mouse model, increased susceptibility to P. aeruginosa burn wound infection was observed. To explain the increased susceptibility to P. aeruginosa, the innate immune response of mice with thermal injuries was investigated.
Materials and methods
Ethics and statistics
The novel burn model was developed in close collaboration with and approved by the Animal Ethics Committee of Denmark. The Danish Animal Committee allowed only a 6% burn area of the total body surface. It was crucial that the animal did not suffer unnecessarily and that the model was useful in a pathophysiological manner to help burn patients.
Statistical analysis of the type of inflammation within all the groups was estimated by Kruskal–Wallis test. Qualitative comparison within two groups was made by Mann–Whitney U-test; the χ2 test was used for comparison of dissemination; and the unpaired t-test was used for cells. All statistics were performed using Statview (Abacus Concepts, Berkely, CA, USA). A probability value of 0·05 was considered statistically significant.
Animals
Specified pathogen-free female 12-week-old C3H/HeN mice (20–25 g) were purchased from M & B Laboratory Animals (Ry, Denmark) and were allowed to acclimatize to the animal facility for at least 1 week prior to experimentation. They were kept in barrier facilities at the Panum Institute, Copenhagen University and had access to chow and water ad libitum.
Induction of thermal injury
The animals were anaesthetized subcutaneously (s.c.) with 0·30 ml Hyp/Mid [2·5 mg/ml Hypnorm (Jansen, Birkeroed, Denmark) and 1·25 mg/ml Midazolan (Roche, Basel, Switzerland) in sterile water]. An electrical clipper was used to shave the hair on the back of each mouse. The mice were placed onto a sledge and covered with a fire blanket with a window (1·7 × 2·6 cm) corresponding to approximately 6% of the TBSA. Above the fire blanket a metal plate with a window (1·7 × 2·6 cm) was placed. The sledge with mice, the fire blanket (Viking, Esbjerg, Denmark) and metal plate were moved into a stream of hot air with a temperature of 330°C delivered by a hot-air blower (Bosch 500-2, Stuttgart, Germany) for 7 s. The temperature was kept constant using the same time schedule. When the procedure was disrupted, the procedure was restarted following a constant time schedule controlling the temperature. This procedure resulted in a third-degree burn and was confirmed by histological examination. Immediately afterwards, the mice received 1·0 ml (SAD, Copenhagen, Denmark) saline and 0·3 ml glucose (SAD) for fluid replacement, and every 8 h 1 ml of saline was given s.c. until the mice were able to drink. As pain therapy, buprenorphin (Schering-Plough, Brussels, Belgium) was given every 8 h during the whole experiment. During the first 24 h of recovery the mice were kept under heating lamps. The burn surface was calculated using the Meeh formula: A = KW2/3, where A = body surface area, K = 9 and W = weight in grams [6,7].
Study design
The experimental groups of mice are specified as (see Table 1):
Table 1.
Design of the study.
| Time of killing (h) |
|||||||
|---|---|---|---|---|---|---|---|
| Experiment | Group | 24 | 48 | 72 | 96 | Investigation | Number of mice |
| Concentration of leucocytes | CG | 6 | – | – | – | FACS on blood | CG (n = 6) |
| SG (n = 18) | |||||||
| BG (n = 18) | |||||||
| Total number: 42 | |||||||
| SG | 6 | 6 | 6 | – | |||
| BG | 6 | 6 | 6 | – | |||
| Infection | CG | 6 | – | – | – | FACS on blood | CG (n = 6) |
| SG (n = 48) | |||||||
| BG (n = 63) | |||||||
| BIG (n = 24) | |||||||
| IG (n = 24) | |||||||
| Total number: 165 | |||||||
| SG | 12 | 12 | 12 | 12 | |||
| BG | 16 | 16 | 15 | 16 | |||
| BIG | – | – | 15 | 9 | |||
| IG | – | – | 16 | 8 | |||
| Dissemination | IG | – | – | 7 | – | Culture of swabs from beneath the skin | CG (n = 7) |
| Culture of blood, liver, and spleen. | SG (n = 7) | ||||||
| Total number: 14 | |||||||
| BIG | – | – | 7 | – | |||
| Histopathology | SG | 6 | 7 | 6 | 6 | Microscopic evaluation of the skin and lungs | CG (n = 25) |
| BG | 8 | 8 | 8 | 8 | SG (n = 32) | ||
| BIG | – | – | 8 | 9 | BG (n = 17) | ||
| IG | – | – | 8 | 8 | BIG (n = 16) | ||
| Total number: 90 | |||||||
| Function of PMNs | SG | – | 6 | – | – | FACS on blood | SG (n = 6) |
| Phagocytosis test | BG (n = 5) | ||||||
| Respiratory burst test | Total number: 11 | ||||||
| BG | 5 | – | – | ||||
Data in the infection experiment were collected from two individual experiments. CG, control group; SG, shave group; BG, burn group; BIG, burn infection group; IG, infection group; PMN, polymorphonuclear neutrophil granulocytes; FACS, fluorescence activated cell sorter.
Control group: mice were unshaved and received only fluid replacement and pain therapy.
Shave group (SG): mice were shaved and received fluid replacement and pain therapy.
Burn group (BG): mice were shaved, burned and received fluid replacement and pain therapy.
Burn infection group (BIG): mice shaved, burned and received fluid replacement and pain therapy. Furthermore, the mice were infected with P. aeruginosa beneath the burn wound.
Infection group (IG): mice shaved and received fluid replacement and pain therapy. Furthermore, the mice were infected with P. aeruginosa beneath the burn wound.
Preparation of challenge solution
The wild-type P. aeruginosa PAO1 strain was obtained from the Pseudomonas Genetic Stock Center (http://www.ecu.edu/Pseudomonas;PAO0001). P. aeruginosa was grown in 100 ml filtered oxbroth overnight at 37°C on a shaker. Strain PAO1 was used in the burn model. To establish a standard curve, the microbial density was estimated by colorimeter followed by serial 10-fold dilution. One hundred µl of the aliquots were plated on Blue plates (a modified Conradi–Drigalski diagnostic substrate selective for Gram-negative rods; Statens Serum Institut, Copenhagen, Denmark) and 24 h later the colony-forming units (CFU) were counted.
Challenge procedure
The mice were injected s.c. with 100 µl challenge solution (infection dose 107 CFU/ml) with a 27 G needle (Terumo Europe NV, Leuven, Belgium) beneath the burn wound.
Blood samples
Fifty µl of blood was obtained by cardiac puncture and plated immediately onto Blue plates. In addition, 100 µl of blood was put into vials. The vials were incubated overnight at 37°C in a shaking incubator.
Spleen and liver samples
Spleen and liver were removed aseptically and stored in saline on ice until homogenizing. Serial 10-fold dilutions of homogenates from 10−1 to 10−4 were prepared in saline. Aliquots of 0·1 ml of each dilution were spread onto a Blue plate.
Skin samples from burn wound
Swabs from beneath the burn eschar or from the site of infection were carried out immediately after killing the mice. The swabs were plated onto Blue plates and the presence or absence of bacteria was registered 24 h later. The plates were incubated overnight at 37°C.
Lungs
To estimate sequestration of polymorphonuclear neutrophil granulocytes (PMNs), the lungs were removed aseptically from mice and fixed immediately in 4% paraformaldehyde (Bie & Berntsen, Copenhagen, Denmark).
Histopathology
For microscopy, two slides with two tissue sections mounted on each were used for evaluation. Regarding the skin samples, an overview at low magnification was provided (62.5) and detailed studies of five different areas of each tissue section using high magnification (500). Regarding the lungs, the entire lung slide was scanned at low magnitude and from an average evaluation of a minimum of five representative areas at higher magnitude (250 and 500). For both the lungs and skin, the type of inflammation was typed as acute (> 90% PMNs), chronic [> 90% mononuclear cells (MN)], both types present, neither dominating (PMN/MN) or no inflammation (NI). The degree of inflammation was scored as 0 (NI), 1 (mild focal inflammation), 2 (moderate to severe focal inflammation) or 3 (severe inflammation with necrosis or with severe inflammation throughout the skin) [8].
Flow cytometry
All monoclonal antibodies were purchased from BD Bioscience (La Jolla, CA, USA), except for antibodies against F4/80, which were obtained from Serotec (Bergen, Norway).
Monocytes and PMNs were identified using fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse F4/80 (CL:A3-1, IgG2b), phycoerythrin-conjugated rat anti-mouse Ly-6G, allophycocyanin-conjugated rat anti-mouse CD11b (M1/70, IgG2b) and peridinin chlorophyll-conjugated rat anti-mouse CD45 (30-F11, IgG2b).
In each fluorescence activated cell sorter (FACS) tube, 75 µl of blood was incubated with 20 µl antibody solution on ice for 30 min on a shaker in the dark. To lyse erythrocytes, 5 ml of haemolysis buffer was added to each sample and incubated for 5 min on ice on a shaker in the dark. After centrifugation (350 g for 7 min at 5°C) supernatants were discarded and pellets were resuspended with 4·5 ml phosphate-buffered saline (PBS). Finally, the samples were centrifuged (350 g for 7 min at 5°C) and the supernatants discarded. One hundred and fifty µl cold PBS with 2% phosphonoformic acid was added to the resuspended cells, and the samples were stored on ice in the dark until FACS analysis.
Counting of leucocytes was carried out by adding 50 µl of blood to Trucount tubes (340334; BD Bioscience), followed by adding FACS lysing solution (349202; Becton Dickinson) to the samples. The samples were kept on ice until analysed by FACS [9].
Functional analysis of PMNs
Phagocytosis tests were carried out using a Phagotest kit (Orpegen Pharma, Heidelberg, Germany). One hundred µl mixed heparinized blood was added to a test-tube (Falcon tube 2052, Falcon labware, Cockeysville, MD, USA). Twenty µl of pre-cooled FITC-labelled opsonized Escherichia coli were added and the blood was incubated in a water bath at 37°C for 40 min. To stop the test, the samples were placed on ice. One hundred µl of pre-cooled quenching solution was added to each sample and mixed. Three ml of washing solution was added to each tube and mixed and centrifuged for 7 min at 350 g at 4°C. The supernatant was discarded and the washing procedure was repeated. One ml of lysing solution was added and mixed. The samples were incubated for 20 min at 20°C and centrifugated for 7 min at 350 g at 4°C and the supernatant was discarded. Three ml of washing solution was added to each tube and mixed. The samples were centrifuged for 7 min at 350 g at 4°C and the supernatant discarded. Finally, 200 µl of PBS with 200 µg/ml propidium iodide (PI) (P-4170; Sigma, St Louis, MO, USA) solution was added to each sample and mixed. The samples were kept on ice for 10 min before analysis using FACS analysis [9].
A respiratory burst-test (Burstest kit, Orpegen Pharma) was performed with FACS. One hundred µl heparinized blood was added to a test tube (Falcon 2052) and incubated on ice for 10 min. Twenty µl of pre-cooled stimuli solution (E. coli) was added and incubated at 37°C for 20 min in a water bath. Ten µl of substrate solution was added to each test-tube for another 20 min at 37°C. To stop the reaction, the samples were placed on ice. Two ml of lysing solution was added and the samples were mixed and incubated at 20°C for 20 min in the dark. The samples were centrifuged for 5 min at 350 g at 4°C and the supernatant discarded. Three ml of PBS was added, mixed and centrifuged for 7 min at 350 g at 4°C. Finally, 200 µl of PBS with 200 µg/ml PI solution was added to each sample. The samples were mixed and incubated for 10 min on ice before analysis using FACS.
The samples were analysed using FACSort (Becton Dickinson, La Jolla, CA, USA) equipped with a 15 Mw-argon-ion laser tuned at 488 nm for excitation. Light scatter and logarithmically amplified fluorescence parameters from 10 000 events were recorded in list mode after gating on forward light scatter and PI fluorescence to avoid debris, cell aggregates and bacteria [9].
Results
Leucocytes
At 48 h, the concentration of leucocytes in the peripheral blood in the BG was reduced compared with the SG (Fig. 1a) (P < 0·05). Furthermore, differential counting revealed that both PMNs and monocytes were reduced at 48 h, compared with the SG (P < 0·05) (Fig. 1b and c). Based on this observation, the time for induction of the burn wound infection was determined to be at 48 h.
Fig. 1.
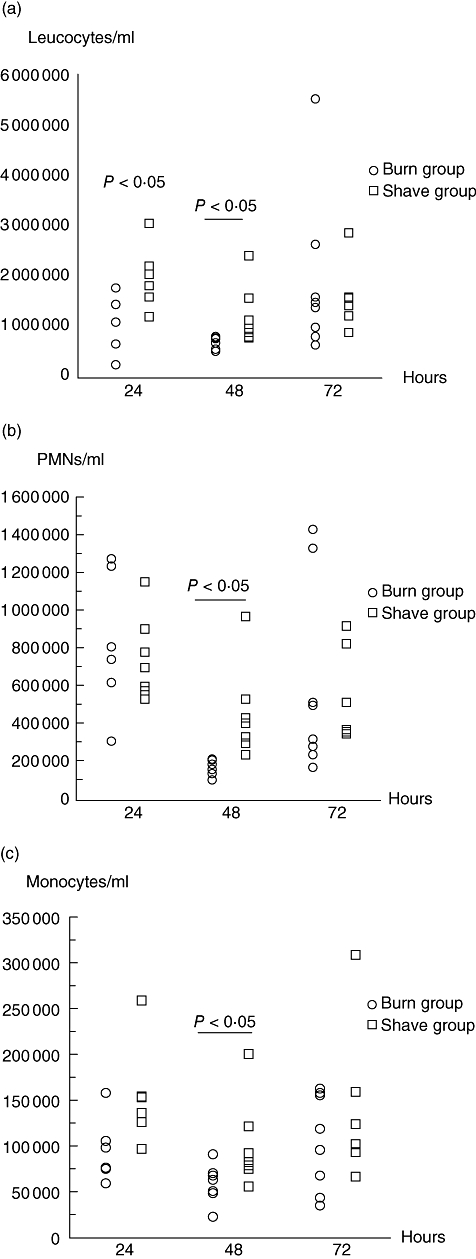
(a) The number of leucocytes in peripheral blood from C3H/HeN mice in the burn group (BG) compared with the shave group (SG) during 72 h. An unpaired t-test was used for statistical analysis. Each circle/square represents one mouse. (b) The number of polymorphonuclear neutrophil granulocytes (PMNs) in peripheral blood from C3H/HeN mice in the BG compared with the SG during 72 h. An unpaired t-test was used for statistical analysis. Each circle/square represents one mouse. (c) The number of monocytes in peripheral blood from C3H/HeN mice in the BG compared with the SG during 72 h. An unpaired t-test was used for statistical analysis. Each circle/square represents one mouse.
Skin culture
Swabs from beneath the burn eschar showed that in the BIG all the samples had P. aeruginosa cultured compared with the IG, where none had P. aeruginosa cultured (P < 0·03) (Fig. 2a).
Fig. 2.
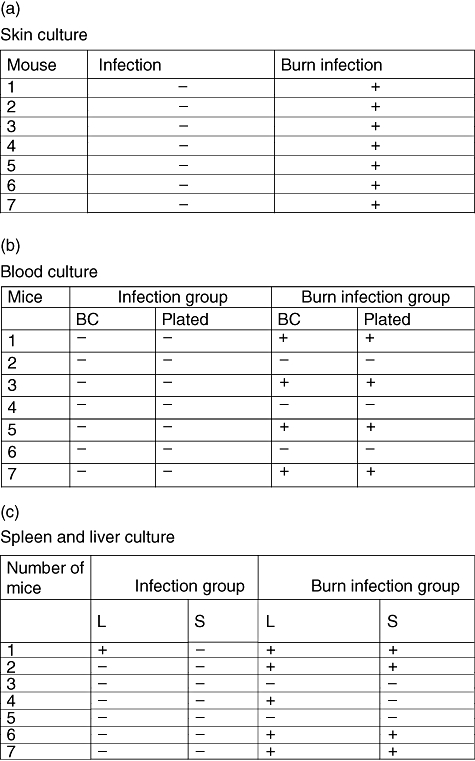
(a) Culture from beneath the skin of C3H/HeN mice in the burn infection group (BIG) compared with the infection group (IG), when infected with Pseudomonas aeruginosa beneath the skin; +/- indicates the presence or absence of P. aeruginosa beneath the skin. The samples were obtained 72 h after induction of the burn wound and 24 h after the infection. (b) Blood culture from C3H/HeN mice in the BIG compared with the IG, when infected with P. aeruginosa beneath the skin; +/- indicates the presence or absence of P. aeruginosa in the blood. The samples were obtained 72 h after induction of the burn wound and 24 h after the infection. (c) Culture of liver and spleen from C3H/HeN mice in the BIG compared with the IG, when infected with P. aeruginosa beneath the skin; +/- indicates the presence or absence of P. aeruginosa in liver or spleen. The samples were obtained 72 h after induction of the burn wound and 24 h after the infection.
Blood culture
Five of seven mice in the BIG had P. aeruginosa cultured from the blood compared with the IG, where none had P. aeruginosa cultured from blood (P < 0·03) (Fig. 2b).
Spleen and liver culture
Twenty-four hours after infection, P. aeruginosa was cultured from the spleen in four of seven mice in the BIG compared with the IG, where no mice had P. aeruginosa cultured (P < 0·07). Concerning the liver culture, five of seven mice had P. aeruginosa cultured in the BIG compared with the IG, where only one mouse had P. aeruginosa cultured (P < 0·04) (Fig. 2c).
Lungs
Lungs were without signs of sequestration of PMNs (data not shown).
Type of inflammation in skin samples
The type of inflammation was PMN-dominated in seven of eight mice 24 h after induction of the burn. This type of inflammation changed gradually to a mixed inflammation type, involving both MN and PMNs. However, a more PMN-dominated inflammation was observed in the BIG compared with the BG at 24 h after infection (P < 0·01). The type of inflammation in the BIG was similar to the type of inflammation in the IG (Table 2). At 96 h (48 h after infection) significantly more mice in the BIG had histological signs of inflammation compared with the IG (P < 0·05) (Table 2).
Table 2.
Type of inflammation in skin samples.
| 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|
| Group | Type of inflammation | |||
| Shave | 2 PMN | 2 PMN | 1 PMN | 0 PMN |
| 0 MN/PMN | 0 MN/PMN | MN/PMN | 0 MN/PMN | |
| 0 MN | 0 MN | MN | 0 MN | |
| 4 NI | 5 NI | 5 NI | 6 NI | |
| (6 mice)* | (7 mice) | (6 mice) | (6 mice) | |
| Burn | 7 PMN | 2 PMN | 0 PMN | 0 PMN |
| 0 MN/PMN | 6 MN/PMN | 8 MN/PMN | 8 MN/PMN | |
| 0 MN | 0 MN | 0 MN | 0 MN | |
| 1 NI | 0 NI | 0 NI | 4 NI | |
| (8 mice) | (8 mice) | (8 mice) | (8 mice) | |
| Burn infection | 5 PMN | 0 PMN | ||
| 3 MN/PMN | 9 MN/PMN | |||
| 0 MN | 0 MN | |||
| 0 NI | 0 NI | |||
| (8 mice) | (9 mice) | |||
| Infection | 7 PMN | 1 PMN | ||
| 1 MN/PMN | 4 MN/PMN | |||
| 0 MN | 0 MN | |||
| 0 NI | 3 NI | |||
| (8 mice) | (8 mice) | |||
Total number of mice in each group. Histopathological evaluation of the type of inflammation in the skin, burn wound, infected burn wound or infected skin from mice. The samples were obtained 24 h, 48 h, 72 h and 96 h after induction of the burn wound. χ2 test was used for statistical analysis. Type of inflammation was classified as acute [> 90% polymorphonuclear neutrophil granulocytes (PMNs)], chronic (> 90% MN), both types present, neither dominating (PMN/MN) or no inflammation (NI).
The degree of inflammation in the skin samples
The degree of inflammation was increased significantly in the BG compared with the BIG at 72 h (P < 0·001) (Table 3). No difference was observed between the BIG and the IG.
Table 3.
The degree of inflammation in the skin samples.
| 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|
| Group | Degree of inflammation | |||
| Shaved | 0 +++ | 0 +++ | 0 +++ | 0 +++ |
| 0 ++ | 0 ++ | 1 ++ | 0 ++ | |
| 2 + | 2 + | 0 + | 0 + | |
| 4 0 | 5 0 | 5 0 | 6 0 | |
| (6 mice)* | (7 mice) | (6 mice) | (6 mice) | |
| Burned | 1 +++ | 0 +++ | 0 +++ | 0 +++ |
| 3 ++ | 8 ++ | 8 ++ | 4 ++ | |
| 3 + | 2 + | 0 + | 4 + | |
| 1 0 | 0 0 | 0 0 | 0 0 | |
| (8 mice) | (8 mice) | (8 mice) | (8 mice) | |
| Burn-infected | 0 +++ | 0 +++ | ||
| 4 ++ | 9 ++ | |||
| 4 + | 0 + | |||
| 0 0 | 0 0 | |||
| (8 mice) | (9 mice) | |||
| Infected | 0 +++ | 0 +++ | ||
| 2 ++ | 0 ++ | |||
| 6 + | 5 + | |||
| 0 0 | 3 0 | |||
| (8 mice) | (8 mice) | |||
Total number of mice in each group. Histopathological evaluation of the degree of inflammation in the skin, burn wound, infected burn wound or infected skin from mice. The samples were obtained 24 h, 48 h, 72 h and 96 h after induction of the burn wound. Mann-Whiney U-test was used for statistical analysis. The degree of inflammation was scored as 0 (no inflammation), 1 (mild focal inflammation), 2 (moderate to severe focal inflammation) or 3 (severe inflammation with necrosis or with severe inflammation throughout the skin).
The infection experiment
In this study only total leucocyte counts and PMNs were investigated, and at 24 h post-burn a significant increase in the leucocytes was observed in the BG compared with the SG. In contrast, but in concordance with the previous experiment, a significant reduction in the leucocytes was observed at 48 h post-burn and again at 96 h post-burn in the burn group. Comparison between the BG and the BIG showed that the concentration of leucocytes in the BIG was depressed compared with the concentration of leucocytes in the BG at 72 h (Fig. 3a) (P < 0·01). However, at 96 h the opposite was registered, as the concentration of leucocytes was depressed in the BG compared with the BIG. Although the concentration of leucocytes did not differ between the BIG and the IG, the concentration of PMNs was reduced in the BG compared with the IG at 96 h (Fig. 3b) (P < 0·02).
Fig. 3.
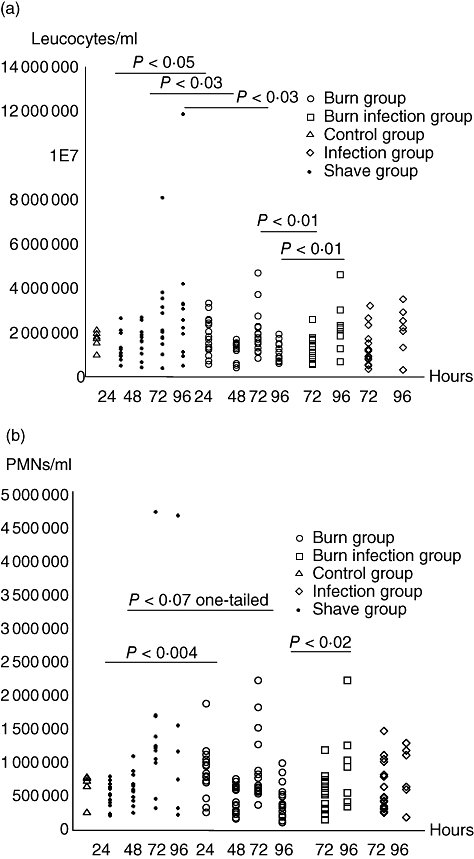
(a,b) The number of leucocytes (a) and polymorphonuclear neutrophil granulocytes (PMNs) (b) in peripheral blood from C3H/HeN mice at five different settings. An unpaired t-test was used for statistical analysis. Each circle/square/triangle represents one mouse.
The respiratory burst test showed significantly reduced burst of PMNs isolated from BG at 48 h compared with the SG [mean ± standard deviation (s.d.) 4·0 ± 3·7/mean ± s.d. 13·3 ± 7·6 respectively] (P < 0·05) (Fig. 4a).
Fig. 4.
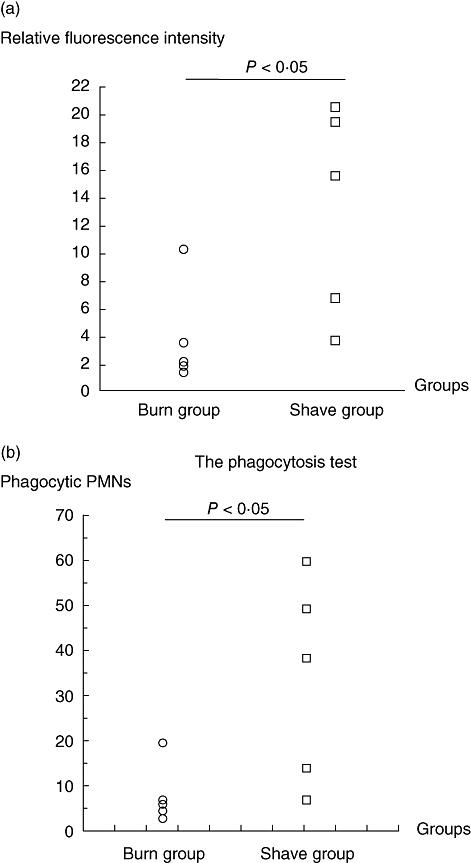
(a) Oxidative burst in polymorphonuclear neutrophil granulocytes (PMNs) at 48 h post-burn from from C3H/HeN mice in the burn group (BG) compared with mice in the shave group (SG). Each circle/square represents one mouse. (b) Phagocytic capacity in PMNs at 48 h post-burn from C3H/HeN mice in the BG compared with mice in the SG. Each circle/square represents one mouse.
With regard to the phagocytosis test, the capacity of PMNs from BG with perform phagocytosis was reduced significantly compared with the ability of PMNs in the SG to perform phagocytosis at 48 h (mean ± s.d. 8·1 ± 6·7/mean ± s.d. 33·8 ± 22·6 respectively) (P < 0·03) (Fig. 4b).
Discussion
In the present study, we introduce a novel animal model of thermal injury. Thermal injury was found to suppress the innate immune response. Immunosuppression was observed as a decrease in the concentration of leucocytes in peripheral blood as well as reduced function of the PMNs. By combining the burn wound with infection, the immunosuppression verified an impaired ability to control the burn wound infection.
The present model, using a hot-air blower to obtain a relatively minor burn area of 6% TBSA, was a compromise with the Danish Animal Experimental Committee, with whom the model was developed. Even with this minor thermal injury, a significant impact on the immune system was observed. Apart from temporary leucocytosis at 24 h a reduction in peripheral blood leucocytes was observed. The temporary peak in the concentration of leucocytes could be caused by an increased release of PMNs from the bone marrow and shifting of the marginal granulocyte pool into the circulation, as described previously [10].
The subsequent reduction in peripheral blood leucocytes has also been reported in another mouse model [11]. The reduction of PMNs could be due to diminished production in the bone marrow, exhaustion of the pool of PMNs, sequestration of PMNs in the liver, lungs, spleen or burn wound or influx of PMNs to the lesion.
In our model, the influx of PMNs into the burn wound could be the cause of neutropenia. No sequestration of PMNs in the lungs was seen in this study, which may be due to the minor total body surface area burned (TBSAB). However, Ozkan et al. have reported that an immunosuppressive glycopeptide in patients with 40% TBSAB is capable of reducing the chemotaxia of PMNs. Furthermore, the glycopeptide was also capable of suppression of T lymphocytes [12]. It cannot be excluded that a depression of the bone marrow has occurred, as Asko-Seljavaara and co-workers investigated the bone marrow and found a depression of the DNA synthesis in the granulocytes [13].
A PMN-dominated inflammation was observed 24 h after the induction of the thermal injury. In burn patients with severe burns of 30% or more a delayed influx of PMNs was observed [14]. Whether such a phenomenon could be observed in our model is less likely, because of the limitations of 6% TBSA. After 48 hours an influx of MNs was observed, and after 72 h the burn wound contained a mixture of PMNs and MNs. When the burn wound was superimposed with an infection the PMN-dominated response continued, probably because of the stimulus from the infection, and this response was comparable with the IG. The degree of inflammation was reduced gradually in the BG. Surprisingly, introduction of local infection resulted in a significantly reduced degree of inflammation in both infection groups. However, the day after, at 96 h, the degree of inflammation was increased significantly in the BIG, reflecting the lack of infection control. Despite the first hit in terms of the thermal insult, it seems that at 96 h the body could restore the leucocyte pool by increasing the concentration of PMNs in the blood. In contrast, it has been observed that superimposing burn wounds with P. aeruginosa infection leads to a reduction in the PMNs in the blood. However, these observations were obtained in mice with a much larger TBSAB (> 30%) [15].
Mice with thermal injury showed a reduced ability to mount phagocytosis and oxidative burst. Humane observations have shown that the ability of PMNs to ingest and their capacity to kill bacteria occurred at day 5, independently of the burn size. When patients were discharged they had regained phagocytosis ability and killing capacity. It was also observed that, in patients with impaired killing capacity, susceptibility to infection was increased compared with patients with normal function. The explanation seemed to be burn-induced reduction of myeloperoxidase, lactoferrin and chemotrypsin-like cationic protein [16].
We examined the ability of focal infection to spread by culturing blood, liver and spleen. We found dissemination in the BIG, but not the IG. In accordance, Barnea et al. observed in their model that when using the spleen as an indicator for spread, bacterial dissemination was beginning at 6 h post-infection and dissemination was seen only in the BIG [17].
In conclusion, the present novel mouse model of minor thermal lesions demonstrated a suppression of the innate immune response comparable with observations in patients suffering from burn wounds. The consequence of immunosuppression is an increased susceptibility to infections originating from colonization of the thermal injury. Our experiments suggest that treatment with granulocyte colony-stimulating factor to restore the early neutropenia may be beneficial for the patient with thermal injury to contain the infection.
Acknowledgments
This work was supported by the Danish Research Council, Rigshospitalet HS, fra A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fonden til almene formaal, Illum Fondet. We are grateful to Jette Petersen for her excellent technical help.
References
- 1.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37:543–50. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 2.Tang K, Jian L, Qin Z, Zhenjiang L, Gomez M, Beveridge M. Characteristics of burn patients at a major burn center in Shanghai. Burns. 2006;32:1037–43. doi: 10.1016/j.burns.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan ST, O'Connor TPF. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–23. doi: 10.1016/s0007-1226(97)90507-5. [DOI] [PubMed] [Google Scholar]
- 4.Edwards-Jones V, Greenwood JE. What's new in burn microbiology? Burns. 2003;29:15–24. doi: 10.1016/s0305-4179(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 5.Tredget EE, Shankowsky HA, Rennie R, Burrel RE, Logsetty S. Pseudomonas aeruginosa in the thermally injured patient. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns. 1996;22:607–11. doi: 10.1016/s0305-4179(96)00064-2. [DOI] [PubMed] [Google Scholar]
- 7.Clancy KD, Lorenz K, Dries D, Gamelli RL, Hahn EL. Chlorpromazine modulates cytokine expression in the liver and lung after burn injury and endotoxemia. J Trauma. 2000;48:215–23. doi: 10.1097/00005373-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Johansen HK. Potential of preventing Pseudomonas aeruginosa lung infections in cystic fibrosis: experimental studies in animals. APMIS. 1996;104(Suppl.)(63):1–42. doi: 10.1111/j.1600-0463.1996.tb05581.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen P. Denmark: Copenhagen University; 2003. Characterization and modulation of the innate immune response during Pseudomonas aeruginosa lung infection in patients with cystic fibrosis: an experimental and clinical study. PhD thesis. [Google Scholar]
- 10.Graddock CG. Production, distribution, and fate of granulocytes. In: Williams WJ, Beutler E, Erslev AJ, et al., editors. Hematology. New York: McGraw-Hill; 1972. pp. 607–18. [Google Scholar]
- 11.Pallua N, Heimburg DV. Pathogenic role of interleukin-6 in the development of sepsis. Part 1. Study in a standardized contact burn murine model. Crit Care Med. 2003;31:1490–4. doi: 10.1097/01.CCM.0000065724.51708.F5. [DOI] [PubMed] [Google Scholar]
- 12.Ozkan AX, Ninnemann JL, Sullivan J. Progress in the characterization of immunosuppressive glycopeptide (8SAP) from patients with major thermal injuries. J Burn Care. 1986;7:388–97. doi: 10.1097/00004630-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Asko-Seljavaara S. Granulocyte kinetics in burned mice. Inhibition in granulocyte growth studied in vivo and in vitro. Scand J Plast Reconstr Surg. 1974;8:185–91. doi: 10.3109/02844317409084392. [DOI] [PubMed] [Google Scholar]
- 14.Peng D, Huang W, Ai S, Wang S. Clinical significance of leukocyte infiltrative response in deep wound of patients with major burns. Burns. 2006;3:946–50. doi: 10.1016/j.burns.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Shoup M, Weisenberger JM, Wang JL, Pyle JM, Gamelli RL, Shankar R. Mechanism of neutropenia involving myeloid maturation arrest in burn sepsis. Ann Surg. 1998;228:112–22. doi: 10.1097/00000658-199807000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arturson G. Neutrophil granulocyte functions in severely burned patients. Burns Incl Therm Inj. 1985;11:309–19. doi: 10.1016/0305-4179(85)90093-2. [DOI] [PubMed] [Google Scholar]
- 17.Barnea Y, Carmeli Y, Kuzmenko B, Eyal G, Hammer-Munz O, Navon-Venezia S. The establishment of a Pseudomonas aeruginosa-infected burn-wound sepsis model and the effect of imipenem treatment. Ann Plast Surg. 2006;56:674–9. doi: 10.1097/01.sap.0000203984.62284.7a. [DOI] [PubMed] [Google Scholar]


