Abstract
Corticothalamic fibres, which originate from layer VI pyramidal neurons in the cerebral cortex, provide excitatory synaptic inputs to both thalamic relay neurons and reticular neurons; reticular neurons in turn supply inhibitory inputs to thalamic relay neurons. Pyramidal cells in layer VI in the mouse somatosensory cortex highly express mRNA encoding kainate receptors, which facilitate or depress transmitter release at several synapses in the central nervous system. We report here that contrary modulation of transmitter release from corticothalamic fibres onto thalamic relay and reticular neurons is mediated by activation of kainate receptors in mouse thalamic ventrobasal complex and thalamic reticular nucleus. Exogenous kainate presynaptically depresses the synaptic transmission at corticothalamic synapses onto thalamic relay neurons, but facilitates it at corticothalamic synapses onto reticular neurons. Meanwhile, the lemniscal synaptic transmission, which sends primary somatosensory inputs to relay neurons, is not affected by kainate. In addition, GluR5-containing kainate receptors are involved in the depression of corticothalamic synaptic transmission onto relay neurons, but not onto reticular neurons. Furthermore, synaptically activated kainate receptors mimic these effects; high-frequency stimulation of corticothalamic fibres depresses synaptic transmission onto relay neurons, but facilitates it onto reticular neurons. Our results suggest that the opposite sensitivity of kainate receptors at the two corticothalamic synapses is governed by cortical activity and regulates the balance of excitatory and inhibitory inputs to thalamic relay neurons and therefore their excitability.
Corticothalamic (CT) fibres originating from layer VI pyramidal neurons in the cerebral cortex supply glutamatergic synaptic inputs to both thalamic relay neurons (thalamocortical (TC) neurons) and reticular (RT) neurons; RT neurons in turn supply inhibitory inputs to TC neurons (Baughman & Gilbert, 1981; Jones, 1985; Ohara & Lieberman, 1985; Bourassa et al. 1995; Eaton & Salt, 1996; Kao & Coulter, 1997; Cox et al. 1997; Kim & McCormick, 1998; Golshani et al. 1998). Anatomically, a single CT axon originating from a layer VI pyramidal neuron innervates TC neurons and its axonal collaterals also innervate RT neurons (Deschenes et al. 1998). The balance of excitatory and inhibitory inputs is considered to be critical for the control of TC neurons and therefore the processing of sensory information and modification of sensory receptive field properties (Sillito et al. 1983; Holdefer et al. 1989; Norton & Godwin, 1992; Zhu & Lo, 1998). The CT inputs, interacting with the RT neuron-mediated inhibitory inputs, are also implicated in the synchronization of intrathalamic oscillatory activities, which is associated with the arousal state (Steriade et al. 1993; von Krosigk et al. 1993; Destexhe et al. 1998) and certain neurological disorders such as absence epilepsy (Avoli & Kostopoulos, 1982; Bal et al. 2000; Blumenfeld & McCormick, 2000; Bessaih et al. 2006; Zhu et al. 2006; Alexander & Godwin, 2006).
It has been reported that activation of groups II and III metabotropic glutamate receptors (mGluRs) decreases the release of transmitter from CT synapse to TC neurons in the dorsal lateral geniculate nucleus (dLGN) (Turner & Salt, 1999; Alexander & Godwin, 2005). Furthermore, endogenous glutamate released by high frequency stimulation of CT fibres activates group II mGluRs to attenuate CT synaptic transmission (Alexander & Godwin, 2005). Despite the previous studies on mGluRs, however, little is known regarding the role that ionotropic glutamate receptors play in the presynaptic modulation of CT synapses onto TC neurons (CT–TC synapses). As for the CT synapses on RT neurons (CT–RT synapses), it is unknown even whether there is presynaptic modulation mediated by ionotropic glutamate receptors.
Activation of presynaptic kainate receptors (KARs), one of the ionotropic glutamate receptors, is known to regulate transmitter release from excitatory and inhibitory nerve terminals at various synapses, although there is limited anatomical evidence that KARs are localized at presynaptic terminals (Darstein et al. 2003). Endogenous glutamate released by synaptic activation induces KAR-mediated presynaptic modulation, suggesting that presynaptic KARs function as autoreceptors at the same synapse from which transmitter release occurs (Lauri et al. 2001a,b; Schmitz et al. 2001b; Delaney & Jahr, 2002; Kidd et al. 2002).
There are five KAR subunits (GluR5, GluR6, GluR7, KA1 and KA2) that can coassemble to form heteromeric KARs (Cui & Mayer, 1999; Paternain et al. 2000). Layer VI of cerebral cortex, as compared with the supragranular layer, expresses a high level of mRNAs of KARs in the rodent brain in in situ hybridization studies. mRNAs of all five KAR subunits exist in layer VI cortical neurons. In particular, GluR7 and KA2 mRNAs are highly expressed in layer VI (Wisden & Seeburg, 1993; Bischoff et al. 1997). Immunohistochemical studies demonstrate that cortical pyramidal neurons in layer VI are labelled by anti-GluR6/7 and/or KA2 antibodies (Petralia et al. 1994). And, antibody of anti-GluR5, 6 and 7 subunits is labelled at CT–TC and CT–RT synapses, although the exact location of KARs at CT synapses onto TC and RT neurons remains to be identified (Bolea et al. 2001).
In the present study, we showed contrary effects of KAR activation on the transmitter release at CT–TC and CT–RT synapses in the thalamic ventrobasal complex (VB) and thalamic reticular nucleus of mouse. Exogenous kainate presynaptically depresses CT synaptic transmission onto TC neurons, whereas it potentiates the synaptic transmission onto RT neurons. In addition, we also found that GluR5-containing KARs are involved in the depression of CT–TC synaptic transmission, but not CT–RT synaptic transmission. Finally, we showed that synaptic release of endogenous glutamate, by stimulation of high-frequency trains to CT fibres, mimics the KAR-mediated presynaptic modulation. Taken together, these results suggest that the excitability of TC neurons is regulated by cortical activity. Under the condition that CT fibres were stimulated at high frequency, depressed CT–TC EPSCs along with enhanced RT-TC IPSCs that result from the facilitation of CT–RT EPSCs lead to the decrease in the excitability of TC neurons.
Methods
All experiments were carried out on mice in accordance with the institutional guidelines for animal experimentation, and approved by the animal research committee of the National Institute for Physiological Sciences. C57BL/6 Cr mice of both sexes (postnatal day p14–23, 106 mice) were deeply anaesthetized with halothane and decapitated, and brains were dissected rapidly. Horizontal slices 300 μm thick (Castro-Alamancos, 2002) were cut in ice-cold, continuously oxygenated (95% O2–5% CO2) sucrose Ringer solution using a vibrating microtome (VT1000S; Leica, Nussloch, Germany) and kept in a submerged chamber for more than 1 h with 95% O2–5% CO2-saturated ACSF at room temperature. The sucrose Ringer solution contained (in mm): 234 sucrose, 2.5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 25 NaHCO3 and 10 glucose. ACSF contained (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3 and 20 glucose. ACSF was equilibrated with 95% O2–5% CO2 (pH 7.3) and infused at a rate of 3.0 ml min−1. During recordings, ACSF containing 10 μm (–)-bicuculline methobromide (Tocris Cookson, Avonmouth, UK) and 5 μm CGP 55348 (Tocris Cookson) was used for blocking GABAA and GABAB receptors, respectively. All experiments were performed at 30–32°C.
TC and RT neurons in VB and thalamic reticular nucleus were visualized under a microscope (BX50WI; Olympus, Tokyo, Japan) equipped with an infrared differential interference contrast video system (C2400–79H; Hamamatsu Photonics, Hamamatsu, Japan). Whole-cell recordings were carried out using 2–4.5 MΩ recording pipettes containing (in mm): 120 caesium methane-sulphonate, 10 Hepes, 1 EGTA, 2 MgCl2, 0.1 CaCl2, 20 NaCl, 5 QX-314, 2 ATP-Na2, and 0.5 GTP-Na (pH 7.3 with CsOH, 298–310 mosmol l−1) for voltage-clamp recordings.
An EPC9 double patch-clamp amplifier (HEKA, Lambrecht, Germany) was used for voltage-clamp whole-cell recordings. EPSCs were recorded with filtering at 3–10 kHz and digitized at 50 kHz. Series resistances were monitored online and the uncompensated series resistance was typically less than 15 MΩ. A series resistance compensation of 70% was used during recordings from the two types of neurons. Most of the synaptic responses were recorded as NMDAR-mediated EPSCs, which were measured at +40 mV of holding potential in the presence of a selective AMPAR antagonist, GYKI 53655 (50 μm), to block only AMPARs, but not KARs. Synaptic responses were evoked using a concentric electrode (tip diameter, 25 μm; Inter Medical, Nagoya, Japan) placed on the internal capsule for recording CT responses, because all CT fibres pass though the internal capsule. We first made whole-cell patch recording from a RT neuron in the thalamic reticular nucleus, and confirmed that CT–RT EPSCs were elicited by stimulating CT fibres. We then searched for a TC neuron (with a second recording electrode) in the VB, from which CT–TC EPSCs were evoked by the same CT fibre stimulation. Typically, the two neurons were aligned along a straight line which intersected at almost a right angle with an imagery curved line running through the anteromedial and posterolateral extremes of the thalamic reticular nucleus in our horizontal slice orientation. For recording lemniscal responses, the electrode was placed on the medial lemniscus, as reported previously (Miyata & Imoto, 2006). The stimulus consisted of a 100 μs bipolar pulse of constant current steps (< 100 μA) delivered using a biphasic isolator (BAK electronics, Oxford, UK). Lemniscal synaptic responses were recorded as non-NMDAR-mediated EPSCs, which were measured at −70 mV of holding potential. The stimulus was delivered at 0.05 Hz. Data were acquired using the PULSE program (HEKA, version 8.54). Pulse Fit (HEKA, version 8.54) and Igor Pro (Wavemetrics, Lake Oswego, OR, USA) were used to analyse the obtained data. The statistical significance was determined using Student's t test for unpaired or paired data depending on the experimental design; the paired t test was used unless stated otherwise. Data shown are means ±s.d. unless otherwise stated.
Drugs
dl-2-Amino-5-phosphonopentanoic acid (dl-AP5), [R-(R*, S*)]-5-(6,8-dihydro-8-oxofuro[3,4-e]-1,3-benzo-dioxol-6-yl)-5, 6, 7, 8-tetrahydro-6,6-dimethyl-1, 3-dioxolo[4,5-g]isoquinolinium bromide ((–)-bicuculline methobromide), (2S)-3-[[(1S)-1-(3, 4-dichlorophenyl) ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid (CGP55845), 2, 3-dioxo-6-nitro-1, 2, 3, 4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX disodium salt), (RS)-a-cyclopropyl-4-phosphonophenylglycine (CPPG), (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5,10-imine hydrogen maleate (MK-801), (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), and (RS)-α-methyl-4-carboxyphenylglycine (MCPG) were purchased from Tocris Cookson (Avonmouth, UK). GYKI 53655 was purchased from Sigma-Aldrich (St Louis, MO, USA). LY382884 was a gift from Eli Lilly and Company.
Results
CT–TC and CT–RT EPSCs are differently altered by applied kainate
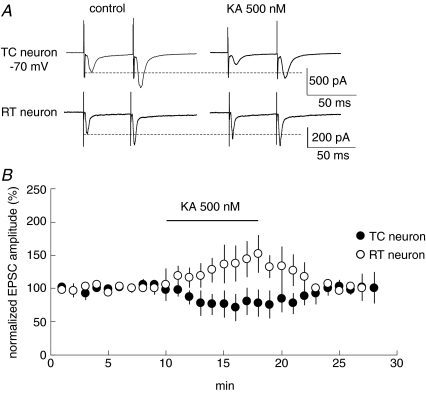
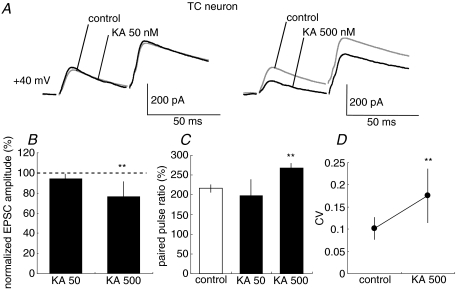
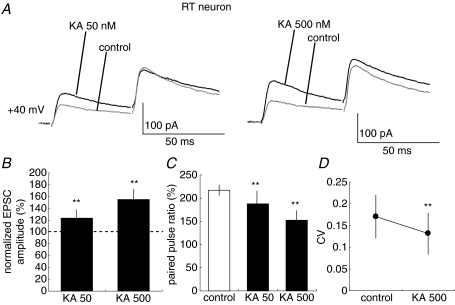
We first recorded AMPA receptor (AMPAR)-mediated CT-EPSCs simultaneously from both TC and RT neurons in the VB and thalamic reticular nucleus. CT–TC EPSCs and CT–RT EPSCs showed similar synaptic properties to those reported previously (de Curtis et al. 1994; Turner & Salt, 1998; von Krosigk et al. 1999; Granseth et al. 2002; Alexander et al. 2006; Miyata & Imoto, 2006). First, these EPSCs increase monotonically with stimulus intensity, indicating that they are composed of a large number of small unitary events. Second, the synaptic responses display paired-pulse facilitation when the same stimulus is delivered twice at a short interval (Fig. 1A). We then examined whether KAR activation alters CT-EPSCs in TC and RT neurons. In TC neurons, the peak amplitude of EPSCs decreased to 76.7 ± 20.0% (P < 0.01, n= 10) 5 min after the bath-application of 500 nm kainic acid (KA: kainate). In contrast, even in the case of stimulation of the same root of CT fibres, KA increased the peak amplitude of EPSCs to 136.8 ± 27.0% (P < 0.01, n= 10) in RT neurons. However, KA at these concentrations had no effect on the holding currents (99.2 ± 2.9% of that in control, P > 0.05) of the two types of neurons. Thus, these changes of EPSC amplitudes are not likely to result from the direct postsynaptic effect of KA. Because NMDARs are insensitive to KA, we examined the effect of KA on synaptic transmission by monitoring NMDAR-mediated EPSCs. NMDAR-mediated currents were pharmacologically isolated by bath-application of GYKI53655 (50 μm), a specific antagonist of AMPAR, and observed at +40 mV of holding potential. The application of GYKI53655 at 50 μm, a concentration commonly used in other studies (Vignes et al. 1998; Bureau et al. 1999; Lauri et al. 2001a; Li et al. 2001; Rebola et al. 2007), obviously abolished AMPAR-mediated EPSCs in CT–TC and CT–RT synapses (see Supplementary Fig. 1). KA at several concentrations was bath-applied during stimulation (Fig. 4). A low concentration of KA (50 nm) had no effect on the amplitude of NMDAR-mediated EPSPs in CT–TC synapses, whereas a high concentration of KA (500 nm) evidently decreased NMDAR-mediated EPSCs in CT–TC synapses (Fig. 2A and B, 85.0 ± 13.0%, P < 0.01, n= 20). KA at both concentrations, however, increased the CT–RT EPSCs (Fig. 3A and B, 123.5 ± 14.0%, P < 0.01, n= 20 at 50 nm; 155.2 ± 17.0%, P < 0.01, n= 20 at 500 nm). These changes in amplitude of EPSCs were associated with clear changes in paired pulse ratio, which is classically considered to inversely correlate with release probability at synaptic terminals (Dobrunz & Stevens, 1997; Zucker & Regehr, 2002). The paired pulse ratio was increased from 216.1 ± 21.7% to 268.3 ± 15.3% (P < 0.01) by KA (500 nm) in CT–TC synapses (Fig. 2C). In contrast, the ratio of CT–RT EPSCs was significantly decreased from 217.9 ± 45.5% to 187.8 ± 36.2% (P < 0.01) and to 153.4 ± 20.6% (P < 0.01) by KA at 50 nm and 500 nm, respectively (Fig. 3C). We further examined the changes in the coefficient of variation (CV) of EPSCs, which is also generally used to monitor changes in release probability (Del Castillo & Katz, 1954), following the bath-application of KA in CT–TC and CT–RT synapses. The bath-application of 500 nm KA increased the CV of CT–TC EPSCs from 0.10 ± 0.02 to 0.17 ± 0.06 (P < 0.01), but decreased the CV of CT–RT EPSCs from 0.17 ± 0.04 to 0.13 ± 0.05 (P < 0.01, Figs 2D and 3D).
Figure 1. The bath-application of KA induces contrarily alternations of the amplitude of CT-EPSCs onto TC and RT neurons.
A, simultaneous recordings of CT-EPSCs (Vh=−70 mV, n= 10) from TC and RT neurons in 500 nm kainate treatments. B, effects of 500 nm KA on amplitudes of CT–TC (•) and CT–RT EPSCs (○) and average of five recordings normalized to average control period amplitude. Values are means ±s.d.
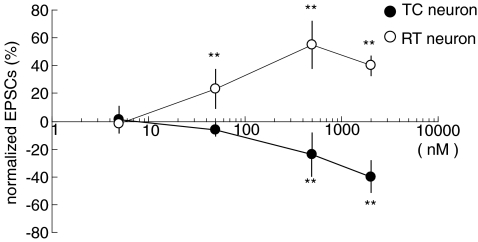
Figure 4. Dose-dependent modulation of CT–TC and CT–RT EPSCs by KA.
Effects of 5, 50 and 500 nm, and 2 μm KA on NMDAR-EPSCs in CT–TC and CT–RT synapses (n= 7, 20, 20, 5 for CT–TC EPSCs, n= 5, 20, 20, 5 for CT–RT EPSCs, respectively; **P < 0.01). Values are means ±s.d.
Figure 2. CT–TC EPSCs are decreased by KA application.
A, traces show NMDAR-EPSCs (Vh=+40 mV) in CT–TC synapses with the paired pulse stimulation at 50 ms interval in control and KA treatments (50 nm, left traces; 500 nm, right traces). Each trace is the average of traces evoked by five stimuli at 0.05 Hz. B, effects of 50 (KA 50) and 500 nm KA (KA 500) on amplitudes of CT–TC EPSCs (**P < 0.01, n= 20 and 20, respectively). C, summary of effects of 50 and 500 nm KA on paired-pulse ratio. D, summary of effects of 50 and 500 nm KA on coefficient of variation of EPSCs. Values are means ±s.d.
Figure 3. CT–RT EPSCs are increased by KA application.
A, traces show NMDAR EPSCs (Vh=+40 mV) in CT–RT synapses with paired pulse stimulation at 50 ms intervals in control and KA treatments (50 nm, left traces; 500 nm, right traces). Each trace is average of traces evoked by five stimuli at 0.05 Hz. B, effects of 50 and 500 nm KA on amplitudes of CT–RT EPSCs (**P < 0.01, n= 20 and 20, respectively). Values are means ±s.d.C, summary of effects of 50 and 500 nm KA on paired-pulse ratio. Values are means ±s.d.D, summary of effects of 50 and 500 nm KA on coefficient of variation of EPSCs. Values are means ±s.d.
Comparison of the dose–response relationships for the effect of KA on the amplitudes of CT–TC and CT–RT EPSCs showed that KA distinctly affects CT–TC and CT–RT synaptic transmission (Fig. 4). We did not observe the dose-dependent crossover from facilitation to depression of EPSCs in the two types of neuron as previously observed in the cerebellum and hippocampus (Schmitz et al. 2001a; Delaney & Jahr, 2002). KA at concentrations lower than 1 μm had no marked effect on the holding currents of the two types of neurons at +40 mV (less than ∼30 pA). However, KA at 2 μm increased the holding current to ∼110 pA at +40 mV. An antagonist of AMPAR/KAR, NBQX (5 μm), blocked the effects of KA in both types of synaptic transmission. In CT–TC synapses, amplitude of the EPSCs was not affected by KA in the presence of NBQX (100.0 ± 6.0% at 50 nm and 98.8 ± 4.2% of control at 500 nm, P > 0.05, n= 7). Moreover, CT–RT EPSCs were not altered either (98.6 ± 12.0% at 50 nm and 101.0 ± 4.2% of control at 500 nm, P > 0.05, n= 8) by KA with NBQX. Taken together, these results indicated that KA contrarily modulated CT–TC and CT–RT synaptic transmissions, which were accompanied by clear changes in presynaptic parameters, such as paired pulse ratio and CV.
Presynaptic modulation by KARs is not observed at lemniscal synapses
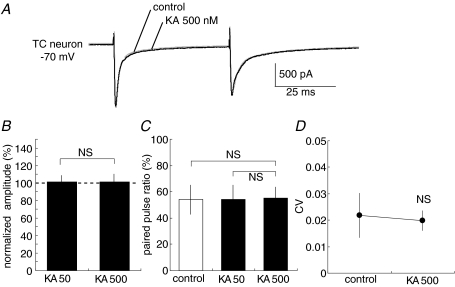
We then examined whether the presynaptic modulation of KARs exists at lemniscal synapses, which convey somatosensory inputs to TC neurons. We distinguished lemniscal–TC EPSCs from CT–TC EPSCs on the basis of their characteristics. First, lemniscal EPSCs in the vast majority of the neurons exhibited all-or-none responses and the threshold stimulation resulted in a unitary response that always had the same amplitude. Second, all lemniscal EPSCs led to paired-pulse depression (Fig. 5A). These synaptic properties have been previously reported in detail (Miyata & Imoto, 2006). In contrast to CT–TC synapses, lemniscal EPSPs were unaffected by applied KA. The amplitude of lemniscal EPSPs was not changed by KA (101.2 ± 7.4% of that in control at 50 nm, P > 0.05, n= 7; 101.3 ± 8.7% at 500 nm, P > 0.05, n= 7, Fig. 5A and B). Furthermore, the paired pulse ratio did not significantly change following KA bath-application (paired pulse ratio was 53.9 ± 11.2% for control; 53.9 ± 8.2% at 50 nm, P > 0.05; 55.2 ± 8.1% at 500 nm, P > 0.05, Fig. 5C). CV was also not affected by KA (P > 0.05, Fig. 5D). These results are consistent with the previous observation in field EPSPs (Binns et al. 2003).
Figure 5. Medial lemniscal fibre–TC neuron EPSCs are not affected by KA application.
A, leminiscal EPSCs (Vh=−70 mV) with the paired pulse stimulation at 50 ms intervals for control and 500 nm KA treatments on TC neurons. Each trace is average of traces evoked by five stimuli at 0.05 Hz. B, effects of 50 and 500 nm KA on amplitudes of lemniscal EPSCs (n= 7; NS, not significant). Values are means ±s.d.C, summary of effects of 50 and 500 nm KA on paired-pulse ratio. Values are means ±s.d.D, summary of effects of 50 and 500 nm KA on coefficient of variation of EPSCs. Values are means ±s.d.
It was previously reported that postsynaptic AMPARs at retinogeniculate synapses, which are the primary sensory synapses in LGN and have a similar structure to lemniscal synapses, are desensitized under normal synaptic transmission (Chen et al. 2002). To exclude the possibility that the desensitized AMPARs may mask the effect of KARs at lemniscal synapses, the same experiment was also performed in the presence of bath-applied cyclothiazide (CTZ), an inhibitor of AMPAR desensitization. CTZ (75 μm) increased the peak amplitude of AMPAR-mediated EPSCs (111.0 ± 8.5% of control; n= 4) and paired pulse depression ratio (53.9 ± 11.2% for control, 76.2 ± 2.0% with CTZ). Under this condition, the subsequent application of KA (500 nm) did not alter the peak amplitude of EPSCs (102.0 ± 2.3% of that without KA, P > 0.05, n= 4) and paired pulse ratio (101.8 ± 2.0% of that without KA, P > 0.05), indicating that KARs did not contribute to transmitter release in lemniscal synapses.
Contrary roles of KA in release probability measured by MK-801
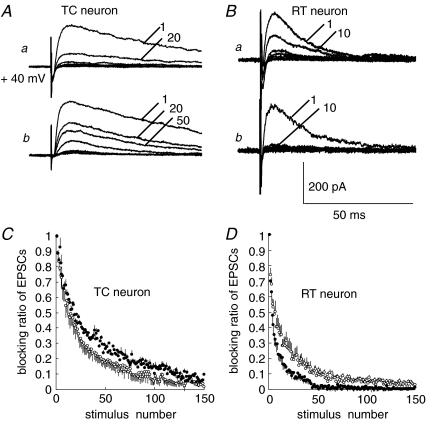
To confirm the presynaptic action of KARs, we examined release probability more directly using a standard assay; the rate of blockade of NMDAR-EPSCs was measured using an irreversible, open-channel blocker of NMDAR, MK-801 (Hessler et al. 1993; Rosenmund et al. 1993). We compared the rate of progressive NMDAR blockade in the absence and presence of 500 nm KA. The NMDAR-EPSC amplitude was set at about 200 pA in all experiments. After obtaining a stable baseline, MK-801 (40 μm) was added to the bath, and stimulation was paused for 10 min to allow MK-801 to equilibrate in the slice. Then, the resumed stimulation (at 15 s intervals) progressively blocked NMDAR-EPSCs. The rate of blockade of NMDAR-EPSCs by MK-801 was significantly decreased by 500 nm KA in CT–TC synapses (decay time constant (τ) of stimulus number; τ= 23.8 ± 5.9 versus 32.3 ± 7.9; n= 10, P < 0.05, unpaired t test, Fig. 6A and B). In contrast, the rate of blockade was increased in CT–RT synapses (τ= 15.0 ± 9.6 versus 5.23 ± 2.9; n= 7, P < 0.05, Fig. 6B and D). Because NMDARs are insensitive to KA (Hollmann & Heinemann, 1994), these results showed directly that the activation of KARs causes contrasting changes in the probability of glutamate release in CT–TC and CT–RT synapses. The MK-801 blocking effects in the presence of NBQX (10 μm) were not significantly different from those measured in the presence of GYKI53655 (τ= 23.8 ± 5.9 versus 23.4 ± 7.0 in CT–TC synapses, n= 7; τ= 15.0 ± 9.6 versus 14.5 ± 10.2 in CT–RT synapses, n= 7, P > 0.05), indicating that presynaptic KARs were not tonically activated by ambient glutamate level at CT–TC and CT–RT synapses.
Figure 6. KA contrarily modulates release probability of CT synapses onto the two types of neurons.
A, traces show MK-801-mediated blockade of synaptic transmission at CT–TC synapses. a, normal ACSF; b, in the presence of 500 nm KA. Added numbers on traces indicate the stimulus number during MK-801 application. B, traces show MK-801-mediated blockade of synaptic transmission at CT–RT synapses. a, control; b, in the presence of 500 nm KA. C, average blocking ratio in EPSC amplitude (mean ±s.e.m.) in normal ACSF (○) and in the presence of 500 nm KA (•) at CT–TC synapses. D, average blocking ratio in EPSCs amplitude in normal ACSF (○) and in the presence of 500 nm KA (•) at CT–RT synapses.
GluR5-containg KARs are involved in presynaptic modulation in CT–TC synapses, but not in CT–RT synapses
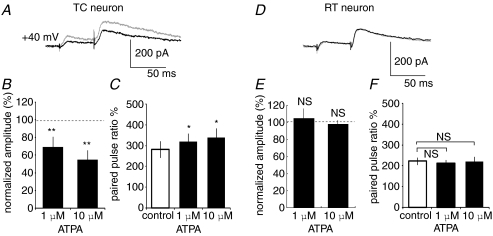
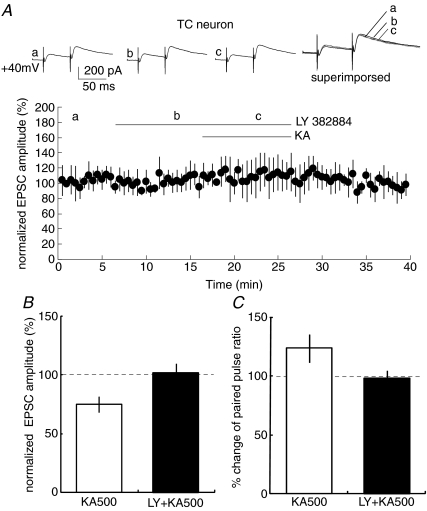
The target neuron-specific difference in presynaptic modulation by KA in CT synapses might originate from different KAR subunit compositions at the two presynaptic terminals onto TC and RT neurons. To examine this hypothesis, we bath-applied ATPA, a GluR5 agonist (Clarke et al. 1997), and then observed NMDAR-mediated EPSCs in CT–TC and CT–RT synapses. We applied ATPA at 1 and 10 μm, which are commonly used concentrations in hippocampal slices (Lauri et al. 2001b; Clarke & Collingridge, 2002). ATPA application mimicked the effect of KA in CT–TC synapses, but not in CT–RT synapses. ATPA significantly decreased NMDAR-mediated EPSCs (69.4 ± 11.5% and 54.9 ± 10.6% at 1 and 10 μm, respectively, P < 0.01 at both concentrations, n= 7 at each concentration, Fig. 7A and B) with increasing paired pulse ratio in CT–TC synapses (from 282.2 ± 40.5% to 320.2 ± 38.0% and 340.2 ± 43.5% at 1 and 10 μm, respectively, P < 0.05, Fig. 7C). In contrast, ATPA did not affect amplitudes of NMDAR-mediated EPSCs in CT–RT synapses (changed to 105 ± 11.3% and 98 ± 4.5% at 1 and 10 μm, respectively, n= 7, Fig. 7D and E) and paired pulse ratio (225.1 ± 6.5% for control, 212.1 ± 6.9% and 222.3 ± 9.6% at 1 and 10 μm, respectively, P > 0.05 at the both concentrations, Fig. 7F). These results suggest that GluR5-containing KARs contributed to the presynaptic modulation in CT–TC synapses, but not in CT–RT synapses. We therefore examined whether the KAR-mediated effect is indeed sensitive to LY382884, an antagonist of GluR5 in CT–TC synapses (Fig. 8). The bath-application of LY382884 (10 μm) alone did not affect CT–TC EPSCs, suggesting that GluR5 was not tonically activated by normal synaptic transmission (Fig. 8A). However, the preincubation of neurons with LY382884 completely blocked the inhibitory effect of KA on CT–TC synaptic transmission. In the case of application of LY382884 with KA (500 nm), the amplitude and paired pulse ratio of CT–TC EPSCs did not significantly change (101.5 ± 8.34% and 98.5 ± 6.2% of the control, percentage change of EPSC amplitude and paired pulse ratio, respectively, n= 6, P > 0.05, Fig. 8A–C). These results demonstrated that GluR5-containing KARs are involved in the inhibition of transmitter release at CT–TC synapses.
Figure 7. ATPA, an agonist of GluR5-containing KARs, mimics the effect of KA in CT–TC but not in CT–RT synapses.
A, CT–TC EPSCs with paired pulse stimulation at 50 ms interval in control (grey) and ATPA application (black line). Each trace is the average of traces evoked by five stimuli at 0.05 Hz. B, effects of ATPA on amplitudes of CT–TC EPSCs (mean ±s.d.; **P < 0.01, n= 10). C, summary of effects of ATPA on paired-pulse ratio in CT–TC EPSCs (mean ±s.d.; *P < 0.05, n= 7). D, CT–RT EPSCs with paired pulse stimulation at 50 ms intervals in control (grey) and ATPA application (black line). E, effects of ATPA on amplitudes of CT–RT EPSCs (NS, not significant; n= 10). F, summary of effects of ATPA on paired-pulse ratio in CT–RT EPSCs (NS, not significant; n= 7).
Figure 8. Pretreatment with GluR5 antagonist abolishes the effect of KA in CT–TC synapses.
A, upper traces show EPSCs for control (a), in the presence of LY382884 (10 μm) (b), and in the presence of LY 382884 with KA (500 nm) (c). Graph shows that pretreatment with LY382884 (10 μm) abolishes the depression of EPSCs induced by KA in CT–TC synapses. B, effects of LY 382884 on KA-induced depression of amplitudes of CT–TC EPSCs (mean ±s.d.; *P < 0.05, n= 20 and 6, respectively). C, comparison of paired-pulse ratio of CT–TC EPSCs in the presence of KA with and without LY 382884 (*P < 0.05, n= 20 and 6, respectively). KA500 and LY + KA500 indicate the application of KA in the absence and presence of LY 382884, respectively.
Synaptically released glutamate induces KARs-mediated presynaptic modulation
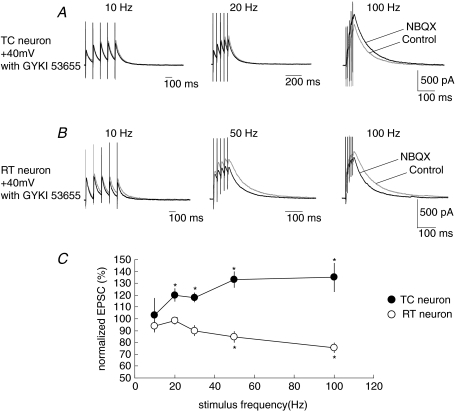
Is KAR-mediated presynaptic modulation of CT synaptic transmission induced by endogenous glutamate released from CT synaptic terminals? To answer this question, we stimulated CT fibres at a high frequency to raise the ambient glutamate concentration at synaptic clefts. It has been reported that the high-frequency stimulation of CT synapses activates several metabotropic glutamate receptors (mGluRs). For example, mGluR1, a member of Group I mGluRs, is activated by high-frequency stimulation of CT fibres and produces slow EPSPs (Godwin et al. 1996; Lee & McCormick, 1997; Golshani et al. 1998; Turner & Salt, 1998; von Krosigk et al. 1999). Moreover, high frequency stimulation of CT fibres activates presynaptic mGluR2, a member of Group II mGluRs, which inhibits CT–TC synaptic transmission (Alexander & Godwin, 2005). Thus, to exclude the effects of mGluRs on transmitter release during high-frequency stimulation, we observed the EPSCs in the presence of a group I/II mGluR antagonist, (RS)-MCPG (0.5 mm), and a group III mGluR antagonist, CPPG (0.5 mm). NMDAR-mediated EPSCs evoked with a single stimulus of CT fibres were not affected by blocking KARs with NBQX (100.2 ± 2.3% of that in the control at 10 μm, n= 7), indicating that the ambient concentration of glutamate under a single stimulus was not sufficiently high to activate KARs. However, both CT–TC and CT–RT synapses were affected by NBQX depending on the stimulus frequency of CT fibres train stimulation. Train stimulation of five pulses was carried out from 20 to 100 Hz. CT–TC EPSCs (NMDAR-mediated EPSCs at 50 μm GYKI53655) were facilitated by NBQX when EPSCs were stimulated at a stimulus frequency higher than 20 Hz. The average change in peak amplitude of the fifth EPSC in the presence of NBQX significantly increased to 120 ± 5.4% (mean ±s.e.m., P < 0.05, n= 7), 133 ± 6.6% (P < 0.05) and 135 ± 11.9% at 20 Hz, 50 Hz and 100 Hz, respectively (Fig. 9A and C). In contrast to the facilitatory effect on CT–TC EPSCs, the amplitudes of CT–RT EPSCs were decreased by NBQX at stimulus frequencies higher than 30 Hz. NBQX decreased the peak amplitudes of the fifth EPSC to 84.6 ± 4.5% and 75.4 ± 4.5% at 50 Hz (P < 0.05, n= 7) and 100 Hz (P < 0.05), respectively (Fig. 9B and C).
Figure 9. Synaptically released glutamate induces KAR-mediated presynaptic modulation.
A, CT–TC EPSCs stimulated at 20 s intervals with trains of five stimuli at 10–100 Hz for control and with the addition of 10 μm NBQX. B, CT–RT EPSCs with trains of five stimuli at 10–100 Hz for control and with the addition of 10 μm NBQX. C, average alternation of peak amplitude (mean ±s.e.m.) after fifth stimulus by NBQX at stimulus frequencies in CT–TC (n= 10) and CT–RT synapses (n= 7; *P < 0.05).
Discussion
We report here that the activation of presynaptic KARs can contrarily alter the strength of glutamatergic synaptic transmission at the two types of CT synapses onto TC and RT neurons in mouse VB and thalamic reticular nucleus. Our major findings are as follows. (1) Activation of KARs presynaptically depresses CT–TC synaptic transmission, but facilitates CT–RT synaptic transmission. (2) In contrast to CT synapses, lemniscal synaptic transmission is not influenced by KA. (3) GluR5-containing KARs contribute to the KAR-mediated presynaptic modulation in CT–TC synapses, but not in CT–RT synapses. (4) High-frequency train stimulation of CT fibres mimics the KAR-mediated modulation of CT synaptic transmissions. A presynaptic locus of KAR regulation of CT synaptic transmission is explainable on the basis of three things. First, accompanying changes in EPSC amplitude induced by the bath-application of KA, the paired pulse facilitation ratio increases in CT–TC synapses, but decreases in CT–RT synapses. Second, the CV of EPSCs, another parameter of presynaptic release probability, increases in CT–TC synapses, but decreases in CT–RT synapses. Third, the rate of blockade of NMDAR-EPSCs using MK801 is decreased in CT–TC synapses, but increased in CT–RT synapses in the presence of KA.
Bolea and colleagues have reported that an exogenous application of kainate has no effect on frequency of miniature EPSCs in TC and RT neurons, suggesting the lack of presynaptic effect of kainate at CT synapses (Bolea et al. 2001). The discrepancy between their study and ours may be attributed to the differences in experimental conditions; miniature EPSCs contain synaptic inputs from all excitatory synapses onto TC and RT neurons, which may mask the component of CT synaptic input. In addition, they recorded EPSCs at a lower temperature (22–25°C), which is known to reduce kainate receptor-mediated currents (Kidd & Isaac, 2001).
Target neuron-specific presynaptic modulation of KARs
Our results demonstrated that KARs display a target neuron-specific modulation of transmitter release at CT synapses. To our knowledge, this is the first report of the existence of such a modulation in the corticothalamic system. The target neuron-specific modulation of KARs has also been observed in the cerebellum and hippocampus. KAR activation presynaptically facilitates synaptic transmission of parallel fibre synapses onto Purkinje cells, but depresses transmission of parallel fibre synapses onto stellate cells (Delaney & Jahr, 2002). In the hippocampus, presynaptic KARs facilitate glutamate release at Schaffer collateral synapses onto somatostatin-positive (SOM) interneurons, but not onto non-SOM interneurons (Sun & Dobrunz, 2006). The anatomical evidence that a single CT fibre from a pyramidal neuron in layer VI of the barrel cortex innervating both types of TC and RT neurons in VB and thalamic reticular nucleus (Deschenes et al. 1998) raises the possibility that distinct subunits of KARs may exist at the two types of terminals innervating TC and RT neurons, respectively. It is also possible that this difference stems from the types of CT fibres targeting TC and RT neurons. CT fibres are anatomically distinct and classified into at least two types. One type originates from pyramidal neurons located in the upper part of layer VI in a barrel column of the cortex and restrictedly terminates at a barreloid in the VB. The other type originates from the lower part of layer VI pyramidal neurons in a barrel column and broadly projects over the thalamic ventroposterial medial nucleus and the thalamic posterior nucleus (Deschenes et al. 1998). Both types of CT fibres have collaterals terminating RT neurons. However, the patterns of their projections to the thalamic reticular nucleus are not homogeneous (Deschenes, 1998). In addition, pyramidal neurons in layer VI, a source of CT fibres, are classified into several subtypes on the basis of their dendritic morphology and excitatory inputs (Zarrinpar & Callaway, 2006). Thus, the difference in KA sensitivity between TC and RT neurons may be attributed to the predominance of different types of CT fibres. However, we cannot distinguish these two possibilities because in our study we stimulated a bundle of CT fibres, instead of a single CT fibre.
Role of KAR subunits in CT synapses
Our results shown in Fig. 7 suggest strongly that GluR5-containing KARs contribute to presynaptic modulation in CT–TC synapses. The property of GluR5-containing KARs to depress transmitter release has also been observed in the hippocampus (Chittajallu et al. 1996; Vignes et al. 1998; Clarke & Collingridge, 2002) and cortex (Campbell et al. 2007). Two mechanisms underlying this depression have been considered: the inhibition of presynaptic Ca2+ influx induced by depolarization block of presynaptic fibres (Kamiya & Ozawa, 1998) and depolarization-independent metabotropic action (Frerking et al. 2001; Lauri et al. 2006; Vesikansa et al. 2007). It should be noted that GluR6 containing KARs may also contribute to depress the transmitter release in hippocampal CA1 region (Kamiya & Ozawa, 1998). Our results do not rule out the possible role for GluR6 subunit in the depression of transmitter release at CT–TC synapses, as GluR5 and GluR6 can form functional heteromeric receptors (Paternain et al. 2000). As for the facilitation effect at CT–RT synapses, our results indicate that other subunits of KARs, except GluR5, are involved. The presynaptic role for the facilitation effect has been well studied in the hippocampal CA3 region using subunit-specific knockout mice of GluR6, GluR7 and KA2 (Contractor et al. 2003; Breustedt & Schmitz, 2004; Pinheiro & Mulle, 2006; Pinheiro et al. 2007). Homometric or heterometoric KARs of these subunits may be involved in the facilitation of transmitter release at CT–RT synapses. Bolea and colleague have reported that anti-GluR5, 6, and 7 subunit antibody is labelled at CT–TC and CT–RT synapses by post-embedding immunogold labelling (Bolea et al. 2001). Although 20% of gold particles are located in postsynaptic site in this study, more than 60% of gold particles are located in the synaptic cleft, making it difficult to ascertain the exact location (i.e. at the presynaptic site or postsynaptic site). The native receptor composition and their specific localization as well as density of the receptor on axon terminals of CT fibres remain to be identified. Further studies are needed to precisely determine their subunit composition at the two types of terminals of CT fibres.
Relation to developmental changes of KARs
It has been reported that the expression pattern of mRNAs of KAR subunits changes during development. In layer VI of rat cerebral cortex, mRNA of GluR5 is detectable during postnatal day P12, but only weakly detectable in adult (Bahn et al. 1994; Binns et al. 2003). In the thalamus, mRNA of GluR5 is highly expressed around birth with a strong decline after postnatal day P12 (Bahn et al. 1994; Binns et al. 2003). In physiological studies, presynaptic KARs that mediate synaptic depression at TC synapses are lost by the end of postnatal day P7 in layer IV of cerebral cortex (Kidd et al. 2002). It has also been reported that presynaptic KARs tonically inhibit glutamate release onto CA1 pyramidal cells during postnatal day P7 (Lauri et al. 2006). Our results were obtained from juvenile animals (P14–23). It is unknown at the moment if the effect observed in our study is maintained in the adult.
Functional implication of KAR-mediated presynaptic modulation in thalamic synapses
KAR-mediated presynaptic modulation is dependent on stimulus frequency of CT fibre train stimulation, suggesting that KARs are activated by augmenting the accumulation of glutamate at CT synapses. The difference in stimulus frequency-dependent KAR modulation between CT–TC and CT–RT synapses provides a mechanism for manipulating the output of TC neurons, depending on cortical activity. That is to say, KAR-mediated presynaptic modulation would finally suppress the excitability of TC neurons when CT fibres are activated at high frequency; along with the depression of CT–TC EPSCs, GABAergic inhibition of TC neurons as a result of the facilitation of CT–RT EPSCs would be enhanced.
CT feedback activities involving the thalamic reticular nucleus underlie thalamic synchrony and slow oscillation as in the case of absence epilepsy. Experimental observations reveal that spindle oscillations can undergo transition to a hyper-synchronous rhythm in the thalamus through high-frequency stimulation of CT fibres (Deschenes et al. 1998; Destexhe, 1998; Bal et al. 2000; Blumenfeld & McCormick, 2000). In addition, it has been reported that genetic juvenile absence epilepsy is due to the linkage of GluR5 gene mutation in humans (Sander et al. 1997; Izzi et al. 2002). Thus, frequency-dependent KAR-mediated modulation of CT synaptic transmissions may prevent the abnormal thalamic synchrony and/or hyperexcitability in absence epilepsy. Unlike CT synapses, lemniscal synaptic transmission is not influenced by activation of KARs, mGluRs and acetylcholine receptors (Castro-Alamancos, 2002; Alexander & Godwin, 2005), thereby ensuring primary sensory information is precisely preserved.
Acknowledgments
We thank Eli Lilly and Company for providing pharmacological tools. We also appreciate Drs Y. Fukazawa and K. Cheng for helpful discussion. This work was in part supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Nos 18500316 and 20021029).
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.164996/DC1
References
- Alexander GM, Fisher TL, Godwin DW. Differential response dynamics of corticothalamic glutamatergic synapses in the lateral geniculate nucleus and thalamic reticular nucleus. Neuroscience. 2006;137:367–372. doi: 10.1016/j.neuroscience.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Presynaptic inhibition of corticothalamic feedback by metabotropic glutamate receptors. J Neurophysiol. 2005;94:163–175. doi: 10.1152/jn.01198.2004. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Avoli M, Kostopoulos G. Participation of corticothalamic cells in penicillin-induced generalized spike and wave discharges. Brain Res. 1982;247:159–163. doi: 10.1016/0006-8993(82)91042-3. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, Debay D, Destexhe A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. J Neurosci. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman RW, Gilbert CD. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci. 1981;1:427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessaih T, Bourgeais L, Badiu CI, Carter DA, Toth TI, Ruano D, Lambolez B, Crunelli V, Leresche N. Nucleus-specific abnormalities of GABAergic synaptic transmission in a genetic model of absence seizures. J Neurophysiol. 2006;96:3074–3081. doi: 10.1152/jn.00682.2006. [DOI] [PubMed] [Google Scholar]
- Binns KE, Turner JP, Salt TE. Kainate receptor (GluR5)-mediated disinhibition of responses in rat ventrobasal thalamus allows a novel sensory processing mechanism. J Physiol. 2003;551:525–537. doi: 10.1113/jphysiol.2003.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Barhanin J, Bettler B, Mulle C, Heinemann S. Spatial distribution of kainate receptor subunit mRNA in the mouse basal ganglia and ventral mesencephalon. J Comp Neurol. 1997;379:541–562. doi: 10.1002/(sici)1096-9861(19970324)379:4<541::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Liu XB, Jones EG. Kainate receptors at corticothalamic synapses do not contribute to synaptic responses. Thalamus Related System. 2001;1:187–196. [Google Scholar]
- Bourassa J, Pinault D, Deschenes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Breustedt J, Schmitz D. Assessing the role of GLUK5 and GLUK6 at hippocampal mossy fiber synapses. J Neurosci. 2004;24:10093–10098. doi: 10.1523/JNEUROSCI.3078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci. 1999;19:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Mathew SS, Hablitz JJ. Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology. 2007;53:37–47. doi: 10.1016/j.neuropharm.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol. 2002;87:946–953. doi: 10.1152/jn.00426.2001. [DOI] [PubMed] [Google Scholar]
- Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron. 2002;33:779–788. doi: 10.1016/s0896-6273(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Clarke VR, Collingridge GL. Characterisation of the effects of ATPA, a GLU (K5) receptor selective agonist, on excitatory synaptic transmission in area CA1 of rat hippocampal slices. Neuropharmacology. 2002;42:889–902. doi: 10.1016/s0028-3908(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/−mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Natl Acad Sci U S A. 1997;94:8854–8859. doi: 10.1073/pnas.94.16.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Arcelli P, De Biasi S, Spreafico R, Avanzini G. Ultrastructural features of the isolated guinea-pig brain maintained in vitro by arterial perfusion. Neuroscience. 1994;59:775–788. doi: 10.1016/0306-4522(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–482. doi: 10.1016/s0896-6273(02)01008-5. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Destexhe A. Spike-and-wave oscillations based on the properties of GABAB receptors. J Neurosci. 1998;18:9099–9111. doi: 10.1523/JNEUROSCI.18-21-09099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Eaton SA, Salt TE. Role of N-methyl-D-aspartate and metabotropic glutamate receptors in corticothalamic excitatory postsynaptic potentials in vivo. Neuroscience. 1996;73:1–5. doi: 10.1016/0306-4522(96)00123-6. [DOI] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3 – >CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci. 2001;21:2958–2966. doi: 10.1523/JNEUROSCI.21-09-02958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin DW, Van Horn SC, Eriir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci. 1996;16:8181–8192. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P, Warren RA, Jones EG. Progression of change in NMDA, non-NMDA, and metabotropic glutamate receptor function at the developing corticothalamic synapse. J Neurophysiol. 1998;80:143–154. doi: 10.1152/jn.1998.80.1.143. [DOI] [PubMed] [Google Scholar]
- Granseth B, Ahlstrand E, Lindstrom S. Paired pulse facilitation of corticogeniculate EPSCs in the dorsal lateral geniculate nucleus of the rat investigated in vitro. J Physiol. 2002;544:477–486. doi: 10.1113/jphysiol.2002.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Norton TT, Godwin DW. Effects of bicuculline on signal detectability in lateral geniculate nucleus relay cells. Brain Res. 1989;488:341–347. doi: 10.1016/0006-8993(89)90727-0. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Izzi C, Barbon A, Kretz R, Sander T, Barlati S. Sequencing of the GRIK1 gene in patients with juvenile absence epilepsy does not reveal mutations affecting receptor structure. Am J Med Genet. 2002;114:354–359. doi: 10.1002/ajmg.10254. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York, NY: Plenum Press; 1985. [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol. 1998;509(3):833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CQ, Coulter DA. Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro. J Neurophysiol. 1997;77:2661–2676. doi: 10.1152/jn.1997.77.5.2661. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Coumis U, Collingridge GL, Crabtree JW, Isaac JTR. A presynaptic kainate receptor is involved in regulating the dynamic properties of thalamocortical synapses during development. Neuron. 2002;34:635–646. doi: 10.1016/s0896-6273(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JTR. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J Neurophysiol. 2001;86:1139–1148. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- Kim U, McCormick DA. The functional influence of burst and tonic firing mode on synaptic interactions in the thalamus. J Neurosci. 1998;18:9500–9516. doi: 10.1523/JNEUROSCI.18-22-09500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Monckton JE, Reiner PB, McCormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience. 1999;91:7–20. doi: 10.1016/s0306-4522(98)00557-0. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JTR, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001a;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Delany C, Vr JC, Bortolotto ZA, Ornstein PL, Isaac JTR, Collingridge GL. Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology. 2001b;41:907–915. doi: 10.1016/s0028-3908(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Modulation of spindle oscillations by acetylcholine, cholecystokinin and 1S,3R-ACPD in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuroscience. 1997;77:335–350. doi: 10.1016/s0306-4522(96)00481-2. [DOI] [PubMed] [Google Scholar]
- Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci. 2001;4:612–620. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- Miyata M, Imoto K. Different composition of glutamate receptors in corticothalamic and lemniscal synaptic responses and their roles in the firing responses of ventrobasal thalamic neurons in juvenile mice. J Physiol. 2006;575:161–174. doi: 10.1113/jphysiol.2006.114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Godwin DW. Inhibitory GABAergic control of visual signals at the lateral geniculate nucleus. Prog Brain Res. 1992;90:193–217. doi: 10.1016/s0079-6123(08)63615-8. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J Neurocytol. 1985;14:365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J Comp Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Sachidhanandam S, Perrais D, Cunha RA, Mulle C. Short-term plasticity of kainate receptor-mediated EPSCs induced by NMDA receptors at hippocampal mossy fiber synapses. J Neurosci. 2007;27:3987–3993. doi: 10.1523/JNEUROSCI.5182-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Sander T, Hildmann T, Kretz R, Furst R, Sailer U, Bauer G, Schmitz B, Beck-Mannagetta G, Wienker TF, Janz D. Allelic association of juvenile absence epilepsy with a GluR5 kainate receptor gene (GRIK1) polymorphism. Am J Med Genet. 1997;74:416–421. [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2001a;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001b;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Berardi N. The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN) Brain Res. 1983;280:299–307. doi: 10.1016/0006-8993(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sun HY, Dobrunz LE. Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci. 2006;26:10796–10807. doi: 10.1523/JNEUROSCI.2746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. J Physiol. 1998;510(3):829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Group III metabotropic glutamate receptors control corticothalamic synaptic transmission in the rat thalamus in vitro. J Physiol. 1999;519(Part 2):481–491. doi: 10.1111/j.1469-7793.1999.0481m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikansa A, Sallert M, Taira T, Lauri SE. Activation of kainate receptors controls the number of functional glutamatergic synapses in the area CA1 of rat hippocampus. J Physiol. 2007;583:145–157. doi: 10.1113/jphysiol.2007.133975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Clarke VR, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology. 1998;37:1269–1277. doi: 10.1016/s0028-3908(98)00148-8. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Callaway EM. Local connections to specific types of layer 6 neurons in the rat visual cortex. J Neurophysiol. 2006;95:1751–1761. doi: 10.1152/jn.00974.2005. [DOI] [PubMed] [Google Scholar]
- Zhu L, Blethyn KL, Cope DW, Tsomaia V, Crunelli V, Hughes SW. Nucleus- and species-specific properties of the slow (<1 Hz) sleep oscillation in thalamocortical neurons. Neuroscience. 2006;141:621–636. doi: 10.1016/j.neuroscience.2006.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Lo FS. Control of recurrent inhibition of the lateral posterior-pulvinar complex by afferents from the deep layers of the superior colliculus of the rabbit. J Neurophysiol. 1998;80:1122–1131. doi: 10.1152/jn.1998.80.3.1122. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.