Abstract
Chronic hypoxia has been proposed to induce a closer coupling in human skeletal muscle between ATP utilization and production in both lowlanders (LN) acclimatizing to high altitude and high-altitude natives (HAN), linked with an improved match between pyruvate availability and its use in mitochondrial respiration. This should result in less lactate being formed during exercise in spite of the hypoxaemia. To test this hypothesis six LN (22–31 years old) were studied during 15 min warm up followed by an incremental bicycle exercise to exhaustion at sea level, during acute hypoxia and after 2 and 8 weeks at 4100 m above sea level (El Alto, Bolivia). In addition, eight HAN (26–37 years old) were studied with a similar exercise protocol at altitude. The leg net lactate release, and the arterial and muscle lactate concentrations were elevated during the exercise in LN in acute hypoxia and remained at this higher level during the acclimatization period. HAN had similar high values; however, at the moment of exhaustion their muscle lactate, ADP and IMP content and Cr/PCr ratio were higher than in LN. In conclusion, sea-level residents in the course of acclimatization to high altitude did not exhibit a reduced capacity for the active muscle to produce lactate. Thus, the lactate paradox concept could not be demonstrated. High-altitude natives from the Andes actually exhibit a higher anaerobic energy production than lowlanders after 8 weeks of acclimatization reflected by an increased muscle lactate accumulation and enhanced adenine nucleotide breakdown.
Lactate metabolism during exercise at altitude has frequently been the focus in studies encompassing both lowlanders at acute and chronic exposure to altitude and high-altitude natives. The predominant view, which has emerged, is that in spite of the hypoxaemia, less lactate is accumulating in blood during exercise after a prolonged exposure to altitude, the phenomenon referred to as the lactate paradox. Different definitions of the altitude ‘lactate paradox’ have been proposed over the years (West, Hochacka, Reeves). West (1986) defined the lactate paradox as ‘Paradoxically, blood lactate for a given work rate at high altitude in acclimatized subjects is essentially the same as at sea level. Because work capacity decreased markedly with increasing altitude, maximal blood lactate also falls’. However, the most commonly used definition is from Reeves et al. (1992). They defined the lactate paradox as the situation: ‘in which blood lactate accumulation during exercise is increased on arrival at high altitude but falls with acclimatization the paradox being that this occurs without a change in muscle oxygen delivery’. A perhaps more accurate description is from Kayser (1996), ‘In acclimatized humans at high altitude the reduction, compared to acute hypoxia, of the blood lactate concentration at any absolute oxygen uptake 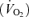 , as well as the reduction of maximal lactate accumulation after exhaustive exercise, compared to both acute hypoxia or normoxia, have been considered paradoxical, and these phenomena have therefore become known as the lactate paradox'. Moreover, it was suggested that high-altitude natives accumulate less lactate than normoxia lowlanders during incremental exhaustive exercise which was persistent after 6 weeks of de-acclimatization at sea level and it was concluded that it was, therefore, an expression of a fixed metabolic feature (‘the perpetual lactate paradox’) (Hochachka, 1989). Hochachka (1989) defined the lactate paradox in highlanders as; ‘during incremental aerobic exercise tests to fatigue under hypoxic condition they (Quechuas, high-altitude natives of the Andes) form less lactate and accumulate to lower levels than normoxic lowlanders'. The proposed mechanisms for this adaptation brought forward by Hochachka and colleagues (Hochachka, 1998; Hochachka et al. 1991) should be that at altitude glycolysis becomes properly matched to the rate by which pyruvate is decarboxylated and utilized for oxidative phosphorylation. The underlying mechanisms are a low ADP/ATP ratio during exercise and less inosine monophosphate (IMP) formed. The reduced excess of pyruvate results in less lactate being produced, the ‘altitude lactate paradox’.
, as well as the reduction of maximal lactate accumulation after exhaustive exercise, compared to both acute hypoxia or normoxia, have been considered paradoxical, and these phenomena have therefore become known as the lactate paradox'. Moreover, it was suggested that high-altitude natives accumulate less lactate than normoxia lowlanders during incremental exhaustive exercise which was persistent after 6 weeks of de-acclimatization at sea level and it was concluded that it was, therefore, an expression of a fixed metabolic feature (‘the perpetual lactate paradox’) (Hochachka, 1989). Hochachka (1989) defined the lactate paradox in highlanders as; ‘during incremental aerobic exercise tests to fatigue under hypoxic condition they (Quechuas, high-altitude natives of the Andes) form less lactate and accumulate to lower levels than normoxic lowlanders'. The proposed mechanisms for this adaptation brought forward by Hochachka and colleagues (Hochachka, 1998; Hochachka et al. 1991) should be that at altitude glycolysis becomes properly matched to the rate by which pyruvate is decarboxylated and utilized for oxidative phosphorylation. The underlying mechanisms are a low ADP/ATP ratio during exercise and less inosine monophosphate (IMP) formed. The reduced excess of pyruvate results in less lactate being produced, the ‘altitude lactate paradox’.
Not all data in the literature conform to the lowland or high-altitude native lactate paradox concept and the existence or absence of the lactate paradox is the topic of a lively debate (Brooks, 2007; Mazzeo et al. 2007; van Hall, 2007a, van Hall, 2007b; West, 2007a,West, 2007b). The study of Dill et al. (1931), used by Reeves et al. (1992) as the first report of the lactate paradox, ‘blood lactate during exercise initially rose higher in one subject (THE, Edwards), than at sea level, but after several months’ residence, the concentrations were similar to those at sea level'. However, two other subjects, Talbot and Dill, did not show a change in sub-maximal and peak lactate blood accumulation with acclimatization. Secondly, and more importantly, Edwards’ maximal lactate was actually unaffected by acclimatization and the reason for his sub-maximal lactate levels nearing sea level values with duration of acclimatization was simply due to a shift of the lactate curves to the right caused by training. This meant that he was able to do similar work after 8 weeks of acclimatization to that at sea level, which is actually discussed in the paper. Therefore, the Leadville expedition provides good support for the non-existence of the lactate paradox and it shows that at altitude the normal lactate to relative workload relationship is maintained, including that of Edwards, and not changed according to West's lactate paradox definition (West (1986)). In the 1960s Klausen and coworkers performed two studies looking at blood lactate accumulation during sub-maximal and maximal exercise in the course of 1–35 days of acclimatization to 3800 m (White Mountain) (Klausen et al. (1966)). They did not find any change in blood lactate accumulation between acute hypoxia and acclimatization and, if any, there was a tendency for an increased lactate accumulation with acclimatization. Dempsey et al. (1972) did not find a change in lactate between acute hypoxia and in the course of acclimatization up to 45 days. In addition, it was shown that maximal lactate accumulation was similar in Caucasian lowlanders 45 days after the sojourn to 3100 m, native lowlanders who had to move to Leadville as children, and native residence to 3100 m. Other studies on high-altitude natives showed considerable elevations in blood lactate (Favier et al. 1995; Kayser et al. 1996), indeed in the same ranges as when sea level natives are exposed acutely to hypoxia. More recently, we showed that in Chacaltaya (Bolivia, 5260 m) the lactate concentration and leg net lactate release were higher at sub-maximal but similar at maximal workloads compared to sea level, and the results of acute exposure to hypoxia were similar to those at 9 weeks of acclimatization. However, we wondered whether the altitude above that of permanent habitation (see also discussion in van Hall, 2007a,b; West, 2007a,b), or longer duration of acclimatization compared to most studies might have caused the lactate response to be similar to that of acute hypoxia after initially reduced levels. Therefore, the primary aim of the present study was to test this hypothesis as well as the one that a more tight match develops while at altitude between rates of glycolysis and oxidation, thereby causing lactate production and blood lactate accumulation to become reduced. Bolivian high-altitude natives from Aymara ancestry (HAN), who were lifetime residents of La Paz/El Alto (3700–4100 m) were invited to participate in the study along with male native lowlanders (LN). They were studied at 4100 m during graded incremental bicycle exercise until exhaustion. In LN the same protocol was applied at sea level while breathing ambient air or a low oxygen gas mixture equivalent to the oxygen content at 4100 m to simulate acute hypoxia.
Methods
Subjects
We studied six male lowlanders from Denmark and eight male Bolivian altitude natives of Aymara ancestry, who were lifetime residents of La Paz/El Alto (3700 to 4100 m). The HAN were all physically active, primarily in karate, soccer or running. The LN were physical education students, participating in a variety of sports and outdoors recreational activities, and they maintained their physical activity level while at altitude. Height and body mass of the HAN (mean (range): 163 cm (157–170); 63 kg (52–70)) were lower compared to the LN (187 cm (175–191); 82 kg (75–91)) with no change in LN while at altitude. All subjects received written and oral information about the study in their native language and gave their written informed consent to the protocols. The study was performed according to the Declaration of Helsinki. The protocol for the Danish subjects was approved by the Copenhagen–Frederiksberg Review Committee (KF 11-050/01) and the protocol for the Danish and Bolivian subjects was approved by El Tribunal de Honor del Colegio Médico Departamental La Paz, and the Ministerio De Previsión Social y Salud Pública, La Paz, Bolivia.
Acclimatization of the lowlanders
Each LN underwent six incremental bicycle exercise tests on a cycle ergometer. The subjects were tested twice at sea level (Copenhagen, Denmark) while breathing ambient air (Sea Level) or a hypoxic gas mixture, 12% O2 in N2 (Acute Hypoxia), with ∼3 weeks between the trials. Approximately 1 month after the tests at sea level, the subjects were flown to La Paz, Bolivia. Upon arrival, they spent ∼4 days in La Paz (∼3700 m), and then moved to El Alto (4100 m) for the remaining 8 weeks of the study. The subjects shared an apartment and prepared their own meals. They maintained their habitual Danish diet, including an occasional visit to a fast food restaurant. Between weeks 3 and 7 of the acclimatization period they went on short excursions, but never below 3700 m. The first incremental bicycling exercise test at altitude was performed between days 11 and 17 after arrival in La Paz (2 weeks Chronic Hypoxia). The second exercise test at altitude was performed between days 52 and 60 after arrival and conducted identically to the first test (8 weeks Chronic Hypoxia).
Protocol measurements
The experiments for LN at sea level and altitude were similar to those performed on HAN. The subjects reported to the laboratory at 8 am. Catheters were placed under local anaesthesia in a femoral artery and vein for blood sampling. The tip of the arterial catheter (18 G, Ohmeda, Wiltshire, UK) was advanced to 6 cm proximal to the inguinal ligament using the Seldinger technique. A thermistor was inserted through the end hole of the venous catheter (a radiopack teflon catheter with side holes; Cook, Bjaerverskov, Denmark) for blood flow measurements by the constant infusion thermodilution technique (Andersen & Saltin, 1985). After placement of the catheters, the subjects remained supine for at least 30 min. Then a biopsy was taken from the vastus lateralis under local anaesthesia (lidocaine (lignocaine), 20 mg ml−1) of the skin and fasciae. Subjects were then seated on a bicycle ergometer (Monark 824E, Varberg, Sweden). In preparation for the study, sample lines were secured, leads were attached to record heart rate and ECG (BIO amp, ADInstruments, UK), arterial blood pressure (BPamp, ADinstruments, UK), and femoral blood and saline infusate temperatures via an interface (PowerLab/16SP, ADInstruments, UK). The above variables were displayed and recorded via data acquisition software (Chart 4, ADInstruments, UK). Finally, subjects were fitted with mouthpiece and nose clip to enable measurements of ventilation 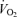 , and
, and 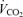 from expired gas (Oxygen Analyzer S-3A/I, Ametek, USA; LB-2, Beckman, USA; VRDC/HC-1, Parvo Medics, USA). Resting measurements were initiated 10 min after placement of the mouthpiece and the resting blood samples were obtained. Exercise consisted of a 15 min warm-up at 100 W for LN and at 80 W for HAN, these workloads representing approximately 35% of
from expired gas (Oxygen Analyzer S-3A/I, Ametek, USA; LB-2, Beckman, USA; VRDC/HC-1, Parvo Medics, USA). Resting measurements were initiated 10 min after placement of the mouthpiece and the resting blood samples were obtained. Exercise consisted of a 15 min warm-up at 100 W for LN and at 80 W for HAN, these workloads representing approximately 35% of 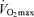 for both groups under hypoxic conditions. After the warm-up, the workload for LN was increased every 2.5 min with 40 W until exhaustion. The first 2.5 min increment for the HAN after the warm-up was 20 W, followed by 40 W increments until exhaustion. After 2 min at each increment blood flow was measured, start of blood sampling exactly after 2 min 15 s and hereafter an additional blood flow measurement was performed. Muscle biopsies were taken at rest, after the 15 min warm-up only in the LN, and when the subjects stopped at their peak workload. The blood was sampled anaerobically in heparinized syringes and analysed for haemoglobin, oxygen saturation (OSM3 Hemoxymeter, Radiometer, Copenhagen, Denmark), blood pH, CO2 and O2 tension (ABL5, Radiometer), glucose, lactate and electrolytes (EML105, Radiometer). The blood from a second sample was collected in ice-cold tubes containing 10 μl 0.33 m EDTA per millilitre of blood, quickly centrifuged within 1 min at maximum speed, and the plasma immediately removed and stored at −50°C until analysis. Plasma lactate was determined in duplicate by an enzymatic method on an automatic analyser (Cobas Fara, Roche, Switzerland). Muscle biopsies were obtained within 5 s of termination of exercise, immediately frozen in liquid nitrogen and stored at −50°C for approximately 2 weeks. Upon arrival in Copenhagen the biopsies were stored at −80°C. Muscle biopsies were cut at −20°C, and ∼15 mg was freeze-dried and dissected under a stereomicroscope to remove blood and visible connective tissue. Hydrochloric acid (1 m) was added to 1–2 mg of muscle fibres and then boiled for 3 h at 100°C. The mixture was centrifuged and the supernatant was neutralized with 0.12 m tris-hydroxylaminoethane/2.1 m potassium hydroxide and analysed for glucose. The remainder of the fibres was extracted with 3 m perchloric acid and centrifuged, and the supernatant was neutralized with 2 m potassium bicarbonate and analysed fluorometrically for lactate, adenine nucleotide, and inosine monophosphate (IMP) by HPLC (Van Der Vusse et al. 1989), and for creatine phosphate (PCr) and creatine (Cr) by an enzymatic method on an automatic analyser (Cobas Fara, Roche, Switzerland) (Harris et al. 1974).
for both groups under hypoxic conditions. After the warm-up, the workload for LN was increased every 2.5 min with 40 W until exhaustion. The first 2.5 min increment for the HAN after the warm-up was 20 W, followed by 40 W increments until exhaustion. After 2 min at each increment blood flow was measured, start of blood sampling exactly after 2 min 15 s and hereafter an additional blood flow measurement was performed. Muscle biopsies were taken at rest, after the 15 min warm-up only in the LN, and when the subjects stopped at their peak workload. The blood was sampled anaerobically in heparinized syringes and analysed for haemoglobin, oxygen saturation (OSM3 Hemoxymeter, Radiometer, Copenhagen, Denmark), blood pH, CO2 and O2 tension (ABL5, Radiometer), glucose, lactate and electrolytes (EML105, Radiometer). The blood from a second sample was collected in ice-cold tubes containing 10 μl 0.33 m EDTA per millilitre of blood, quickly centrifuged within 1 min at maximum speed, and the plasma immediately removed and stored at −50°C until analysis. Plasma lactate was determined in duplicate by an enzymatic method on an automatic analyser (Cobas Fara, Roche, Switzerland). Muscle biopsies were obtained within 5 s of termination of exercise, immediately frozen in liquid nitrogen and stored at −50°C for approximately 2 weeks. Upon arrival in Copenhagen the biopsies were stored at −80°C. Muscle biopsies were cut at −20°C, and ∼15 mg was freeze-dried and dissected under a stereomicroscope to remove blood and visible connective tissue. Hydrochloric acid (1 m) was added to 1–2 mg of muscle fibres and then boiled for 3 h at 100°C. The mixture was centrifuged and the supernatant was neutralized with 0.12 m tris-hydroxylaminoethane/2.1 m potassium hydroxide and analysed for glucose. The remainder of the fibres was extracted with 3 m perchloric acid and centrifuged, and the supernatant was neutralized with 2 m potassium bicarbonate and analysed fluorometrically for lactate, adenine nucleotide, and inosine monophosphate (IMP) by HPLC (Van Der Vusse et al. 1989), and for creatine phosphate (PCr) and creatine (Cr) by an enzymatic method on an automatic analyser (Cobas Fara, Roche, Switzerland) (Harris et al. 1974).
The cross-sectional area of the right thigh was analysed with magnetic resonance imaging (MRI) only in the lowlander before and within 1 day of the return to sea level after the altitude sojourn. Twenty-eight images were taken with 15 mm between each section. The section closest to the midpoint between basis patellae and trochanter major was used for analysis. Lean area was defined as muscle bone and vessels.
Calculations
The measured pH, 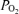 and
and 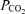 were corrected for temperature using the blood temperature as measured in the femoral vein. Plasma bicarbonate was calculated according to Siggaard-Andersen (1977). All muscle metabolites values for a given individual were expressed relative to the average total creatine content (PCr + Cr) for that individual for each trial.
were corrected for temperature using the blood temperature as measured in the femoral vein. Plasma bicarbonate was calculated according to Siggaard-Andersen (1977). All muscle metabolites values for a given individual were expressed relative to the average total creatine content (PCr + Cr) for that individual for each trial.
Muscle pH and free ADP and AMP estimations
Since ADP and AMP are largely bound, the free concentration of these nucleotides have been calculated using the reactants and equilibrium constants of the near-equilibrium reaction catalysed by creatine kinase and adenylate kinase, respectively. Free ADP (ADPf) and AMP (AMPf) content was calculated by assuming equilibrium of the creatine kinase and adenylate kinase reactions (Lawson & Veech, 1979; Dudley et al. 1987). Specifically, ADPf was calculated using the measured ATP, Cr, PCr, estimated H+ concentration (Sahlin et al. 1975), and the creatine kinase constant of 1.66 × 109. AMPf was calculated from the estimated ADPf and measured ATP content using the adenylate kinase equilibrium constant of 1.05. Free inorganic phosphate (Pi) was calculated by adding the estimated resting free phosphate of 10.8 mmol (kg dry wt)−1 (Dudley et al. 1987) to the difference in PCr content (Δ[CP]) between rest and exhaustion. A critical assumption is that the intracellular buffer capacity is the same in the course of acclimatization as that at sea level in LN and similar in HAN so the relationship between lactate and pH as established by Sahlin et al. (1975) is correct. It is unlikely that this assumption is entirely correct; however, modest differences in actual and estimated pH would have relatively little impact on the estimated free ADP and AMP concentrations.
Statistical analysis
All data are presented as mean ± standard error of the mean. To determine the statistical significance between the means in LN at Sea Level, Acute Hypoxia, and 2 week and 8 week Chronic Hypoxia, a repeated-measures analysis of variance was performed; Tukey's post hoc test was utilized to indicate where significant differences occurred. To determine the statistical significance of differences between two means of HAN and LN, a Student's paired t test was performed. The level of significance was set at P < 0.05.
Results
Subject description, exercise performance and baseline blood and metabolic parameters (Table 1)
Table 1.
Subject description, maximal exercise performance, pulmonary and leg oxygen uptake, and blood parameters under resting conditions
| Danish lowlanders | |||||
|---|---|---|---|---|---|
| 0 m |
4100 m |
High-altitude natives |
|||
| Sea Level | Acute Hypoxia | 2 weeks Chronic Hypoxia | 8 weeks Chronic Hypoxia | Ambient air | |
| Age (years) | 26 (22–31) | 31 (26–37) | |||
| Height (cm) | 187 (175–191) | 163 (157–170)§ | |||
| Body mass (kg) | 82 (75–91) | 79 (72–91) | 78 (68–90) | 63 (52–70)§ | |
| Wmax (W) | 393 (380–412) | 307 (300–340)* | 285 (276–315)* | 296 (288–340)* | 215 (190–247)§ |
| Wmax (W kg−1) | 4.8 (4.3–5.3) | 3.7 (3.6–4.0)* | 3.6 (3.4–3.8)*# | 3.8 (3.6–4.0)* | 3.5 (3.1–4.7) |
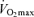 max (l) max (l) |
4.6 (4.1–5.0) | 3.7 (3.6–4.0)* | 3.5 (3.2–3.9)*# | 3.7 (3.3–4.2)* | 2.7 (2.4–3.1)§ |
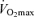 max (ml kg−1) max (ml kg−1) |
56 (52–60) | 44 (42–48)* | 44 (41–46)* | 47 (42–51)* | 43 (31–52) |
| Heart ratemax (beats min−1) | 183 (177–196) | 170 (162–177)* | 171 (157–187)* | 175 (161–190)* | 184 (182–202)§ |
| Leg blood flowmax (l min−1) | 12.3 (11.1–13.5) | 11.0 (9.6–12.2)* | 9.6 (7.4–12.5)* | 8.7 (7.1–11.5)* | 6.9 (5.3–11.0)§ |
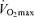 max (l min−1) max (l min−1) |
2.19 (1.82–2.35) | 1.53 (1.34–1.76)* | 1.48 (1.24–1.70)* | 1.44 (1.19–1.82)* | 1.14 (0.9–1.4)§ |
| Hb (g dl−1) | 13.9 (12.9–14.6) | 13.1 (12.2–14.9) | 16.3 (15.4–17.4)* | 15.9 (14.4–16.7)* | 16.7 (15.5–18.2) |
| pH | 7.41 (7.39–7.44) | 7.43 (7.42–7.45) | 7.45 (7.44–7.48)* | 7.47 (7.43–7.49)* | 7.44 (7.41–7.45)§ |
| O2 saturation (%) | 98.4 (98.1–98.8) | 87.9 (87.1–89.6)* | 89.7 (86.5–90.9)* | 90.9 (89.8–92.3)*#& | 87.7 (85.6–89.1)§ |
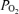 (mmHg) (mmHg) |
98.8 (102.1–93.6) | 48.7 (44.4–50.9)* | 55.1 (54.6–59.1)*# | 58.4 (54.9–62.1)*# | 52.5 (49.1–57.2)§ |
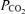 (mmHg) (mmHg) |
39.4 (34.8–41.6) | 36.7 (35.8–39.0) | 26.4 (25.4–27.9)*# | 25.9 (24.5–27.3)*# | 31.3 (28.1–32.0)§ |
| Lactate (mmol l−1) | 0.8 (0.4–1.4) | 0.8 (0.4–1.2) | 0.8 (0.4–1.2) | 1.0 (0.9–1.2) | 0.7 (0.4–1.0) |
| Base deficit (mmol l−1) | −0.5 (2.3 to –1.6) | −0.2 (2.3 to –1.6) | 3.9 (2.2–6.1)* | 3.8 (2.0–6.2)* | 1.3 (0.5–2.2)§ |
| Adrenaline (nmol l−1) | 0.3 (0.1–0.4) | 0.6 (0.1–1.2) | 1.5 (0.7–2.5)* | 1.6 (0.7–2.2)* | 0.7 (0.2–1.1)§ |
| Noradrenaline (nmol l−1) | 0.6 (0.3–0.9) | 0.7 (0.5–1.0) | 4.0 (2.7–5.7)* | 3.5 (1.6–5.2)* | 1.6 (0.8–2.4)§ |
Values are means of male lowlanders (n= 6) and high-altitude natives breathing ambient air (n= 7).
Significantly different from Sea Level,
significantly different from Acute Hypoxia.
Significantly different from lowlanders/8 weeks Chronic Hypoxia.
Significant differences between 2 weeks and 8 weeks Chronic Hypoxia.
Maximal oxygen consumption was reduced in LN under hypoxic conditions, but did not change during the stay at altitude. Maximal power output and the associated maximal oxygen uptake were lower in HAN than in LN during hypoxic conditions. However, if expressed per kilogram body mass both groups had similar maximal oxygen uptake and power output. LN body mass remained unchanged under the various conditions as well as the right thigh muscle mass with 2.2 ± 0.1 and 2.1 ± 0.1 kg (P= 0.18) before and after 8 weeks at high altitude, respectively. Acclimatization caused haemoglobin levels in LN to increase within 2 weeks at altitude with no further increase at 8 weeks. At 8 weeks Chronic Hypoxia resting blood pH, oxygen saturation and oxygen tension were lower in HAN than in LN. In addition, catecholamine levels were elevated under chronic hypoxic conditions as compared to Sea Level and Acute Hypoxia and higher than in HAN. Detailed information and discussion of leg blood flow and oxygen uptake during exercise can be found in Lundby et al. (2006).
Lactate
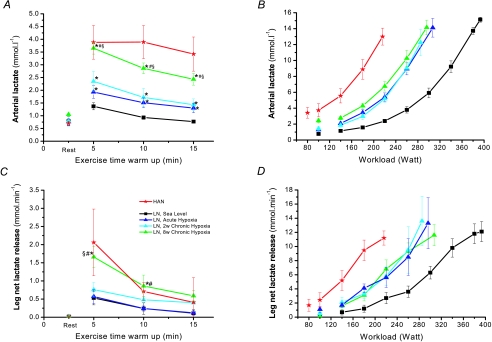
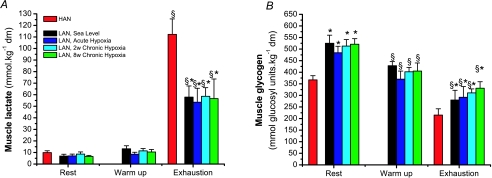
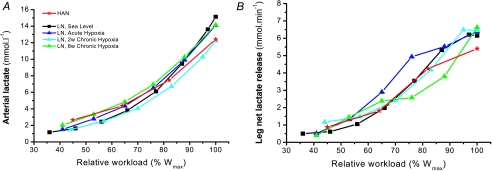
Bicycle exercise started with a warm-up period of 15 min, which caused the arterial lactate concentration and leg net lactate release to increase under hypoxic compared to sea level conditions (Fig. 1A and C). However, after 8 weeks of altitude exposure the initial plasma lactate concentration and leg net lactate release was significantly higher compared to Acute Hypoxia and 2 weeks Chronic Hypoxia. Muscle lactate content and accumulation (Fig. 3A) was similar for all hypoxic conditions, which agrees with the similar leg net lactate release after 15 min of warm-up (Fig. 1C). These differences were not apparent during the incremental exercise until exhaustion (Fig. 1B and D). Peak arterial lactate concentration and the leg peak net lactate release were similar in HAN and LN in the various trials reaching 13–15 mmol l−1 and 11–13 mmol min−1, respectively. In LN, Acute and Chronic Hypoxia arterial lactate levels as well as leg net lactate release were similarly elevated at sub-maximal workloads as compared to Sea Level. Interestingly, when these two lactate variables are related to the relative exercise intensity, they follow a common ‘line’ regardless of condition and ethnic group (Fig. 2). Skeletal muscle lactate content in LN was similar in all trials (Fig. 3A). However, the HAN skeletal muscle lactate content at the moment of exhaustion was markedly higher than in LN.
Figure 1. Arterial and leg net lactate release during warm-up (A and C) and incremental bicycle exercise (B and D) in high-altitude natives (HAN) and lowlanders (LN) in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). Significant differences (P≤ 0.05) during warm-up from *Sea Level, #Acute Hypoxia and §2 weeks Chronic Hypoxia. No differences during incremental bicycling exercise were found in LN for arterial lactate concentration and leg net lactate release under hypoxic conditions but all were different from Sea Level. In addition, HAN had higher arterial lactate concentration and leg net lactate release compared LN under all conditions.
Figure 3. Vastus lateralis lactate content (A) and glycogen (B) at rest, warm-up and at the moment of exhaustion from incremental bicycle exercise in HAN and LN in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). §Denotes a significant change in muscle content between rest and exhaustion from incremental bicycle exercise. No differences were found between different conditions in LN. *Significant differences between LN and HAN.
Figure 2. Arterial (A) and leg net lactate release (B) as a function of relative work during incremental bicycle exercise in HAN and LN in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). No significant differences were found either between conditions in LN or between LN and HAN.
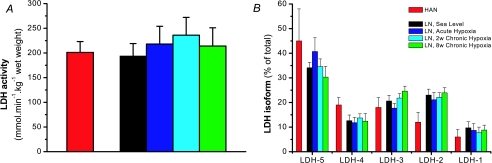
No differences were observed between any of the trials in LN and when comparing HAN with LN in total lactate dehydrogenase (LDH) activity or LDH isoform distribution (Fig. 4A and B), or myosin heavy chain isoform distribution, between any of the trials in LN and when comparing HAN with LN (Juel et al. 2003).
Figure 4. Vastus lateralis lactate dehydrogenase activity (A) and relative isoform content (B) in HAN and LN in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). No significant differences were found either between conditions in LN or between LN and HAN.
Muscle glycogen (Fig. 3B)
The pre-exercise muscle glycogen content was similar for all conditions in LN. In addition, muscle glycogen utilization in LN was similar under all hypoxic conditions during incremental bicycling. HAN natives had substantially lower muscle glycogen stores as compared to LN. Nevertheless, the overall muscle glycogen utilization during exercise was similar to LN.
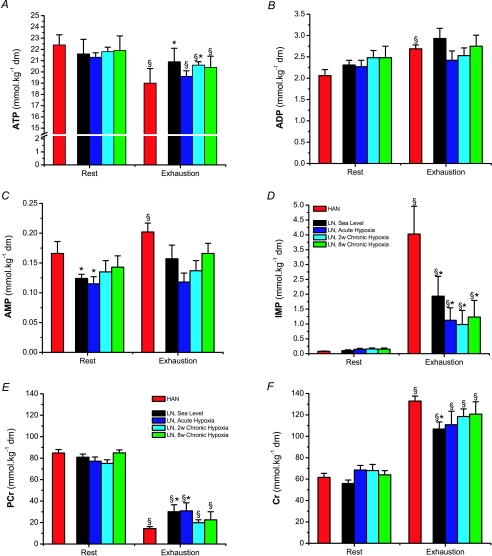
Muscle creatine phosphate (PCr), creatine (Cr), adenine nucleotides and inosine monophosphate (IMP)
Muscle adenine nucleotide levels were similar in LN and HAN at rest. At the moment of exhaustion, a significant decrease in total adenine nucleotide could only be found in HAN. The muscle ATP content was decreased and the ADP and the AMP contents were elevated (Fig. 5A, B and C). Furthermore, a pronounced increase in IMP was observed (Fig. 5D). In LN, the differences observed in muscle ADP and AMP content going from rest to exhaustion were not significant, whereas muscle IMP content was increased, although far less pronounced than in HAN. Total muscle creatine (PCr + Cr) was 137 ± 4, 145 ± 5, 142 ± 6, 146 ± 3 and 146 ± 4 mmol (kg dm)−1 for LN at Sea Level, Acute Hypoxia, 2 weeks Chronic Hypoxia, 8 weeks Chronic Hypoxia and HAN, respectively. At the moment of exhaustion the HAN tended to have a lower muscle content of PCr, higher Cr and hence a higher Cr/PCr ratio compared to LN, albeit not significantly different from LN 8 weeks Chronic Hypoxia (Fig. 5E and F). However, the Cr/PCr ratio was higher in HAN compared to LN under all conditions. No changes in PCr, Cr or Cr/PCr ratio were observed in the course of acclimatization in LN.
Figure 5. Vastus lateralis adenine nucleotides, IMP, creatine phosphate and creatine content at rest and at the moment of exhaustion of incremental exercise in HAN and LN in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). §Denotes a significant change in muscle content between rest and exhaustion from incremental bicycle exercise. No differences were found between different conditions in LN. *Significant differences between LN and HAN.
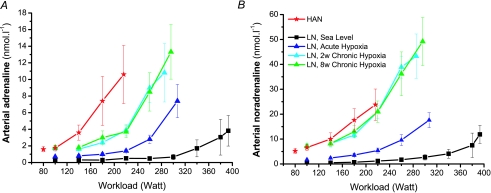
Catecholamines
Resting arterial catecholamine levels were somewhat higher at altitude in LN and similar to those of HAN (Table 1). During the incremental exercise in acute hypoxia and even more so at 2 weeks and 8 weeks at altitude, both adrenaline and noradrenaline levels were markedly elevated at the submaximal and maximal levels (Fig. 6). HAN also had a high concentration during exercise, but without reaching as high a level for noradrenaline as LAN at exhaustion. The adrenaline concentration in HAN was higher than in LN at the submaximal exercise intensity, but similar to LN at exhaustion.
Figure 6. Arterial adrenaline (A) and noradrenaline (B) concentrations during incremental exercise in HAN and LN in the course of acclimatization to high altitude.
Values are mean ±s.e.m. of LN (n= 6) and HAN (n= 7). During incremental bicycling exercise in LN arterial adrenaline and noradrenaline were significantly higher under Acute Hypoxic conditions compared to Sea Level and both conditions had lower concentrations compared to 2 weeks and 8 weeks Chronic Hypoxia. At the moment of exhaustion HAN had a similar adrenaline concentration compared to LN under hypoxic conditions. However, HAN's peak noradrenaline concentration was lower compared to LN after 2 weeks and 8 weeks Chronic Hypoxia.
If the catecholamine levels during exercise in HAN and LN are related to the relative exercise intensity they approach a common line, but far from as tight as is the case for blood lactate.
Discussion
The findings of the present study do not conform to the hypothesis that in skeletal muscle there is a close match between pyruvate production and its oxidation by the mitochondria during chronic hypoxic conditions. Neither sea-level residents adapting for 8 weeks to quite severe hypoxia nor high-altitude natives showed any sign of less reliance on glycolysis to lactate during intense exercise. Indeed, the high-altitude natives appeared to depend more on an anaerobic energy yield in an exhaustive effort, a notion supported by the higher muscle lactate, Cr/PCr ratio, and IMP content. Moreover, during the 15 min warm-up at moderate work intensity LN had a higher leg lactate release after 8 weeks of acclimatization compared to Acute Hypoxia and 2 weeks Chronic Hypoxia. Thus, the lactate paradox could not be demonstrated in any of the groups. Furthermore, high-altitude natives have a more pronounced mismatch between pyruvate formation and utilization in the mitochondria than low-altitude natives.
As it was claimed that one of the features of acclimatization to chronic exposure to hypoxia lead to a closer match of glycolytic rate and oxidative phosphorylation and one of the key features of adaptation seen in high-altitude natives from the Andes (Hochachka, 1989), we measured arterial lactate concentration and net leg lactate release, and the muscle lactate, adenine nucleotides, IMP, CrP and Cr concentrations. Since the actual activators of glycolysis are not the total muscle ADP, AMP and Pi but their free forms, we have calculated these parameters according to Dudley et al. (1987), and these can be seen in Table 2 together with arterial ammonia concentration and leg ammonia release as indicators of adenine nucleotide deaminase activity. An assumption is that the muscle pH–lactate relationship for intracellular H+ concentrations as established by Sahlin et al. (1975) is similar for LN in the course of acclimatization and HAN. However, these calculations are little effected in case the pH–lactate relationship would be somewhat different. The overwhelming picture is that HAN are more glycolytic than LN. After acclimatization to high altitude the LAN and HAN arterial lactate and leg net lactate release were very similar at sub-maximal and maximal relative workloads (Fig. 2). However, muscle lactate accumulation was nearly doubled in HAN as compared to LN. The density of skeletal muscle transporters involved in lactate and proton transport was similar in HAN and LN (Juel et al. 2003), which might explain the similar leg lactate release despite the much higher muscle lactate content. HAN total lactate dehydrogenase (LDH) activity and isoform pattern was not significantly different from LN. However, this is not in disagreement with the higher muscle lactate accumulation in HAN since we argue that the rate of lactate formation is not dependent on total activity or isoform of the equilibrium enzyme LDH but on the local intracellular pyruvate and NADH concentration (van Hall, 2000; van Hall et al. 2003). This suggestion is best illustrated by the fact that muscle subunit LDH-deficient patients produce substantial quantities of lactate especially compared to their low total activity (Kanno & Maekawa, 1995; Van Hall, 2000). The HAN's higher muscle IMP, ammonia, PCr utilization and Cr/PCr ratio, and the calculated free ADP, AMP and Pi at exhaustion suggest that 6-phosphofructo 1-kinase was more activated. Therefore, presumably the rate of glycolysis was higher in HAN compared to LAN causing high local pyruvate concentration which pushes the LDH equilibrium to lactate formation resulting in the twofold higher muscle lactate accumulation. The hypothesis put forward by Hochachka (1989), suggesting a reduced glycolytic potential and tighter coupling between ATP production and utilization, is the exact opposite of our findings. Hochachka et al. (1991) observed that Quechuas (high-altitude natives in the Andes) accumulated less lactate in blood for a given amount of work when studied at sea level and that they had a lower muscle LDH activity (Hochachka et al. 1991, 1992). However, these observations have been questioned as the subjects were anaemic and it was suggested that the metabolic adaptation observed was linked to anaemia rather than to genetic or developmental hypoxia-induced adaptations (Favier et al. 1995). Since Aymaras and Quechuas are closely related it seems highly unlikely that genetic differences explain the discrepancy in the observed blood lactate responses (Favier et al. 1995; Kayser et al. 1995).
Table 2.
The estimated intracellular muscle pH, free ADP, AMP, Pi and the arterial and net leg ammonia release at rest and at the moment of exhaustion
| Danish lowlanders |
||||||
|---|---|---|---|---|---|---|
| 0 m |
4100 m |
High-altitude natives |
||||
| Sea Level | Acute Hypoxia | 2 weeks Chronic Hypoxia | 8 weeks Chronic Hypoxia | Ambient air | ||
| pH | Rest | 7.04 ± 0.02 | 7.00 ± 0.02 | 6.98 ± 0.02 | 7.03 ± 0.02 | 7.01 ± 0.02 |
| Exhaustion | 6.77 ± 0.04 | 6.74 ± 0.06 | 6.65 ± 0.05 | 6.67 ± 0.08 | 6.60 ± 0.03#1,2 | |
| ADPf (μmol (kg dm)−1) | Rest | 98 ± 5 | 110 ± 7 | 121 ± 6 | 107 ± 8 | 98 ± 5 |
| Exhaustion | 315 ± 49 | 289 ± 51 | 363 ± 34 | 410 + 41*# | 472 ± 31#1,2,3 | |
| AMPf (μmol (kg dm)−1) | Rest | 0.47 ± 0.03 | 0.60 + 0.07 | 0.71 ± 0.06 | 0.57 ± 0.09 | 0.46 ± 0.04 |
| Exhaustion | 5.97 ± 2.12 | 5.33 ± 1.88 | 7.05 ± 1.33 | 10.01 ± 2.70*# | 12.29 ± 2.07#1,2,3,4 | |
| Pi (mmol (kg dm)−1) | Exhaustion | 62 ± 5 | 61 ± 7 | 66 ± 5 | 73 ± 9 | 81 ± 4#1,2,3 |
| NH3 (μmol l−1) | Rest | 39 ± 8 | 60 ± 8 | 75 ± 11 | 82 ± 14 | 83 ± 16 |
| Exhaustion | 195 ± 21 | 172 ± 33 | 201 ± 16 | 236 ± 13 | 289 ± 20#1,2,34 | |
| NH3 release (μmol min−1) | Rest | −6 ± 3 | −9 ± 6 | −6 ± 6 | −2 ± 6 | −7 ± 5 |
| Exhaustion | 107 ± 16 | 75 ± 33 | 105 ± 18 | 95 ± 21 | 145 ± 19#1,2,4 | |
Values are means of male lowlanders (n= 6) and high-altitude natives/ambient air (n= 7).
Significantly different from Sea Level,
significantly different from Acute Hypoxia.
Significantly different from lowlanders 1at Sea Level, 2under Acute Hypoxia, 32 weeks Chronic Hypoxia, 48 weeks Chronic Hypoxia.
Significant differences between 2 weeks and 8 weeks Chronic Hypoxia. pH was calculated according to Sahlin et al. (1975). Free ADP (ADPf) was calculated by assuming equilibrium of the creatine kinase reaction and using the measured ATP, PCr, Cr and the estimated pH (Dudley et al. 1987) For the creatine kinase equilibrium constant 1.66 × 109 l mol−1 was used. Free AMP (AMPf) was estimated assuming equilibrium of the adenylate kinase reaction using the measured ATP and estimated ADPf and the adenylate kinase equilibrium constant of 1.05. Inorganic phosphate (Pi) at exhaustion was estimated as the resting free phosphate of 10.8 mmol (kg dm)−1) and the difference in creatine phosphate between exhaustion and rest.
In the present study the glycolytic potential during incremental exercise to exhaustion seemed unchanged in the course of acclimatization of LN in terms of muscle lactate production and glycogen utilization. In addition, the muscle adenine nucleotides and IMP content at the moment of exhaustion were similar in all trials. Green and colleagues made similar measurements during the Operation Everest II study (Green et al. 1989). They observed a reduction in muscle IMP and lactate at the moment of exhaustion, which seemed to support the hypothesis of a tighter coupling between ATP production and utilization and a decreased glycolytic potential. Of note, however, is that Green et al. (1989) observed a high and unchanged glycogen utilization during exercise at altitude, which was of the same magnitude as observed in the present study. They claimed their high glycogen utilization at altitude to be due to an analytical error; a statement that might be questioned not only based on the observations from the present study, but also because the analysis is quite uncomplicated compared to lactate and IMP. The fact that in Operation Everest II the volunteers were continuously exposed to progressive hypobaria over 40 days (Houston et al. 1987) and thus, never acclimatized to chronic hypoxia, might have impaired their ability to push themselves further during exercise which is mandatory in order to produce IMP and high lactate levels in skeletal muscle.
As mentioned above, it seemed that glycolytic potential was unchanged in the course of acclimatization; however, two measurements may suggest otherwise. Firstly, a markedly higher arterial lactate and net lactate release were observed during 15 min warm-up at 100 W, and secondly, the calculated free ADP and AMP concentrations were significantly higher at exhaustion after 8 weeks of acclimatization. Moreover, the subjects of this investigation were also studied at sea level, acute hypoxic exposure and after 4 weeks of acclimatization during a 1 h bicycle exercise at both the relative (50%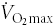 ) and absolute workload (150 W) of sea level. Venous lactate concentrations were elevated after 15 min of exercise at both relative and absolute workload after 4 weeks of acclimatization. Therefore, it seems that upon the initiation of low to moderate continuous exercise glycolysis is more activated after acclimatization. At the start of exercise, the muscle ATP requirement for contraction is instantaneously many-fold increased whereas the increase in muscle oxygen utilization is somewhat delayed (Bangsbo et al. 2000; Krustrup et al. 2004; Grassi, 2005). Since muscle ATP levels are virtually unchanged that means that ATP hydrolysis is matched by re-synthesis via PCr and glycolysis, the latter quantitatively most important. These processes are crucial to match ATP demand until ATP synthesis via oxidative phosphorylation or ATP transfer via creatine kinase convection from mitochondria to myofibrils become optimal (Hochachka, 2003; Kaasik et al. 2003; Grassi, 2005). Thus, the rate of glycolysis and lactate formation ceases when exercise at a constant workload continues. Although lower compared to the initial phase of exercise, a substantial rate of glycolysis close to the myofibrils remains mandatory. However, the net lactate release becomes small due to the simultaneous lactate uptake from the circulation and subsequent oxidation (van Hall et al. 2002, 2003). A possible explanation for the higher lactate concentration and net release at the start of exercise in acclimatized LN may lay in the observation that with duration of acclimatization the factional oxygen extraction, skeletal muscle capillary O2 conductance and estimated muscle diffusion capacity was found reduced at maximal exercise, albeit even after 8 weeks of acclimatization still higher in LN as compared to HAN (for detailed discussion see Lundby et al. 2006). This may imply that upon the start of exercise there is a longer lag phase between optimal oxidatively produced ATP and demand at the myofibril. This results in an enhanced reliance on PCr and possibly ATP hydrolysis causing higher local Pi, ADP and AMP levels and consequently a more pronounced activation of glycolysis and lactate formation. Moreover, the very much higher levels of these activators of glycolysis in connection with a more reduced leg oxygen extraction in HAN as compared to LN may explain the nearly double lactate accumulation in HAN muscle at the moment of exhaustion. Thus, the higher glycolytic potential may be the result of a more pronounced activation of glycolysis and/or an increase in glycolytic enzymes. Indeed, mountaineers after the expedition to Mounts Everest and Lhotse had increased levels of enzymes involved in glycolysis, whereas those of aerobic–oxidative metabolism had decreased (Howald et al. 1990). Moreover, skeletal muscle of rats chronically exposed to hypoxia showed an up-regulation of glycolytic enzymes and deaminases involved in ATP and AMP breakdown and a down-regulation of enzymes involved in the TCA cycle, ATP production and the electron transport chain (DePalma et al. 2007).
) and absolute workload (150 W) of sea level. Venous lactate concentrations were elevated after 15 min of exercise at both relative and absolute workload after 4 weeks of acclimatization. Therefore, it seems that upon the initiation of low to moderate continuous exercise glycolysis is more activated after acclimatization. At the start of exercise, the muscle ATP requirement for contraction is instantaneously many-fold increased whereas the increase in muscle oxygen utilization is somewhat delayed (Bangsbo et al. 2000; Krustrup et al. 2004; Grassi, 2005). Since muscle ATP levels are virtually unchanged that means that ATP hydrolysis is matched by re-synthesis via PCr and glycolysis, the latter quantitatively most important. These processes are crucial to match ATP demand until ATP synthesis via oxidative phosphorylation or ATP transfer via creatine kinase convection from mitochondria to myofibrils become optimal (Hochachka, 2003; Kaasik et al. 2003; Grassi, 2005). Thus, the rate of glycolysis and lactate formation ceases when exercise at a constant workload continues. Although lower compared to the initial phase of exercise, a substantial rate of glycolysis close to the myofibrils remains mandatory. However, the net lactate release becomes small due to the simultaneous lactate uptake from the circulation and subsequent oxidation (van Hall et al. 2002, 2003). A possible explanation for the higher lactate concentration and net release at the start of exercise in acclimatized LN may lay in the observation that with duration of acclimatization the factional oxygen extraction, skeletal muscle capillary O2 conductance and estimated muscle diffusion capacity was found reduced at maximal exercise, albeit even after 8 weeks of acclimatization still higher in LN as compared to HAN (for detailed discussion see Lundby et al. 2006). This may imply that upon the start of exercise there is a longer lag phase between optimal oxidatively produced ATP and demand at the myofibril. This results in an enhanced reliance on PCr and possibly ATP hydrolysis causing higher local Pi, ADP and AMP levels and consequently a more pronounced activation of glycolysis and lactate formation. Moreover, the very much higher levels of these activators of glycolysis in connection with a more reduced leg oxygen extraction in HAN as compared to LN may explain the nearly double lactate accumulation in HAN muscle at the moment of exhaustion. Thus, the higher glycolytic potential may be the result of a more pronounced activation of glycolysis and/or an increase in glycolytic enzymes. Indeed, mountaineers after the expedition to Mounts Everest and Lhotse had increased levels of enzymes involved in glycolysis, whereas those of aerobic–oxidative metabolism had decreased (Howald et al. 1990). Moreover, skeletal muscle of rats chronically exposed to hypoxia showed an up-regulation of glycolytic enzymes and deaminases involved in ATP and AMP breakdown and a down-regulation of enzymes involved in the TCA cycle, ATP production and the electron transport chain (DePalma et al. 2007).
It has been hypothesized that the lactate paradox is transient in the course of chronic exposure to hypoxia (Van Hall et al. 2001). This was based on findings from several studies with reduced blood lactate concentration with short-term acclimatization. We also observed a somewhat lower capillary peak blood lactate concentration after 2 weeks compared to 5 weeks at 5400 m in a field study at the base camp of Mt Everest (Lundby et al. 2000). However, the present study clearly demonstrates that no lactate paradox exists during exercise early (2 weeks) or late during acclimatization (8 weeks) in motivated healthy lowlanders. The protocol and subjects of the present study were carefully chosen in order to overcome all potential adverse effects of a stay at altitude. Possible explanations for the findings of reduced lactate levels during exercise with chronic exposure to hypoxia in other studies might be that: (1) some studies were field studies or semi-field studies implying a risk of underfeeding, possibly in combination with gastro-intestinal-related problems, which may cause low muscle glycogen stores and thus, a reduced lactate accumulation; (2) some studies were conducted at altitudes far above that of permanent human habitations, which potentially caused patho-physiological conditions with reduced lactate levels during exercise up to resting levels (Cerretelli & Samaja, 2003; van Hall, 2007b); (3) other studies have not included arterial samples or arterialized blood which may cause an artificially lower lactate concentration, especially when using the same incremental exercise protocol at sea level compared to altitude; (4) the lactate paradox has occasionally been visualized by combining different studies or studies that used discontinuous exercise protocols (West, 1986; Bender et al. 1989; Hochachka et al. 1991; Reeves et al. 1992); (5) it has been suggested that the observation of the lactate paradox is dependent on exercise intensity and duration. Grassi and co-workers observed that after 10 days of acclimatization to 5050 m blood lactate accumulation was unaffected during short supra-maximal exercise (Grassi et al. 1995, 2001). However, peak blood accumulation was decreased during 20–25 min of incremental exercise to exhaustion (Grassi et al. 2001). In view of the findings of the present study and Lundby & van Hall (2002) it is not a likely explanation for the existence/absence of the lactate paradox.
In the studies where a lower blood lactate concentration has been observed during exercise, it has been linked with lower blood adrenaline levels as adrenaline levels were higher in acute compared to chronic hypoxia (Brooks et al. 1992; Pronk et al. 2003). However, a role for adrenaline was disputed as an effective β−adrenergic blockade did not affect the blood lactate response during exercise after 3 weeks of acclimatization (Mazzeo et al. 1994). In the present study adrenaline levels were substantially higher during the acute hypoxic condition compared to sea level with a further elevation during the prolonged exposure to hypoxia. These findings imply that under chronic hypoxic conditions the sympathoadrenal response is significantly enhanced and that no direct relation exists between arterial adrenaline and lactate concentrations.
In summary, sea-level residents in the course of acclimatization to high altitude did not exhibit a reduced capacity for the active muscle to produce lactate. Thus, the lactate paradox concept could not be demonstrated. High-altitude natives from the Andes actually exhibit a higher anaerobic energy production than lowlanders after 8 weeks of acclimatization reflected by an increased muscle lactate accumulation and enhanced adenine nucleotide breakdown.
Acknowledgments
We thank Drs Monroy Traverso, Dr Quevedo, and Dr Straatman from the Hospital Municipal Boliviano Hollandés for their invaluable help in the organization of this study, and extend our gratitude to Dr Cabezas and her staff from Maternidad Maria Madre de Dios, El Alto, where the experiments were conducted for all their help and support. We would also like to thank Professor Carlos Aguirre and Ing. Ismael Montes de Oca from the Academia Nacional de Ciencas de Bolivia for invaluable help. We are grateful to Radiometer (Copenhagen, Denmark) and Monark (Sweden) for placing valuable equipment at our disposal. The Copenhagen Muscle Research Centre is receiving a grant from the Danish National Research Foundation (grant no. 504-14). J. A. L. Calbet was on leave from the Department of Physical Education at the University of Las Palmas de Gran Canaria, Spain.
Glossary
Abbreviations
- Cr
creatine
- HAN
high-altitude natives
- IMP
inosine monophosphate
- LDH
lactate dehydrogenase
- LN
lowland natives
- PCr
creatine phosphate
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Bender PR, Groves BM, McCullough RE, McCullough RG, Trad L, Young AJ, Cymerman A, Reeves JT. Decreased exercise muscle lactate release after high altitude acclimatization. J Appl Physiol. 1989;67:1456–1462. doi: 10.1152/jappl.1989.67.4.1456. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Comments on point:counterpoint: “The lactate paradox does/does not occur during exercise at high altitude”. J Appl Physiol. 2007;102:2408. doi: 10.1152/japplphysiol.00287.2007. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman A, Mazzeo RS, Sutton JR, Wolfe RR, Reeves JT. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol. 1992;72:2435–2445. doi: 10.1152/jappl.1992.72.6.2435. [DOI] [PubMed] [Google Scholar]
- Cerretelli P, Samaja M. Acid-base balance at exercise in normoxia and in chronic hypoxia. Revisiting the ‘lactate paradox’. Eur J Appl Physiol. 2003;90:431–448. doi: 10.1007/s00421-003-0928-x. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Forster HV, Birnbaum ML, Reddan WG, Thoden J, Grover RF, Rankin J. Control of exercise hypernea under varying durations of exposure to moderate hypoxia. Resp Physiol. 1972;16:213–231. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- DePalma S, Ripamonti M, Vigano A, Moriggi M, Capitanio D, Samaja M, Milano G, Cerretelli P, Wait R, Gelfi C. Metabolic modulation induced by chronic hypoxia in rats using a comparative proteomic analysis of skeletal muscle tissue. J Proteome Res. 2007;6:1974–1984. doi: 10.1021/pr060614o. [DOI] [PubMed] [Google Scholar]
- Dill DB, Edwards HT, Fölling A, Oberg SA, Pappenheimer AM, Tabott JH. Adaptations of the organism to changes in oxygen pressure. J Physiol. 1931;71:47–63. doi: 10.1113/jphysiol.1931.sp002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987;262:9109–9114. [PubMed] [Google Scholar]
- Favier R, Spielvogel D, Ferretti G, Kayser B, Hoppler H. Maximal exercise performance in chronic hypoxia and normoxia in high altitude natives. J Appl Physiol. 1995;78:1868–1874. doi: 10.1152/jappl.1995.78.5.1868. [DOI] [PubMed] [Google Scholar]
- Grassi B. Delayed metabolic activation of oxidative phosphorylation in skeletal muscle at exercise onset. Med Sci Sports Exerc. 2005;37:1567–1573. doi: 10.1249/01.mss.0000177472.67419.0a. [DOI] [PubMed] [Google Scholar]
- Grassi B, Ferretti G, Kayser B, Marzorati M, Colombini A, Marconi C, Cerretelli P. Maximal rate of blood lactate accumulation during exercise at altitude in humans. J Appl Physiol. 1995;79:331–339. doi: 10.1152/jappl.1995.79.1.331. [DOI] [PubMed] [Google Scholar]
- Grassi B, Mognoni P, Mazorati M, Mattiotti S, Marconi C, Cerretelli P. Power and peak blood lactate at 5050 m with 10 and 30 s ‘all out’ cycling. Acta Physiol Scand. 2001;172:189–194. doi: 10.1046/j.1365-201x.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- Green HJ, Sutton J, Young P, Cymerman A, Houston CS. Operation Everest II: muscle energetics during maximal exhaustive exercise. J Appl Physiol. 1989;66:142–150. doi: 10.1152/jappl.1989.66.1.142. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjö LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Hochachka PW. The lactate paradox: analysis of underlying mechanisms. Ann Sports Med. 1989;4:184–188. [Google Scholar]
- Hochachka PW. Mechanism and evolution of hypoxia-tolerance in humans. J Exp Biol. 1998;201:1243–1254. doi: 10.1242/jeb.201.8.1243. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. Intracellular convection, homeostasis and metabolic regulation. J Exp Biol. 2003;206:2001–2009. doi: 10.1242/jeb.00402. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Stanley C, McKenzie DC, Villena A, Monge C. Enzyme mechanisms for pyruvate-to-lactate flux attenuation: a study of sherpas, Quenchuas, and hummingbirds. Int J Sports Med. 1992;13(Suppl. 1):S119–S122. doi: 10.1055/s-2007-1024613. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Stanley C, Matheson GO, McKenzie DC, Allen PS, Parkhouse WS. Metabolic and work efficiencies during exercise in Andean natives. J Appl Physiol. 1991;70:1720–1730. doi: 10.1152/jappl.1991.70.4.1720. [DOI] [PubMed] [Google Scholar]
- Houston CS, Sutton JR, Cymerman A, Reeves JT. Operation Everest II: man at extreme altitude. J Appl Physiol. 1987;63:877–882. doi: 10.1152/jappl.1987.63.2.877. [DOI] [PubMed] [Google Scholar]
- Howald H, Pette D, Simoneau J-A, Uber A, Hoppeler H, Cerretelli P. III. Effects of chronic hypoxia and muscle enzyme activities. Int J Sports Med. 1990;11(Suppl. 1):S10–S14. doi: 10.1055/s-2007-1024847. [DOI] [PubMed] [Google Scholar]
- Juel C, Lundby C, Sander M, Calbet JAL, van Hall G. Human skeletal muscle and erythrocyte proteins involved in acid–base homeostasis: adaptations to chronic hypoxia. J Physiol. 2003;548:639–648. doi: 10.1113/jphysiol.2002.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Ventura-Clapier R. From energy store to energy flux: a study in creatine kinase deficient fast skeletal muscle. FASEB J. 2003;17:708–710. doi: 10.1096/fj.02-0684fje. [DOI] [PubMed] [Google Scholar]
- Kanno T, Maekawa M. Lactate dehydrogenase M-subunit deficiencies: clinical features, metabolic background, and genetic heterogeneities. Muscle Nerve. 1995;(Suppl. 3):S54–S60. doi: 10.1002/mus.880181413. [DOI] [PubMed] [Google Scholar]
- Kayser B. Lactate during exercise at high altitude. Eur J Appl Physiol. 1996;74:195–205. doi: 10.1007/BF00377441. [DOI] [PubMed] [Google Scholar]
- Kayser B, Favier R, Ferretti G, Desplanches D, Spielvogel H, Koubi H, Sempore B, Hoppeler H. Lactate and epinephrine during exercise in altitude natives. J Appl Physiol. 1996;81:2488–2494. doi: 10.1152/jappl.1996.81.6.2488. [DOI] [PubMed] [Google Scholar]
- Kayser B, Narici MV, Cibella F. Fatigue and performance at high altitude. In: Sutton JR, Houston CS, Coates G, editors. Hypoxia and Molecular Medicine. Burlington: Queen City; 1995. pp. 222–234. [Google Scholar]
- Klausen K, Robinson S, Micahel ED, Myhre LG. Effect of high altitude on maximal working capacity. J Appl Physiol. 1966;21:1191–1194. doi: 10.1152/jappl.1966.21.4.1191. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lundby C, Saltin B, van Hall G. The ‘lactate paradox’, evidence for a transient change in the course of acclimatization to severe hypoxia in lowlanders. Acta Physiol Scand. 2000;170:265–269. doi: 10.1046/j.1365-201x.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- Lundby C, van Hall G. Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand. 2002;176:195–201. doi: 10.1046/j.1365-201X.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- Lundby C, Sander M, van Hall G, Saltin B, Calbet JAL. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol. 2006;573:535–547. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA, Butterfield GE, Cymerman A, Roberts AC, Selland M, Wolfel EE, Reeves JT. β-Adrenergic blockade does not prevent the lactate response to exercise after acclimatization to high altitude. J Appl Physiol. 1994;76:610–615. doi: 10.1152/jappl.1994.76.2.610. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Secher NH, Rasmussen P, Kayser B, Wagner PD, Samaja M, Guazzi M, Grassi B, Marzorati M, Marconi C, Cerretelli P, Gladden LB. Comments on point:counterpoint: “The lactate paradox does/does not occur during exercise at high altitude”. J Appl Physiol. 2007;102:2403–2405. [Google Scholar]
- Pronk M, Tiemessen I, Hupperest MDW, Kennedy BP, Powell FL, Hopkins SR, Wagner PD. Persistence of the lactate paradox over 8 weeks at 3800 m. High Alt Med Biol. 2003;4:431–443. doi: 10.1089/152702903322616182. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Wolfel EE, Green HJ, Mazzeo RS, Young AJ, Sutton JR, Brooks GA. Oxygen transport during exercise at altitude and the lactate paradox: lessons from Operation Everest II and Pikes Peak. Exerc Sport Sci Rev. 1992;20:275–296. [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Hultman E. Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J. 1975;152:173–180. doi: 10.1042/bj1520173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The van Slyke equation. Scand J Clin Lab Invest Suppl. 1977;37:15–20. doi: 10.3109/00365517709098927. [DOI] [PubMed] [Google Scholar]
- Van Der Vusse GJ, Janssen GME, Coumans WA, Kuipers H, Does RJMM, Ten Hoor F. Effect of training and 15-, 25-, and 42-km contests on the skeletal muscle content of adenine and guanine nucleotides, creatine phosphate, and glycogen. Int J Sports Med. 1989;10:S146–S152. doi: 10.1055/s-2007-1024963. [DOI] [PubMed] [Google Scholar]
- van Hall G. Lactate as a fuel for mitochondrial respiration. Acta Physiol Scand. 2000;168:643–656. doi: 10.1046/j.1365-201x.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- van Hall G. Counterpoint: The lactate paradox does not occur during exercise at high altitude. J Appl Physiol. 2007a;102:2399–2401. doi: 10.1152/japplphysiol.00039a.2007. [DOI] [PubMed] [Google Scholar]
- van Hall G. Rebuttal from Dr. van Hall. J Appl Physiol. 2007b;102:2402. [Google Scholar]
- van Hall G, Calbet JAL, Søndergaard H, Saltin B. The re-establishment of the normal blood lactate response to exercise in humans after prolonged acclimatization to altitude. J Physiol. 2001;563:963–975. doi: 10.1111/j.1469-7793.2001.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Calbet JAL, Søndergaard H, Saltin B. Skeletal muscle carbohydrate and lactate metabolism after 9 weeks of acclimatization to 5260 m. Am J Physiol Endocrinol Metab. 2002;283:E1203–E1213. doi: 10.1152/ajpendo.00134.2001. [DOI] [PubMed] [Google Scholar]
- van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg H-C, Saltin B, Calbet JAL. Leg and arm lactate and substrate metabolism during exercise. Am J Physiol Endocrinol Metab. 2003;284:E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]
- West JB. Lactate during exercise at extreme altitude. Fed Proc. 1986;45:2953–2957. [PubMed] [Google Scholar]
- West JB. Point: The lactate paradox does/does not occur during exercise at high altitude. J Appl Physiol. 2007a;102:2398–2399. doi: 10.1152/japplphysiol.00039.2007. [DOI] [PubMed] [Google Scholar]
- West JB. Rebuttal from Dr. West. J Appl Physiol. 2007b;102:2401. [Google Scholar]