Abstract
Recent studies have demonstrated deposition of secretory immunoglobulin A (sIgA) in glomeruli of some patients with IgA nephropathy (IgAN). The aim of this study is to investigate the levels of urinary sIgA in IgAN patients with different pathological phenotypes and whether it could be used as a non-invasive biomarker for assessment of kidney injury in IgAN. Urine samples from 202 patients with IgAN were collected on the day of renal biopsy. Forty-eight fulfilled the histopathological criteria of Haas-I or II (group 1), 60 fulfilled Haas-III (group 2) and 94 patients fulfilled Haas-IV or V (group 3). Urine samples from 60 healthy sex- and age-matched volunteers with negative urinalysis were collected as normal controls. Urinary sIgA was detected by sandwich enzyme-linked immunosorbent assay and was corrected by urinary creatinine. In comparison with normal controls, the levels of urinary sIgA were significantly higher in IgAN [2·22 (0–43·82) μg/mg Cr versus 1·08 (0–16·49) μg/mg Cr, P < 0·001]. The levels of urinary sIgA were significantly higher in group 3 than that in group 2 and group 1 [3·54 (0–43·82) μg/mg Cr versus 1·63 (0–15·88) μg/mg Cr versus 0·91 (0–11·79), P < 0·001], and group 2 than group 1 (P = 0·014). The levels of urinary sIgA were associated positively with proteinuria (r = 0·443, P < 0·001), serum creatinine (r = 0·376, P < 0·001) and histopathological parameters, such as ratio of global sclerosis (r = 0·356, P < 0·001), ratio of total crescents (r = 0·339, P < 0·001) and ratios of cellular crescents (r = 0·231, P < 0·001). The levels of urinary sIgA were associated closely with histopathological phenotypes of IgAN and might be used as a non-invasive biomarker to evaluate kidney injury in IgAN.
Keywords: biomarker, IgA nephropathy, sIgA
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis worldwide, with a prevalence of 20–45% in primary glomerular disease [1–3]. Substantial evidence suggests that IgAN is a heterogeneous disease with different clinical and pathological phenotypes. More than one-third of patients with IgAN could progress to end-stage renal failure after 20–25 years, eventually requiring renal replacement therapy [4]. Thus, sequential assessment of kidney injury and disease progression is crucial during follow-up of patients with IgAN. Repeated renal biopsy is not practical in the clinical setting as an invasive technique. Although serum creatinine (SCr) and creatinine clearance (Ccr) are used to reflect renal injury, non-invasive and more reliable biomarkers are still needed. Recently, more attention has been paid to the urinary biomarkers, which would be useful in pharmaceutical intervention and evaluation of the effectiveness of therapy.
Secretory IgA (sIgA) is a dominant Ig in external mucosal secretions. Next to its presence in mucosal secretions, small amounts of sIgA can also be found in human serum. It has been suggested that sIgA might play a pathogenic role in IgAN; substantial evidence has demonstrated that serum sIgA was elevated in patients with IgAN [5–10] and, recently, studies including ours revealed successfully the deposition of sIgA in glomeruli of some patients with IgAN [11–13]. However, only one study of 31 patients with chronic glomerulonephritis, including 10 patients with IgAN, found that the level of urinary sIgA was significantly higher than normal controls [14].
In this study, we investigated whether urinary sIgA could be used as a non-invasive biomarker for assessment of kidney injury in patients with IgAN. We first detected sIgA in urine from a large cohort of patients with IgAN with different histopathological phenotypes. We then analysed the associations between the levels of urinary sIgA and serological biomarkers of renal function, as well as histological parameters of IgAN.
Materials and methods
Patients
To detect urinary sIgA, morning urine samples from 202 patients with IgAN were collected on the day of renal biopsy, and morning urine samples from 60 healthy sex- and age-matched normal controls with normal urinalysis were also collected. The severity of renal lesions of patients with IgAN was graded according to Haas's classifications [15]. Among the 202 patients, 48 patients fulfilling the histopathological criteria of Haas-I or II were defined as mild renal lesions (group 1), 60 patients with Haas-III as moderate renal lesions (group 2) and 94 patients with Haas-IV or V as severe renal lesions (group 3). Clinical parameters such as mean arterial pressure (MAP), routine urinalysis, SCr, Ccr and 24-h urinary protein, etc. were obtained immediately before renal biopsy. The clinical parameters of patients with IgAN were collected retrospectively. Informed consent was obtained for sampling prior to renal biopsy.
Evaluation of renal histopathological damage
Sections were stained routinely for haematoxylin and eosin, periodic-acid Schiff and/or periodic-acid-silver methenamine for histopathology. Two pathologists who were blinded to patients’ data evaluated the slides separately. For glomerular lesions, mesangial proliferation was scored as 1+ for mild (i.e. between four and six cells per mesangial area) and 2+ for moderate and intense (more than six cells per mesangial area). Global and segmental sclerosis, total crescents, cellular crescents, cellular and fibrous crescents were calculated as percentages of the total number of glomeruli respectively. For tubulointerstitial lesions, tubular atrophy, interstitial fibrosis and interstitial inflammatory cell infiltration were scored 0 as absent, 1+ as mild (involving < 25% of the interstitium and tubules), 2+ as moderate (involving 25–50% of the interstitium and tubules) and 3+ as intense (involving > 50% of the interstitium and tubules).
Urine samples
Spot morning urine samples from patients were collected on the same day of renal biopsy. All samples were centrifuged at 769 g for 10 min to remove cellular components, and the supernatant was kept at −70°C until use. Spot morning urine samples from normal controls were also collected and prepared in the same manner as for patients.
Detection of urinary sIgA by enzyme-linked immunosorbent assay
According to previous studies, a sandwich enzyme-linked immunosorbent assay was used [7,12]. Briefly, mouse monoclonal anti-human secretory component (SC) (Sigma, St Louis, MO, USA) was diluted to 5·1 µg/ml with 0·05 mol/l bicarbonate buffer pH 9·6 and was coated onto the wells of one-half of a polystyrene microtitre plate (Costar, Corning, NY, USA). The wells in the other half were coated with bicarbonate buffer alone to act as antigen-free wells. Incubation was carried out for 1 h at 37°C. Free binding sites were blocked for a further 1 h at 37°C with phosphate-buffered saline containing 0·1% (vol/vol) Tween-20 (PBST)/1% (10 mg/ml) bovine serum albumin (BSA). Urine samples were diluted to 1:10 in PBST/BSA. The volumes of this and subsequent steps were 100 µl and all incubations were carried out at 37°C for 1 h. The plates were washed three times with PBST. Each plate contained a blank control (PBST/BSA) and a positive control. After incubation and washing, rabbit polyclonal anti-human IgA (Dako, Glostrup, Denmark) with a dilution of 1:5000 was added. The binding was detected by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG with a dilution of 1/10 000 (gibco BRL, Grand Island, NY, USA). The HRP substrate O-phenylenediamine was used at 0·4 mg/ml in 0·1 mmol/l citrate phosphate buffer (pH 5·0). The reaction was stopped by 1·0 mol/l H2SO4 and results were recorded as the net optical density 490 nm. SIgA purified and isolated from human colostrums (Serotec, Oxford, UK) was used to establish standard curve and the level of urinary sIgA was calculated.
Detection of urinary creatinine
Urinary creatinine was detected (using Synchron LX20; Beckman Coulter, Clinical Systems, Fullerton, CA, USA) in the same urine samples of patients and controls to correct urinary sIgA levels. The adjusted urinary sIgA was expressed as a concentration of sIgA/concentration of creatinine (µg/mg Cr).
Statistical analysis
For statistical analysis, statistical software spss version 10·0 (SPSS, Inc., Chicago, IL, USA) was employed. Quantitative data were expressed as mean ± standard deviation and median with range (minimum–maximum). For comparison between patients and controls, and comparison of clinical features and the patients’ pathological data, Student's t-test, one-way anova and the Mann–Whitney U-test were used. Spearman's correlation was used to analyse correlation. Statistical significance was considered as P < 0·05.
Results
Demographic data of patients with IgAN
The clinical parameters of patients with IgAN with various glomerular and tubulointerstitial lesions are shown in Table 1. Compared with patients with tubular atrophy and interstitial fibrosis grade 0, the levels of MAP, SCr and proteinuria were significantly higher in patients with grades 2 and 3 and the level of Ccr was significantly lower in patients with grades 1, 2 and 3.
Table 1.
Clinical parameters of patients with immunoglobulin A nephropathy with different histopathological phenotypes.
|
P-value (between groups) |
||||||
|---|---|---|---|---|---|---|
| Group 1 n = 48 | Group 2 n = 60 | Group 3 n = 94 | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| Male/female | 28/20 | 31/29 | 57/37 | 0·489 | 0·791 | 0·273 |
| Age (years) | 30·98 ± 8·72 | 32·88 ± 9·69 | 34·65 ± 11·58 | 0·346 | 0·049* | 0·307 |
| Disease duration (months) | 25·87 (0·3–396) | 22·93 (0·25–300) | 13·53 (0·3–108) | 0·713 | 0·094 | 0·170 |
| MAP (mmHg) | 90·38 ± 11·68 | 91·27 ± 18·28 | 101·26 ± 21·50 | 0·807 | 0·001* | 0·001* |
| History of URT infection | 22/48 | 19/60 | 18/94 | 0·132 | 0·001* | 0·076 |
| History of macrohaematuria | 20/48 | 21/60 | 18/94 | 0·478 | 0·004* | 0·027* |
| Serum IgA (mg/ml) | 3·15 ± 1·04 | 3·42 ± 1·30 | 3·20 ± 1·01 | 0·272 | 0·834 | 0·294 |
| Serum complement 3 (mg/ml) | 1·01 ± 0·23 | 1·00 ± 0·22 | 0·99 ± 0·18 | 0·799 | 0·547 | 0·757 |
| Serum creatinine (µmol/l) | 83·30 ± 16·91 | 90·31 ± 34·29 | 189·58 ± 169·85 | 0·759 | < 0·001* | < 0·001* |
| Creatinine clearance (ml/min) | 103·01 ± 28·84 | 93·02 ± 26·47 | 58·67 ± 32·75 | 0·108 | < 0·001* | < 0·001* |
| Proteinuria (g/24 h) | 0·97 ± 1·47 | 1·49 ± 1·26 | 2·57 ± 1·95 | 0·11 | < 0·001* | < 0·001* |
Group 1, patients with mild lesions; group 2, patients with moderate lesions; group 3, patients with severe lesions; MAP, mean arterial pressure; URT, upper respiratory tract.
P < 0·05.
The histopathological parameters are shown in Table 2; the parameters included extent of mesangial proliferation, ratio of segmental sclerosis, ratio of global sclerosis, ratio of crescents, endocapillary proliferation and infiltration of interstitial mononuclear cells.
Table 2.
Histopathological characteristics of patients with immunoglobulin A nephropathy with different histopathologic phenotypes.
|
P-value (between groups) |
||||||
|---|---|---|---|---|---|---|
| Group 1 n = 48 | Group 2 n = 60 | Group 3 n = 94 | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| Mesangial proliferation score 1 | 47/48 | 39/60 | 48/94 | 0·193 | 0·021* | 0·414 |
| Mesangial proliferation score 2 | 1/48 | 21/60 | 46/94 | < 0·001* | < 0·001* | 0·293 |
| Patients with EC proliferation | 1/48 | 11/60 | 29/94 | 0·008* | < 0·001* | 0·103 |
| Ratios of segmental sclerosis | 1·92 ± 5·99 | 0·72 ± 2·52 | 2·20 ± 6·65 | 0·273 | 0·773 | 0·113 |
| Ratios of global sclerosis | 6·22 ± 28·88 | 4·62 ± 6·66 | 23·30 ± 21·91 | 0·045* | < 0·001* | < 0·001* |
| Ratios of total crescents | 0·12 ± 0·87 | 9·8 ± 11·64 | 21·49 ± 127·39 | < 0·001* | < 0·001* | 0·061 |
| Ratios of cellular crescents | 0·06 ± 0·44 | 2·65 ± 5·76 | 5·69 ± 10·16 | < 0·001* | < 0·001* | 0·06 |
| Interstitial fibrosis score 0 | 19/48 | 5/60 | 8/94 | 0·003* | 0·001* | 1 |
| Interstitial fibrosis score 1 | 29/48 | 49/60 | 21/94 | 0·367 | 0·004* | < 0·001* |
| Interstitial fibrosis score 2 | 0/48 | 6/60 | 29/94 | 0·039* | < 0·001* | 0·018* |
| Interstitial fibrosis score 3 | 0/48 | 0/60 | 36/94 | > 1 | < 0·001* | < 0·001* |
| Interstitial infiltration score 0 | 23/48 | 16/60 | 5/94 | 0·137 | < 0·001* | 0·002* |
| Interstitial infiltration score 1 | 25/48 | 37/60 | 25/94 | 0·632 | 0·062 | 0·007* |
| Interstitial infiltration score 2 | 0/48 | 7/60 | 38/94 | 0·040* | < 0·001* | 0·004* |
| Interstitial infiltration score 3 | 0/48 | 0/60 | 26/94 | > 1 | < 0·001* | < 0·001* |
Group 1, patients with mild lesions; group 2, patients with moderate lesions; group 3, patients with evere lesions; EC, endocapillary;
P < 0·05.
Both clinical and histopathological parameters were compared among different groups of patients.
Levels of urinary sIgA from patients with IgAN and controls
The levels of urinary sIgA in normal controls and patients with IgAN were 1·08 (0–16·49) μg/mg Cr and 2·22 (0–43·82) μg/mg Cr respectively. In comparison with normal controls, urinary sIgA was significantly higher in patients with IgAN (P < 0·001) (Fig. 1). More importantly, after stratification of the patients by different histopathology phenotypes, the levels of urinary sIgA were significantly higher in group 3 than that in groups 2 and 1 [3·54 (0–43·82) μg/mg Cr versus 1·63 (0–15·88) μg/mg Cr versus0·91 (0–11·79), P < 0·001], and in group 2 than that in group 1 (P = 0·014) (Fig. 2).
Fig. 1.
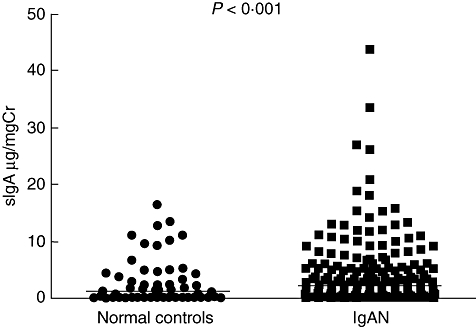
Levels of urinary urinary secretory immunoglobulin A (sIgA) in patients with IgA nephropathy (IgAN) and normal controls. The horizontal solid lines indicate the median values for each group.
Fig. 2.
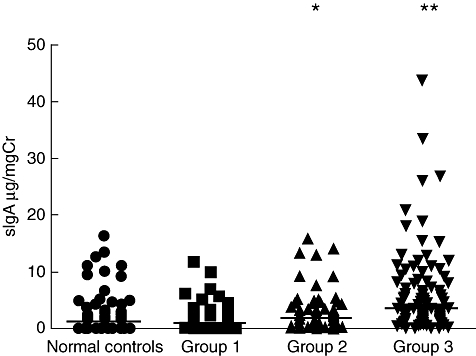
Levels of urinary urinary secretory immunoglobulin A (sIgA) in IgA nephropathy (IgAN) patients with different histopathological phenotypes and normal controls. Group 1: patients with mild lesions; group 2: patients with moderate lesions; group 3: patients with severe lesions (group 2 versus normal controls and group 1 respectively, *P < 0·05; group 3 versus normal controls, groups 1 and 2, **P < 0·05). The horizontal solid lines indicate the median values for each group.
Associations between clinical parameters and urinary sIgA
The levels of urinary sIgA were correlated positively with the levels of SCr and proteinuria correlated negatively with CCr in patients with IgAN. However, there was no correlation between the levels of urinary sIgA and levels of serum IgA (Fig. 3). No difference in urinary sIgA was found between patients with or without history of upper respiratory tract infection or history of macrohaematuria respectively [2·15 (0–26·91) μg/mg Cr versus 2·22 (0–43·82) μg/mg Cr, P = 0·403; 2·37 (0–43·82) μg/mg Cr versus 1·65 (0–33·6) μg/mg Cr, P = 0·076].
Fig. 3.
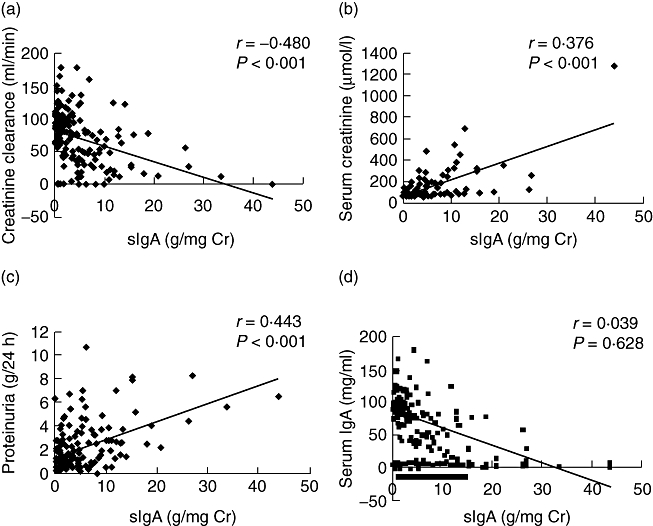
Associations between levels of urinary urinary secretory immunoglobulin A (sIgA) and clinical parameters of IgA nephropathy (IgAN) patients. (a) Correlation between levels of urinary sIgA and creatinine clearance; (b) correlation between levels of urinary sIgA and serum creatinine; (c) correlation between levels of urinary sIgA and the quantity of proteinuria; (d) correlation between levels of urinary sIgA and serum IgA.
Associations between histopathological parameters and urinary sIgA
The levels of urinary sIgA were significantly higher in patients with mesangial proliferation score 2 than those with score 1 [4·30 (0–43·82) μg/mg Cr versus 1·50 (0–33·60) μg/mg Cr, P < 0·001]. The levels of urinary sIgA were significantly higher in patients with crescents than those without crescents [1·64 (0–26·18) μg/mg Cr versus 2·89 (0–43·82) μg/mg Cr, P = 0·009]. The levels of urinary sIgA were elevated in patients with higher scores of tubular atrophy and interstitial fibrosis, and interstitial inflammatory cell infiltration. In patients with a score of 3, the levels of urinary sIgA were significantly higher than those with scores of 0, 1 and 2 respectively (Figs 4 and 5). Finally, the urinary sIgA was correlated positively with the ratios of global sclerosis, total crescents and cellular crescents (Table 3).
Fig. 4.
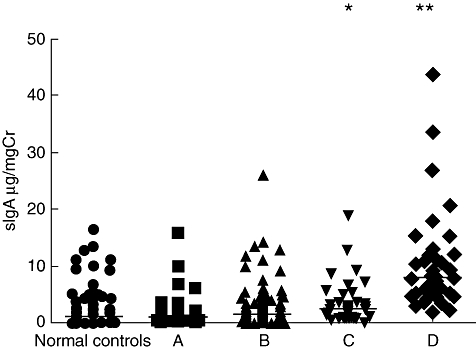
Levels of urinary urinary secretory immunoglobulin A (sIgA) in IgA nephropathy (IgAN) patients with different scores of tubular atrophy and interstitial fibrosis. (A) patients with tubular atrophy and interstitial fibrosis score 0; (B) patients with tubular atrophy and interstitial fibrosis score 1; (C) patients with tubular atrophy and interstitial fibrosis score 2; (D) patients with tubular atrophy and interstitial fibrosis score 3 (compared with normal controls, *P < 0·05; compared with normal controls, A, B and C, **P < 0·05). The horizontal solid lines indicate the median values for each group.
Fig. 5.
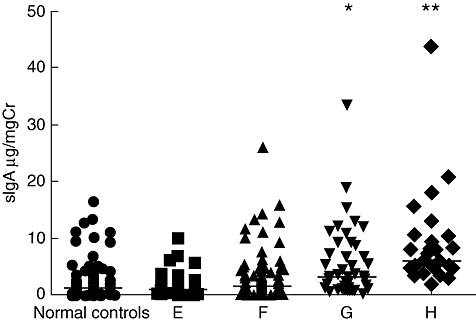
Levels of urinary urinary secretory immunoglobulin A (sIgA) in IgA nephropathy (IgAN) patients with different scores of interstitial inflammatory infiltration. (E) Patients with interstitial inflammatory infiltration score 0; (F) patients with interstitial inflammatory infiltration score 1; (G) patients with interstitial inflammatory infiltration score 2; (H) patients with interstitial inflammatory infiltration score 3 (compared with normal controls, E and F, *P < 0·005, compared with normal controls, E, F and G, **P < 0·05). The horizontal solid lines indicate the median values for each group.
Table 3.
Association between levels of urinary secretory immunoglobulin A (sIgA) and histopathological parameters of patients with immunoglobulin A nephropathy.
| Urinary sIgA (n = 202) | ||
|---|---|---|
| Parameters | r-value | P-value |
| Ratios of global sclerosis | 0·356 | < 0·001* |
| Ratios of total crescents | 0·339 | < 0·001* |
| Ratios of cellular crescents | 0·231 | < 0·001* |
Discussion
The IgAN is the most common form of glomerulonephritis worldwide. This is the first study to investigate whether urinary sIgA could be a reliable non-invasive biomarker for monitoring renal injury and disease progression in patients with IgAN. We show that the levels of urinary sIgA are higher in patients with IgAN than that in healthy individuals. The higher the urinary sIgA, the more pronounced will be the kidney injury. Furthermore, the levels of urinary sIgA were associated with levels of clinical parameters of renal function, such as SCr, quantity of proteinuria and CCr in patients with IgAN. These data indicate that the urinary sIgA parallel with the severity of renal injury and disease progression.
Previous studies have shown that levels of serum sIgA and saliva sIgA were elevated in patients with IgAN, and the elevation of serum sIgA was associated with haematuria. Meanwhile, sIgA exhibits stronger binding and stimulation to mesangial cells than serum IgA. More production of interleukin-6 were detected after the stimulation of sIgA, which could not be blocked by free SC [7,10]. More importantly, recent studies from Oortwijn et al. and ours have demonstrated sIgA deposition in glomeruli of some patients with IgAN. The presence of sIgA in the biopsies showed a strong correlation and co-localization with deposition of mannose-binding lectin and C4d, which indicates that mesangial IgA was originated, at least partly, from mucosal immune sites and its appearance induces more severe injuries [11–13]. This study confirms further that sIgA may play an important role in the pathogenesis of IgAN. The mechanisms of increasing urinary sIgA in IgAN might be as follows: (i) excessive production of sIgA in submucosal tissue with partial backward flow to blood, which passes through the filter barrier of injury glomeruli, as we found that serum levels of sIgA were correlated with urinary sIgA levels (data were not shown); (ii) the free SC secreted by renal tubular epithelial cells binding with polymeric IgA originating from bone marrow, spleen or lymphoid tissues, which passes through the filter barrier of injury glomeruli in urine [16–18]; and (iii) secretion of sIgA by mucosa of urinary tract [14,19]. However, the exact reason for elevated urinary sIgA in IgAN is still unknown, and further study is needed.
Studies indicate that sIgA is associated closely with mucosal immunity, and the levels of urinary sIgA elevated in the patients with urinary tract infection [14,19]. However, our patients were excluded for urinary tract infection before renal biopsy. To explore the possible link between mucosal immunity and the development of IgAN, we examined the association between levels of urinary sIgA and history of upper respiratory tract infection and history of macrohaematuria, but no association was found.
Our study has some limitations. First, the morning spot urine sample might not be appropriate to represent total sIgA secretion in urine; however, it is well accepted that protein/creatinine ratio in spot urine may represent 24-h proteinuria [20–22], therefore it is rational to use the sIgA/creatinine ratio to reflect total sIgA secretion in urine. Secondly, the best way to evaluate disease progression in the histopathology of patients with IgAN is to perform repeat renal biopsy periodically when patients have signs of disease progression clinically; however, repeat renal biopsy is not acceptable clinically. Therefore, in the current study, we stratified our patients into three groups according to disease severity in histopathology. We hope the stratification of patients may reflect disease progression clinically as well as histopathologically.
In conclusion, we found the level of urinary sIgA was elevated in patients with IgA nephropathy and was associated with pathological phenotypes of the disease. These findings support the notion that sIgA might play an important role in the pathogenesis of IgAN. The urinary sIgA could be used as a reliable non-invasive biomarker for monitoring renal injury and disease progression in patients with IgAN.
Acknowledgments
This work was supported by grants from the ‘985’ project from Peking University and National Key Technology R&D Programme (2007BAI04B10), People's Republic of China.
References
- 1.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. see comment. [DOI] [PubMed] [Google Scholar]
- 2.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–3. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Ma XZ, Zou WZ, Wang M, Wang HY. The prevalence of biopsy-proven renal diseases in adults between two periods over the past 10 years. J Clin Intern Med. 2004;21:834–8. [Google Scholar]
- 4.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–37. doi: 10.1053/ajkd.2000.8966. see comment. [DOI] [PubMed] [Google Scholar]
- 5.Oortwijn BD, Eijgenraam JW, Rastaldi MP, Roos A, Daha MR, van Kooten C. The role of secretory IgA and complement in IgA nephropathy. Semin Nephrol. 2008;28:58–65. doi: 10.1016/j.semnephrol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Oortwijn BD, Roos A, Royle L, et al. Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol. 2006;17:3529–39. doi: 10.1681/ASN.2006040388. [DOI] [PubMed] [Google Scholar]
- 7.Oortwijn BD, van der Boog PJ, Roos A, et al. A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 2006;69:1131–8. doi: 10.1038/sj.ki.5000074. see comment. [DOI] [PubMed] [Google Scholar]
- 8.Rostoker G, Terzidis H, Petit-Phar M, et al. Secretory IgA are elevated in both saliva and serum of patients with various types of primary glomerulonephritis. Clin Exp Immunol. 1992;90:305–11. doi: 10.1111/j.1365-2249.1992.tb07947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson RA, Asquith P, Cooke WT. Secretory IgA in the serum. Lancet. 1969;2:517–19. doi: 10.1016/s0140-6736(69)90217-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamabe H, Ozawa K, Fukushi K, et al. Elevated serum secretory IgA in patients with IgA nephropathy. Nephron. 1989;51:499–501. doi: 10.1159/000185383. [DOI] [PubMed] [Google Scholar]
- 11.Oortwijn BD, Rastaldi MP, Roos A, Mattinzoli D, Daha MR, van Kooten C. Demonstration of secretory IgA in kidneys of patients with IgA nephropathy. Nephrol Dial Transplant. 2007;22:3191–5. doi: 10.1093/ndt/gfm346. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JJ, Xu LX, Liu G, Zhao MH, Wang HY. The level of serum secretory IgA of patients with IgA nephropathy is elevated and associated with pathological phenotypes. Nephrol Dial Transplant. 2008;23:207–12. doi: 10.1093/ndt/gfm492. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Kobayashi H, Sato H, Arakawa M. Immunohistochemical characterization of glomerular IgA deposits in IgA nephropathy. Clin Nephrol. 1990;33:66–71. see comment. [PubMed] [Google Scholar]
- 14.Marx M, Weber M, Schafranek D, Wandel E, Meyer zum Buschenfelde KH, Kohler H. Secretory immunoglobulin A in urinary tract infection, chronic glomerulonephritis, and renal transplantation. Clin Immunol Immunopathol. 1989;53:181–91. doi: 10.1016/0090-1229(89)90048-2. [DOI] [PubMed] [Google Scholar]
- 15.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 16.Abramowsky CR, Swinehart GL. Secretory immune responses in human kidneys. Am J Pathol. 1986;125:571–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrin RS, Knudson FE, Michael AF. The secretory immune system and renal disease. Clin Exp Immunol. 1975;21:318–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Rice JC, Spence JS, Megyesi J, Goldblum RM, Safirstein RL. Expression of the polymeric immunoglobulin receptor and excretion of secretory IgA in the postischemic kidney. Am J Physiol. 1999;276:F666–73. doi: 10.1152/ajprenal.1999.276.5.F666. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DB, Katz R, McIntosh RM. Secretory IgA in urinary tract infections. BMJ. 1970;4:463–5. doi: 10.1136/bmj.4.5733.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cote AM, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein:creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ. 2008;336:1003–6. doi: 10.1136/bmj.39532.543947.BE. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National. Kidney Foundation. Am J Kidney Dis. 2007;50:169–80. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Lane C, Brown M, Dunsmuir W, Kelly J, Mangos G. Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? Nephrology. 2006;11:245–9. doi: 10.1111/j.1440-1797.2006.00564.x. [DOI] [PubMed] [Google Scholar]


