Abstract
Whole-body heat stress reduces orthostatic tolerance via a yet to be identified mechanism(s). The reduction in central blood volume that accompanies heat stress may contribute to this phenomenon. The purpose of this study was to test the hypothesis that acute volume expansion prior to the application of an orthostatic challenge attenuates heat stress-induced reductions in orthostatic tolerance. In seven normotensive subjects (age, 40 ± 10 years: mean ±s.d.), orthostatic tolerance was assessed using graded lower-body negative pressure (LBNP) until the onset of symptoms associated with ensuing syncope. Orthostatic tolerance (expressed in cumulative stress index units, CSI) was determined on each of 3 days, with each day having a unique experimental condition: normothermia, whole-body heating, and whole-body heating + acute volume expansion. For the whole-body heating + acute volume expansion experimental day, dextran 40 was rapidly infused prior to LBNP sufficient to return central venous pressure to pre-heat stress values. Whole-body heat stress alone reduced orthostatic tolerance by ∼80% compared to normothermia (938 ± 152 versus 182 ± 57 CSI; mean ±s.e.m., P < 0.001). Acute volume expansion during whole-body heating completely ameliorated the heat stress-induced reduction in orthostatic tolerance (1110 ± 69 CSI, P < 0.001). Although heat stress results in many cardiovascular and neural responses that directionally challenge blood pressure regulation, reduced central blood volume appears to be an underlying mechanism responsible for impaired orthostatic tolerance in the heat-stressed human.
The ability of the cardiovascular system to maintain arterial blood pressure during orthostatic stress is critical. Numerous investigations have demonstrated that during whole-body heat stress, one's ability to maintain arterial blood pressure and, ultimately, cerebral blood flow, in the face of an orthostatic challenge is impaired during perturbations such as upright tilting (Lind et al. 1968; Wilson et al. 2002b), gravitational acceleration (Allan & Crossley, 1972) and lower-body negative pressure (LBNP) (Johnson et al. 1973; Cui et al. 2004b; Wilson et al. 2006). Under such conditions, the maintenance of arterial blood pressure is dependent on the ability of the cardiovascular system to increase total peripheral resistance and attenuate the reduction in cardiac output, despite the relative displacement of blood from the central circulation (Crandall et al. 2008). While selected studies have investigated acute countermeasures to attenuate heat stress-induced reductions in orthostatic tolerance, such as acute skin surface cooling (Wilson et al. 2002b) and suprasystolic leg cuff occlusion during head-up tilt (Lind et al. 1968), the precise mechanisms causing this reduction in tolerance have not been fully elucidated.
Whole-body heating, itself, results in multiple cardiovascular and neural adjustments, including increased cardiac output (Rowell et al. 1969; Minson et al. 1998, 1999; Wilson et al. 2007), marked decreases in cutaneous vascular resistance (Lewis & Pickering, 1931; Johnson & Proppe, 1996), splanchnic vasoconstriction (Rowell et al. 1971; Minson et al. 1999) and increased muscle sympathetic nerve activity (Niimi et al. 1997; Crandall et al. 1999a; Yamazaki et al. 2003; Cui et al. 2004a; Keller et al. 2006a). These responses occur in a coordinated effort to redistribute cardiac output to the cutaneous circulation to aid in appropriate heat dissipation (i.e. body temperature regulation). Despite the large decrease in cutaneous vascular resistance and consequent decrease in total peripheral resistance during whole-body heat stress (Rowell et al. 1969, 1970, 1971; Wilson et al. 2007), arterial blood pressure is well-maintained or only slightly decreased in supine individuals (Rowell et al. 1969 1970, 1971; Johnson et al. 1973; Wilson et al. 2007).
An orthostatic challenge results in many directionally similar reflex-mediated cardiovascular responses, including vasoconstriction of non-cutaneous vascular beds (Fadel et al. 2004) and increased muscle sympathetic nerve activity (Fu et al. 2004b; Convertino et al. 2006). While increased heart rate is classically observed in response to orthostatic challenge, cardiac output is often decreased as a result of diminished stroke volume (Levine et al. 1991; Harms et al. 2003; Fu et al. 2004a, 2005). To that end, another hallmark response to whole-body heat stress in humans is a reduction in indices of ventricular filling pressures (Rowell et al. 1969; Minson et al. 1998, 1999; Wilson et al. 2007; Crandall et al. 2008) and a concomitant decrease in central blood volume (Crandall et al. 2008). Although these reductions in ventricular filling pressures and central blood volume are the result of normal thermoregulatory responses, they may ultimately play a principal role in diminished orthostatic tolerance during whole-body heat stress. When an orthostatic challenge is imposed upon a heat-stressed individual, the competition between adequate thermoregulatory responses and the maintenance of arterial pressure may overwhelm the cardiovascular system by way of inadequate cardiac filling.
Although factors such as impaired arterial baroreflex control of blood pressure (Crandall, 2000) and impaired cutaneous vasoconstrictor responsiveness (Wilson et al. 2002a) during whole-body heating may contribute to impaired orthostatic tolerance, the additive effects of orthostatic challenge and heat stress on central blood volume is likely to be instrumental in the development of heat stress-induced orthostatic intolerance. Therefore, the aim of this study was to test the hypothesis that acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans.
Methods
Seven individuals (5 men and 2 women) voluntarily participated in the investigation (age, 40 ± 10 years; height, 175 ± 12 cm; mass, 79 ± 8 kg; mean ±s.d.). All procedures conformed to the standards set by the Declaration of Helsinki. Each subject signed an informed consent that was approved by the institutional review boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas. Prior to participation, all subjects were familiarized with the testing protocols. Subjects were healthy, non-smokers, free of known cardiovascular and respiratory diseases, and not using prescription or over-the-counter medications. Subjects were advised to not consume alcohol for 24 h before any of the scheduled experiments. Subjects were also asked to refrain from consuming caffeinated beverages for the 12 h period prior to the scheduled experiments.
Instrumentation
At the beginning of each experimental day, subjects were dressed in a tube-lined perfusion suit enabling the control of skin and core temperature (Tcore) via changing the temperature of the water perfusing the suit. Tcore was measured using a telemetry temperature pill swallowed 2–3 h before data collection (HQ Inc., Palmetto, FL, USA). Whole-body mean skin temperature (Tsk) was measured from the electrical average of six thermocouples (Taylor et al. 1989) fixed to the skin with porous adhesive tape. Non-invasive measures of arterial blood pressure were continuously obtained using finger cuff photoplethysmography (Finometer Pro, FMS, Amsterdam, the Netherlands). Arterial blood pressure was also measured by auscultation of the brachial artery (Tango, Suntech Medical Instruments, Raleigh, NC, USA). Brachial artery blood pressure was used for data analysis while measures from the Finometer were used to monitor beat-to-beat blood pressure to aid in the detection of ensuing syncope. Heart rate was collected from an electrocardiogram (ECG) signal (Agilent, Munich, Germany) interfaced with a cardiotachometer (1 000 Hz sampling rate, CWE, Ardmore, PA, USA). A laser Doppler flowmeter probe (Perimed, North Royalton, OH, USA) was placed on dorsal forearm skin midway between the wrist and the elbow on the forearm not exposed to the tube-lined suit.
On experimental day 1, subjects were instrumented with a central venous catheter advanced into the superior vena cava via the basilic vein. Adequate position of the catheter was established using the following criteria: (1) distance the catheter was advanced into the body, (2) adequate pressure waveforms, and (3) rapid rise and fall in venous pressure in response to Valsalva and Mueller manoeuvres, respectively. The catheter was connected to a pressure transducer and zero-referenced to the midaxillary line. The catheter remained in each subject for three consecutive days and was removed upon completion of experimental day 3. Each day, following all experimental testing procedures, the catheter was filled with heparinized saline. At the beginning of each experimental day, the specific gravity of the subject's urine was determined using a digital refractometer. If the specific gravity for a given subject was greater than 1.020, the subject consumed a small amount of water, and urine specific gravity was re-tested at 30 min intervals until specific gravity was below this threshold.
Orthostatic tolerance testing (LBNP)
Orthostatic tolerance was determined using graded LBNP testing. Beginning at −20 Torr, 3 min stages of LBNP were applied, progressing by −10 Torr for each subsequent stage (i.e. −20, −30, −40, −50 Torr, etc.). Test termination was based upon the following criteria: continued self-reporting by the subject of feeling faint or feeling like he/she could no longer tolerate LBNP, pallor, diaphoresis, rapid and progressive decrease in blood pressure resulting in systolic blood pressure being less than 70 mmHg, and/or relative bradycardia accompanied with narrowing of pulse pressure. Typically, a combination of the aforementioned conditions was observed at the cessation of the tolerance test with a reduction in pulse pressure along with relative bradycardia being the most common conditions.
If subjects endured to −100 Torr, that stage continued until subjects presented with symptoms of ensuing pre-syncope. The total time of each test was measured and used to determine a cumulative stress index (CSI). CSI was calculated by summing the product of the negative pressure and duration, in minutes and fraction of minutes, at each stage of negative pressure (e.g., 20 Torr × 3 min + 30 mmHg × 3 min + 50 mmHg × 3 min, etc.) until the test was terminated.
Experimental protocol
All testing was conducted in the morning of each experimental day. Furthermore, for each experimental day the subject was supine for a similar duration between experimental days such that each LBNP challenge was performed at approximately the same time between the three testing days. Experimental day 3 was always the volume expansion day, while experimental days 1 and 2 were randomized between normothermia and whole-body heating days. This design was chosen to eliminate any potential long-term effects (i.e. greater than 24 h) of dextran infusion on the obtained data.
Normothermia day
Water at 34°C was perfused through the tube-lined water perfusion suit for baseline data collection and throughout LBNP testing.
Whole-body heating day
Following ∼1 h exposure to 34°C water (and baseline data collection), ∼48°C water was perfused through the suit sufficient to increase Tcore by ∼1.5°C. As Tcore approached this temperature, the temperature of the water perfusing the suit was decreased to ∼44°C to attenuate further increases in Tcore. Upon achieving an increase in Tcore of 1.5°C, pre-LBNP baseline data were obtained followed by the LBNP challenge.
Heat stress plus volume expansion day
The protocol for this day was identical to the whole-body heating day. However, after achieving an increase in Tcore of ∼1.3°C, a rapid infusion of 500 ml warmed dextran 40 (n= 6) was administered followed by saline (if necessary) until central venous pressure returned to the value prior to heat stress. One to two minutes prior to dextran 40 infusion, 20 ml of dextran 1 (Promit) was administered intravenously as a prophylaxis for anaphylactic reactions associated with the infusion of dextran 40 (Ljungstrom et al. 1993). The infusion rate of the dextran was approximately 40 ml min−1. In an effort to avoid potential ‘overshoot’ of central venous pressure, the rate of dextran infusion was reduced as central venous pressure approached pre-heat stress values. In one subject, 500 ml of dextran was not necessary to restore central venous pressure. In two subjects, the infusion of 500 ml of dextran did not completely restore central venous pressure to pre-heat stress values. In these subjects, saline was administered at a similar rate (i.e. ∼40 ml min−1) for a few additional minutes after the completion of the dextran infusion. In all subjects, once central venous pressure reached pre-heat stress values, the rate of saline infusion was reduced to a minimal drip. One subject (the first trial) received saline only to return central venous pressure to pre-heat stress values. However, the volume of saline required to restore (and maintain) central venous pressure prior to LBNP testing was greater than 2 l (however, this subject's orthostatic tolerance was similarly improved following saline infusion compared to the remaining 6 subjects). In an effort to minimize the total infused volume, the remaining subjects were given dextran as the ‘priming’ dose to restore central venous pressure (dextran stays in the vascular space more effectively than saline). Based upon preliminary observations, each bag of dextran and saline was heated such that the temperature of the solution at the end of the catheter was ∼39°C. Warming these solutions eliminated any potential effect of cooling the subject during the infusion. The LBNP tolerance test began following cessation of rapid volume infusion and after central venous pressure plateau for ∼3 min.
Data analysis
Steady-state haemodynamic and thermal variables were determined from 45 s averages during normothermic and whole-body heating conditions, as well as during the final minute of respective LBNP stages. CSI was determined for LBNP tolerance tests on each experimental day. Cutaneous vascular conductance was calculated as the ratio of laser-Doppler flux and the difference between mean arterial blood pressure and central venous pressure.
Statistical analysis
Comparison of orthostatic tolerance, as measured via CSI, between experimental days was evaluated using a one-way ANOVA with repeated measures. The probability of tolerating a given stage of LBNP during the graded orthostatic tests was compared between experimental days using Kaplan–Meier survival analysis with log-rank tests (analysis of ‘survival,’ i.e., ‘tolerance probability,’ was considered the stage during which each subject presented with pre-syncopal symptoms). Comparisons of steady-state physiological variables between experimental conditions during respective normothermic baseline conditions were made using a one-way ANOVA with repeated measures. When the assumptions of normality were not met, non-parametric analyses were used. Comparisons of the effect of whole-body heating (normothermia versus heat stress) between whole-body heating experimental day and volume expansion experimental day after dextran infusion, but prior to LBNP, were made using a two-way ANOVA with repeated measures. Comparisons of steady-state physiological variables during whole-body heating prior to dextran infusion were not included in the statistical comparison between experimental heat stress days because prior to dextran administration internal temperature was lower during the volume infusion day relative to the whole-body heat stress day (see protocol above). However, separate one-way repeated measures ANOVA was used to illustrate the effect of dextran infusion on the volume expansion day (i.e., normothermic, heat stress pre- and post-volume infusion conditions). Post hoc pairwise multiple comparisons were made using Holm–Sidak analysis. Student's paired t test was used to compare cutaneous vascular conductance prior to and following dextran infusion during heat stress. Statistical significance was set at P < 0.05. Unless noted, data are reported as means ±s.e.m.
Results
Cardiovascular and thermal variables immediately prior to LBNP
Heat stress caused similar increases in Tcore (∼1.5°C) on the whole-body heating and the volume expansion day, compared to respective normothermic conditions (P < 0.001). Furthermore, whole-body heating was accompanied by an increase in heart rate (∼44 bpm, P < 0.001) on both heat stress days, while mean arterial pressure was unchanged by heat stress. Central venous pressure was similar during normothermic baseline on both heat stress days (see Table 1), while heat stress decreased this variable ∼4.3 mmHg (P < 0.001).
Table 1.
Group averaged variables between experimental days
| Whole-body heating day | Volume expansion day | ||||
|---|---|---|---|---|---|
| Normothermia day | NT baseline | preLBNP | NT baseline | preLBNP | |
| Heart rate (bpm) | 58 ± 3 | 59 ± 3 | 99 ± 7* | 59 ± 3 | 106 ± 8* |
| SBP (mmHg) | 112 ± 4 | 110 ± 4 | 114 ± 4 | 110 ± 6 | 116 ± 5 |
| DBP (mmHg) | 68 ± 3 | 70 ± 4 | 62 ± 4 | 65 ± 3 | 61 ± 1 |
| MAP (mmHg) | 83 ± 3 | 83 ± 4 | 79 ± 2 | 80 ± 3 | 79 ± 3 |
| CVP (mmHg) | 6.6 ± 0.9 | 6.7 ± 0.8 | 2.4 ± 1.0* | 7.5 ± 1.2 | 8.1 ± 1.1† |
| Core temp (°C) | 36.9 ± 0.1 | 36.9 ± 0.1 | 38.4 ± 0.1* | 36.8 ± 0.1 | 38.4 ± 0.1* |
| Skin temp (°C) | 34.5 ± 0.1 | 34.5 ± 0.2 | 38.4 ± 0.1* | 34.6 ± 0.1 | 38.6 ± 0.2* |
| Skin blood flux (AU) | 15 ± 3 | 14 ± 2 | 93 ± 10* | 17 ± 1 | 114 ± 7*† |
| CVC (AU (100/mmHg)–1) | 20 ± 4 | 18 ± 2 | 120 ± 10* | 25 ± 2 | 161 ± 7*† |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; CVP, central venous pressure; CVC, cutaneous vascular conductance. *Significantly different from respective normothermic (NT) baseline. †Significantly different from preLBNP of the whole-body heating day. P < 0.05.
Cardiovascular and thermal variables on heat stress plus volume expansion day
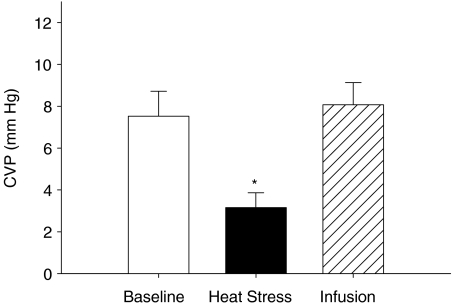
Prior to the infusion of dextran, whole-body heating reduced central venous pressure from 7.5 ± 1.2 mmHg during normothermic baseline to 3.2 ± 0.7mmHg (P < 0.001, see Fig. 1). Infusion of dextran restored central venous pressure (8.1 ± 1.1 mmHg) to values similar to that observed during normothermic baseline (P= 0.64). Mean arterial pressure was unchanged throughout the experimental protocol, including dextran infusion (P= 0.70). Furthermore, systolic (P= 0.230) and diastolic (P= 0.11) blood pressures were also unchanged throughout the experimental protocol. Heart rate was increased from normothermic baseline (59 ± 3 beats min−1) during whole-body heating (100 ± 5 beats min−1, P < 0.001). There was no further increase in heart rate following dextran infusion (106 ± 8 beats min−1, P= 0.62).
Figure 1. Group averaged (±s.e.m.) central venous pressure (CVP) during normothermia (Baseline), heat stress and heat stress plus volume expansion (Infusion) prior to lower-body negative pressure on the volume expansion day.
*Significantly different from baseline and infusion (P < 0.05).
Heat stress increased Tcore from 36.8 ± 0.1°C during normothermic baseline to 38.2 ± 0.1°C (P < 0.001). Following dextran infusion and prior to LBNP, Tcore had further increased to 38.4 ± 0.1°C (P < 0.001). Cutaneous vascular conductance increased during heat stress (141 ± 5 mmHg−1) compared to normothermic baseline (22 ± 2 AU mmHg−1, P < 0.001). Following infusion of dextran, cutaneous vascular conductance was further increased (161 ± 7 AU mmHg−1, P < 0.001).
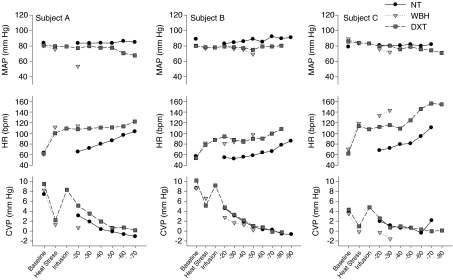
Figure 2 depicts mean arterial pressure, heart rate and central venous pressure during each condition (i.e. baseline, heat stress, volume infusion, LBNP stage, etc.) on all three experimental days for three representative subjects. Thermal and hemodynamic responses during LBNP were not statistically analysed due to the large discrepancy between the LBNP stages completed for each subject between experimental days.
Figure 2. Responses from three representative subjects on each experimental day.
NT, normothermia day; WBH, whole-body heating day; DXT, heat stress plus volume expansion day. The data depict responses from the LBNP stage prior to the final LBNP stage that resulted in syncopal symptoms and subsequent cessation of the LBNP challenge. MAP, mean arterial pressure; HR, heart rate; CVP, central venous pressure. LBNP, lower-body negative pressure.
Orthostatic tolerance testing between experimental days
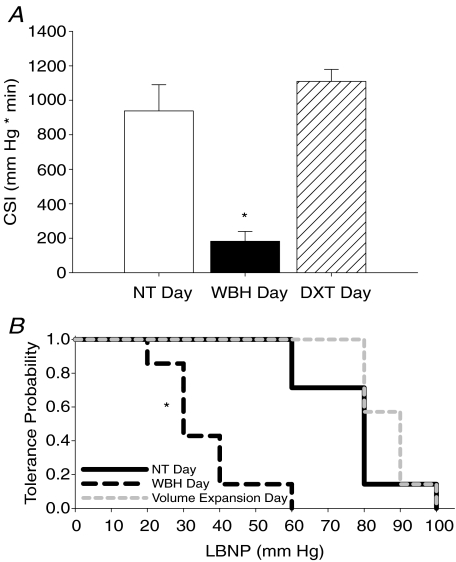
On the normothermic experimental day, the group average orthostatic tolerance was 988 ± 152 CSI. Orthostatic tolerance was markedly reduced on the whole-body heating experimental day (182 ± 57 CSI) compared to normothermic day values (P < 0.001). As hypothesized, orthostatic tolerance was restored on the volume expansion day (1110 ± 69 CSI, see Fig. 3A) despite similar increases in Tcore between heat stress days (i.e. whole-body heating day and heat stress plus volume expansion day; see Table 1).
Figure 3. Cumulative stress index and tolerance probability curves.
A, group averaged (±s.e.m.) cumulative stress index (CSI) as a quantitative measure of orthostatic tolerance during graded lower-body negative pressure on each experimental day. NT, normothermia day; WBH, whole-body heating day; DXT, heat stress plus volume expansion day. *Significantly different from NT and DXT days (P < 0.05). B, ‘Tolerance probability’ curves for each experimental day. Tolerance probability for each stage of lower body negative pressure (LBNP) depicted as a ratio of subjects that completed the respective stage of LBNP; *significantly different from NT and DXT days (P < 0.05).
The tolerance probability curve for LBNP was significantly different on the whole-body heating experimental day (mean survival LBNP 35.7 Torr; 26.3 and 45.1 lower and upper 95% confidence intervals) compared to both the normothermic (mean survival LBNP 77.1 Torr; 66.9 & 87.4 lower and upper 95% confidence intervals) and volume expansion experimental days (mean survival LBNP 87.1 Torr; 81.5 and 92.7 lower and upper 95% confidence intervals) (see Fig. 3B, P < 0.05).
Discussion
The primary finding from this investigation is that during heat stress, acute volume expansion completely reversed heat stress-induced reductions in orthostatic tolerance. Passive whole-body heat stress, to the extent utilized in this investigation (ΔTcore∼1.5°C), markedly reduced orthostatic tolerance compared to normothermic conditions (∼80% reduction based upon the CSI, see Fig. 3A). Despite the potential for multiple central and peripheral mechanisms to contribute to reduced orthostatic tolerance during heat stress, restoration of central venous pressure and presumably, central blood volume, can entirely compensate for reduced orthostatic tolerance during heat stress.
During whole-body heat stress, the ability to maintain cerebral perfusion during orthostatic challenge is impaired (Wilson et al. 2006). While heat stress-induced reductions in orthostatic tolerance have been demonstrated using multiple experimental models, such as upright tilting (Lind et al. 1968; Wilson et al. 2002b), gravitational acceleration (Allan & Crossley, 1972) and lower-body negative pressure (LBNP) (Johnson et al. 1973; Cui et al. 2004b; Wilson et al. 2006), the underlying mechanisms for this intolerance remain unclear. Lind et al. (1968) investigated the hypothesis that heat stress-induced reductions in orthostatic tolerance were due to excessive pooling of blood in the legs during these combined stressors. This was accomplished by inflating cuffs placed on the thighs to super-systolic pressures in heat-stressed subjects prior to and throughout an orthostatic challenge. Despite prevention of blood pooling in the lower limbs via this cuff inflation, orthostatic tolerance remained impaired relative to without cuff inflation. They concluded that pooling of blood in the legs was not the primary mechanism for heat-induced reductions in orthostatic tolerance. Although those findings do not discount the role of increased leg blood volume in adversely affecting the maintenance of arterial blood pressure during all conditions, they do support the idea that other mechanisms may contribute to orthostatic intolerance in the heat-stressed human. Furthermore, it remains possible that excessive pooling of blood in other dependent vascular beds (e.g. the splanchnic and/or cutaneous vasculature) contributes to reduced orthostatic tolerance during heat stress. The application of LBNP in the supine position does not result in the same hydrostatic gradient distribution as exposure to upright tilting (i.e. the gradient produced by LBNP is specific to the waist seal and below and is not graded, but equal across the entire lower body) (Taneja et al. 2007). Although there are obvious differences between the orthostatic challenges utilized in the present study and the work of Lind et al., findings from the current study demonstrate the importance of the resulting central pressure and, presumably central blood volume, on orthostatic tolerance during heat stress.
Wilson et al. (2002b) used acute skin surface cooling in otherwise heat-stressed humans as a potential countermeasure to heat stress-induced orthostatic intolerance and demonstrated improved orthostatic tolerance in some subjects. Increases in central venous pressure, and presumably central blood volume (Cui et al. 2005), accompanying skin surface cooling may be the primary mechanism for improved tolerance in those subjects. However, the extent to which skin surface cooling altered central venous pressure and central blood volume of the heat-stressed individuals investigated by Wilson et al. is unknown. Wilson and colleagues did not perform a true orthostatic tolerance test, but rather used 10 min of head-up tilt during combinations of normothermic and heat stress conditions, both with and without acute skin surface cooling. In the current study, orthostatic tolerance tests were not limited by a pre-determined test time, but were continued until each subject presented with signs of ensuing syncope. Furthermore, the mechanisms by which orthostatic tolerance was improved between the current study and the work of Wilson et al. (2002b) may be different. Skin surface cooling of the heat-stressed individual reduced cutaneous vascular conductance prior to upright tilt. In the current study, cutaneous vascular conductance was increased by acute volume expansion of the heat-stressed individual, similar to previous findings by Crandall et al. (1999b) who demonstrated increased cutaneous vascular conductance following saline infusion in otherwise heat-stressed individuals. This observation demonstrates clear differences in the contribution of different vascular beds and, potentially, differing neural adjustments between skin surface cooling and acute volume expansion as countermeasures to heat stress-induced orthostatic intolerance.
It is well-established that in hypovolaemic conditions induced by spaceflight (Bungo et al. 1985) and prolonged bed rest (Haruna et al. 1998), as well as during dehydration (Harrison et al. 1986; Davis & Fortney, 1997), restoration of blood volume improves orthostatic tolerance. Furthermore, it has been shown that plasma volume positively correlates and predicts time to presyncope during LBNP (Ludwig & Convertino, 1994). The current investigation extends these observations to the acute, central hypovolaemia induced by whole body heating. Therefore, despite the marked redistribution of cardiac output to the cutaneous vasculature during whole-body heating, as well as other physiological factors such as impaired baroreflex function and impaired cutaneous vascular vasoconstrictor responsiveness observed during whole-body heating, it appears that the decrease in central blood volume and central venous pressure related to whole-body heat stress is likely to be a key variable in the accompanying orthostatic intolerance.
In the current study, the effect of acute volume expansion on total peripheral resistance and cardiac output was not assessed. Therefore, future studies are warranted to examine the precise cardiovascular mechanisms (e.g. preservation of cardiac output and/or elevated total peripheral resistance) responsible for the restored blood pressure and orthostatic tolerance following acute volume expansion in heat-stressed subjects.
Other factors, such as impaired arterial baroreflex control of blood pressure (Crandall, 2000) and impaired cutaneous vasoconstrictor responsiveness (Wilson et al. 2002a) observed during whole-body heating have been proposed as potential contributors to the heat stress-induced reduction in orthostatic tolerance. Crandall (2000) demonstrated reduced maximal gain of the carotid-vasomotor function curve in heat-stressed subjects, while carotid-cardiac baroreflex function was not appreciably changed in that study. Considering that during passive whole-body heat stress, the fraction of cardiac output directed to the cutaneous vasculature can exceed 50% of the prevailing cardiac output (Rowell et al. 1969), a relative lack of carotid baroreflex control of the cutaneous sympathetic nerves and associated cutaneous vasculature (Wallin et al. 1975; Crandall et al. 1996) may be fundamental in the reduced carotid-vasomotor reflex, although the carotid baroreflex may exhibit some dynamic control of the cutaneous vasculature (Keller et al. 2006b). Furthermore, diminished cutaneous vasoconstrictor responsiveness has been proposed as a potential contributor to reduced orthostatic tolerance during heat stress (Wilson et al. 2002a). Together, both diminished arterial baroreflex function associated with heat stress and reduced cutaneous vascular responsiveness may pose significant challenges to the cardiovascular system during combined heat stress and orthostatic challenge. However, the present data clearly demonstrate that restoration of central venous pressure, and presumably central blood volume, can overcome these and, perhaps, other factors associated with heat stress that contribute to heat stress induced orthostatic intolerance.
Limitations
The effect of volume infusion on central blood volume was not directly assessed; rather, central venous pressure was used as a marker of the volume load administered. It is recognized that changes in central blood volume and central venous pressure are not consistently proportional, as the linearity of the relationship between these variables has been questioned (Marik et al. 2008). However, the direction of the relationship is positive in nature; that is, increases in central venous pressure are accompanied by increases in central blood volume. Volume infusion restored central venous pressure to pre-heat stress values prior to the application of LBNP during whole-body heating. This approach was chosen so that each subject had a reference marker (pre-heat stress baseline central venous pressure) reflective of the volume load administered. It is possible that whole-body heat stress altered the central venous pressure–volume relationship so that a given central blood volume resulted in a different venous pressure. Therefore, it remains possible that acute volume expansion used in this study resulted in a relative volume over- or under-expansion compared to normothermic conditions. Regardless, the restoration of orthostatic tolerance observed following dextran infusion clearly demonstrates the importance of the central reservoir in the development of heat stress-induced orthostatic intolerance. Future studies to assess the relationship between changes in central blood volume and central venous pressure in heat-stressed individuals may help to better understand underlying mechanisms responsible for the improved orthostatic tolerance following acute volume expansion.
In summary, heat stress-induced reductions in orthostatic tolerance were ameliorated following restoration of central venous pressure using acute infusion of dextran prior to orthostatic testing. Such a finding demonstrates the importance of central venous pressure and presumably, central blood volume, on the development of heat stress-induced orthostatic intolerance. Although other factors may be important contributors to heat stress-induced orthostatic intolerance, acute volume restoration can override these potential factors so that the ability to withstand gravitational challenge is preserved.
Acknowledgments
The authors would like to thank all of the volunteer subjects that participated in this investigation. The authors would also like to thank Amanda Fralin, R.N. for her support and clinical expertise. This work was supported by the National Institutes of Health, grants NIH-HL61388, HL84072 and HL082426.
References
- Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol. 1972;33:418–420. doi: 10.1152/jappl.1972.33.4.418. [DOI] [PubMed] [Google Scholar]
- Bungo MW, Charles JB, Johnson PC., Jr Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med. 1985;56:985–990. [PubMed] [Google Scholar]
- Convertino VA, Cooke WH, Holcomb JB. Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J Trauma. 2006;61:629–634. doi: 10.1097/01.ta.0000196663.34175.33. [DOI] [PubMed] [Google Scholar]
- Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2000;279:H1955–1962. doi: 10.1152/ajpheart.2000.279.4.H1955. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 1999a;277:H2348–2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol. 1996;81:2192–2198. doi: 10.1152/jappl.1996.81.5.2192. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999b;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol. 2005;289:H2429–2433. doi: 10.1152/ajpheart.00383.2005. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol. 2004a;96:2103–2108. doi: 10.1152/japplphysiol.00717.2003. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004b;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Davis JE, Fortney SM. Effect of fluid ingestion on orthostatic responses following acute exercise. Int J Sports Med. 1997;18:174–178. doi: 10.1055/s-2007-972615. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Keller DM, Watanabe H, Raven PB, Thomas GD. Noninvasive assessment of sympathetic vasoconstriction in human and rodent skeletal muscle using near-infrared spectroscopy and Doppler ultrasound. J Appl Physiol. 2004;96:1323–1330. doi: 10.1152/japplphysiol.01041.2003. [DOI] [PubMed] [Google Scholar]
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004a;286:H449–457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004b;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Harms MP, van Lieshout JJ, Jenstrup M, Pott F, Secher NH. Postural effects on cardiac output and mixed venous oxygen saturation in humans. Exp Physiol. 2003;88:611–616. doi: 10.1113/eph8802580. [DOI] [PubMed] [Google Scholar]
- Harrison MH, Hill LC, Spaul WA, Greenleaf JE. Effect of hydration on some orthostatic and haematological responses to head-up tilt. Eur J Appl Physiol Occup Physiol. 1986;55:187–194. doi: 10.1007/BF00715003. [DOI] [PubMed] [Google Scholar]
- Haruna Y, Takenaka K, Suzuki Y, Kawakubo K, Gunji A. Effect of acute saline infusion on the cardiovascular deconditioning after 20-days head-down tilt bedrest. J Gravit Physiol. 1998;5:P45–46. [PubMed] [Google Scholar]
- Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Blatteis C, Fregly M, editors. Handbook of Physiology: section 4, Environemntal Physiology. Bethesda, MD USA: American Physiological Society; 1996. pp. 215–243. [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006a;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Davis SL, Low DA, Shibasaki M, Raven PB, Crandall CG. Carotid baroreceptor stimulation alters cutaneous vascular conductance during whole-body heating. J Physiol. 2006b;577:925–933. doi: 10.1113/jphysiol.2006.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- Lewis T, Pickering GW. Vasodilation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart. 1931;16:33–51. [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Ljungstrom KG, Willman B, Hedin H. Hapten inhibition of dextran anaphylaxis. Nine years of post-marketing surveillance of dextran 1. Ann Fr Anesth Reanim. 1993;12:219–222. doi: 10.1016/S0750-7658(05)81033-0. [DOI] [PubMed] [Google Scholar]
- Ludwig DA, Convertino VA. Predicting orthostatic intolerance: physics or physiology? Aviat Space Environ Med. 1994;65:404–411. [PubMed] [Google Scholar]
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol. 1970;28:415–420. doi: 10.1152/jappl.1970.28.4.415. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man: Role of falling blood pressure. J Appl Physiol. 1971;31:864–869. doi: 10.1152/jappl.1971.31.6.864. [DOI] [PubMed] [Google Scholar]
- Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol. 2007;292:H1420–1426. doi: 10.1152/ajpheart.01096.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002a;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002b;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol. 2007;585:279–285. doi: 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki F, Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Shiraki K. Whole body heating reduces the baroreflex response of sympathetic nerve activity during Valsalva straining. Auton Neurosci. 2003;103:93–99. doi: 10.1016/s1566-0702(02)00140-6. [DOI] [PubMed] [Google Scholar]