Abstract
Dendritic cells (DCs) have been described as initiators and modulators of the immune response. Recently we have shown a predominant production of interleukin-10 cytokine, low levels of interferon-γ and inefficient T cell proliferation in patients with severe forms of chromoblastomycosis. Chromoblastomycosis starts with subcutaneous inoculation of Fonsecaea pedrosoi into tissue where DCs are the first line of defence against this microorganism. In the present study, the interaction of F. pedrosoi and DCs obtained from patients with chromoblastomycosis was investigated. Our results showed that DCs from patients exhibited an increased expression of human leucocyte antigen D-related (HLA-DR) and co-stimulatory molecules. In the presence of conidia, the expression of HLA-DR and CD86 was up-regulated by DCs from patients and controls. Finally, we demonstrate the reversal of antigen-specific anergy and a T helper type 1 response mediated by DCs incubated with F. pedrosoi conidea.
Keywords: chromoblastomycosis, DC, IL-10, IL-12, immune regulation, Th1/Th2
Introduction
Chromoblastomycosis is a human chronic fungal disease limited usually to subcutaneous tissues. Infection is initiated by traumatic inoculation of mycelium fragments or conidial cells into the skin by thorns or wood splinters [1]. Chromoblastomycosis usually affects agricultural workers who are not protected adequately while handling soil, vegetables and decomposing organic matter, which are natural habitats of the fungus [2,3]. It occurs worldwide, but is observed more frequently in tropical countries such as Brazil [4,5]. This mycosis is caused by several species of dematiaceous hyphomycetes: Fonsecaea pedrosoi, Phialophora verrucosa, Cladophialophora carrionii and Rhinocladiella aquaspersa[6]. F. pedrosoi is considered its most frequent aetiological agent in Brazil. Clinically, chromoblastomycosis is characterized by a slow development of polymorphic skin lesions where there are usually erythematous papules, which enlarge gradually to display varying morphologies (nodules, verrucas, plaques and scar tissue) [6]. Inside the host, infectious propagules adhere to epithelial cells and differentiate into sclerotic forms, which effectively resist destruction by host effector cells and allow chronic disease to establish. The disease is usually insidious and the lesions increase slowly but progressively, not responding to the usual treatments and quite often reappearing.
Dendritic cells (DCs) have been described as initiators and modulators of the immune response [7]. Mature DCs are able to prime naive lymphocytes and polarize them towards a T helper type 1 (Th1) response, whereas immature DCs have been shown to induce tolerance [8]. Immature DCs, which reside in most tissues and organs, capture and process antigens actively. Mature DCs decrease antigen uptake, undergo a change in chemokine receptor expression that regulates major histocompatibility complex (MHC), co-stimulatory and adhesion molecules, and secrete chemokines and interleukin (IL)-12. This cytokine plays a key role in inducing cell-mediated immunity to intracellular pathogens by triggering the production of interferon (IFN)-γ by natural killer and T cells [7]. Several studies have shown that different DC subtypes are specialized to make different cytokines and drive distinct T cell differentiation [9,10]. For instance, human myeloid DCs (mDCs) stimulated via CD40L produce IL-12 and promote Th1 responses while CD40L-activated plasmacytoid DCs fail to produce IL-12 and promote Th2 responses or tolerance [11]. A second model is that DCs are flexible and induce Th1 or Th2 responses depending upon the nature of the maturation stimulus and kinetics of activation [12–14]. Indeed, the ability of DCs to produce IL-12 and prime Th1 responses can be modulated by hormones, cytokines or microbial products [15–17]. DCs are uniquely able to decode the fungus-associated information and translate it into qualitatively different Th immune responses. Murine and human DCs phagocytose conidia and hyphae of Aspergillus fumigates or Candida albicans through distinct recognition receptors. The engagement of distinct receptors translates subsequently into disparate downstream signalling events, ultimately affecting cytokine production and co-stimulation [18]. Recently, Siegemund and Alber demonstrated that C. neoformans induces the up-regulation of MHC-II and CD86 on conventional DCs rather than on plasmacytoid bone marrow-derived DCs, and that this induction depends on MyD88 [19].
In vitro studies have demonstrated that mature DCs pulsed with antigen can also stimulate naive lymphocytes [20]. These human studies demonstrate that DCs are able to initiate and modulate lymphocytes in both infectious diseases and cancer. To our knowledge, interaction of DCs with F. pedrosoi has not been studied previously.
In the present study, we investigated the interaction of F. pedrosoi with DCs obtained from patients with chromoblastomycosis. We evaluated the influence of infection of DCs by F. pedrosoi on the ability of those cells to secrete cytokines and to express human leucocyte antigen D-related (HLA-DR) and co-stimulatory molecules. Finally, presentation of antigens by DCs pulsed with conidia of F. pedrosoi was investigated using autologous CD4 T cells from patients with severe forms of chromoblastomycosis.
Material and methods
Patients
Seven patients (five men and two women; age range: 40–70 years) with clinical diagnoses of severe form of chromoblastomycosis, attending the Department of Pathology, Federal University of Maranhão or at the Department of Dermatology, Medical School of the University of São Paulo, were included in the present study. Chromoblastomycosis lesions were classified according to criteria as described by Queiroz-Telles et al. [21]. Briefly, severe forms were characterized by lesions including those of the tumoral and cicatricial types covering extensive cutaneous regions. All diagnoses were confirmed by direct mycological examination, culture and histopathogy. In all cases, F. pedrosoi fungus was isolated. Healthy individuals (nine individuals: seven men and two women; age range: 35–75 years) living in an area that was non-endemic for chromoblastomycosis (São Paulo City) were used as controls. This study was approved by Ethical Committee of Faculty of Pharmacy – University of Sao Paulo, protocol no. 373.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation over Ficoll-Hypaque (400 g, 30 min). The phase containing white cells was removed and washed in RPMI-1640. For positive selection of CD14+ monocytes, PBMCs were mixed with anti-human CD14 antibody-conjugated microbeads (Miltenyi Biotec, Auburn, CA, USA). CD14+ monocytes were separated from other cells using a magnetic separation column under a magnetic field (Miltenyi Biotec), according to the manufacturer's instructions, and washed thoroughly to remove any non-specific binding to the beads. CD14+ cells were then eluted in phosphate-buffered saline (PBS) containing fetal calf serum (2%). Isolated CD14+ cells were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 (50 and 1 ng/ml respectively; 7 days). Cultures were fed every 2 days with GM-CSF and IL-4; the cells generated were denominated as immature DCs [22]. DCs thus obtained were more than 95% as assessed by flow cytometry analysis. Part of DCs was matured with 500 IU/ml tumour necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN, USA) for 24 h and denominated as mDCs [22].
Microorganism
An isolate of F. pedrosoi (American Type Culture Collection 46428) was used in the present investigation. Stock cultures were maintained on Sabouraud dextrose agar under oil (4°C) and transferred every 6 months.
Conidia of fungus
In order to obtain a large number of conidia, fungal fragments were scraped off the Sabouraud agar culture plates and incubated in potato-broth medium [50 g potatoes, 5 g glucose (Merck, Darmstadt, Germany) and 500 ml distilled water] under constant rotation (25°C, 4 days). The fungal suspensions were then filtered through a sterile micropore Whatman no. 1 filter to remove hyphae fragments but not microconidia. Conidia were then washed with PBS and counted in a haemocytometer.
Analysis of expression of surface molecule by DCs and cytokine production
The effects of the presence of F. pedrosoi on expression of cell-surface molecules by DCs were investigated by incubating (24 h) these cells with conidia of F. pedrosoi (conidia : cell ratio = 3:1). Phenotypes of DCs were analysed by flow cytometry with a fluorescence activated cell sorter (FACScan, San Jose, CA, USA) flow cytometer (Becton Dickinson). To determine the expression of HLA-DR and co-stimulatory molecules, phycoerythrin-labelled monoclonal antibodies were used against CD80, CD83, HLA-DR and CD86 or isotype controls. Supernatants from cultures of DCs stimulated with conidia of F. pedrosoi were assayed for TNF-α, IL-12 and IL-10 by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (BD-Pharmingen, San Jose, CA, USA).
Assays of antigen presentation
Immature and matured DCs were prepared as described above and incubated (24 h) with conidia of F. pedrosoi (conidia : cell ratio = 3:1) in the presence or absence of TNF-α (500 IU/ml). Afterwards, DCs were irradiated (3000 rad, 60Co source) and plated (2 × 104 cells/well in triplicate) on a 96-well flat-bottomed plate. Autologous CD4+ T cells were purified from non-adherent PBMCs using positive separation with anti-human CD4 antibody-conjugated microbeads and added (2 × 105 cells/well). Proliferation of CD4 T cells was evaluated after 5 days of culture by measuring [3H]-thymidine uptake during the last 16 h of the assay. The supernatant of this assay was harvested and assayed for IFN-γ, IL-4, IL-17 and IL-10 by ELISA, according to the manufacturer's instructions (BD-Pharmingen).
Statistical analysis
Statistical comparisons were made using the Kruskal–Wallis non-parametric test. The significance level was defined as P < 0·05.
Results
Production of cytokines and expression of co-stimulatory molecules by human DCs exposed to F. pedrosoi in vitro
In order to assess the pattern of cytokine production and expression of co-stimulatory molecules by DCs upon phagocytosis of the fungus, monocyte-derived DCs from patients and normal individuals were exposed to the fungus. Cytokines were determined by ELISA and expression of co-stimulatory molecules was performed using flow cytometry. First, we observed that expression of CD80, CD86, HLA-DR and CD83 by immature DCs from patients was higher than by DCs from normal individuals (Figs 1 and 2a). In the presence of conidia, no change was observed in the expression of co-stimulatory molecules by cells from patients. However, an increase in the expression of CD86 and HLA-DR was observed in cells from normal individuals when cells were cultivated in presence of the fungus (Figs 1 and 2a). No differences were observed in matured DCs from patients or controls, but these cells expressed high levels of co-stimulatory molecules (Fig. 2b). On the other hand, it was observed that in the presence of fungus, immature DCs from patients increased the fluorescence intensity of CD86, CD83 and HLA-DR and cells from normal individuals increased the intensity of CD86 and HLA-DR (Fig. 3).
Fig. 1.
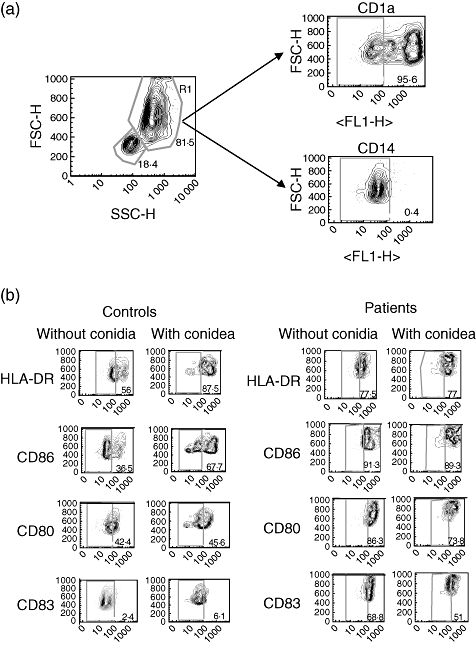
Flow cytometry of dendritic cells (DCs) from patients and controls in the presence or absence of Fonsecaea pedrosoi. Monocyte-derived DCs were obtained by culture of CD14+ with granulocyte–macrophage colony-stimulating factor and interleukin (IL)-4 as described in Material and methods. After 7 days of culture more than 95% of cells were positive for CD1a and negative for CD14 (a). Monocyte-derived DCs from patients and controls were co-cultured with F. pedrosoi (parasite : cell ratio, 3:1) for 24 h and stained with antibodies against human leucocyte antigen D-related, CD86, CD80 and CD83 for 30 min (b). The quadrant represents the negative control (DCs unstained). The y-axis represents forward scatter (FSC)-H and the x-axis represents the intensity of fluorescence with specific antibody. Numbers indicate the percentage of specific expression on DCs. Results of one representative experiment of three are shown.
Fig. 2.
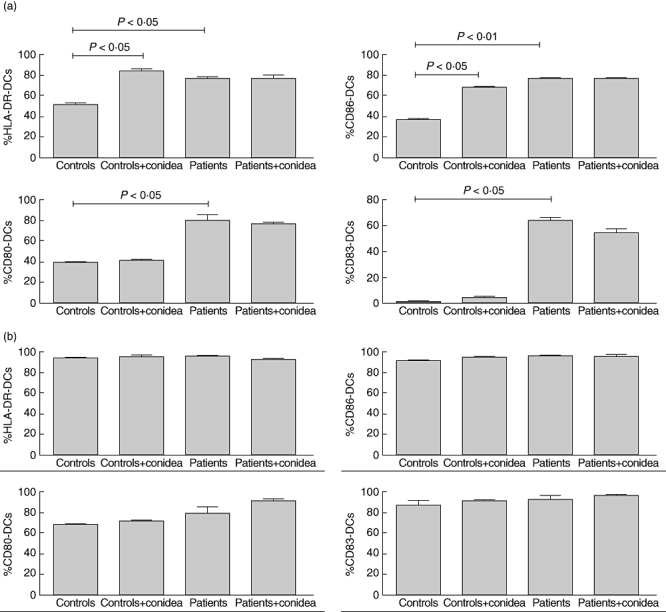
Percentage of expression of human leucocyte antigen D-related (HLA-DR), CD86, CD80 and CD83 on dendritic cells (DCs) from patients or controls in the presence or absence of Fonsecaea pedrosoi. Immature monocyte-derived DCs (a) and tumour necrosis factor (TNF)-α matured monocyte-derived DCs (b) from patients and controls were co-cultured with F. pedrosoi (parasite : ratio, 3:1) for 24 h and stained with antibodies against human HLA-DR, CD86, CD80 and CD83 for 30 min. The results represent the mean ± standard deviation of three independent experiments, where the blood was collected from each study subject (seven patients and nine controls) at three different times. No significant differences were observed in the variability intra-subject regarding DC phenotype when comparing samples collected on different days. *P< 0·05 was considerate significant.
Fig. 3.
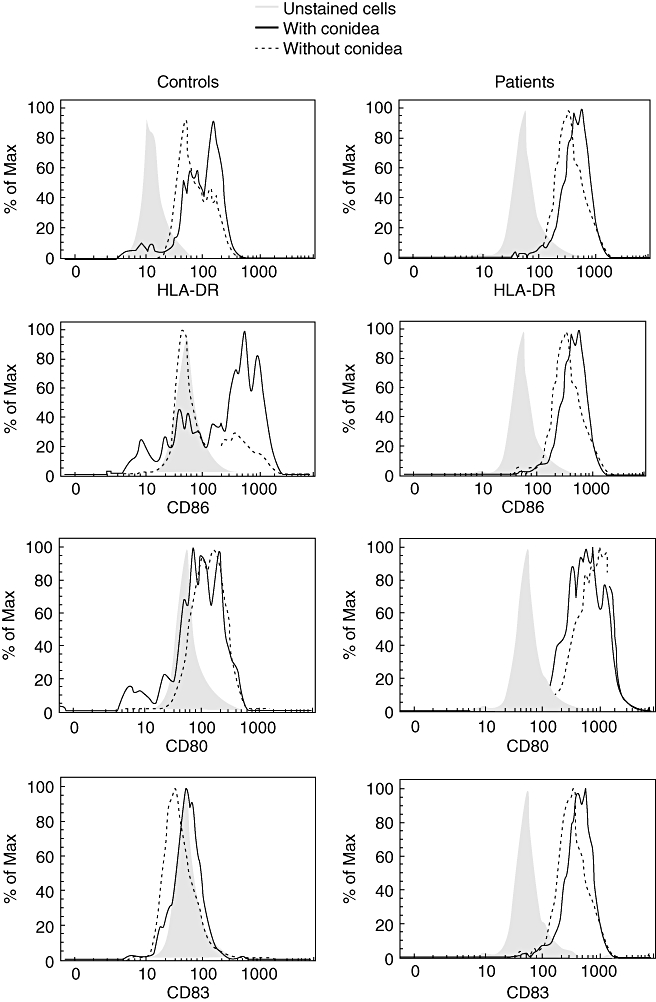
Intensity of fluorescence of human leucocyte antigen D-related, CD86, CD80 and CD83 on immature dendritic cells (DCs) from patients and controls in the presence or absence of Fonsecaea pedrosoi. Monocyte-derived DCs from patients and controls were co-cultured with F. pedrosoi (parasite : cell ratio, 3:1) for 24 h and stained with antibodies against human HLA-DR, CD86, CD80 and CD83 for 30 min. Results of one representative experiment of three are shown.
Cytokine analysis showed that only basal levels of IL-12, IL-10 and TNF-α were produced by immature DCs from patients and controls without stimulus. However, high levels of IL-12, IL-10 and TNF-α were produced when cells from control individuals and patients were stimulated with conidia (Fig. 4). The same patterns of cytokine production were observed in the matured DCs (data not shown).
Fig. 4.
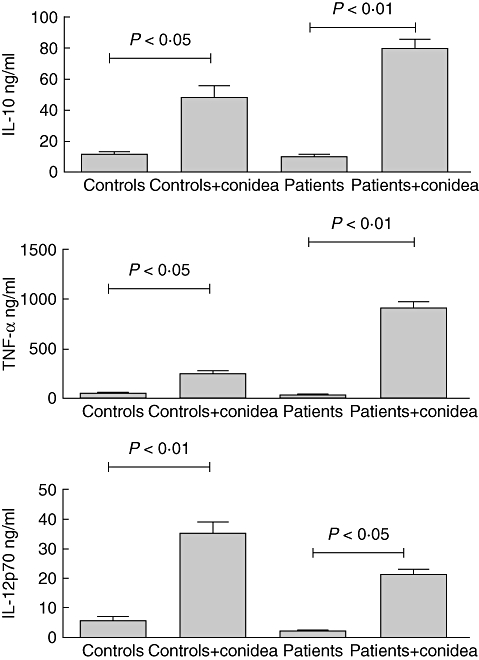
Cytokine production by immature dendritic cells (DCs) from patients and controls in the presence or absence of Fonsecaea pedrosoi. Monocyte-derived DCs from patients and controls were co-cultured with F. pedrosoi (parasite : cell ratio, 3:1) for 24 h. After 24 h the supernatant was harvested and cytokine determined. The results represent the mean ± standard deviation of three independent experiments, where the blood was collected from each study subject (seven patients and nine controls) at three different times. No significant differences were observed in the variability intra-subject regarding dendritic cell phenotype when comparing samples collected on different days. *P < 0·05 was considerate significant.
Proliferation of T cells
The effective generation of DCs from anergic patients with severe forms of chromoblastomycosis allowed us to evaluate autologous T cell responses to DCs incubated with F. pedrosoi conidea. DCs incubated with conidea, without maturing stimulus (TNF-α), were able to induce autologous T cell proliferation in anergic patients but not in healthy non-immune individuals (Fig. 5). Interestingly, TNF-α-mDCs incubated with conidea induced higher levels of proliferation, in both anergic patients and non-immune individuals (Fig. 5). Cytokine analysis showed a predominant Th1 pattern (Fig. 6). The proliferation of CD4 T cells and IFN-γ production shown in Fig. 6 indicates that DCs from patients with severe forms of chromoblastomycosis can reverse lymphocyte non-responsiveness, as observed in vitro.
Fig. 5.
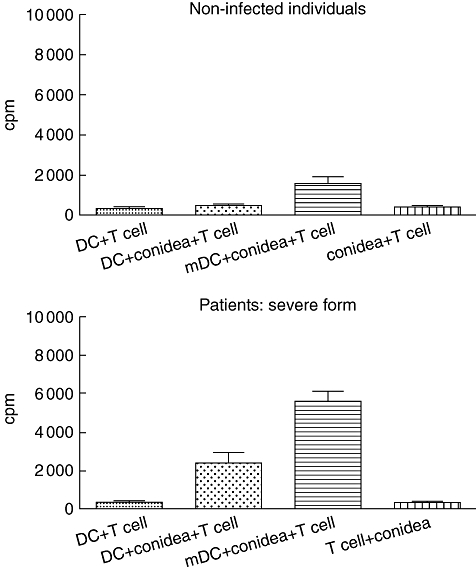
Proliferation of CD4+ T cells in the presence of Fonsecaea pedrosoi conidea-pulsed dendritic cells (DCs) (conidia : cell ratio = 3:1) from patients or non-infected individuals. Immature DCs were prepared as described in Materials and methods and pulsed with or without conidia of F. pedrosoi on day 7. On day 7, matured DCs (mDCs) were incubated with 500 IU/ml of tumour necrosis factor (TNF)-α by 24 h and pulsed with or without conidia of F. pedrosoi (conidia : cell ratio = 3:1). Afterwards, pulsed DCs were plated at 2 × 104 in triplicate wells of a 96-well flat-bottomed plate and irradiated with 3000 rad from a 60Co source. Autologous CD4+ T cells were added at 2 × 105 cells/well. Proliferation of CD4 T cells was evaluated after 5 days by measuring [3H]-thymidine uptake and results were expressed as counts per minute (CPM). The results represent the mean ± standard deviation of three independent experiments, where the blood was collected from each study subject (seven patients and nine controls) at three different times. No significant differences were observed in the variability intra-subject regarding DC phenotype when comparing samples collected on different days. *P < 0·05 was considerate significant.
Fig. 6.
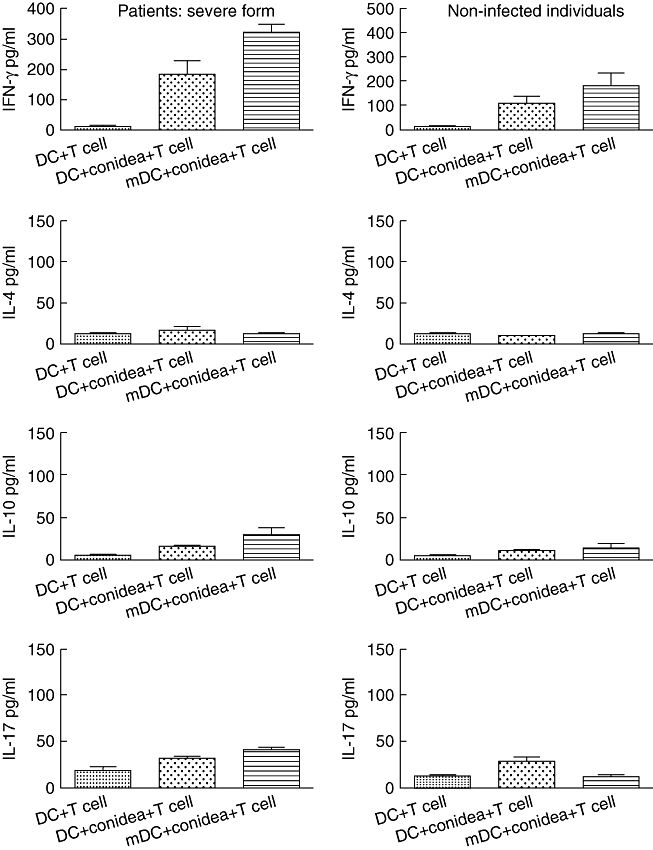
Cytokine production by CD4+ T cells in the presence of dendritic cells (DCs) or tumour necrosis factor (TNF)-α-matured DCs (mDCs) and Fonsecaea pedrosoi conidia (conidia : cell ratio = 3:1). After 5 days of proliferation the supernatant was harvested and cytokines determined. The results represent the mean ± standard deviation of three independent experiments, where the blood was collected from each study subject (seven patients and nine controls) at three different times. No significant differences were observed in the variability intra-subject regarding DC phenotype when comparing samples collected on different days. *P < 0·05 was considerate significant.
Discussion
Chromoblastomycosis starts with subcutaneous inoculation of F. pedrosoi into tissues where DCs are in the first line of defence against this microorganism. We thus hypothesized that DCs are important for phagocytosis of F. pedrosoi and presentation of antigens to T cells. In the present study, we show for the first time that phenotypically and functionally normal monocyte-derived DCs can be generated from patients with severe forms of chromoblastomycosis. Moreover, monocyte-derived DCs generated from patients presented higher levels of co-stimulatory expression. We also demonstrate the reversal of antigen-specific PBMC anergy mediated by DCs incubated with F. pedrosoi conidea.
As the most potent antigen-presenting cells, DCs play an important role in initiating or modulating the immune response [23]. To understand more clearly the role of DCs in human chromoblastomycosis immunity, DCs from anergic patients with severe forms of disease were generated.
During maturation, DCs lose their ability to capture and process antigens, decreasing the number of receptors involved in phagocytosis (such as mannose receptors), and increase their expression of class II MHC co-stimulatory (CD40, CD80, CD86) and adhesion (CD54) molecules [24,25]. Our results show that DCs from patients presented a frequency of expression of CD86, HLA-DR and CD83 higher than that observed in the controls. On the other hand, we demonstrate that fluorescence intensity of HLA-DR and CD86 in DCs from both individuals was increased in the presence of fungus, thus indicating that interaction of fungus with DCs could induce alteration in the expression of co-stimulatory molecules. The mechanism of maturation of DCs observed by us is unknown, but recent evidence suggests that innate immune receptors, such as those of the Toll-like receptor family, may induce maturation of DCs in response to microbial products [26]. Induction of maturation of human DCs by fungal agents, including Coccidioides spherules, Aspergillus conidia, Candida and Malassezia yeast cells, and mannoproteins derived from Cryptococcus, has been described in other studies [27–32]. DC–fungus interactions reported in these studies have been linked to recognition of pathogens by pathogen-recognition receptors such as DC-specific intercellular adhesion molecule-3 grabbing non-integrin and mannose receptors. Viriyakosol et al. demonstrated that both Toll-like receptor 2 and Dectin-1 are important for the recognition of C. posadasii by using an in vitro murine macrophage model [33].
It was observed that DCs from controls and patients produced TNF-α, IL-10 and IL-12 in presence of conidia, as analysed by cytokine production.
Recently, we showed a high production of IL-10 and low levels of IFN-γ by cells from humans with severe forms of the disease after stimulation with specific antigen [34]. T cells from these individuals fail to proliferate in vitro after induction with chromoantigen. In contrast, patients with mild forms of disease produce predominantly IFN-γ and low levels of IL-10 together with an efficient T cell proliferation. These results suggest that T cells secreting IFN-γ (Th1 response) are required to induce protective immunity against F. pedrosoi.
An inappropriate T cell response may exacerbate diseases caused by F. pedrosoi and other pathogens. Given the crucial role of DCs in the T cell-mediated immune response, we investigated the ability of DCs generated from anergic patients to induce T cell activation. Our results show that DCs incubated with F. pedrosoi conidia induced T cell activation efficiently. Moreover, proliferation of T cells was increased when TNF-α-matured DCs were used. Interestingly, this protocol also induces activation of T cells from non-infected individuals. The ability of DCs pulsed with F. pedrosoi conidia to stimulate non-immune lymphocyte proliferation is supported by previous findings, which demonstrated that DCs pulsed with keyhole limpet haemocyanin, sperm-whale myoglobin, human immunodeficiency virus gp160 or in the coccidioidal model also induced proliferation of lymphocytes from non-immune individuals [22,23,35,36]. Cytokine analysis of proliferation demonstrated polarization towards Th1 immunity.
Induction of CD4 T cell proliferation and a Th1 response suggests that DCs can stimulate an appropriate immune response against F. pedrosoi in patients with severe forms of chromoblastomycosis in vitro. However, the finding that DCs activate anergic PBMCs from patients with severe forms of disease might indicate a role for those cells in the treatment of chromoblastomycosis. Clinical trials using DCs in cancer patients support this use [37,38]. On the other hand, the nature of the mechanisms which DCs can activate anergic T cells, observed by us in this work, is a complex issue. Nevertheless, three non-mutually exclusive hypotheses could be proposed. First, DC function in vivo may be suppressed in an antigen-specific manner by certain unknown suppressive F. pedrosoi components. Secondly, the level of plasmocytoid DCs could be increased in the severe form of chromoblastomycosis, inducing regulatory T cells and consequently anergy. Thirdly, inhibition of DCs trafficking from the skin during chronic infection could induce an inefficient activation of immune response. These possibilities are currently under investigation in our laboratory. However, understanding the functions of DCs and their in vivo potential provides important information to deal with the complex mechanisms involved in the differential evolution of chromoblastomycosis disease.
Acknowledgments
This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP Process: 05/58753-8) and CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico).
References
- 1.Salgado CG, da Silva JP, Diniz JA, et al. Isolation of Fonsecaea pedrosoi from thorns of Mimosa pudica, a probable natural source of chromoblastomycosis. Rev Inst Med Trop Sao Paulo. 2004;46:33–6. doi: 10.1590/s0036-46652004000100006. [DOI] [PubMed] [Google Scholar]
- 2.Burks JB, Wakabongo M, McGinnis MR. Chromoblastomycosis A fungal infection primarily observed in the lower extremity. J Am Podiatr Med Assoc. 1995;85:260–4. doi: 10.7547/87507315-85-5-260. [DOI] [PubMed] [Google Scholar]
- 3.Marques SG, Pedrozo Silva CM, Resende MA, Andreata LS, Costa JM. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Med Mycol. 2004;42:261–5. doi: 10.1080/13693780310001597700. [DOI] [PubMed] [Google Scholar]
- 4.Esterre P, Andriantsimahavandy A, Ramarcel ER, Pecarrere JL. Forty years of chromoblastomycosis in Madagascar: a review. Am J Trop Med Hyg. 1996;55:45–7. doi: 10.4269/ajtmh.1996.55.45. [DOI] [PubMed] [Google Scholar]
- 5.Minotto R, Bernardi CD, Mallmann LF, Edelweiss MI, Scroferneker ML. Chromoblastomycosis: a review of 100 cases in the state of Rio Grande do Sul, Brazil. J Am Acad Dermatol. 2001;44:585–92. doi: 10.1067/mjd.2001.112220. [DOI] [PubMed] [Google Scholar]
- 6.Bonifaz A, Carrasco-Gerard E, Saul A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1–7. doi: 10.1046/j.1439-0507.2001.00613.x. [DOI] [PubMed] [Google Scholar]
- 7.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–9. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 9.Moser M, Murphy KM. Dendritic cell regulation of Th1–Th2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 11.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 12.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 13.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and nonpolarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 15.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 16.Hochrein H, O'Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–33. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinski P, Smits HH, Schuitemaker JH, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–81. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 18.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 19.Siegemund S, Alber G. Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol. 2008;52:417–27. doi: 10.1111/j.1574-695X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. 2007;19:48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Queiroz-Telles F, Purim KS, Fillus JN, et al. Itraconazole in the treatment of chromoblastomycosis due to Fonsecaea pedrosoi. Int J Dermatol. 1992;31:805–12. doi: 10.1111/j.1365-4362.1992.tb04252.x. [DOI] [PubMed] [Google Scholar]
- 22.Richards JO, Ampel NM, Lake DF. Reversal of coccidioidal anergy in vitro by dendritic cells from patients with disseminated coccidioidomycosis. J Immunol. 2002;169:2020–5. doi: 10.4049/jimmunol.169.4.2020. [DOI] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia A. Dendritic cell maturation and generation of immune responses. Haematologica. 1999;84(Suppl EHA-4):23–5. [PubMed] [Google Scholar]
- 25.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348–54. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 26.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Dendritic cells at the end of the millennium. Immunol Cell Biol. 1999;77:404–10. doi: 10.1046/j.1440-1711.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 27.Dionne SO, Podany AB, Ruiz YW, Ampel NM, Galgiani JN, Lake DF. Spherules derived from Coccidioides posadasii promote human dendritic cell maturation and activation. Infect Immun. 2006;74:2415–22. doi: 10.1128/IAI.74.4.2415-2422.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31:1583–93. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 29.Cambi A, Gijzen K, de Vries JM, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–8. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 30.Persat F, Noirey N, Diana J, et al. Binding of live conidia of Aspergillus fumigatus activates in vitro-generated human Langerhans cells via a lectin of galactomannan specificity. Clin Exp Immunol. 2003;133:370–7. doi: 10.1046/j.1365-2249.2003.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005;73:820–7. doi: 10.1128/IAI.73.2.820-827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrano-Gomez D, Dominguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA, Corbi AL. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. J Immunol. 2004;173:5635–43. doi: 10.4049/jimmunol.173.9.5635. [DOI] [PubMed] [Google Scholar]
- 33.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun. 2005;73:1553–60. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazo Favero Gimenes V, Da Gloria de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708–13. doi: 10.1016/j.micinf.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Steinman RM, Sapp M, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–80. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurner B, Haendle I, Roder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell–dendritic cell hybrids. Nat Med. 2000;6:332–6. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]


