Abstract
Phasic activity in supraoptic nucleus vasopressin neurones is characterized by alternating periods of activity (bursts) and silence. During bursts, activation of a medium afterhyperpolarization induces spike frequency adaptation. Antagonism of A1 adenosine receptors within the supraoptic nucleus decreases spike frequency adaptation and prolongs phasic bursts in vivo, indicating that endogenous adenosine contributes to spike frequency adaptation. Here we used sharp electrode intracellular recordings from supraoptic nucleus neurones in hypothalamic explants to show that endogenous adenosine increases medium afterhyperpolarization amplitude to enhance spike frequency adaptation during phasic bursts. Superfusion of the A1 receptor antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT, 10 μm) increased intraburst firing rate of phasic neurones (by 2.0 ± 0.7 spikes s−1, P= 0.03) and burst duration (by 141 ± 113 s, P= 0.03). The CPT-induced increase in intraburst firing rate developed over the first few seconds of firing and persisted thereafter. In a separate series of experiments, CPT reduced the amplitude of the medium afterhyperpolarization evoked by a 1 s 20 Hz spike train (by 0.8 ± 0.3 mV, P < 0.001) in supraoptic nucleus neurones; this inhibition was not prevented by 3 mm CsCl (0.8 ± 0.1 mV decrease, P < 0.01) to block the afterdepolarization (which overlaps temporally with the medium afterhyperpolarization). In the presence of apamin to block the medium afterhyperpolarization, CPT did not alter afterdepolarization amplitude. Taken together, these data show that endogenous adenosine enhances medium afterhyperpolarization amplitude to contribute to spike frequency adaptation in phasic supraoptic nucleus neurones.
Vasopressin neurones are principally located within the hypothalamic paraventricular nucleus and supraoptic nucleus and project to the posterior pituitary gland where they release vasopressin (the antidiuretic hormone) into the general circulation to maintain plasma osmolality and blood pressure by promoting antidiuresis and vasoconstriction (Holmes et al. 2001). Vasopressin neurone axon terminals do not sustain intrinsic repetitive firing (Bourque, 1990) and so vasopressin secretion is largely determined by action potential (spike) discharge initiated at the soma. In vivo, vasopressin neurones display a range of spike discharge patterns under basal conditions: some are silent, some are irregular, some display rhythmic ‘phasic’ activity and some are continuously active (Brown et al. 1998, 2007).
Phasic activity is highly efficient for secretion of vasopressin into the circulation (Leng et al. 1999) and is characterized by spike discharge in periods of firing (bursts) that each last tens of seconds, separated by silent periods (interburst intervals) of similar duration (Brown, 2004; Brown & Bourque, 2006). Phasic bursts are sustained by plateau potentials that are generated by temporal summation of non-synaptic afterdepolarizations (ADPs) that follow each spike (Andrew & Dudek, 1983; Armstrong et al. 1994; Li et al. 1995; Li & Hatton, 1997; Ghamari-Langroudi & Bourque, 1998; Brown & Bourque, 2004). Phasic activity is not simply an on–off pattern; spike discharge within bursts undergoes spike frequency adaptation, whereby there is a progressive decrease in firing rate over the first few seconds of bursts (to a steady state rate that is maintained until burst termination) due to activation of a medium afterhyperpolarization (mAHP) (Kirkpatrick & Bourque, 1996).
Vasopressin neurones contain large amounts of neuropeptide in their soma and dendrites from which vasopressin is secreted by exocytosis (Pow & Morris, 1989). This somato-dendritic release is important for modulating phasic activity; vasopressin administration excites irregular or weakly phasic vasopressin neurones (Gouzenes et al. 1998) but inhibits vasopressin neurones displaying robust phasic spike discharge or continuous spike discharge (Ludwig & Leng, 1997; Gouzenes et al. 1998). Hence, somato-dendritic vasopressin might function as a ‘population feedback signal’ to equalize the activity level amongst vasopressin neurones (Moos et al. 1998).
Vasopressin neurones also synthesize and secrete several other factors that have been implicated in modulation of phasic spike discharge in vasopressin neurones (Brown et al. 2008), including the κ-opioid peptide dynorphin (Watson et al. 1982); bursts are terminated by autocrine feedback inhibition of ADPs by somatodendritic dynorphin (Brown & Bourque, 2004; Brown et al. 2006), as well as by activation of a slow afterhyperpolarization (sAHP) (Greffrath et al. 1998; Ghamari-Langroudi & Bourque, 2004), each of which reduce the probability of spontaneous spikes firing as bursts progress (Brown et al. 2006).
Vasopressin neurosecretory granules also contain ATP (Poisner & Douglas, 1968), which is presumably co-released upon somato-dendritic exocytosis. Although ATP induces vasopressin secretion from hypothalamic explants, these effects are truncated by rapid catabolism to adenosine in the extracellular space (Kapoor & Sladek, 2000); this adenosine also influences the spike discharge of vasopressin neurones; in anaesthetized rats, the major effects of endogenous adenosine on spike discharge are to enhance spike frequency adaptation and to reduce burst duration in phasic vasopressin neurones (Bull et al. 2006), suggesting that these effects of endogenous adenosine are activity dependent. A2A receptors are also expressed by supraoptic nucleus neurones (and glia) and activation of these receptors induces depolarization of supraoptic neurones to increase spike discharge (Ponzio et al. 2006). Nevertheless, the major functional role of endogenous adenosine appears to be inhibition (via A1 receptor activation) because inhibition of adenosine uptake reduces spike discharge (Ponzio & Hatton, 2005).
As the major in vivo effects of endogenous adenosine (Bull et al. 2006) are similar to those induced by activation of the mAHP in vitro (Kirkpatrick & Bourque, 1996), we used intracellular sharp electrode recordings from supraoptic nucleus neurones in hypothalamic explants to test the hypothesis that endogenous adenosine increases mAHP amplitude to enhance spike frequency adaptation during spontaneous phasic bursts in supraoptic nucleus neurones.
Methods
Electrophysiology
Conscious female Sprague–Dawley rats (∼200–250 g) were restrained in a soft plastic cone (5–10 s), decapitated and the brains rapidly removed according to a procedure approved by the University of Otago Animal Ethics Committee. A block of tissue 8 mm × 8 mm × 2 mm containing the basal hypothalamus was excised using razor blades and pinned, ventral surface up, to the silicone elastomer base of a superfusion chamber. Within 2–3 min, the excised hypothalamic explant was superfused (at 0.5–1.0 ml min−1 at 32–33°C) with carbogenated (95% oxygen; 5% carbon dioxide) artificial CSF (aCSF; see below) delivered via tubing placed over the medial tuberal region. The arachnoid membrane overlying the supraoptic nucleus was removed using fine forceps, and a tissue paper wick was placed at the rostral tip of the explant to facilitate aCSF drainage. The aCSF (pH 7.4; 295 ± 3 mosmol kg−1) was comprised of (in mm): 120 NaCl, 3 KCl, 1.2 MgCl2, 26 NaHCO2, 2.5 CaCl2 and 10 glucose (Sigma).
Intracellular recordings were made using sharp micropipettes prepared from glass capillaries (1.5 mm O.D. 0.86 mm I.D) pulled on a P-97 Flaming-Brown puller (Sutter Instruments, Novato, CA, USA). Micropipettes were filled with 2 m potassium acetate to yield DC resistances of 70–160 MΩ to a Ag–AgCl wire electrode immersed in aCSF. Voltage recordings were obtained using an Axoclamp 2B amplifier (Molecular Devices, Foster City, CA, USA) in continuous current-clamp (‘bridge’) mode. Digitized signals (5 kHz; DigiData 1320 Interface, Molecular Devices) were stored on a personal computer running pClamp 9.2 (Molecular Devices) and analysed offline.
Recordings were made from supraoptic nucleus neurones impaled with sharp electrodes in superfused hypothalamic explants. The cells had resting membrane potentials more negative than –50 mV, input resistances of > 150 MΩ, and spike amplitudes of > 60 mV when measured from baseline. Each cell displayed frequency-dependent spike broadening and transient outward rectification when depolarized from initial membrane potentials more negative than −75 mV (characteristics specific to supraoptic nucleus neurones (Renaud & Bourque, 1991)). Vasopressin neurones were differentiated from oxytocin neurones by applying increasing hyperpolarizing pulses of 1.5 s duration from a membrane potential of about −50 mV; using this protocol most oxytocin but no vasopressin neurones express a sustained outward rectification and a rebound depolarization (Stern & Armstrong, 1995); none of the neurones reported here expressed a sustained outward rectification and so it is likely that most, if not all, of the neurones tested contained vasopressin.
The adenosine A1 receptor antagonists, 8-cyclopentyl-1,3-dimethylxanthine (CPT; Sigma) and 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; Sigma), were prepared (10 mm) in deionized water, stored frozen until the day of use and bath applied after dilution to 10 μm in aCSF. Apamin (1 mm; Sigma) was prepared in deionized water and bath applied after dilution to 1 μm in aCSF. CsCl (3 m) was prepared in deionized water and bath applied after dilution to 3 mm in aCSF.
Spontaneous phasic activity data analysis
Spikes were identified during spontaneous phasic bursts using the threshold search function on Clampfit (pClamp 9.2) and bursts identified using the ‘bursts analysis’ function with the minimum events set at five spikes.
Intraburst firing rate was measured in 1 s bins. Spike frequency adaptation was calculated as the change of firing rate from the maximum intraburst firing rate to the mean steady-state firing rate between 10 and 15 s after the maximum (expressed as a percentage of maximum intraburst firing rate).
The probability of spike firing (hazard) was calculated from the interspike interval distribution of individual cells using the formula:
where h[i−1,i] is the hazard at interval i, n[i−1,i] is the number of spikes in interval i, n[0,i−1] is the total number of spikes preceding the current interval and N is the total number of spikes in all intervals. This gives the inferred probability (as a decimal) of a cell firing a subsequent spike in any interval after a spike (at time 0), given that another spike has not occurred earlier.
Statistics
All statistical comparisons were completed using SigmaStat software (SPSS Science, Chicago, IL, USA). Non-parametric analyses were used where data failed normality and/or equal variance tests. Where analysis of variance (ANOVA) was used, post hoc Students–Newman–Keuls tests were used only when the F ratio of the ANOVA was significant. P≤ 0.05 was considered statistically significant.
Results
Endogenous adenosine decreases intraburst firing rate, burst duration and increases spike frequency adaptation during spontaneous phasic activity
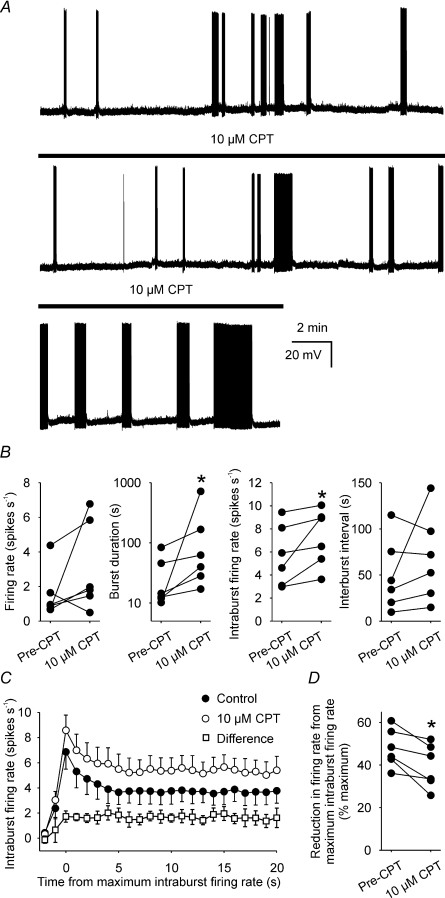
A1 adenosine receptor antagonism with CPT increases intraburst firing rate and burst duration, and decreases spike frequency adaptation of spontaneously phasic supraoptic nucleus neurones in vivo (Bull et al. 2006). To determine whether these effects are also evident in vitro, we recorded the activity of six supraoptic nucleus neurones displaying spontaneous phasic bursts in hypothalamic explants (e.g. Fig. 1A).
Figure 1. A1 receptor antagonism increases phasic firing in vitro.
A, current-clamp recording of the membrane potential of a supraoptic nucleus neurone during spontaneous phasic activity, before and during superfusion of 10 μm CPT (bar, lower two panels). Note that CPT increased the duration of spontaneous phasic bursts, typical of all six neurones tested. B, the firing rates, burst durations, intraburst firing rates and interburst intervals of six spontaneously phasic neurones before (Pre-CPT) and during superfusion of 10 μm CPT. C, the mean intraburst firing rate of the 6 neurones in B (averaged in 1 s bins over the course of bursts and aligned to peak intraburst firing rate) before (Pre-CPT) and during superfusion of 10 μm CPT. Note that the CPT-induced increase in firing rate (Difference) developed over the first 3 s of bursts and remained relatively constant thereafter. D, reduction in intraburst firing rate from maximum intraburst firing rate (spike frequency adaptation) of the 6 neurones in B. *P < 0.05 Wilcoxon signed rank test.
Under basal conditions, the mean (±s.e.m.) firing rate of these neurones was 1.5 ± 0.6 spikes s−1 (measured over ≥ 10 min), and this was not altered by superfusion of 10 μm CPT (P= 0.16, Wilcoxon signed rank test). Nevertheless, 10 μm CPT consistently increased the intraburst firing rate from 5.7 ± 1.1 spikes s−1 to 7.7 ± 0.8 spikes s−1 (P= 0.03) and the burst duration from 29.8 ± 12.2 s to 171.7 ± 111.1 s (P= 0.03), but did not change interburst interval (49.8 ± 16.0 s and 68.5 ± 19.2 s, before and during CPT administration, respectively; P= 0.47; Fig. 1B).
Detailed analysis of the temporal evolution of activity over the course of bursts by calculation of the firing rate in 1 s bins from the onset of burst firing revealed that the CPT-induced increase in firing rate was not evident at the onset of burst firing but increased progressively over the first 3 s of bursts and persisted thereafter (Fig. 1C), indicating that endogenous adenosine inhibition of spike firing is absent at burst onset but rapidly reaches a maximum within the first few seconds of spike firing during spontaneous bursts.
Spike frequency adaptation (calculated by the change of firing rate from the maximum intraburst firing rate to the mean steady-state firing rate between 10 and 15 s after the maximum) was reduced from 48 ± 4% change in firing rate to 39 ± 4% change (P= 0.03, Wilcoxon signed rank test; Fig. 1D).
Endogenous adenosine decreases post-spike excitability during spontaneous phasic bursts in vitro
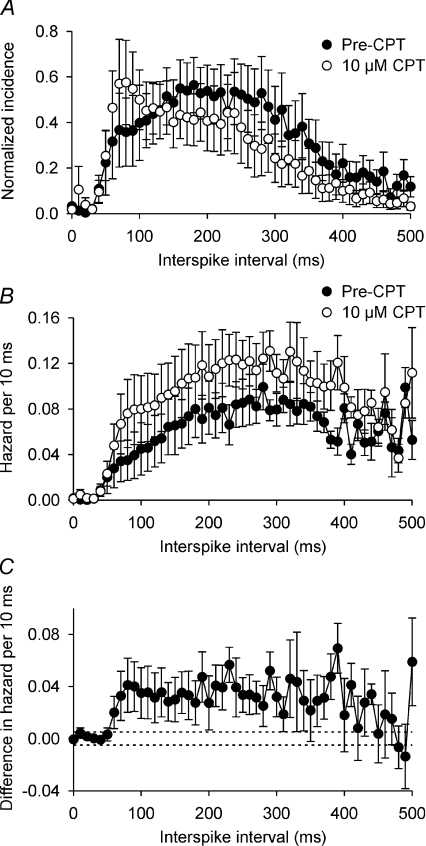
CPT increases post-spike excitability during phasic bursts in vivo (Bull et al. 2006), and this has been inferred to reflect changes in the post-spike potentials that underpin the plateau potential (Brown et al. 2004, 2006; Sabatier et al. 2004). Accordingly, we calculated the post-spike excitability of supraoptic nucleus neurones during spontaneous phasic bursts in vitro using plots of the hazard function (Brown & Leng, 2000; Sabatier et al. 2004; Brown et al. 2006), which reflect the changes in excitability of neurones after spontaneous spikes by displaying the inferred probability of spike firing with time after the preceding spike, calculated from the interspike interval distribution (Fig. 2A and B). CPT increased the post-spike excitability from ∼50 to 400 ms after each spike (Fig. 2B and C), indicating that endogenous adenosine decreases the probability of spike firing in the period 50–400 ms after each spike during spontaneous phasic bursts.
Figure 2. A1 receptor antagonism increases post-spike excitability during phasic bursts in vitro.
A, consensus mean interspike interval distribution (±s.e.m.) of the 6 supraoptic nucleus neurones in Fig. 1 before (•) and during (○) superfusion of 10 μm CPT. Distributions were normalized to the height of the mode for each neurone and averaged across the group. B, the mean hazard functions calculated from the interspike interval distributions used to generate the data shown in A, showing that 10 μm CPT (○) increased the probability of spike firing (hazard) after each spike. C, subtraction plot of the difference in hazard before and during 10 μm CPT superfusion, showing that the CPT-induced increase in the probability of spike firing was most prominent in the period ∼50–400 ms after each spike. The dashed lines show the 95% confidence interval of the difference in hazard plotted against the x-axis to illustrate the separation of the CPT-sensitive hazard from zero.
Endogenous adenosine increases mAHP amplitude in supraoptic nucleus neurones
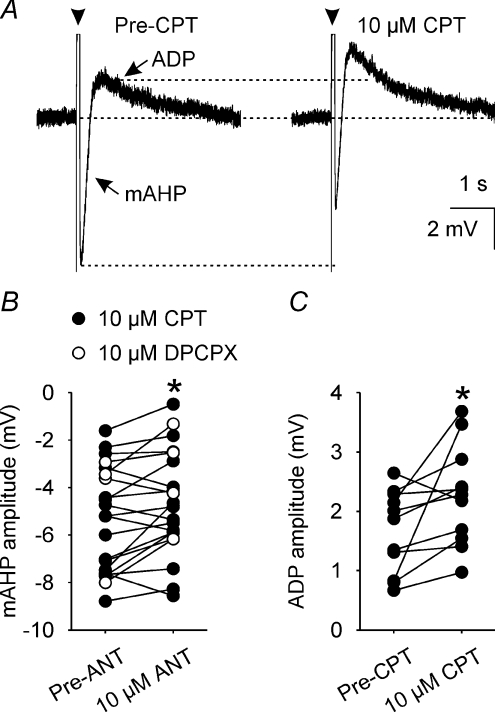
Activation of the mAHP causes spike frequency adaptation (Kirkpatrick & Bourque, 1996) and because we observed That CPT reduces spike frequency adaptation during spontaneous phasic bursts, we determined the effects of 10 μm CPT (n= 18) or 10 μm DPCPX (n= 4) (to confirm that the effects of CPT were mediated by antagonism of A1 adenosine receptors) on the amplitude of the mAHP elicited in supraoptic nucleus neurones every 20 s (using trains of 5–7 spikes evoked by an 80 ms depolarizing current pulse, with the number of spikes kept constant for each neurone; Fig. 3A). Two-way repeated measures ANOVA showed that the effects of CPT and DPCPX on mAHP amplitude were indistinguishable (P= 0.28). Under these conditions, 10 μm CPT/DPCPX decreased mAHP amplitude by 0.7 ± 0.2 mV (P= 0.02, Student–Newman–Keuls test, n= 22, Fig. 3B).
Figure 3. A1 receptor antagonism reduces mAHP amplitude.
A, mAHPs and ADPs (averages of 5 traces) that each follow a 5-spike train (arrowheads, spikes truncated) evoked by a 80 ms depolarizing pulse (+200 pA) before (Pre-CPT, left) and during superfusion of 10 μm CPT (right). B, mAHP amplitude in supraoptic nucleus neurones (n= 22) before (Pre-ANT, left) and during (10 μm ANT, right) superfusion of 10 μm of the A1 receptors antagonists CPT (•, n= 18) or DPCPX (○, n= 4), showing that A1 adenosine receptor antagonism reduces mAHP amplitude. *P < 0.05, two-way repeated measures ANOVA followed by Student–Newman–Keuls test. C, ADP amplitude in supraoptic nucleus neurones (n= 11) before (Pre-CPT, left) and during (right) superfusion of 10 μm CPT, showing that CPT increases ADP amplitude concomitant with its reduction of mAHP amplitude (note that none of the cells recorded in the presence of DPCPX displayed a measurable ADP). *P < 0.05, paired t test.
CPT/DPCPX did not alter the time course of the mAHP. Using pClamp, a standard exponential was fitted to the membrane potential for 1 s after the nadir of the mAHP (the correlation coefficient for all fitted curves was ≥ 0.99); the time constant for the decay of the mAHP was 166.5 ± 15.1 ms and 182.0 ± 32.2 ms, before and during 10 μm CPT/DPCPX administration, respectively (P= 0.44, paired t test, n= 22).
Concomitant with a decreased mAHP amplitude, CPT also increased ADP amplitude in neurones in which this potential was evident (none of the cells tested with DPCPX exhibited an ADP) from 1.7 ± 0.2 mV to 2.3 ± 0.3 mV (P= 0.04, paired t test, n= 11, Fig. 3C).
Endogenous adenosine enhancement of the mAHP is activity dependent
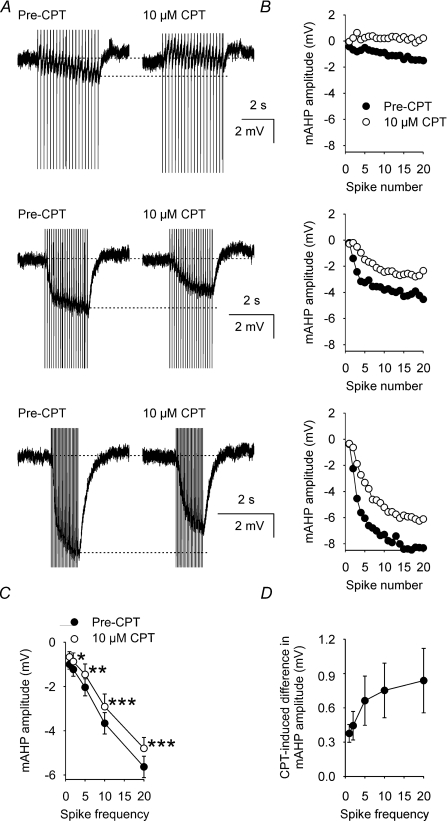
To determine whether the CPT-induced inhibition of mAHP amplitude was activity dependent, we evoked mAHPs using trains of 20 spikes at 1, 2, 5, 10 and 20 Hz in identified supraoptic nucleus neurones (e.g. Fig. 4A and B). CPT (10 μm) decreased the amplitude of the mAHP measured from supraoptic nucleus neurones after 2, 5, 10 and 20 Hz trains (two-way repeated measures ANOVA followed by Student–Newman–Keuls tests, n= 14, Fig. 4C) by 0.4 ± 0.1 mV (P= 0.03), 0.7 ± 0.2 mV (P= 0.003), 0.8 ± 0.2 mV (P= 0.001) and 0.8 ± 0.3 mV (P < 0.001), respectively, but not after a 1 Hz train (0.4 ± 0.1 mV decrease, P= 0.06). Indeed, there was a significant correlation between the frequency of stimulation and the CPT-induced decrease in mAHP amplitude (P= 0.04, Pearson product moment correlation, Fig. 4D), indicating that endogenous adenosine causes an activity-dependent increase in mAHP amplitude in these neurones.
Figure 4. A1 receptor antagonist inhibition of mAHP amplitude is activity dependent.
A, mAHPs (averages of 5 traces) that follow 20 spike trains (evoked by a corresponding number of 5 ms, +500 pA depolarizing pulses) at 5 Hz (top), 10 Hz (middle) and 20 Hz (bottom) in a supraoptic nucleus neurone before (Pre-CPT, left) and during (right) superfusion of 10 μm CPT, showing that CPT inhibition of the mAHP increases as the frequency of stimulation is increased. B, mAHP amplitude measured after each evoked spike in A during 5 Hz (top), 10 Hz (middle) and 20 Hz (bottom) stimulation. C, mean mAHP amplitude (n= 14) after the final evoked spike of 20 spike trains delivered at 1, 2, 5, 10 or 20 Hz before (•) and during (○) superfusion of 10 μm CPT. Two-way repeated measures ANOVA revealed significant CPT effects (P= 0.002), frequency effects (P < 0.001) and a significant interaction between the effects of CPT and frequency (P < 0.001). *P < 0.05, **P < 0.01 and ***P < 0.001, Student–Newman–Keuls tests. D, subtraction plot of the mean CPT-induced difference in mAHP amplitude, showing a progressive increase in the effect of CPT as spike frequency increases (Pearson product moment correlation coefficient = 0.90, P= 0.04).
Blockade of the ADP does not alter endogenous adenosine enhancement of the mAHP
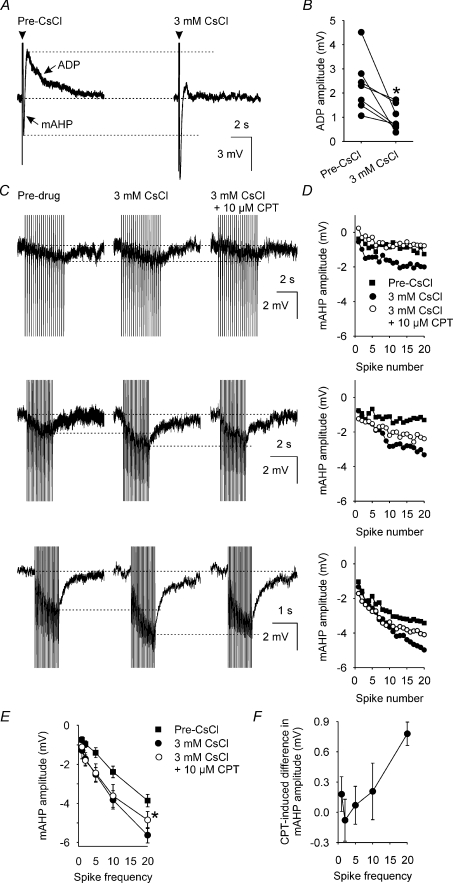
The mAHP and ADP overlap temporally (Greffrath et al. 1998) and so it is possible that the observed CPT-induced decrease in mAHP amplitude might result from an offset of the mAHP by an increase in the amplitude of the concomitant ADP. Consequently, we used 3 mm CsCl to block the ADP (Ghamari-Langroudi & Bourque, 1998) to determine whether blocking the ADP would prevent CPT actions on the mAHP in supraoptic nucleus neurones.
In seven supraoptic nucleus neurones in which a clear ADP was present (> 0.5 mV), 3 mm CsCl reduced ADP amplitude from 2.3 ± 0.42 mV to 1.0 ± 0.2 mV (P= 0.02, paired t test; Fig. 5A and B). In eight neurones, the mAHP amplitude after trains of 20 spikes at 1, 2, 5, 10 and 20 Hz was increased by 3 mm CsCl by 0.6 ± 0.1 mV (P= 0.02), 0.7 ± 0.1 mV (P= 0.01), 0.8 ± 0.2 mV (P= 0.003), 1.1 ± 0.2 mV (P < 0.001) and 1.4 ± 0.3 mV (P < 0.001), respectively (Fig. 5C and D).
Figure 5. Activity-dependent A1 receptor antagonist inhibition of mAHP amplitude is independent of ADP modulation.
A, mAHPs and ADPs (averages of 5 traces) that each follow a 5-spike train (arrowheads, spikes truncated) evoked by a 80 ms depolarizing pulse (+200 pA) before (Pre-CsCl, left) and during superfusion of 3 mm CsCl (right). B, ADP amplitude in supraoptic nucleus neurones (n= 7) before (Pre-CsCl, left) and during (right) superfusion of 3 mm CsCl, showing that CsCl decreases ADP amplitude. *P < 0.05, paired t test. C, mAHPs (averages of 5 traces) that follow 20-spike trains (evoked by a corresponding number of 5 ms, +500 pA depolarizing pulses) at 5 Hz (top), 10 Hz (middle) and 20 Hz (bottom) in a supraoptic nucleus neurone before (Pre-drug, left), during superfusion of 3 mm CsCl alone (middle) and superfusion of 10 μm CPT in the continued presence of 3 mm CsCl (right), showing that Cs inhibition of the ADP increases mAHP amplitude. D, mAHP amplitude measured after each evoked spike in A during 5 Hz (top), 10 Hz (middle) and 20 Hz (bottom) stimulation. E, mean mAHP amplitude (n= 4) after the final evoked spike of 20-spike trains delivered at 1, 2, 5, 10 or 20 Hz before (▪) and during superfusion of 3 mm CsCl (•) and of 10 μm CPT in the presence of 3 mm CsCl (○). Two-way repeated measures ANOVA on the mAHP amplitudes before and during superfusion of 10 μm CPT revealed a significant effect of frequency (P < 0.001) and a significant interaction between the effects of CPT and frequency (P= 0.03) in the presence of 3 mm CsCl. F, subtraction plot of the mean CPT-induced difference in mAHP amplitude recorded in the presence of 3 mm CsCl (n= 4), showing a progressive increase in the effect of CPT as spike frequency increases (Pearson product moment correlation coefficient = 0.91, P= 0.03).
For four of these neurones, recordings were maintained for long enough to determine the effects of 10 μm CPT on mAHP amplitude after trains of 20 spikes at 1, 2, 5, 10 and 20 Hz in 3 mm CsCl. In 3 mm CsCl, 10 μm CPT reduced mAHP amplitude only after the 20 Hz train (by 0.8 ± 0.1 mV, P= 0.003, two-way repeated measures ANOVA followed by Student–Newman–Keuls tests; Fig. 5E). Nevertheless, in 3 mm CsCl, there was a significant correlation between the frequency of stimulation and the CPT-induced decrease in mAHP amplitude (P= 0.03, Pearson product moment correlation, Fig. 5F), indicating that endogenous adenosine causes an activity-dependent increase in mAHP amplitude by an action on the mAHP itself rather than on the concomitant ADP.
Endogenous adenosine does not alter ADP amplitude
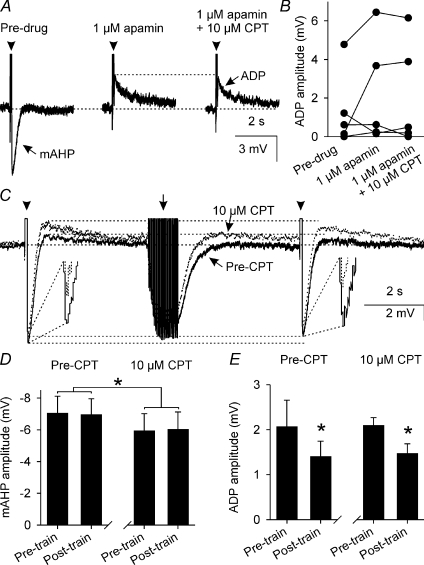
In five neurones, we used 1 μm apamin (which we estimated to be ∼5–10 times greater than the IC50 for apamin against the mAHP in this preparation (Kirkpatrick & Bourque, 1996)) to reduce mAHP amplitude (from −4.0 ± 0.9 mV to −0.8 ± 0.4 mV, P= 0.03, one-way repeated measures ANOVA followed by Student–Newman–Keuls test) and tested the effects of 10 μm CPT on ADP amplitude (Fig. 6A). In the continued presence of 1 μm apamin, 10 μm CPT did not further alter mAHP amplitude (P= 0.70) and did not affect ADP amplitude (P= 0.40, Fig. 6B), confirming that endogenous adenosine does not increase mAHP amplitude via actions on the ADP.
Figure 6. A1 receptor antagonism does not affect the ADP.
A, mAHPs and ADPs (averages of 5 traces) that each follow a 5-spike train (arrowheads, spikes truncated) evoked by a 80 ms depolarizing pulse (+170 pA) before (Pre-drug, left) and during superfusion of 1 μm apamin (middle) and 10 μm CPT in the continued presence of 1 μm apamin (right). B, ADP amplitude in supraoptic nucleus neurones (n= 5) before (Pre-drug, left) and during superfusion of 1 μm apamin (middle) and 10 μm CPT in the continued presence of 1 μm apamin (right), showing that A1 adenosine receptor antagonism does not affect ADP amplitude when the mAHP is reduced by apamin. C, mAHPs and ADPs (average of 5 traces) that each follow a 5-spike train (arrowheads) evoked 9 s apart by a 80-ms depolarizing pulse (+200 pA) before (Pre-CPT, black) and during (dotted line) superfusion of 10 μm CPT. A conditioning train (arrow) of 25 spikes (at 25 Hz) was evoked by a corresponding number of 5 ms, +500 pA DC pulses. D, bar graphs showing mean mAHP amplitude (n= 8) before (Pre-train) and after (Post-train) the 25-spike conditioning train before (Pre-CPT, left) and during superfusion of 10 μm CPT (right). Two-way repeated measures ANOVA showed that the conditioning train had no effect on mAHP amplitude (P= 0.97) but that CPT reduced mAHP amplitude (P= 0.05), independent of the conditioning train (P= 0.51). *P < 0.05, two-way repeated measures ANOVA. E, bar graphs showing mean ADP amplitude (n= 4) before (Pre-train) and after (Post-train) the 25 spike conditioning train before (Pre-CPT, left) and during superfusion of 10 μm CPT (right), showing that activity-dependent inhibition of the ADP was not altered by 10 μm CPT. A separate two-way repeated measured ANOVA showed no effect of CPT on ADP amplitude (P= 0.92) but revealed a significant inhibition by the conditioning train (P= 0.01) that was independent of CPT treatment (P= 0.92). *P < 0.05, Student–Newman–Keuls test.
We have previously demonstrated that endogenous activation of κ-opioid receptors causes activity-dependent inhibition of the ADP in supraoptic nucleus neurones (Brown & Bourque, 2004). To determine whether endogenous adenosine modulates activity-dependent inhibition of ADP amplitude, we evoked pairs of ADPs 9 s apart every 60 s and interposed a 25-spike conditioning train between the pairs of ADPs (Fig. 6C); this protocol elicits activity-dependent inhibition of the ADP, without altering mAHP amplitude (Brown & Bourque, 2004). CPT (10 μm) reduced mAHP amplitude (Fig. 6D) but did not alter activity-dependent ADP inhibition (Fig. 6E), indicating that endogenous adenosine does not contribute to activity-dependent inhibition of ADP amplitude evoked in this protocol.
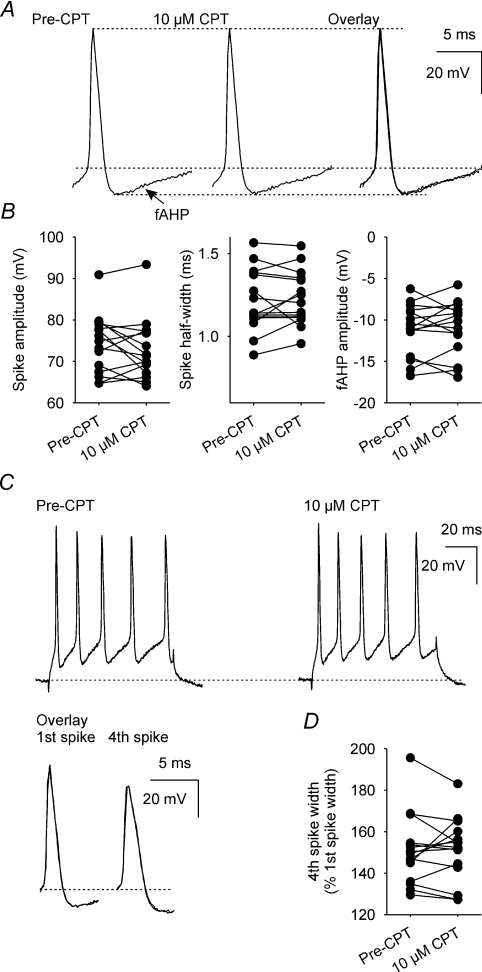
Endogenous adenosine does not alter spike parameters
To determine whether CPT affects spikes, we measured spike amplitude and half-width, and fast AHP (fAHP) amplitude (Fig. 7A), as well as activity-dependent spike broadening (Fig. 7C) in data used to generate the mAHP and ADP (all n= 15); none of these parameters was altered by superfusion of 10 μm CPT (Fig. 7B and D).
Figure 7. A1 receptor antagonism does not affect spike amplitude or half-width, fAHP amplitude, or activity-dependent spike broadening.
A, individual spikes, with their associated fAHP, recorded before (Pre-CPT) and during superfusion of 10 μm CPT. B, spike amplitude, spike half-width and fAHP amplitude before and during superfusion of 10 μm CPT (all n= 15); there was no significant effect of CPT on each of these three parameters (P= 0.26, P= 0.65 and P= 0.72, respectively, paired t tests). C, individual 5-spike trains (evoked by a 80 ms depolarizing current injection) before (Pre-CPT) and during superfusion of 10 μm CPT. The overlay shows the first and fourth spikes of the evoked trains before (black) and during superfusion of 10 μm CPT (dotted line). D, spike half-width of the fourth spike in the evoked train, relative to first spike width, before and during superfusion of 10 μm CPT (n= 14); CPT did not alter spike broadening (P= 0.82, paired t test).
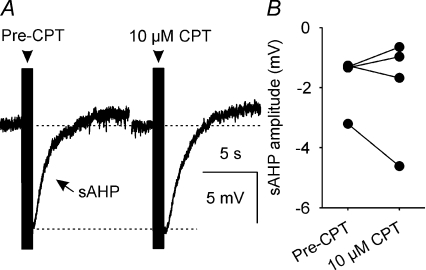
Endogenous adenosine does not alter the slow AHP
To determine whether CPT affects the slow AHP (sAHP), we measured the amplitude of sAHPs that followed a 50-spike train (Fig. 8A); superfusion of 10 μm CPT did not alter peak sAHP amplitude (−0.4 ± 0.7 mV change; n= 4, P= 0.86, Wilcoxon signed rank test) or the amplitude of the sAHP measured between 1 and 2 s after the end of the spike train (−0.2 ± 0.5 mV change; n= 4, P= 0.86, Wilcoxon signed rank test; Fig. 8B), when potential confounding effects of the much faster mAHP would not be observed.
Figure 8. A1 receptor antagonism does not affect sAHP amplitude.
A, sAHPs (average of 5 traces) that each follow a 50-spike train (arrowhead) evoked by a corresponding number of 5 ms, +500 pA DC pulses. B, sAHP amplitude measured between 1 and 2 s after the 50-spike train before and during superfusion of 10 μm CPT (n= 4); CPT did not alter sAHP amplitude (P= 0.86, Wilcoxon signed rank test).
Discussion
Here, we used intracellular sharp electrode recordings from supraoptic nucleus neurones in hypothalamic explants to show that antagonism of A1 receptors reduced the amplitude of evoked mAHPs in these neurones and decreased spike frequency adaptation during spontaneous phasic bursts. Endogenous adenosine enhancement of the mAHP was not mediated by actions on the ADP because the amplitude of the ADP was not affected by CPT when the mAHP was reduced by apamin. In the presence of CsCl to block the ADP, CPT appeared marginally less effective at inhibiting the mAHP after lower frequency spike trains. CsCl also blocks the hyperpolarization-activated inward current (IH) and the leakage K+ current in supraoptic nucleus neurones (Ghamari-Langroudi & Bourque, 2001), which might account for the reduced effectiveness of CPT in the presence of CsCl.
Antagonism of A1 receptors also increased the duration of spontaneous phasic bursts and this was associated with increased post-spike excitability during bursts. Taken together, our results indicate that endogenous adenosine increases mAHP amplitude to enhance spike frequency adaptation and reduce the probability of spike firing during spontaneous phasic bursts in vasopressin neurones.
Generation of endogenous adenosine from somato-dendritic ATP release
As the effects of CPT on both spontaneous phasic spike discharge and the mAHP were activity dependent, the simplest explanation for the effects of CPT is that endogenous adenosine accumulates in an activity-dependent manner during burst firing. ATP and adenosine triphosphatase are contained within vasopressin neurosecretory vesicles (Poisner & Douglas, 1968) and so will be released with vasopressin into the supraoptic nucleus during somato-dendritic exocytosis of vasopressin (Ludwig, 1998; Ludwig & Leng, 2006). Thus the main source of extracellular adenosine might be the vasopressin neurones themselves, via a combination of adenosine secretion and the rapid catabolism of exocytosed ATP (Song & Sladek, 2005).
Somato-dendritic release of vasopressin is activity dependent (Brown et al. 2004) and capacitance measurements from isolated somata suggest that exocytosis can be evoked by a single spike (de Kock et al. 2003). Consistent with this observation, enhancement of the mAHP by endogenous adenosine was revealed by CPT after only the second spike of a 20-spike train in our current experiments (e.g. Fig. 4A and B), indicating that a single spike might be adequate to generate sufficient adenosine to enhance mAHP amplitude. However, in the presence of CsCl to block the ADP, greater numbers of spikes at higher frequencies were required to reveal the effect of CPT on the mAHP. As CsCl also blocks IH and the leakage K+ current (Ghamari-Langroudi & Bourque, 2001), it is possible that the effects of CPT at lower frequencies might result from interactions with these other targets of CsCl. Calculation of the rate of somato-dendritic secretion required to maintain an effective concentration of vasopressin within the hypothalamus in vivo indicates that (on average) each vasopressin neurone is required to release less than one vesicle every second under basal conditions (Brown et al. 2007), suggesting that high firing rates might be required to provoke an increase in somato-dendritic exocytosis. We have previously suggested that endogenous adenosine might be constitutively present in the extracellular space, but that its effects might not be exposed until after activity-dependent activation of the mAHP (Bull et al. 2006). The molar ratio of vasopressin to ATP is 20: 1 in vasopressin neurosecretory vesicles (Poisner & Douglas, 1968), which might be able to constitutively maintain an effective concentration of adenosine within the extracellular space of the supraoptic nucleus that requires spike discharge for its effects to be exposed. Alternatively, the more rapid effects of endogenous adenosine might result from kiss-and-run exocytotic events because these would not be expected to release vasopressin and dynorphin from the dense core vesicles but might release ATP, which could then be converted to adenosine. However, the adenosine triphosphatase within the neurosecretory vesicles would presumably require full exocytosis for release and so such a mechanism would require ambient adenosine triphosphatase in the extracellular environment.
Vasopressin neurones are not the only potential source of adenosine within the supraoptic nucleus. Noradrenaline (norepinephrine) triggers release of glial ATP to increase postsynaptic efficacy (Gordon et al. 2005) and dendritically released vasopressin facilitates noradrenaline release into the supraoptic nucleus (Ludwig et al. 2000). Hence, it is possible that somato-dendritic vasopressin release also increases the concentration of noradrenaline within the supraoptic nucleus, which in turn releases glial ATP that upon conversion to adenosine then activates A1 receptors on the vasopressin neurones to enhance mAHP amplitude. The effects of endogenous vasopressin on vasopressin neurones are constitutively present in vivo (Brown et al. 2004) and so neighbouring glia are likely to be continuously bathed in vasopressin. Hence, adenosine generated from glia would be expected to be constitutively present in the extracellular space, again requiring spike discharge for its effects to be exposed.
Implications of endogenous A1 receptor activation for spontaneous phasic activity
Phasic bursting in vasopressin neurones involves both extrinsic and intrinsic processes. Bursts are initiated by extrinsic inputs to vasopressin neurones, when summation of synaptic potentials causes membrane potential to cross the threshold for spike generation. Once firing is initiated, both extrinsic and intrinsic mechanisms are required to maintain further firing. When consecutive spikes occur close enough together, their associated non-synaptic ADPs summate to generate a persistent plateau potential that sustains further firing by bringing the membrane potential closer to threshold for spike initiation (Andrew & Dudek, 1983), which increases the probability that on-going synaptic potentials will reach threshold and generate further spikes (Brown & Bourque, 2006; Brown et al. 2006). During bursts, the fAHP increases spike discharge via activation of IH (Ghamari-Langroudi & Bourque, 2000), the mAHP induces spike frequency adaptation over the course of bursts (Kirkpatrick & Bourque, 1996; Greffrath et al. 2004) and the sAHP gradually decreases plateau potential amplitude (Greffrath et al. 1998; Ghamari-Langroudi & Bourque, 2004). In addition, activity-dependent inhibition of ADPs (Brown & Bourque, 2004) reduces the probability of spontaneous spikes firing as bursts progress to increase the probability of burst termination (Brown et al. 2006). Thus, in a spontaneous burst, the firing rate settles to a stable level that reflects a dynamic equilibrium between activity-dependent depolarizing mechanisms and hyperpolarizing influences.
Both the synaptic and non-synaptic mechanisms that generate phasic firing are modified by substances released by somata and dendrites of vasopressin neurones. Autocrine feedback by somato-dendritic vasopressin reduces vasopressin neurone firing rate during bursts (Ludwig & Leng, 1997); this vasopressin inhibition is evident from the onset of bursts (Brown et al. 2004), indicating that endogenous vasopressin constitutively inhibits phasic vasopressin neurones, probably by reducing the amplitude of EPSCs (Kombian et al. 2000) and by increasing IPSC frequency (Hermes et al. 2000). Nitric oxide is also generated by vasopressin neurones and inhibits firing (Liu et al. 1997; Srisawat et al. 2000), probably by increasing IPSC frequency and amplitude (Stern & Ludwig, 2001). During phasic bursts, ADPs are progressively inhibited by somato-dendritic dynorphin over tens of seconds to increase the likelihood of burst termination (Brown & Bourque, 2004; Brown et al. 2004, 2006). Here, we have shown that the autocrine somato-dendritic processes that generate phasic spike discharge in vasopressin neurones also include adenosine enhancement of the mAHP to increase spike frequency adaptation over the first few seconds of bursts. Hence, the activity-dependent inhibition of phasic firing via A1 receptors reaches a maximum over a much shorter time-course (several seconds) than activity-dependent inhibition by somato-dendritic dynorphin (several tens of seconds) (Brown et al. 2008).
The increase in burst duration induced by CPT is in marked contrast to the effects of complete blockade of the mAHP with apamin, which greatly increases firing rate, eliminates spike frequency adaptation and shortens bursts in vitro (Kirkpatrick & Bourque, 1996); the greatly increased firing rate in the presence of apamin might induce over-activation of other activity-dependent mechanisms, such as activation of the sAHP (Greffrath et al. 1998; Ghamari-Langroudi & Bourque, 2004) and inhibition of the ADP (Brown & Bourque, 2004) to shorten bursts. The more subtle inhibition of the mAHP by CPT (to cause a more modest increase in firing rate than apamin) might enhance the plateau potential, increasing the degree of ADP inhibition and sAHP activation required to terminate bursts.
The effects of endogenous adenosine are probably more complex than enhancement of the mAHP alone because endogenous adenosine also contributes to activity-dependent inhibition of the amplitude of evoked IPSCs and EPSCs (Oliet & Poulain, 1999). Hence, the more profound inhibitory effects of endogenous adenosine evident in vivo (Bull et al. 2006) probably reflect (at least) a reduced synaptic input strength combined with an enhanced mAHP amplitude.
Mechanisms of adenosine action
The intracellular mechanisms by which adenosine increases mAHP amplitude are unknown but might involve a reduction of calcium entry because A1 receptor activation reduces calcium currents and spike duration in supraoptic nucleus neurones (Noguchi & Yamashita, 2000; Ponzio & Hatton, 2005). However, we found no evidence that antagonism of endogenous activation of A1 receptors alters spike duration (or fAHP amplitude) and other calcium-dependent processes, such as activity-dependent spike broadening (Kirkpatrick & Bourque, 1991), were unaffected by CPT suggesting that endogenous adenosine probably does not alter global intracellular calcium concentrations. In vasopressin neurones, the mAHP is underpinned by an apamin-sensitive, calcium-activated small-conductance potassium current (Kirkpatrick & Bourque, 1996). A1 receptors can couple to Gi/o-proteins to reduce adenylyl cyclase activity as well as to Gq-proteins to activate Ca2+ release from intracellular stores; this latter effect is known to stimulate a variety of effector proteins, including calcium-activated potassium channels (Ralevic & Burnstock, 1998) and so might account for the enhancement of the mAHP by endogenous adenosine activation of A1 receptors.
Concluding remarks
Phasic spike discharge (periods of activity with marked spike frequency adaptation, separated by periods of silence) releases more vasopressin per spike than continuous activity, with the greatest secretion occurring early in bursts (Bicknell & Leng, 1981; Cazalis et al. 1985). Hence, spike frequency adaptation is important for efficient vasopressin release from the posterior pituitary gland. Spike frequency adaptation in vasopressin neurones is mediated by activation of the mAHP (Kirkpatrick & Bourque, 1996) and we show that mAHP amplitude is enhanced by endogenous activity-dependent activation of adenosine A1 receptors. We further show that this endogenous activity-dependent activation of adenosine A1 receptors increases spike frequency adaptation during spontaneous phasic bursts as well as reducing burst duration. Taken together, these observations suggest that, after conversion to adenosine, dendritic ATP release contributes to the termination of spontaneous phasic bursts in vivo by autocrine enhancement of mAHP amplitude to reduce the probability of synaptic potentials reaching spike threshold to trigger further spike firing as bursts progress.
Acknowledgments
This work was supported by a University of Otago Research Grant and the Otago School of Medical Sciences Bequest Fund.
References
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994;475:115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinol. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH. Rhythmogenesis in vasopressin cells. J Neuroendocrinol. 2004;16:727–739. doi: 10.1111/j.1365-2826.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brown CH, Leng G. In vivo modulation of post-spike excitability in vasopressin cells by k-opioid receptor activation. J Neuroendocrinol. 2000;12:711–714. doi: 10.1046/j.1365-2826.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus κ-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95:3235–3244. doi: 10.1152/jn.00062.2006. [DOI] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. k-Opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–111. doi: 10.1016/j.neuroscience.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–228. doi: 10.1016/S0079-6123(08)00419-6. [DOI] [PubMed] [Google Scholar]
- Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. doi: 10.1042/BST0351236. [DOI] [PubMed] [Google Scholar]
- Bull PM, Brown CH, Russell JA, Ludwig M. Activity-dependent feedback modulation of spike patterning of supraoptic nucleus neurons by endogenous adenosine. Am J Physiol Regul Integr Comp Physiol. 2006;291:R83–R90. doi: 10.1152/ajpregu.00744.2005. [DOI] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol. 1998;510:165–175. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Excitatory role of the hyperpolarization-activated inward current in phasic and tonic firing of rat supraoptic neurons. J Neurosci. 2000;20:4855–4863. doi: 10.1523/JNEUROSCI.20-13-04855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Ionic basis of the caesium-induced depolarisation in rat supraoptic nucleus neurones. J Physiol. 2001;536:797–808. doi: 10.1111/j.1469-7793.2001.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci. 2004;24:7718–7726. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular vasopressin neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol. 2004;16:577–588. doi: 10.1111/j.1365-2826.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- Greffrath W, Martin E, Reuss S, Boehmer G. Components of after-hyperpolarization in magnocellular neurones of the rat supraoptic nucleus in vitro. J Physiol. 1998;513:493–506. doi: 10.1111/j.1469-7793.1998.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ML, Ruijter JM, Klop A, Buijs RM, Renaud LP. Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J Neurophysiol. 2000;83:705–711. doi: 10.1152/jn.2000.83.2.705. [DOI] [PubMed] [Google Scholar]
- Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989–1002. doi: 10.1378/chest.120.3.989. [DOI] [PubMed] [Google Scholar]
- Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci. 2000;20:8868–8875. doi: 10.1523/JNEUROSCI.20-23-08868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Dual role for calcium in the control of spike duration in rat supraoptic neuroendocrine cells. Neurosci Lett. 1991;133:271–274. doi: 10.1016/0304-3940(91)90586-i. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol. 1996;494:389–398. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Hirasawa M, Pittman QJ. Vasopressin preferentially depresses excitatory over inhibitory synaptic transmission in the rat supraoptic nucleus in vitro. J Neuroendocrinol. 2000;12:361–367. doi: 10.1046/j.1365-2826.2000.00462.x. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Li Z, Decavel C, Hatton GI. Calbindin-D28k: role in determining intrinsically generated firing patterns in rat supraoptic neurones. J Physiol. 1995;488:601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Ca2+ release from internal stores: role in generating depolarizing after-potentials in rat supraoptic neurones. J Physiol. 1997;498:339–350. doi: 10.1113/jphysiol.1997.sp021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Jia YS, Ju G. Nitric oxide inhibits neuronal activity in the supraoptic nucleus of the rat hypothalamic slices. Brain Res Bull. 1997;43:121–125. doi: 10.1016/s0361-9230(96)00209-2. [DOI] [PubMed] [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Onaka T, Yagi K. Vasopressin regulation of noradrenaline release within the supraoptic nucleus. J Neuroendocrinol. 2000;12:477–479. doi: 10.1046/j.1365-2826.2000.00516.x. [DOI] [PubMed] [Google Scholar]
- Moos F, Gouzenes L, Brown D, Dayanithi G, Sabatier N, Boissin L, Rabie A, Richard P. New aspects of firing pattern autocontrol in oxytocin and vasopressin neurones. Adv Exp Med Biol. 1998;449:153–162. doi: 10.1007/978-1-4615-4871-3_18. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Yamashita H. Adenosine inhibits voltage-dependent Ca2+ currents in rat dissociated supraoptic neurones via A1 receptors. J Physiol. 2000;526:313–326. doi: 10.1111/j.1469-7793.2000.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520:815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisner AM, Douglas WW. Adenosine triphosphate and adenosine triphosphatase in hormone-containing granules of posterior pituitary gland. Science. 1968;160:203–204. doi: 10.1126/science.160.3824.203. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. J Neurophysiol. 2005;93:535–547. doi: 10.1152/jn.01185.2003. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Wang YF, Hatton GI. Activation of adenosine A2A receptors alters postsynaptic currents and depolarizes neurons of the supraoptic nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R359–366. doi: 10.1152/ajpregu.00747.2005. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–111. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA, Leng G. Nitric oxide and the oxytocin system in pregnancy. J Neurosci. 2000;20:6721–6737. doi: 10.1523/JNEUROSCI.20-17-06721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1815–R1822. doi: 10.1152/ajpregu.2001.280.6.R1815. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, van wimersma Griedanus TB. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]