Abstract
Islet or β cell transplantation provides a promising cure for type 1 diabetes patients, but insulin-independency decreases frequently over time. Immunosuppressive regimens are implemented attempting to cope with both auto- and alloimmunity after transplantation. We analysed the influence of different immunotherapies on autoreactive and alloreactive T cell patterns and transplant outcome. Patients receiving three different immunosuppressive regimens were analysed. All patients received anti-thymocyte globulin induction therapy. Twenty-one patients received tacrolimus–mycophenolate mofetil maintenance immunosuppression, whereas the other patients received tacrolimus–sirolimus (SIR, n = 5) or SIR only (n = 5). Cellular autoreactivity and alloreactivity (CTL precursor frequency) were measured ex vivo. Clinical outcome in the first 6 months after transplantation was correlated with immunological parameters. C-peptide levels were significantly different between the three groups studied (P = 0·01). We confirm that C-peptide production was correlated negatively with pretransplant cellular autoreactivity and low graft size (P = 0·001, P = 0·007 respectively). Combining all three therapies, cellular autoimmunity after transplantation was not associated with delayed insulin-independence or C-peptide production. In combined tacrolimus–SIR and SIR-treated patients, CTL alloreactivity was associated with less insulin independence and C-peptide production (P = 0·03). The percentage of donors to whom high CTLp frequencies were measured was lower in insulin-independent recipients (P = 0·03). In this cohort of islet cell graft recipients, clinical outcome in the first 6 months after transplantation correlates with the applied immunosuppressive regimen. An association exists between insulin-independence and lower incidence of CTL alloreactivity towards donor human leucocyte antigen. This observational study demonstrates the usefulness of monitoring T cell reactivity against islet allografts to correlate immune function with graft survival.
Keywords: autoimmunity, β cell transplantation, cytotoxic T cell, islets of Langerhans, type 1 diabetes
Introduction
Pancreas or simultaneous pancreas–kidney transplantation are established and successful therapeutic options to cure type 1 diabetes patients with end-stage renal failure [1,2]. However, in patients not eligible for this major surgical procedure, transplantation of isolated β cells from islets of Langerhans would be favoured [3]. This method is associated with low morbidity while still restoring endogenous insulin production. Short-term results have been very promising, as demonstrated in several studies, and with different isolation protocols and immunosuppressive regimens [4–8]. However, in a large number of patients the long-term outcome is disappointing, with lasting long-term insulin-independence achieved by less than 10% of recipients [9] and occurrence of adverse events related to immunosuppression [10].
Several factors related to donors, grafts, the transplantation procedure and the engraftment can be important for graft survival. The amount of β cells injected was shown previously to correlate with C-peptide release 2 months after transplantation [8]. The successful Edmonton protocol applies daclizumab induction and sirolimus (SIR) and tacrolimus (TAC) maintenance immunosuppressive therapy. However, avoidance of TAC may have some advantages, because of diabetogenic [11] and nephrotoxic [12] effects as well as its interference with tolerance induction [13]. The same holds true for SIR, which is associated with side effects such as mouth ulcers, acne and hypercholesterolaemia as well as impaired engraftment and insulin resistance [9,10,14–16]. Induction with anti-thymocyte globulin (ATG) is as successful as daclizumab in simultaneous pancreas–kidney transplantation [17] and is also reported to be effective in combination with monotherapy of SIR [18]. These arguments validate exploration and comparison of other immunosuppressive regimens in β cell transplantation.
The immunosuppressive regimen used may also affect the success of β cell replacement by its effect on T cell-mediated autoimmunity and allograft rejection. Therefore, analysis of auto- and alloreactivity before and after islet cell transplantation can contribute to identify possible markers for success. The importance of cellular islet-specific autoimmunity in TAC–mycophenolate mofetil (MMF)-treated recipients was revealed previously [19], and analysis of the alloreactive cytotoxic T response has proved useful in other cohorts involving both islet cell (islet alone and islet after kidney [20,21]) and bone marrow transplantation [22]. However, alloreactive CTL responses towards donor human leucocyte antigens (HLA) seemed non-informative in patients transplanted with islets under TAC–MMF immune suppression [19]. Because this might relate to the type of immunosuppression used, we performed a retrospective analysis of patients receiving standardized islet cell grafts and induction therapy (ATG) under three different maintenance immune suppressive protocols: TAC–MMF, TAC–SIR or SIR monotherapy. TAC–MMF therapy has become standard practice in our centre in recent years, while TAC–SIR and SIR therapy were initiated to enable comparison with TAC–MMF in a homogeneous cohort in a single β cell transplantation programme. Clinical outcome in the different patient groups has been published previously [8,10]. The aim of the current study was to evaluate the effect of the different immunosuppressive regimens on clinical parameters such as insulin-independence and C-peptide release. Subsequently, the effect of the different immunosuppressive therapy was correlated with immunological data (auto- and alloreactivity).
Materials and methods
Patient groups
Patients were recruited for islet cell transplantation after signing informed consent and met the following inclusion criteria: long-standing type 1 diabetes, between 18 and 65 years of age, plasma C-peptide < 0·09 ng/ml, large variation in blood glucose levels [coefficient of variation (CV) of fasting glycaemia (CVgl) ≥ 25%], HbA1c concentration > 7% and one or more chronic diabetes lesions. Exclusion criteria included body weight > 90 kg, active smoking, pregnancy, disturbed liver function tests, history of hepatic disease, presence of HLA antibodies or negative Epstein–Barr virus serostatus.
In this study 31 patients who received one (n = 11) or two (n = 20) islet cell grafts in the first 26 weeks after transplantation were analysed. Twenty-one patients analysed were transplanted under ATG induction and TAC–MMF immunosuppression [8,19], five under ATG–TAC–SIR and five under ATG–SIR. All three groups have been described in detail previously [8,10,19]. Given the number limitations inherent to β cell transplantation programmes, group size for the TAC–SIR and SIR groups remained limited. For the TAC–MMF group, the cohort of 21 patients reported on earlier [8] was included in the current study. Patients’ baseline characteristics were not different (Table 1). However, patients transplanted under TAC–MMF received a smaller total amount of β cells per kg body weight (P = 0·02), in accordance with the lower number of patients in this group receiving a second graft. The decision to inject a second islet cell graft in the TAC–MMF group was based on insufficient C-peptide levels and/or variation of fasting glycaemia (CVgl > 25%) after the first engraftment [8]. Patients in the TAC–SIR and SIR group always received a second transplant regardless of C-peptide levels or CV. We also compared those patients in the TAC–MMF group receiving two transplants (n = 10) with the TAC–SIR and SIR groups (all receiving two transplants).
Table 1.
Patient characteristics of cohorts transplanted under anti-thymocyte globulin–tacrolimus–mycophenolate mofetil (ATG–TAC–MMF), ATG–TAC–sirolimus (SIR) or ATG–SIR immunosuppression.
| TAC–MMF |
TAC–SIR |
SIR |
||
|---|---|---|---|---|
| Parameter | All n = 21 | n = 5 | n = 5 | P-value |
| Age (years) | 42 (37–49) | 36 (35–40) | 41 (33–47) | 0·31 |
| Gender (male/female) | 13–8 | 4–1 | 4–1 | 0·60 |
| Body weight (kg) | 69 (65–76) | 75 (62–78) | 78 (76–80) | 0·17 |
| Duration of disease (years) | 26 (19–33) | 21 (20–29) | 22 (6–23) | 0·21 |
| Age at onset (years) | 17 (12–24) | 16 (10–19) | 21 (18–25) | 0·41 |
| HbA1c (%) | 7·6 (6·9–8·1) | 8·0 (7·9–8·5) | 7·5 (7·0–7·9) | 0·69 |
| Insulin dose (IU/kg/day) | 0·7 (0·5–0·9) | 0·6 (0·5–0·6) | 0·6 (0·5–0·7) | 0·46 |
| Total injected β cells (106/kg body weight) | 3·8 (2·6–4·9) | 7·0 (4·6–7·4) | 5·4 (4·0–5·8) | 0·02 |
Data present median and interquartile range. P-values are calculated by Kruskal–Wallis test.
Preparation of islet cell grafts
Pancreases from brain-dead heart-beating donors were procured by hospitals affiliated with the Eurotransplant Foundation (Leiden, the Netherlands) according to local medical, legal and ethical guidelines for organ donation. Islet cell-enriched fractions were cultured as described previously by using serum-free Ham's F10 medium/0·5% human albumin/135 mg/dl glucose/2 mM glutamine (50 µl of tissue in 45 ml of medium suspended in a T175 Starsted culture flask with a vented cap). After 2–20 days [median 6 days; interquartile range (IQR) 3–11 days] the preparations were analysed for their β cell number and purity [5,8,10]. Data were used to select preparations that, after combination, would constitute a graft with 0·5–5 × 106β cells/kg of recipient body weight suspended in 40–85 ml of Ham's F10 medium with 0·5% human albumin. The final cellular composition of each β cell graft was determined on samples that were taken just before implantation [5,8,10]. For each preparation, whether taken at the start of culture or during culture, or from the final graft, triplicate samples for DNA assay were taken, each being assayed in duplicate; when calculated for 30 consecutive grafts, the CV among these aliquots was 9% (5–14%), and that among duplicate samples was < 5%. The total number of cells in a fraction was calculated by dividing its DNA content (in picograms) by 6·5 pg per cell, the average cellular DNA content measured in sorted single human adult β cells and duct cells. The number of β cells was then determined on the basis of the percentage of insulin-positive cells counted in duplicate samples of this fraction. The number of donors per graft was four (median; IQR 3–5). Compared with freshly isolated islet fractions [4], these preparations exhibit a higher percentage of β cells and contain virtually no acinar cells. Standardized grafts were injected into the portal vein of the recipient, as described previously [5,8,23,24].
Immunosuppression and clinical follow-up
The ATG induction therapy (Fresenius, Fresenius Hemocare, Redmond, WA, USA) was administered to all patients and started with a single infusion of 9 mg/kg and subsequently with 3 mg/kg for 6 days or until the T lymphocyte count was under 50/mm3. TAC maintenance immunosuppression (Prograft, Astellas Pharma Belux) was dosed according to trough level: 8–10 ng/ml in months 0–3 post-transplantation, 6–8 ng/ml thereafter. SIR (Rapamune; Wyeth Pharmaceuticals, Philadelphia, PA, USA) was administered orally at 0·2 mg/kg/day as a loading dose, 0·1 mg/kg/day thereafter, to achieve through levels of 10–15 ng/ml. Standard MMF (Roche, Vilvoorde, Belgium) dosage was 2000 mg/day. Three hours before an islet cell graft implant, one dose of 500 mg methylprednisolone was given intravenously.
Graft recipients were followed-up regularly regarding plasma C-peptide levels (at glycaemia 120–200 mg/dl) as well as percentage HbA1c. The C-peptide level over 26 weeks was calculated by the area under the curve of available plasma C-peptide values. Insulin dose was adjusted to avoid symptomatic hypoglycaemia, maintain blood glucose levels between 70 and 180 mg/dl and Hba1c levels below 7·0%.
Lymphocyte stimulation test to determine cellular autoreactivity
All cellular reactivity tests were performed blinded from clinical results. Blood was drawn from patients before transplantation and on regular intervals post-transplantation (once every 2–6 weeks). Peripheral blood mononuclear cells (PBMCs) were isolated and processed as described previously [25]. Briefly, 150 000 fresh PBMCs/well were cultured in 96-well round-bottomed plates in Iscove's modified Dulbecco's medium with 2 mmol/l glutamine (Gibco, Paisley, Scotland, UK) and 10% pooled human serum in the presence of antigen, interleukin (IL)-2 (35 U/ml) or medium alone in triplicate. After 5 days, [3H]-thymidine (0·5 µCI per well) was added and [3H]-thymidine incorporation was measured 16 h later. Antigens analysed included islet antigen-2 (IA-2) (10 µg/ml), glutamic acid decarboxylase (GAD65) (10 µg/ml), insulin (25 µg/ml) and tetanus toxoid (‘third-party’ antigen, 1.5 LF/ml). Results were interpreted as stimulation index (SI) compared with medium value, where SI < 3 was considered negative and SI ≥ 3 positive. After transplantation, positivity in the case of incidental SIs between 3 and 5 was defined based on the pattern and frequency of autoreactivity over time, blinded from clinical outcome.
CTLp assay to determine the number of alloreactive T cells
The CTLp assay has been described in detail previously [26]. Briefly, cryopreserved PBMCs from recipients from before and different time-points after transplantation were cultured in a limiting dilution assay (40 000 to 625 cells/well, 24 wells per concentration) with different irradiated stimulator PBMCs expressing HLA class I antigens that are also expressed on the injected islet cell grafts (50 000 cells/well, three to eight different stimulators depending on the number of donors and mismatches). Cells were cultured for 7 days at 37°C in 96-well round-bottomed plates in RPMI-1640 medium with 3 mM l-glutamine, 20 U/ml IL-2 and 10% pooled human serum. Next, Europium-labelled graft HLA-specific target cells (5000 cells/well, four to eight different targets) were added to the stimulator/responder combinations for 4 h. Wells were scored positive if the Europium release through target cell lysis exceeded spontaneous release +3 standard deviations. Quantification of CTLp frequencies was performed by computer software developed by Strijbosch et al.[27]. Cytotoxic alloreactivity in the first 26 weeks after transplantation was analysed blinded from clinical outcome and classified initially as either low or increased, based on the CTLp frequencies against the different mismatch combinations and their evolution over time.
Statistics
Analysis of dichotomous data was performed by two-tailed Fisher's exact test and χ2 test. Quantitative differences between groups were analysed by Mann–Whitney U-test as well as Kruskal–Wallis analysis. Correlations between quantitative variables were calculated by Spearman's rank correlation test. Analyses were performed using GraphPad Prism (version 4·0) and spss (version 14·0) software. P < 0·05 was considered significant.
Results
Clinical outcome
Factors possibly influencing transplant outcome were analysed with respect to two clinically relevant parameters: independence from exogenous insulin and C-peptide production over 26 weeks. C-peptide levels were significantly different between the three groups studied (Fig. 1, P = 0·01). Furthermore, a significant difference in achievement of insulin independence was observed between the groups (P = 0·04; Table 2).
Fig. 1.
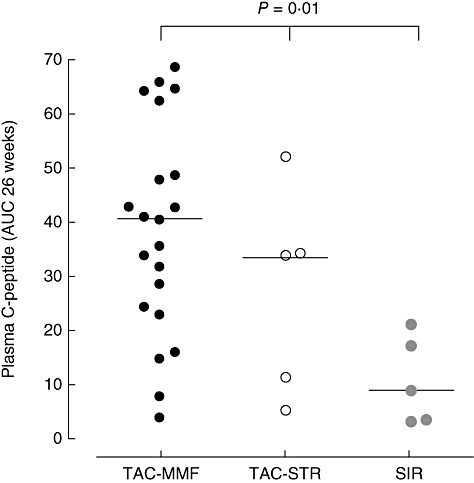
Total C-peptide production over 26 weeks for patients receiving maintenance immunosuppression with tacrolimus (TAC)–mycophenolate mofetil (n = 21), TAC–sirolimus (SIR) (n = 5) or SIR only (n = 5) (P = 0·01, calculated by Kruskal–Wallis test).
Table 2.
Influence of immune parameters on outcome in 31 islet transplant recipients.
| End-point |
Insulin independence |
C-peptide production (AUC) over 26 weeks (weeks × ng/ml) |
|||
|---|---|---|---|---|---|
| Variable | (n) | N (%) | P* | Median (range) | P** |
| Immunosuppressive protocol | TAC–MMF (21) | 13 (62%) | 0·04 | 40·43 (3·56–68·63) | 0·01 |
| TAC–SIR (5) | 3 (60%) | 33·46 (5·12–51·97) | |||
| SIR (5) | 0 (0%) | 8·77 (2·99–20·91) | |||
| All injections ≥ 2·0 × 106β cells/kg | No (10) | 2 (20%) | 0·02 | 40·43 (3·30–68·63) | 0·007 |
| Yes (21) | 14 (67%) | 15·22 (2·99–40·78) | |||
| Pretransplant cellular autoreactivity | No reactivity (11) | 9 (82%) | 0·004 | 51·97 (2·99–68·63) | 0·01 |
| IA-2 or GAD (6) | 4 (67%) | 31·18 (14·59–40·78) | |||
| IA-2 and GAD (6) | 0 (0%) | 12·39 (3·56–42·79) | |||
| Post-transplant cellular autoreactivity | No reactivity (9) | 5 (56%) | 0·15 | 35·45 (22·81–65·85) | 0·62 |
| IA-2 or GAD (12) | 5 (42%) | 33·83 (3·30–68·63) | |||
| IA-2 and GAD (6) | 2 (33%) | 26·33 (2·99–51·97) | |||
| Overall post-transplant cellular alloreactivity (CTLp) | Low (17) | 10 (65%) | 0·46 | 31·75 (3·56–64·19) | 0·74 |
| High (13) | 5 (38%) | 33·83 (2·99–68·63) | |||
| % donors with high CTLp frequency | (30) | 15 (50%) | 0·03** | 33·46 (2·99–68·63) | 0·53*** |
P-values calculated by χ2 test or Fisher's exact test;
calculated by Mann–Whitney U- or Kruskal–Wallis test;
P-value calculated by Spearman's correlation, r = −0·12. Autoreactivity data were unavailable for eight patients, alloreactivity data for one patient. AUC, area under the curve; CTLp, cytotoxic T lymphocyte precursor assay; GAD, glutamic acid decarboxylase; IA-2, islet antigen-2; MMF, mycophenolate mofetil; SIR, sirolimus; TAC, tacrolimus.
Influence of β cell mass, cellular auto- and alloreactivity
In the total patient group the known predictive factors, pretransplant cellular autoreactivity and injected β cell mass, were associated significantly with clinical outcome of islet cell transplantation (Table 2). Post-transplant cellular islet autoreactivity against GAD and/or IA-2, assessed blinded from clinical outcome, did not correlate with insulin independence or C-peptide production (P = 0·15 and 0·62 respectively).
Alloreactivity was analysed by determination of graft HLA-specific CTLp frequencies as well as the percentage of islet donors inducing alloreactivity. The total number of islet donors per patient ranged from two to 10 (mean six) representing nine to 29 (mean 18) HLA class I mismatches per patient (mismatches expressed on more donors were counted separately). In our CTLp analysis we were able to evaluate 79% (429 of 542) of the HLA mismatches using extensive mismatch combinations of a large panel of HLA-typed blood donors. Consequently, we covered alloreactivity to 96% of the grafts. With respect to 59% of the donors, coverage of all HLA mismatches was reached (Fig. 2).
Fig. 2.
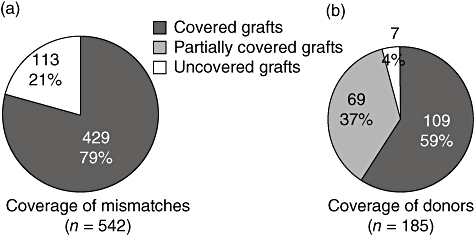
Coverage of islet transplant human leucocyte antigen class I mismatches by cytotoxic T lymphocyte precursor assay.
The donor HLA-specific cytotoxic T cell precursor frequency was analysed blinded over time in the first 26 weeks after transplantation. The general pattern of overall CTLp frequencies after transplantation proved not to indicate clinical outcome in the total patient group (Table 2), nor in the TAC–MMF-treated patients (Fig. 3a and [19]). However, in patients receiving SIR (TAC–SIR/SIR, n = 10), a high donor alloantigen-specific CTLp frequency was associated with significantly lower total C-peptide production compared with patients with a low CTLp frequency (P = 0·03; analysed in TAC–SIR/SIR combined).
Fig. 3.
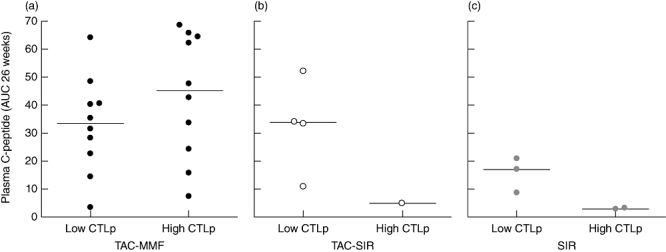
Influence of low or high cytotoxic T lymphocyte precursor assay (CTLp) frequency on C-peptide production is shown for tacrolimus–mycophenolate mofetil (TAC–MMF) (a, n = 20), TAC–sirolimus (SIR) (b, n = 5) or SIR only (c, n = 5) patients. The combined analysis of all non-TAC–MMF patients a high donor alloantigen-specific CTLp frequency was associated with significantly lower C-peptide levels (P = 0·03, Mann–Whitney U-test).
Alloreactivity after second transplant
In patients receiving a second transplant, exposure to new β cell antigens and foreign HLA could lead to changes in auto- and alloimmune reactivity. All patients treated with SIR as immunosuppression (SIR/TAC–SIR) and 10 of the 20 patients in the TAC/MMF group received a second islet infusion. In patients with an increased CTLp frequency after the second transplant under TAC–SIR or SIR immunosuppression (examples in Fig. 4), a significantly lower C-peptide production was observed (P = 0·02, data not shown). Additionally, only one of seven patients with an increased CTLpf became insulin-independent versus eight of 13 of the patients with stable CTLpf (P = 0·07 by Fisher's exact test).
Fig. 4.
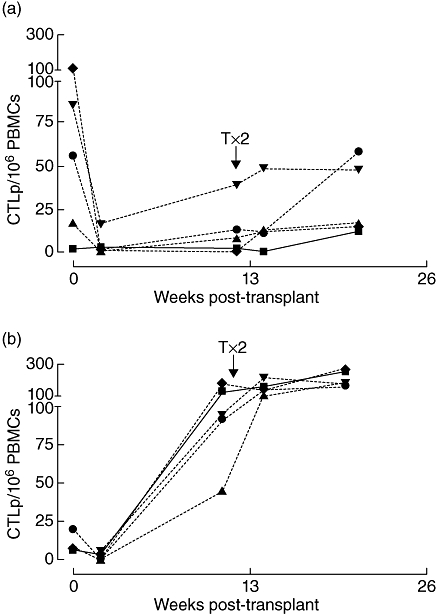
Different patterns of cytotoxic T lymphocyte precursor assay (CTLp) frequency development after first and second islet implantation. The single lines in each graph represent different human leucocyte antigen mismatch-specific stimulator–responder combinations. Straight lines depict combinations specific for the first transplant only, striped lines for the second transplant only and dotted lines for both. Interpretation of cellular alloreactive pattern per patient was performed based on all mismatch combinations and blinded from clinical outcome. Shown are representative examples of patients with low CTLp before and high after second transplant (a) or with high CTLp frequency both before and after (b).
Not every stimulator–target combination induced CTL alloreactivity in every patient [Fig. 4; two of five in panel (a) versus all in panel (b)]. For this reason selective donor-specific correlations between CTL alloreactivity and clinical outcome were assessed further by calculating the fraction of donors against which alloreactive CTLs were induced (‘targeted donors’), ranging from 0% (increased CTLp frequency against none of the donors tested) to 100% (increased CTLp frequencies against all donors tested). In the total patient group, the proportion of targeted donors differed significantly between insulin-dependent and insulin-independent recipients (P = 0·03, Fig. 5).
Fig. 5.
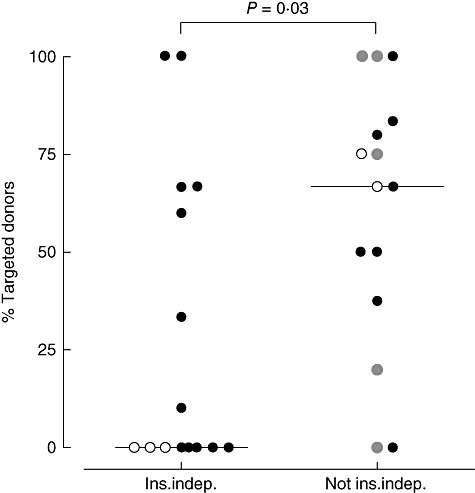
Percentage of islet donors causing high cytotoxic T lymphocyte precursor assay (CTLp) reactivity in the first 26 weeks after islet transplantation, stratified for patients who were (n = 15) or were not (n = 15) insulin-independent after 26 weeks. Filled circles depict patients transplanted under tacrolimus–mycophenolate mofetil (TAC–MMF) immunosuppression, open circles patients transplanted under TAC–sirolimus (SIR) and grey circles patients under SIR only. P-value TAC–MMF: P = 0·35, non-TAC–MMF: P = 0·03.
Discussion
The TAC–MMF protocol has been shown previously to result in considerable islet graft survival in our clinical trial [8]. We recently reported a pilot study evaluating SIR alone or TAC–SIR, which resulted in poor islet graft survival compared with TAC–MMF therapy [10]. In the current study we analysed pre- and post-transplant immune reactivity in these three groups with different immune suppression and investigated their correlation with insulin-independence and C-peptide production.
Analysis of three different immunosuppressive protocols led to a number of potentially valuable observations. First, the applied immunosuppressive regimen was associated significantly with outcome of islet cell transplantation. Furthermore, we confirmed that next to β cell mass, pretransplant cellular autoreactivity correlated with worse transplant outcome in the total cohort of 31 patients. Thirdly, the assessment of the alloreactive CTL response against donor HLA antigens is not an indicator of clinical outcome in the patients receiving TAC–MMF, but alloreactive CTLs may mark poor clinical outcome in patients receiving SIR regardless of combination with TAC. However, as this association was significant only when the TAC–SIR and SIR patient populations were combined, definitive conclusions regarding this matter are precluded. An increased CTL alloreactivity after second transplantation also correlated with clinical outcome under TAC–SIR or SIR.
The SIR monotherapy in particular led to lower C-peptide levels that correlated inversely with cytotoxic alloreactivity. Therefore, the differences between the cohorts may result from the fact that monotherapy with SIR is insufficient to suppress immune reactivity after islet transplantation. However, we cannot exclude that worse islet engraftment and induction of insulin resistance that were shown recently to be associated with SIR therapy also affected clinical and immunological outcome [6,15]. SIR monotherapy following ATG has, none the less, proved to be successful in kidney transplantation [18]. The inadequacy of immunosuppression in the SIR-only group is supported by the significantly higher numbers of CD4+ cells in this group compared with the TAC–SIR group [10], which confirms previous claims that CD4+ counts are reduced by calcineurin inhibitors but not by SIR [28]. Calcineurin inhibitors have been reported to be more potent inhibitors of memory effector T cells that survive depletion regimens, and are therefore useful against acute rejection [29]. Furthermore, it is known that different immunosuppressive therapies can have differential effects on antigen presentation. For instance, SIR does not inhibit major histocompatibility complex-restricted antigen presentation [30] whereas TAC can. Although T cells are the main targets of the calcineurin inhibitors, antigen presentation is also affected [31]. Production of tumour necrosis factor-α by plasmacytoid dendritic cells, a type of antigen-presenting cells, is inhibited by TAC which leads to an impaired T cell response [32].
There are various examples of the value of CTLp measurement in clinical transplantation in the islet cell transplantation setting (both islet alone and islet after kidney [20,21]), as well as in bone marrow transplantation [22]. The presence of CTL against donor HLA class I appears relevant and informative on graft function and survival in the combined SIR protocols, but not in the TAC–MMF group. Although in the separate groups the relationship between CTL measurement and clinical outcome was not significant, this lack of association seems to be attributable mainly to small group size. An alloreactive response after the second transplant correlated with a significantly lower rate of insulin independence and lower C-peptide levels. This effect was again attributable mainly to the SIR and TAC–SIR groups. Patients treated with TAC and MMF may harbour alloreactive CTLs, but the applied immunosuppression apparently appears capable of suppressing these sufficiently in vivo. This would also explain why two patients with increased CTLp frequencies to all donors still managed to achieve insulin-independence. None the less, highly avid CTL may prove particularly important, as has been shown in pre- and post-transplant renal and cardiac transplantation settings [33–36].
The combined usage of TAC and MMF as maintenance therapy appears to be the best option to cope with alloreactivity, as it leads to good clinical outcome regardless of the presence of alloreactive CTLs and often without a need of a second islet transplant. Both TAC–MMF and TAC–SIR contain a calcineurin inhibitor reported to be diabetogenic [11]. Both drugs also contain a cell cycle inhibitor (inhibiting mTOR and mycophenolate respectively). However, in contrast to MMF, SIR has potential side effects that may limit its feasibility in islet cell transplantation, as it was shown to impair islet engraftment [16,33]. Furthermore, SIR-based therapies are associated with considerable side effects that largely disappear after conversion to TAC–MMF. However, even though SIR is not well tolerated by the patients, it may aid the generation of regulatory T cells [10,37,38].
In conclusion, ATG induction and TAC–MMF maintenance therapy seem effective to counter alloreactivity in islet cell transplantation, apart from their limited effect on pre-existent autoimmunity [19]. Donor-specific CTL alloreactivity may be related to islet graft function and clinical outcome, especially in patients receiving SIR or TAC–SIR. The determination of alloreactivity may be useful to predict or guide safe tapering of immunosuppression, but future studies are required to define its feasibility.
References
- 1.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas–kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet. 1999;353:1915–9. doi: 10.1016/S0140-6736(98)07513-8. [DOI] [PubMed] [Google Scholar]
- 3.Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: progress and challenge. Arch Med Res. 2005;36:273–80. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Keymeulen B, Ling Z, Gorus FK, et al. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia. 1998;41:452–9. doi: 10.1007/s001250050929. [DOI] [PubMed] [Google Scholar]
- 6.Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 7.Ricordi C, Inverardi L, Kenyon NS, Goss J, Bertuzzi F, Alejandro R. Requirements for success in clinical islet transplantation. Transplantation. 2005;79:1298–300. doi: 10.1097/01.tp.0000157275.64874.84. [DOI] [PubMed] [Google Scholar]
- 8.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA. 2006;103:17444–9. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 10.Gillard P, Ling Z, Mathieu C, et al. Comparison of sirolimus alone with sirolimus plus tacrolimus in type 1 diabetic recipients of cultured islet cell grafts. Transplantation. 2008;85:256–63. doi: 10.1097/TP.0b013e31815e8926. [DOI] [PubMed] [Google Scholar]
- 11.Maes BD, Kuypers D, Messiaen T, et al. Posttransplantation diabetes mellitus in FK-506-treated renal transplant recipients: analysis of incidence and risk factors. Transplantation. 2001;72:1655–61. doi: 10.1097/00007890-200111270-00014. [DOI] [PubMed] [Google Scholar]
- 12.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35:333–46. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 14.van Gelder T, ter Meulen CG, Hene R, Weimar W, Hoitsma A. Oral ulcers in kidney transplant recipients treated with sirolimus and mycophenolate mofetil. Transplantation. 2003;75:788–91. doi: 10.1097/01.TP.0000056639.74982.F9. [DOI] [PubMed] [Google Scholar]
- 15.Fraenkel M, Ketzinel-Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–57. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Su D, Qu S, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006;55:2429–36. doi: 10.2337/db06-0173. [DOI] [PubMed] [Google Scholar]
- 17.van de Linde P, van de Boog P, Tysma OMH, et al. Immunotherapy with antibodies to protect beta cells in type I diabetic pancreas transplant recipients. Clin Exp Immunol. 2007;149:56–62. [Google Scholar]
- 18.Swanson SJ, Hale DA, Mannon RB, et al. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet. 2002;360:1662–4. doi: 10.1016/S0140-6736(02)11606-0. [DOI] [PubMed] [Google Scholar]
- 19.Huurman VAL, Hilbrands R, Pinkse GGM, et al. Cellular islet autoimmunity associates with clinical outcome of islet transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roep BO, Stobbe I, Duinkerken G, et al. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 1999;48:484–90. doi: 10.2337/diabetes.48.3.484. [DOI] [PubMed] [Google Scholar]
- 21.van Kampen CA, van de Linde P, Duinkerken G, et al. Alloreactivity against repeated HLA mismatches of sequential islet grafts transplanted in non-uremic type 1 diabetes patients. Transplantation. 2005;80:118–26. doi: 10.1097/01.tp.0000164143.22287.e3. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk MB, Roelen DL, Dankers MK, et al. Allogeneic MHC class I molecules with numerous sequence differences do not elicit a CTL response. Hum Immunol. 2005;66:969–76. doi: 10.1016/j.humimm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Maleux G, Gillard P, Keymeulen B, et al. Feasibility, safety, and efficacy of percutaneous transhepatic injection of beta-cell grafts. J Vasc Interv Radiol. 2005;16:1693–7. doi: 10.1097/01.RVI.0000182506.88739.39. [DOI] [PubMed] [Google Scholar]
- 24.Movahedi B, Keymeulen B, Lauwers MH, Goes E, Cools N, Delvaux G. Laparoscopic approach for human islet transplantation into a defined liver segment in type-1 diabetic patients. Transplant Int. 2003;16:186–90. doi: 10.1007/s00147-002-0517-7. [DOI] [PubMed] [Google Scholar]
- 25.Roep BO, Kallan AA, Duinkerken G, et al. T-cell reactivity to beta-cell membrane antigens associated with beta-cell destruction in IDDM. Diabetes. 1995;44:278–83. doi: 10.2337/diab.44.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Bouma GJ, van der Meer-Prins PM, van Bree FP, van Rood JJ, Claas FH. Determination of cytotoxic T-lymphocyte precursor frequencies using europium labeling as a nonradioactive alternative to labeling with chromium-51. Hum Immunol. 1992;35:85–92. doi: 10.1016/0198-8859(92)90015-f. [DOI] [PubMed] [Google Scholar]
- 27.Strijbosch LW, Does RJ, Buurman WA. Computer aided design and evaluation of limiting and serial dilution experiments. Int J Biomed Comput. 1988;23:279–90. doi: 10.1016/0020-7101(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Dong Y, Feng P, Fechner J, Hamawy M, Knechtle SJ. Effect of immunosuppressants on T-cell subsets observed in vivo using carboxy-fluorescein diacetate succinimidyl ester labeling. Transplantation. 2003;75:1075–7. doi: 10.1097/01.TP.0000055832.35337.E6. [DOI] [PubMed] [Google Scholar]
- 29.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–74. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee YR, Yang IH, Lee YH, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–5. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Lee YR, Im SA, et al. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007;179:5711–6. doi: 10.4049/jimmunol.179.9.5711. [DOI] [PubMed] [Google Scholar]
- 32.Naranjo-Gómez M, Climent N, Cos J, et al. Tacrolimus treatment of plasmacytoid dendritic cells inhibits dinucleotide (CpG-)-induced tumour necrosis factor-alpha secretion. Immunology. 2006;119:488–98. doi: 10.1111/j.1365-2567.2006.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roelen D, Datema G, van Bree S, Zhang L, van Rood J, Claas F. Evidence that antibody formation against a certain HLA alloantigen is associated not with a quantitative but with a qualitative change in the cytotoxic T cells recognizing the same antigen. Transplantation. 1992;53:899–903. doi: 10.1097/00007890-199204000-00035. [DOI] [PubMed] [Google Scholar]
- 34.Roelen DL, van Bree S, Witvliet MD, et al. IgG antibodies against an HLA antigen are associated with activated cytotoxic T cells against this antigen, IgM are not. Transplantation. 1994;57:1388–92. doi: 10.1097/00007890-199405150-00018. [DOI] [PubMed] [Google Scholar]
- 35.van Kampen CA, Versteeg-van der Voort Maarschalk MF, Roelen DL, ten Berge IJ, Claas FH. Rejection of a kidney transplant does not always lead to priming of cytotoxic T cells against mismatched donor HLA class I antigens. Transplantation. 2001;71:869–74. doi: 10.1097/00007890-200104150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Cantaluppi V, Biancone L, Romanazzi GM, et al. Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant. 2006;6:2601–11. doi: 10.1111/j.1600-6143.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia M, Stabilini A, Draghici E, et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes. 2006;55:1571–80. doi: 10.2337/db05-1576. [DOI] [PubMed] [Google Scholar]
- 38.Battaglia M, Stabilini A, Draghici E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–9. [PubMed] [Google Scholar]


