Abstract
The expression and localization of the cystic fibrosis transmembrane conductance regulator (CFTR) were determined in four osmoregulatory tissues during the ontogeny of the sea-bass Dicentrarchus labrax acclimated to fresh water and sea water. At hatch in sea water, immunolocalization showed an apical CFTR in the digestive tract and integumental ionocytes. During the ontogeny, although CFTR was consistently detected in the digestive tract, it shifted from the integument to the gills. In fresh water, CFTR was not present in the integument and the gills, suggesting the absence of chloride secretion. In the kidney, the CFTR expression was brief from D4 to D35, prior to the larva–juvenile transition. CFTR was apical in the renal tubules, suggesting a chloride secretion at both salinities, and it was basolateral only in sea water in the collecting ducts, suggesting chloride absorption. In the posterior intestine, CFTR was located differently from D4 depending on salinity. In sea water, the basolateral CFTR may facilitate ionic absorption, perhaps in relation to water uptake. In fresh water, CFTR was apical in the gut, suggesting chloride secretion. Increased osmoregulatory ability was acquired just before metamorphosis, which is followed by the sea-lagoon migration.
Keywords: osmoregulation, ionic regulation, sea-bass, immunofluorescence, cystic fibrosis transmembrane conductance regulator (CFTR), teleost
Introduction
In a water environment, the survival and distribution of organisms are mostly influenced by salinity. Euryhaline teleosts, through osmotic adaptation, maintain an almost constant blood osmolarity at 300–350 mosm kg−1 and are able to live in variable environments, as for instance Fundulus heteroclitus (Griffith, 1974), Sparus aurata (Chervinski, 1984), Oreochromis mossambicus (Hwang, 1987) and Dicentrarchus labrax (Kelley, 1988; Sabriye et al. 1988; Pickett et al. 2004). According to the external salinity, teleosts hyper- or hypo-osmoregulate through four osmoregulatory tissues, whose relative involvement depends on the ontogenetic stage (Hwang, 1989; Gonzalez et al. 1996; Varsamos 2002, 2005; Falk-Petersen, 2005). Integuments limit water and ion fluxes in adult fish (Wales & Tytler, 1996). In early ontogenetical stages, before gills develop, the integument is the major site for respiration (Roberts et al. 1973; Gonzalez et al. 1996) and osmoregulation (Varsamos et al. 2002). Gills, besides their respiratory function, are the predominant organ of ionic exchanges in adults (Evans et al. 1999, 2005). The kidney has a limited osmoregulatory role in sea water (SW), but in freshwater (FW) it produces abundant and hypotonic urine (Nebel et al. 2005). Marine teleosts ingest the medium orally, then in the gut; water absorption coupled to salt uptake compensates for dehydration in sea water (Venturini et al. 1992; Marshall & Grosell, 2005; Giffard-Mena et al. 2006).
Specific cells that are involved in osmoregulation are called mitochondrion-rich cells (MRC), also termed ionocytes or chloride cells (CC) (Rankin & Davenport, 1981; Marshall & Bryson, 1998). In these cells, ion transport is mainly activated by the Na+/K+ ATPase (NKA), which is consistently located in the basolateral membrane (Evans, 1993; Hirose et al. 2003), and generates an electrochemical gradient. Following this gradient, ions are driven according to the expression, location and abundance of different transmembrane proteins (Hirose et al. 2003). One of them, the cystic fibrosis transmembrane conductance regulator (CFTR), has been sequenced from human (Riordan et al. 1989). Dysfunctioning or mislocation of CFTR induces cystic fibrosis (Kleizen et al. 2000; Chen et al. 2001). The CFTR, included in the super-family of ATP-binding cassette (ABC) transporters, is the only ABC transporter described as an ion channel (Devidas & Guggino, 1997). In fish, complete CFTR cDNAs have been sequenced in Takifugu rubripes (GenBank accession no. NM_001048040.1), Fundulus heteroclitus (AF000271.1) and Salmo salar (CFTR I: AF155237.1; CFTR II: AF161070.1). This last species is the only one presenting two isoforms (Chen et al. 2001). CFTR is probably involved in bicarbonate fluxes and acid-base balance (Marshall & Singer, 2002), but its main function is in osmotic regulation, particularly chloride regulation (Singer et al. 1998; Marshall et al. 1999, 2002b; Marshall & Singer, 2002). CFTR has mostly been studied in gills, blood, brain and opercular tissues (Marshall et al. 2002b; Scott et al. 2004; Scott & Schulte, 2005), and in the intestine (Marshall & Singer, 2002, Scott et al. 2006). There is evidence that CFTR is highly expressed in gill ionocytes, with an apical location linked to Cl− excretion in SW (Marshall et al. 1999; Katoh & Kaneko, 2003). However, CFTR may be differently located according to the species: Fundulus heteroclitusCC express a diffuse CFTR in FW (Marshall et al. 2002b), but in other species such as Oreochromis mossambicus, CFTR is not expressed in these cells in FW (Hiroi et al. 2005); in Morone saxatilis, there is no change in the localization and expression of the CFTR according to salinity (Madsen et al. 2007).
The European sea-bass is a euryhaline marine teleost whose adults are able to tolerate salinities ranging from fresh water (FW) to sea water (SW) and hypersaline SW (Pickett & Pawson, 1994). During development, sea-bass live in different habitats and are thus exposed to salinity variations. Reproduction, spawning and the first ontogenetic stages occur in the open sea (Barnabé, 1989, Pickett & Pawson, 1994). Following a passive drift from open sea to coastal areas, larvae metamorphose into juveniles that actively migrate to estuaries and lagoons, where salinity is variable. The capacity to osmoregulate is present at hatch and it increases during ontogeny, sequentially relying on different organs according to the life stage (Varsamos et al. 2001, 2005; Nebel et al. 2005; Giffard-Mena et al. 2006). At the cellular level, the adaptation to salinity during development of the sea-bass is made possible by selective protein expression in ionocytes located first in the integument and then in the gills, intestine and kidney. Among the main proteins, the ontogenetic expressions of the enzyme Na+/K+ ATPase (NKA) and of the co-transporter Na+, K+, 2Cl− (NKCC) have previously been studied in the sea-bass (Varsamos et al. 2005; Lorin-Nebel et al. 2006). Apical chloride channels have also been identified in cultured sea-bass gills but the molecular structure of these channels is still unknown (Duranton et al. 1997).
The objectives of this study were to determine the presence and to evaluate the function of the CFTR channel during the ontogeny of the sea-bass exposed to different salinities, using immunocytochemistry to follow its location in ionocytes of the different involved organs.
Materials and methods
Animals
The different stages of sea-bass (Dicentrarchus labraxLinnaeus, 1758) were provided by the aquaculture farm ‘Les poissons du Soleil’ at Balaruc (Hérault, France) and by the Ifremer station at Palavas (Hérault, France). Sea-bass were transferred to the laboratory and, according to their size, were maintained in 5-, 40- or 3500-L tanks, filled with aerated and filtrated natural sea water (≈ 35‰) from the Mediterranean Sea at 18 °C under a 12 h light/12h dark photoperiod. Four developmental stages were studied. Their choice was based on the sequence of acquisition of osmoregulatory capacities (Varsamos et al. 2001): prelarvae (0–5 days; 3.5–5 mm); larvae (5–40 days; 5–15 mm); late larvae (40–60 days; 15–30 mm) and juveniles (over 60 days; over 30 mm). The stages chosen for observations were: day 1 post-hatch (D1), D4, D35, D50 and juveniles at D400. The fish were fed with Artemia (Artemia salina) nauplii during the larval period, then with fish granules of adequate sizes (Aphymar). Prelarvae and larvae were kept in SW and transferred to FW through a progressive decrease of 5‰ per 2 h. Prelarvae and larvae were maintained in each salinity for 4 days before fixation. Juveniles were progressively transferred (2 weeks) from SW to fresh water (FW ≈ 0.3‰) and maintained in SW and FW for 8 months. Before any experimentation, the fish were anaesthetized with a solution of phenoxy-2-ethanol (0.15 mL L−1). All procedures were conducted according to the French law concerning scientific animal experimentation.
Western blot
The protein extraction and the Western blot technique were adapted according to the protocols of Marshall et al. (2002a,b) and Wilson et al. (2000). Following anaesthesia of the fish, the different organs were dissected on ice and homogenized in ice-cold SEI buffer (100 mmol L−1 imidazole, 300 mmol L−1 sucrose, 20 mmol L−1 EDTA, pH 7.3) containing 75 µL of protease inhibitors (PI) (complete TM, Mini, EDTA-free, Boehringer Mannheim GmbH). After 1 h 30 min in ice, homogenates were centrifuged at 2000 g for 6 min at 4 °C. Pellets were resuspended in 2.4 mmol L−1 sodium deoxycholate in SEI-IP buffer and centrifuged at 2000 for 6 min at 4 °C. The resulting supernatants were stored at −20 °C. The Bradford method (Bradford, 1976) was used to determine the total protein content of each supernatant. After adjusting the protein concentration (30-µg proteins), samples were denatured by addition of 0.33 volumes of loading buffer 4× (250 mmol L−1 Tris, 8.33% SDS, 40% glycerol, 2.8 mβ-mercaptoethanol, 0.02% bromophenol blue) and incubated at 90 °C for 10 min. The proteins were separated by SDS-polyacrylamide gel electrophoresis (7% polyacrylamide) done in duplicate and run for 30 min at 200 V. Each gel was then transferred on PVDF (polyvinylidene difluoride) membrane (0.45 µm) (Schleischer and Schuell, Saint Marcel, France) for 2 h. The membranes were blocked using a solution of PBS (phosphate-buffered saline pH 7.3) containing 5% skimmed milk for 1 h at 37 °C. After three washes of 10 min in PBS buffer containing 0.05% Tween 20 (PBS-T), one of the two membranes was exposed to the first antibody, the monoclonal anti-human CFTR (R&D Systems, Minneapolis, MN, USA), at 0.2 µg mL−1 in PBS-Tween 20 (0.05%) in skimmed milk (0.5%) overnight at 4 °C. This primary antibody has already been used in other species of fish (Squalus acanthias: (Lehrich et al. 1998); Fundulus heteroclitus: (Marshall et al. 2002b); Oreochromis mossambicus: (Hiroi et al. 2005). The second membrane, which was the negative control, was incubated only in the PBS-Tween 20 (0.05%) in skimmed milk (0.5%) overnight at 4 °C, without the first antibody. After two washes in PBS-Tween (0.05%) and one in PBS buffer for 10 min each, the two membranes were incubated with the secondary antibody at 0.1 µg mL−1[peroxidase-conjugated goat anti-mouse IgG (H + L); Jackson ImmunoResearch] in PBS-Tween 20 (0.05%) in skimmed milk (0.5%) for 1 h at room temperature. Following washes, blots were visualized using chemiluminescence (Amersham Pharmacia Biotech) according to the manufacturer's instructions, and pictures were obtained using a Lumi Imager (Roche Applied Science, France).
Histomorphology and immunocytochemistry
The structure of the integument, the gills, the kidney and the digestive tract were studied using light microscopy, and CFTR was immunolocalized in the ionocytes of these four osmoregulatory tissues at the different ontogenetic stages. After anaesthesia and juvenile dissection, the larvae or the organs were fixated in Bouin's liquid for 48 h, and then washed and dehydrated in ascending grades of ethanol. Prior to impregnation and embedding in Paraplast, tissues were immersed in butanol and Histochoice Clearing Agent (Amersco®, USA). For histological observations, serial sections 4 µm thick were dried for 24 h, stained with Masson's trichrome, and observed on a Leica Diaplan light microscope. For immunocytochemistry, after cutting 4-µm-thick sections on a Leitz microtome, and transfer on a slide, slides were incubated in PBS and put in a microwave (80% power for 5 min, two times) to reveal the antigenic sites (Bancroft & Gamble, 2002). The slides were immersed for 10 min into 0.01% Tween 20, 150 mm NaCl in 10 mm PBS, pH 7.3. Slide saturation was performed in a solution of 5% skimmed milk in PBS at room temperature for 20 min. Slides were washed three times in PBS and incubated with a solution of 10 µg mL−1 primary antibody in PBS + 0.5% skimmed milk for 2 h in a moist chamber at room temperature. The primary antibody was the monoclonal anti-human CFTR. Control slides were placed in the same conditions but without primary antibody. After rinsing three times in PBS, the slides were exposed to the secondary anti-mouse antibody (FluoProbes 488 Anti-Mouse; Interchim) (10 µg mL−1 in PBS + 0.5% skimmed milk) for 1 h at room temperature. The slides were washed, mounted in an anti-bleaching medium (Gel/Mount, Permanent Aqueous Mounting Medium, Biomeda) and observed with a Leica Diaplan microscope equipped with a special filter for fluorescence (450–490 nm) together with a Leica DC 300 F digital camera and its software (FW 4000). Particular attention was paid to the localization of the immunostaining, either apical, or basolateral or diffuse, indicating a basolateral localization expanding in the cell.
Results
Western blot analysis
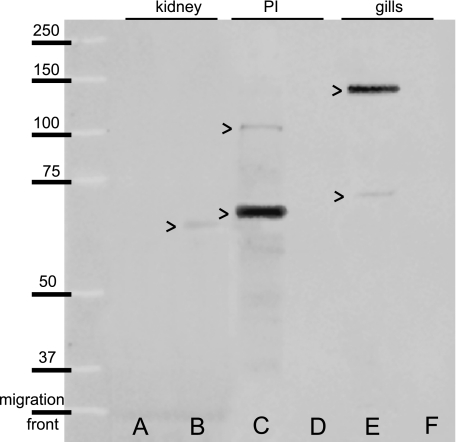
The negative control membrane, without the primary antibody, showed no band (not illustrated). The molecular weight of CFTR was evaluated using Western blot on the gills, posterior intestine and kidney of the sea-bass in SW and FW (Fig. 1). In the kidney, a weak band was found in FW at 65 kDa (Fig. 1B), but none was observed in SW (Fig. 1A). In the posterior intestine, two bands were detected in SW, one strongly stained at 67 kDa, and another weakly stained at 105 kDa (Fig. 1C), whereas none was observed in FW (Fig. 1D). In the gills, a major band was noted at 138 kDa and another one at 71 kDa in SW (Fig. 1E); in FW no immunoreactivity was found (Fig. 1F).
Fig. 1.
Western blot of CFTR from long-term (8 months) acclimated Dicentrarchus labrax juveniles in SW (A,C,E) and FW (B,D,F) in the kidney (A,B), the posterior intestine (PI) (C,D) and the gills (E,F). Molecular weights in kDa are indicated on the left.
Histomorphology
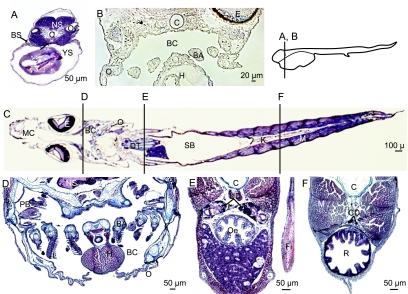
The main osmoregulatory tissues investigated through immunocytochemistry (see below) are briefly described here. At hatch, a major part of the body is occupied by the yolk sac and the branchial slides are developing (Fig. 2A). The digestive tract is a tube made of a single cellular layer (Fig. 2A). At D4, the branchial cavity develops, limited by the opercula and containing branchial arches. Filament and lamellae are not yet present in the gills (Fig. 2B). At D42, sections of the sea-bass display the organization and organs of larvae, similar to those of juveniles (Fig. 2C). The branchial cavity is located between the two opercula and the gills are made of four pairs of arches bearing filaments and lamellae (Fig. 2D). The kidney is represented by the urinary ducts in the anterior part of the body (Fig. 2E) and by the collecting ducts in the posterior part (Fig. 2F). The digestive tract is divided into several segments including the oesophagus (Fig. 2C,F), the stomach, the anterior and the posterior intestine (not illustrated), and the rectum (Fig. 2F).
Fig. 2.
Histomorphology of the sea-bass at different stages, stained with Masson's trichrome (A,C–F) and in phase contrast (B), transverse sections (A,B,D–F) and longitudinal horizontal section (C). The location of transverse sections is indicated beside B and on C. Larvae at hatch (D0) (A), at D4 (B), and at D42 (C–F). At hatch, note the branchial slit (BS) and the straight digestive tract (DT) in an anterior section (A). At D4, the developing branchial cavity (BC) is limited by the developing opercula (O) and it contains the branchial arches (BA). At D42, the branchial cavity is well formed (C,D) with complete gills with arches (BA), filaments (F) and lamellae (L). In the median section (E), the urinary ducts (UD) are in a dorsal position compared to the oesophagus (Oe). In the posterior part of the body (F), the kidney is represented by the collecting ducts (CD) above the rectum (R). BA, branchial arch; BC, branchial cavity; BS, branchial slit; C, chord; CD, collecting duct; DT, digestive tract; E, eye; F, filament; Fi, pectoral fin; H, heart; I, ionocyte; Int, intestine; K, kidney; L: lamellae; M: muscle; MC: mouth cavity; NS: nervous system; O: operculum; Oe: oesophagus; OC: otic cavity; PB, pseudo-branches; PI, posterior intestine; R, rectum; RD, renal duct; SB, swim bladder; T, integument; TI, integumental ionocyte; UB, urinary bladder; UD, urinary duct; YS, yolk sac.
Immunocytochemistry
None of the negative control slides, without the primary antibody, showed immunostaining (not illustrated). However, autofluorescence was noted in the erythrocytes.
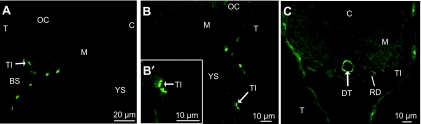
From hatch, in prelarvae at D1, an apical staining of the integumental ionocytes was observed along the branchial slits, on the yolk sac membrane and in the undifferentiated gut (Fig. 3A–C). During formation of the branchial arches, CFTR was only located at D4 at the top of the branchial cavity and in the developing operculum in SW-exposed fish (Fig. 4A). In the anterior part of the body (Fig. 3A,B,B′), the integumental chloride cells (CC) were ovoid in shape, like the gill CC present in later stages. Along the yolk sac, CC seem organized in pairs and their apical pits appeared very close, possibly forming multicellular complexes (Fig. 3B′). The posterior part of the prelarvae presented flatter integumental CC (Fig. 3C). Their apical pit, relatively small, was located in the middle of the cells. At D4, the number of CFTR-positive CC decreased in the integument and most of them were concentrated in the posterior region, with a flat shape (Fig. 4C,E). At hatch, the gut was formed by a straight tube lined by a single layer of cells (Fig. 3C). From D4, the gut started to differentiate into several sections, with an oesophagus, a developing stomach, an anterior and posterior intestine, and a rectum. CFTR was located only in the posterior section of the intestine (Fig. 4C,D). No CFTR immunostaining was observed in the kidney at D1 (Fig. 3C). Where the kidney develops into several urinary and collecting ducts opening into the urinary bladder, a diffuse CFTR staining was observed at D4 in the collecting ducts (Fig. 4E).
Fig. 3.
Immunolocalization of CFTR in 1-day-old prelarvae (D1), transverse sections: (A,B,B′) anterior section; (C) posterior section. (A) Integumental ionocytes (TI) present an apical immunofluorescence around the branchial slits (BS). (B,B′) Apical staining in integumental ionocytes (TI) and on the yolk-sac (YS) membrane. (C) No staining of the renal ducts (RD). (—): no fluorescence; (→): fluorescence.
Fig. 4.
Immunolocalization of CFTR in 4-day-old prelarvae (D4), transverse sections: (A,C,E) SW-acclimated fish, (B,D,F) FW-acclimated fish. (A) Crypt staining of ionocytes (I) on the roof of the branchial cavity (BC). (B) No staining in the branchial cavity. (C) Diffuse staining in intestinal cells. (D) Apical staining on the brush border of the intestine enterocytes. (E) Weak diffuse staining in the collecting ducts. (F) No staining.
From D4 prelarvae, samples were compared according to the salinity acclimation, either SW (Fig. 4A,C,E) or FW (Fig. 4B,D,F). At D4, in the branchial chamber (Fig. 4A,C) and in the integument (Fig. 4C), CFTR staining was only present in SW, restricted to the apical pit of CC, but none was observed in FW samples (Fig. 4B,D). In the posterior intestine, CFTR was diffuse or basolateral in SW enterocytes (Fig. 4C). In FW, CFTR shifted to an apical position in the entire posterior intestine (Fig. 4D). In the kidney, no CFTR was observed in FW (Fig. 4F), but there was a weak diffuse staining in the collecting ducts from SW samples (Fig. 4E).
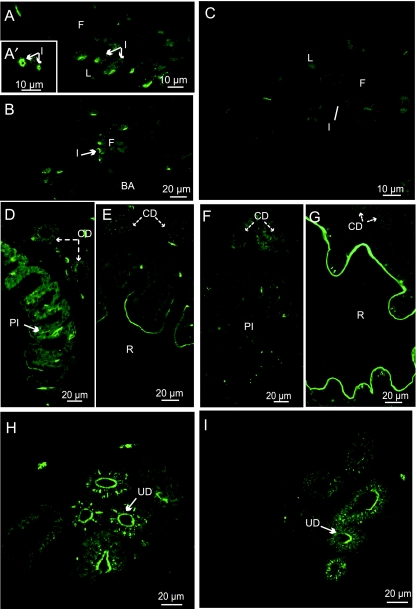
In larvae at D35, gills were formed by four branchial arches bearing filaments and lamellae. In SW, CFTR was located in the apical pits of the filamental CC (Fig. 5A,A′) and apically in the branchial cavity CC (Fig. 5B), whereas in FW, CFTR was not or weakly present in CC of filaments and lamellae (Fig. 5C). At both salinities, CFTR was apically located and diffuse in few cells of the epithelium of the urinary ducts (Fig. 5H,I). In the collecting ducts, there was a weak diffuse CFTR staining in SW (Fig. 5D,E) but no visible staining in FW (Fig. 5,F,G). In the gut, CFTR was only observed in posterior sections (posterior intestine and rectum) (Fig. 5D–G), but not in the oesophagus, stomach and anterior intestine (not illustrated). In the posterior intestine, CFTR was abundantly present at a basal location in SW (Fig. 5D) but not in FW (Fig. 5F). In the rectum at both salinities, CFTR was apically located and abundant in the anterior part (Fig. 5E,G); the posterior part was devoid of fluorescence (not illustrated).
Fig. 5.
Immunolocalization of CFTR in 35-day-old larvae (D35), transverse sections: (A,A’,B,D,E,H) SW-acclimated fish, (C,F,G,I) FW-acclimated fish. (A,A′,B) Crypt staining in ionocytes (I) of the gill filaments (F). (C) No staining in gills. (D) Basolateral staining in the posterior intestine (PI). (D–G) Weak diffuse staining in the collecting ducts. (E) Apical staining of the brush border in the rectum (R). (F) Weak apical staining in the posterior intestine (PI). (G) Apical staining of the brush border in the rectum (R). (H,I) Apical and diffuse staining in the urinary ducts (UD).
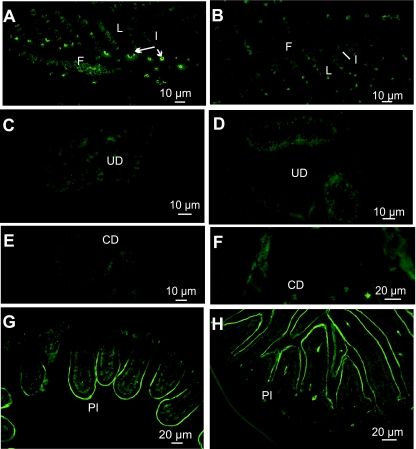
Before metamorphosis, at D50, gills were completely formed. In SW, CFTR was located in inter-lamellae filamentary CC; fluorescence was intense and restricted to the pit (Fig. 6A). In FW, no immunostaining was observed (Fig. 6B). No CFTR staining was observed in the urinary and collecting ducts at either salinity (Fig. 6C–F). Regarding the gut, in the posterior intestine section, fluorescence was apical and basal in SW (Fig. 6G) but in FW, CFTR was located only in the brush-border (Fig. 6H). At both salinities, CFTR was apically located in the anterior part of the rectum, as at D35 (not illustrated). CFTR was not detected in the few remaining integumental CC.
Fig. 6.
Immunolocalization of CFTR in 50-day-old larvae (D50), transverse sections: (A,C,E,G) SW-acclimated fish, (B,D,F,H) FW-acclimated fish. (A) Crypt staining in ionocytes (I) of gill filaments (F). (B) No staining in gills. (C,D–F) No staining in the urinary ducts (UD) and collecting ducts. (G) Apical and basal staining in the posterior intestine (PI). (H) Apical staining of the brush border in the posterior intestine (PI).
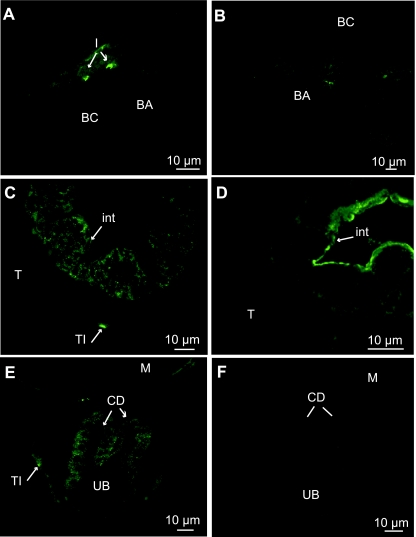
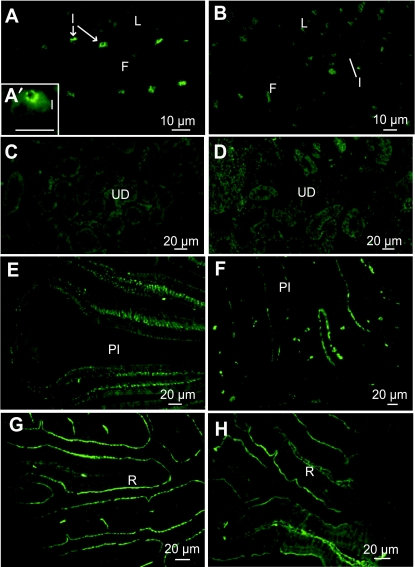
In juveniles, in SW, ionocytes were located in inter-lamellar spaces on the gill filaments, with apical CFTR (Fig. 7A,A′). In FW, CC were more numerous, located on filaments and on lamellae but no fluorescence was observed at this salinity (Fig. 7B). In the kidney, no CFTR staining was observed at either salinity (Fig. 7C,D). Regarding the gut, as in previous stages, CFTR was located differently depending on salinity. In SW, CFTR immunostaining was basal in the posterior intestine (Fig. 7E). In FW, an apical staining was observed and some cells of the lamina propria were also diffusely immunostained (Fig. 7F). In the rectum, CFTR was apically located in SW and FW (Fig. 7G,H).
Fig. 7.
Immunolocalization of CFTR in juveniles (130 mm), transverse sections: (A,A′,C,E,G) SW-acclimated fish, (B,D,F,H) FW-acclimated fish. (A,A’) Crypt staining in ionocytes (I) of the gill filaments (F). (B) No staining in the gills. (C,D) No staining in the urinary ducts (UD). (E) Basal staining in the posterior intestine (PI). (F) Apical staining of the brush border in the posterior intestine. (G,H) Apical staining in the rectum (R).
Discussion
Of the different transmembrane proteins involved in ion transport in sea-bass ionocytes, this study presents new results on the location of CFTR in the osmoregulatory organs of a euryhaline fish ontogeny.
Western blot analysis
Two bands were detected in Western blots in the gills and posterior intestine of SW-exposed sea-bass. The most intense stain in the gills has a molecular weight of 138 kDa, corresponding to the mature CFTR. This weight was lower than the mature CFTR band identified in the mudskipper and killifish, which is around 150 and 160 kDa, respectively (Wilson et al. 2000; Scott et al. 2004). Another weak band identified in the posterior intestine around 104 kDa could be the unglycosylated form of CFTR as detected in human and killifish (Scott et al. 2004; Devuyst et al. 1996). There are three bands around 65 kDa in the three organs with a more intense band in the posterior intestine; these proteins could be, as shown in human kidney (Devuyst et al. 1996), a CFTR truncated form constituted by the TMD1, the NBD1 and the R domain motives. The antibody used in this study is specific to the CFTR protein and is efficient for the sea-bass CFTR, as it was for other teleost species (Hiroi et al. 2005, Marshall et al. 2002a).
Ontogenetic localization of CFTR in the kidney
The results are synthesized in Table 1. CFTR was undetected in ionocytes over the entire length of the straight kidney ducts at hatch, at D50 and in juveniles. Between D4 and D35, CFTR expression was high in the anterior part of the ducts, with an apical location at both salinities. The expression was weak in the posterior part of the kidney, only in SW-acclimated fish.
Table 1.
Summary of the results on immunolocalization of CFTR during the ontogeny of the sea-bass Dicentrarchus labrax in sea water (SW) and fresh water (FW)
| Kidney |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Branchial chamber (BC) and/or gills |
Integument |
Digestive tract (DT) |
Anterior |
Posterior |
||||||
| SW | FW | SW | FW | SW | FW | SW | FW | SW | FW | |
| D0–D1 | +++ A | +++ A | ++ A (Posterior DT) | − | − | |||||
| D4 | +++ A | − | +++ A | − | ++ BL (Posterior DT) | ++ A (Posterior DT) | ++ A | ++ A | + D | |
| MOUTH OPENING | ||||||||||
| D8 | +++ A | − | +++ A | − | ++ BL (Posterior DT) | ++ A (Posterior DT) | ++ A | ++ A | +/− D | − |
| D35 | +++ A | +/− | +++ A | − | PI rectum | PI rectum | +++ A | +++ A | +/− D | − |
| ++ B/A ++ A | ++ B/A ++ A | − | − | − | − | |||||
| D50 | +++ A | − | − | − | ++ B/A ++ A | ++ B/A ++ A | ||||
| METAMORPHIC LARVA−JUVENILE TRANSITION | ||||||||||
| Juveniles | +++ A | − | +++ A +++ B | ++ A ++ A | − | − | − | − | ||
Acclimation time: 4 days. Fluorescence intensity: −, negative; +/−, weak; +, average; ++, strong; +++, very strong. Localization of fluorescence at cellular level: A, apical; B, basal, BL; basolateral; D, diffuse. PI: posterior intestine.
Areas in grey indicate no data.
At hatch, neither CFTR (this study) or the co-transporter Na+, K+, 2Cl− (NKCC) (Lorin-Nebel et al. 2006) are observable in the kidney. Only Na+/K+-ATPase (NKA) is expressed basolaterally (Nebel et al. 2005). From D4 and increasingly up to D35, the kidney may be ready for urinary and osmoregulatory functions, as the three major proteins are present (Hiroi et al. 2005), CFTR mainly in the urinary ducts (this study), NKA, and NKCC in the collecting ducts (Lorin-Nebel et al. 2006; Nebel et al. 2005). The apical location of CFTR in the proximal tubules, more intense than in previous stages, points to an ionic secretion in both FW and SW. Previously, it was suspected in two stenohaline species, Chionodraco hamatus and Trematomus bermacchii, from the basolateral expression of NKCC (Masini et al. 2001). At the basal side, Cl− probably enters the cells through an ion transmembrane protein which is NKCC according to Marshall & Grosell (2005), although this transporter was not determined in urinary ducts of the sea-bass (Lorin-Nebel et al. 2006). The cytosol negative membrane potential, combined with the Cl− concentrations, provides electrochemical gradients favouring outward movement of Cl− from the cell via CFTR or other channels. Cl− secretion in proximal tubules would be coupled with a paracellular Na+ transport (Beyenbach, 2004). In FW, the localization of CFTR suggests ion secretion. This, in addition to the glomerular filtration, could contribute to the elimination of water in the urine, which is hypotonic to blood (Cliff & Beyenbach, 1988; Nebel et al. 2005). In SW, the same localization of the CFTR was observed in FW, suggesting an ion secretion. The urinary ducts would play a role in the regulation of plasma concentrations in marine teleost by the secretion of ions when the glomerular filtration is commonly reduced (Cliff & Beyenbach, 1992; Beyenbach, 2004), although it is well known that urine in teleosts is, at most, isotonic to the blood and thus does not provide for any NaCl secretion (Evans, 1993). The diffuse CFTR in the collecting ducts only in SW corresponds to an uptake of Cl− ions. The association of the diffuse-basal CFTR, the basolateral NKA (Nebel et al. 2005) and the apical NKCC (Lorin-Nebel et al. 2006) points to an ionic absorption, CFTR allowing a Cl− flux from the intracellular compartment to the blood (Singer et al. 1998; Marshall & Singer, 2002; Marshall & Grosell, 2005). This ion uptake could retrieve water by osmosis through aquaporin channels, particularly AQP1, contributing to a decrease in water loss via urine (Ivone Giffard-Mena et al. unpublished observations). The overload of ions would be excreted by the gills (Bodinier et al. unpublished observations). The CFTR involvement in these osmotic functions seems transitory, during the early developmental stages; it apparently disappears (Table 1) when the kidney is completely differentiated (Nebel et al. 2005). Cl− transport could then be managed by other transmembrane proteins such as Cl−/HCO3− exchangers, which are implicated in osmotic and acid-base regulations (Marshall & Grosell, 2005).
Ontogenetic localization of CFTR in the gut
At all stages of the development, no CFTR immunostaining was observed in the oesophagus, stomach and anterior intestine (Table 1). CFTR is not implicated in Cl− uptake in these parts of the gut, whereas NKA is present (Giffard-Mena et al. 2006). The posterior sections of the gut are positively immunostained for CFTR.
At hatch, when the digestive tract starts to differentiate, enterocytes organized in a simple layer express CFTR in the apical brush border. From D1 to D4, in endotrophic prelarvae, CFTR is present before the acquisition of the gut functionality. From D4, in the posterior gut section, CFTR is differently located according to salinity, a change that points to its involvement in osmoregulation. In SW, CFTR is basolaterally located in the posterior section of the gut (D4) and in the posterior intestine and rectum following their differentiation (D35, D50) (Table 1). This location can be related to Cl− uptake to the blood. In FW, the apically located CFTR points to ionic secretion. In the rectum, CFTR is apically located at both salinities. From FW to SW, the CFTR localization changes from basolateral to apical (Table 1). This redistribution has been observed in the gill ionocytes of F. heteroclitus (Marshall et al. 2002b), O. mossambicus (Hiroi et al. 2005) and D. labrax (see next paragraph and Bodinier et al. unpublished observations). The time necessary for the relocalization of the protein in the gut is apparently after 4 days of exposure to salinity. As shown by immunocytochemistry, the CFTR is diffuse after 4 days in SW (Fig. 5D; Fig. 6G) but if the exposure time is longer (Fig. 7E), the CFTR is entirely basal. Thus the apical CFTR found in FW shifts to a basal position in SW long-term acclimated fish. In SW, the basal location of CFTR in enterocytes of the posterior intestine suggests Cl− uptake, in agreement with the apical location of NKCC (Lorin-Nebel et al. 2006) in the sea-bass, and also in F. heteroclitus (Marshall et al. 2002a). The electrochemical gradient generated by NKA induces ion uptake via the apically located NKCC, which operates in parallel with apical anion exchangers to provide Cl− uptake. Once in the cell, Cl− ions diffuse to the blood via the basolaterally located CFTR (Grosell, 2006). This ion absorption, apparently unfavourable in itself in SW, probably results in water uptake through aquaporins (AQP1), which have been detected in the sea-bass (Ivone Giffard-Mena et al. unpublished). This mechanism, previously described in other species (Loretz, 2001; Aoki et al. 2003) is essential to compensate dehydration resulting from immersion in SW (Smith, 1930; Sharratt et al. 1964). In FW, Cl− secretion suggested by the apical location of CFTR is more difficult to explain: at this salinity, the fish must take up ions and not lose them. Moreover, it is generally accepted that in marine teleosts, the intestine is involved only in ion absorption, not in secretion (Smith, 1930; Evans, 1993). If it is associated with apical anion exchangers such as Cl−/HCO3−, CFTR could locally secrete Cl− in FW to favour its re-uptake by the Cl−/HCO3− exchanger in order to facilitate bicarbonate secretion (Grosell, 2006; Taylor & Grosell, 2008). CFTR would thus change its SW-osmotic function in Cl−absorption to an FW-acid-base regulation with a Cl− secretion. In FW, the intestine seems involved in ion and nutrient uptake due to high expression of basal NKA (Varsamos et al. 2002) and apical NKCC (Lorin-Nebel et al. 2006).
Ontogenetic localization of CFTR in the integument and gills
As the integument and gill ionocytes are sequentially involved in osmoregulation during development (Varsamos et al. 2002; Lorin-Nebel et al. 2006), the CFTR expression at these sites will be discussed together.
At hatch, the integument of the prelarvae reveals an apical localization of CFTR in CC, especially along the yolk sac membrane and in the first integumentary folds, which represent the first occurrence of the branchial slits (Varsamos et al. 2002; Lorin-Nebel et al. 2006). At D4, the branchial cavity containing branchial arches starts to differentiate, and CFTR is apically located in integumentary ionocytes in SW (Table 1). When gills differentiate, they bear inter-lamellae CC with apically located CFTR in SW. After D35, CC are located at both integument and gill; their respective involvement in osmoregulation remains to be determined. After D35 the number of CC in the integument decreases until they are completely absent at D50. From D50, only the gill ionocytes retain CFTR in SW. Following our initial experiments of exposure to FW, which was at D4, the cellular presence of CFTR changed according to salinity. In SW, CFTR is located in the apical and subapical pit of ionocytes, first in the integument, then in the gills. In FW, no CFTR presence was observed in the integument and in the gills.
Compared to other species, the development of the branchial cavity in D. labrax occurs late. In another species such as Oncorhynchus mykiss, it starts during the embryonic phase (Gonzalez et al. 1996). The presence of CFTR (this study) associated with NKCC (Lorin-Nebel et al. 2006) in the integumentary CC reveals that these ionocytes are functional (Evans et al. 2005; Hiroi et al. 2005), which is essential for osmoregulation from hatch (Varsamos et al. 2005). The CC covering the yolk sac seem to form multicellular complexes with a common apical pit, as previously described in D. labrax (Varsamos et al. 2002). Previous structural and ultrastructural studies (Shiraishi et al. 1997; Sardet, 1980) have shown that junctions between cells of the multicellular complex are shallow and leaky in SW. They would provide a paracellular pathway for sodium excretion (Marshall & Bryson, 1998). CC located in the posterior part of the prelarvae appear different, flat, and they also contain CFTR. Varsamos et al. (2002) hypothesized that this particular shape might originate from the organization of the circulatory network in the vicinity of the integument. When gills start to develop, the integument is probably still the major site of osmoregulation. In the sea-bass, there is a transitory phase between D35 and D50 when the osmoregulatory function shifts from integument to gills, as also shown in the killifish (Katoh et al. 2000). The present CFTR results confirm that at the transition to the juvenile stage, the osmotic function is taken over completely by specific organs, particularly the gills (Katoh et al. 2000; Varsamos et al. 2002). In SW-acclimated juveniles, CFTR is located in the apical and subapical pit of ionocytes. CFTR, coupled with other proteins, mediates chloride secretion. The NKA pump, through the electrochemical gradient generated in the cell, excretes Na+ to the basal part of the cell, part of which re-enters the cell through the basolateral NKCC (Lorin-Nebel et al. 2006), driving Cl− flux into the cell. Cl− ions, in excess concentration in SW fish, are excreted to the surrounding medium through the apical CFTR. This localization reported in D. labraxis in agreement with observations in other species such as F. heteroclitus (Katoh & Kaneko, 2003, Marshall et al. 2002b), O. mossambicus (Hiroi et al. 2005), Periophthalmodonsp. (Wilson et al. 2000) and Stenogobius hawaiiensis (McCormick et al. 2003). In FW, no CFTR has been observed in gill cells of D. labrax, corresponding to the absence of chloride secretion. In contrast, F. heteroclitus gill ionocytes express a diffuse basolateral CFTR, reflecting a chloride uptake (Marshall & Singer, 2002). Although CFTR is the only anion channel yet localized in the basolateral membrane, the anion transfer could be conducted by other anion channels or exchangers (Marshall & Singer, 2002). In the sea-bass, the CFTR observed in SW is thus probably replaced in FW by a different anion channel.
In summary, CFTR is already present at hatch in the integument and gut of the sea-bass. During the larval growth, the gut ionocytes contain CFTR until the juvenile stage. In the integument, the CFTR expression decreases in favour of gills when they develop and grow. In the kidney, the CFTR occurrence is only transitory before the larva-juvenile transition. Juvenile-like osmoregulatory abilities are acquired shortly before metamorphosis, after which the fish is confronted by strong variation of salinity during the sea-lagoon migration.
Acknowledgments
We would like to thank Mr Gilles Maingon from ‘Les Poissons du Soleil’ for generously providing all stages of sea-bass, Eva Blondeau-Bidet and Evelyse Grousset for their technical assistance, and two anonymous referees for contributing to significantly enhance the manuscript.
References
- Aoki M, Kaneko T, Katoh F, Hasegawa S, Tsutsui N, Aida K. Intestinal water absorption through aquaporin 1 expressed in the apical membrane of mucosal epithelial cells in seawater-adapted Japanese eel. J Exp Biol. 2003;206:3495–3505. doi: 10.1242/jeb.00579. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M. Histological Techniques. Oxford: Churchill Livingstone; 2002. Application of microwave technology to histology; pp. 421–464. [Google Scholar]
- Barnabé G. L’élevage du loup et de la daurade. In: Barnabé G, editor. Aquaculture. Paris: Technique et Documentation – Lavoisier; 1989. pp. 675–720. [Google Scholar]
- Beyenbach KW. Kidneys sans glomeruli. Am J Physiol Renal Physiol. 2004;286:F811–827. doi: 10.1152/ajprenal.00351.2003. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J-M, Cutler C, Jacques C, et al. A combined analysis of the cystic fibrosis transmembrane conductance regulator: implications for structure and disease models. Mol Biol Evol. 2001;18:1771–1788. doi: 10.1093/oxfordjournals.molbev.a003965. [DOI] [PubMed] [Google Scholar]
- Chervinski J. Salinity tolerance of young gilthead sea bream Sparus aurataL. Bamidgeh. 1984;36:121–124. [Google Scholar]
- Cliff WH, Beyenbach KW. Fluid secretion in glomerular renal proximal tubules of freshwater-adapted fish. Am J Physiol Regul Integr Comp Physiol. 1988;254:R154–158. doi: 10.1152/ajpregu.1988.254.1.R154. [DOI] [PubMed] [Google Scholar]
- Cliff WH, Beyenbach KW. Secretory renal proximal tubules in seawater- and freshwater-adapted killifish. Am J Physiol Renal Physiol. 1992;262:F108–116. doi: 10.1152/ajprenal.1992.262.1.F108. [DOI] [PubMed] [Google Scholar]
- Devidas S, Guggino WB. CFTR: domains, structure, and function. J Bioenerg Biomembr. 1997;29:443–451. doi: 10.1023/a:1022430906284. [DOI] [PubMed] [Google Scholar]
- Devuyst O, Burrow CR, Schwiebert EM, Guggino WB, Wilson PD. Developmental regulation of CFTR expression during human nephrogenesis. Am J Physiol Renal Physiol. 1996;271:F723–735. doi: 10.1152/ajprenal.1996.271.3.F723. [DOI] [PubMed] [Google Scholar]
- Duranton C, Tauc M, Avella M, Poujeol P. Chloride channels in primary cultures of seawater fish (Dicentrarchus labrax) gill. Am J Physiol-Cell Physiol. 1997;273:C874–C882. doi: 10.1152/ajpcell.1997.273.3.C874. [DOI] [PubMed] [Google Scholar]
- Evans DH. Osmotic and ionic regulation. In: Evans DH, editor. The Physiology of Fishes. Boca Raton, FL: CRC Press; 1993. pp. 315–341. [Google Scholar]
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005;85:97–177. doi: 10.1152/physrev.00050.2003. [DOI] [PubMed] [Google Scholar]
- Evans DH, Piermarini PM, Potts WTW. Ionic transport in the fish gill epithelium. J Exp Zool. 1999;283:641–652. [Google Scholar]
- Falk-Petersen IB. Comparative organ differentiation during early life stages of marine fish. Fish Shellfish Immun – Fish Larval Immun. 2005;19:397–412. doi: 10.1016/j.fsi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Giffard-Mena I, Charmantier G, Grousset E, Aujoulat F, Castille R. Digestive tract ontogeny of Dicentrarchus labrax: Implication in osmoregulation. Dev Growth Differ. 2006;48:139–151. doi: 10.1111/j.1440-169X.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Blanquez MJ, Rojo C. Early gill development in the rainbow trout, Oncorhynchus mykiss. J Morphol. 1996;229:201–217. doi: 10.1002/(SICI)1097-4687(199608)229:2<201::AID-JMOR5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Griffith RW. Environment and salinity tolerance in the genus Fundulus. Copeia. 1974;2:319–331. [Google Scholar]
- Grosell M. Intestinal anion exchange in marine fish osmoregulation. J Exp Biol. 2006;209:2813–2827. doi: 10.1242/jeb.02345. [DOI] [PubMed] [Google Scholar]
- Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T. Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+-ATPase, Na+/K+/2Cl− cotransporter and CFTR anion channel. J Exp Biol. 2005;208:2023–2036. doi: 10.1242/jeb.01611. [DOI] [PubMed] [Google Scholar]
- Hirose S, Kaneko T, Naito N, Takei Y. Molecular biology of major components of chloride cells. Comp Biochem Physiol B. 2003;136(Part B):593–620. doi: 10.1016/s1096-4959(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Hwang PP. Tolerance and ultrastructural responses of branchial chloride cells to salinity changes in the euryhaline teleost Oreochromis mossambicus. Mar Biol. 1987;94:643–649. [Google Scholar]
- Hwang P-P. Distribution of chloride cells in teleost larvae. J Morphol. 1989;200:1–8. doi: 10.1002/jmor.1052000102. [DOI] [PubMed] [Google Scholar]
- Katoh F, Kaneko T. Short-term transformation and long-term replacement of branchial chloride cells in killifish transferred from seawater to freshwater, revealed by morphofunctional observations and a newly established ‘time-differential double fluorescent’ technique. J Exp Biol. 2003;206:4113–4123. doi: 10.1242/jeb.00659. [DOI] [PubMed] [Google Scholar]
- Katoh F, Shimizu A, Uchida K, Kaneko T. Shift of chloride cell distribution during early life stages in seawater-adapted killifish, Fundulus heteroclitus. Zool Sci. 2000;17:11–18. doi: 10.2108/zsj.17.11. [DOI] [PubMed] [Google Scholar]
- Kelley DF. The importance of estuaries for sea-bass, Dicentrarchus labrax(L.) J Fish Biol. 1988;33:25–33. [Google Scholar]
- Kleizen B, Braakman I, de Jonge HR. Regulated trafficking of the CFTR chloride channel. Eur J Cell Biol. 2000;79:544–556. doi: 10.1078/0171-9335-00078. [DOI] [PubMed] [Google Scholar]
- Lehrich RW, Aller SG, Webster P, Marino CR, Forrest JN., Jr Vasoactive intestinal peptide, forskolin, and genistein increase apical CFTR trafficking in the rectal gland of the Spiny Dogfish, Squalus acanthias. Acute regulation of CFTR trafficking in an intact epithelium. J Clin Invest. 1998;101:737–745. doi: 10.1172/JCI803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretz CA. 14th International Congress of Comparative Endocrinology. Italy: Sorrento; 2001. Drinking and alimentary transport in teleost osmoregulation; pp. 723–732. [Google Scholar]
- Lorin-Nebel C, Boulo V, Bodinier C, Charmantier G. The Na+-K+-2Cl−cotransporter in the sea bass Dicentrarchus labraxduring ontogeny: involvement in osmoregulation. J Exp Biol. 2006;209:4908–4922. doi: 10.1242/jeb.02591. [DOI] [PubMed] [Google Scholar]
- Madsen SS, Jensen LN, Tipsmark CK, Kiilerich P, Borski RJ. Differential regulation of cystic fibrosis transmembrane conductance regulator and Na+, K+-ATPase in gills of striped bass, Morone saxatilis: effect of salinity and hormones. J Endocrinol. 2007;192:249–260. doi: 10.1677/JOE-06-0016. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Bryson SE. Transport mechanisms of seawater teleost chloride cells: an inclusive model of a multifunctional cell. Comp Biochem Physiol A. 1998;119:97–106. doi: 10.1016/s1095-6433(97)00402-9. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Grosell M. Ion transport, osmoregulation, and acid–base balance. In: Evans DH, Claiborne J), editors. The Physiology of Fishes. Boca Raton, FL: CRC Press; 2005. pp. 177–230. [Google Scholar]
- Marshall WS, Singer TD. Cystic fibrosis transmembrane conductance regulator in teleost fish. Biochim Biophys Acta – Biomembranes. 2002;1566:16–27. doi: 10.1016/s0005-2736(02)00584-9. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Emberley TR, Singer TD, Bryon SE, Cormick SDM. Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: a multivariable approach. J Exp Biol. 1999;202:1535–1544. doi: 10.1242/jeb.202.11.1535. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Howard JA, Cozzi RRF, Lynch EM. NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. J Exp Zool. 2002a;205:745–758. doi: 10.1242/jeb.205.6.745. [DOI] [PubMed] [Google Scholar]
- Marshall WS, Lynch EM, Cozzi RRF. Redistribution of immunofluorescence of CFTR anion channel and NKCC cotransporter in chloride cells during adaptation of the killifish Fundulus heteroclitusto sea water. J Exp Biol. 2002b;205:1265–1273. doi: 10.1242/jeb.205.9.1265. [DOI] [PubMed] [Google Scholar]
- Masini MA, Sturla M, Prato P, Uva B. Ion transport systems in the kidney and urinary bladder of two Antarctic teleost, Chionodraco hamatusand Trematomus bernacchii. Polar Biol. 2001;24:440–446. [Google Scholar]
- McCormick SD, Sundell K, Björnsson BT, Brown CL, Hiroi J. Influence of salinity on the localisation of Na+/K+-ATPase, Na+/K+/2Cl− cotransporter (NKCC) and CFTR anion channel in chloride cells of the Hawaiian goby (Stenogobius hawaiiensis. J Exp Biol. 2003;206:4575–4583. doi: 10.1242/jeb.00711. [DOI] [PubMed] [Google Scholar]
- Nebel C, Nègre-Sardargues G, Blasco C, Charmantier G. Morphofunctional ontogeny of the urinary system of the European sea bass Dicentrarchus labrax. Anat Embryol (Berl) 2005;209:193–206. doi: 10.1007/s00429-004-0438-6. [DOI] [PubMed] [Google Scholar]
- Pickett GD, Kelley DF, Pawson MG. The patterns of recruitment of sea bass, Dicentrarchus labraxL. from nursery areas in England and Wales and implications for fisheries management. Fish Res. 2004;68:329–342. [Google Scholar]
- Pickett GD, Pawson MG. Biology and ecology. In. In: Pitcher TJ, editor. Sea Bass Biology, Exploitation and Conservation. London: Chapman & Hall; 1994. pp. 1–147. [Google Scholar]
- Rankin JC, Davenport JA. Movement between fresh water and sea water. In: Rankin JC, Davenport JA, editors. Animal Osmoregulation. Glasgow: Blackie & Son Ltd; 1981. pp. 83–100. [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Roberts J, Bell M, Young H. Studies on the skin of plaice (Pleuronectes platessaL.) II. The development of larval plaice skin. J Fish Biol. 1973;5:103–108. [Google Scholar]
- Sabriye AS, Reay PJ, Coombs SH. Sea-bass larvae in coastal and estuarine plankton. J Fish Biol. 1988;33:231–233. [Google Scholar]
- Sardet C. Freeze fracture of the gill epithelium of euryhaline teleost fish. Am J Physiol Regul Integr Comp Physiol. 1980;238:R207–212. doi: 10.1152/ajpregu.1980.238.3.R207. [DOI] [PubMed] [Google Scholar]
- Scott GR, Schulte PM. Intraspecific variation in gene expression after seawater transfer in gills of the euryhaline killifish Fundulus heteroclitus. Comp Biochem Physiol A. 2005;141:176–182. doi: 10.1016/j.cbpb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM. Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitusafter abrupt salinity transfer. Am J Physiol-Cell Physiol. 2004;287:C300–C309. doi: 10.1152/ajpcell.00054.2004. [DOI] [PubMed] [Google Scholar]
- Scott GR, Schulte PM, Wood CM. Plasticity of osmoregulatory function in the killifish intestine: drinking rates, salt and water transport, and gene expression after freshwater transfer. J Exp Biol. 2006;209:4040–4050. doi: 10.1242/jeb.02462. [DOI] [PubMed] [Google Scholar]
- Sharratt BM, Bellamy D, Jones IC. Adaptation of the silver eel (Anguilla anguillaL.) to sea water and to artificial media together with observations on the role of the gut. Comp Biochem Physiol. 1964;11:19–30. doi: 10.1016/0010-406x(64)90092-1. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Kaneko T, Hasegawa S, Hirano T. Development of multicellular complexes of chloride cells in the yolk-sac membrane of tilapia (Oreochromis mossambicus) embryos and larvae in seawater. Cell Tissue Res. 1997;288:583–590. doi: 10.1007/s004410050844. [DOI] [PubMed] [Google Scholar]
- Singer TD, Tucker SJ, Marshall WS, Higgins CF. A divergent CFTR homologue: highly regulated salt transport in the euryhaline teleost F. heteroclitus. Am J Physiol-Cell Physiol. 1998;274C:715–723. doi: 10.1152/ajpcell.1998.274.3.C715. [DOI] [PubMed] [Google Scholar]
- Smith HW. The absorption and excretion of water and salts by marine teleosts. Am. J. Physiol. 1930;93:480–505. [Google Scholar]
- Taylor J, Grosell M. Basolateral NBC is the hinge of a mechanism serving both osmoregulation and acid-base balance in the marine teleost intestine. Comp Biochem Physiol A Mol Integr Physiol. 2008;150(suppl 1):S57–58. [Google Scholar]
- Varsamos S, Connes R, Diaz JP, Barnabe G, Charmantier G. Ontogeny of osmoregulation in the European sea bass Dicentrarchus labraxL. Mar Biol. 2001;138:909–915. [Google Scholar]
- Varsamos S, Diaz JP, Charmantier G, Blasco C, Connes R, Flik G. Location and morphology of chloride cells during the post-embryonic development of the European sea bass, Dicentrarchus labrax. Anat Embryol (Berl) 2002;205:203–213. doi: 10.1007/s00429-002-0231-3. [DOI] [PubMed] [Google Scholar]
- Varsamos S, Nebel C, Charmantier G. Ontogeny of osmoregulation in postembryonic fish: A review. Comp Biochem Physiol A Mol Integr PhysiolMol Integr Physiol. 2005;141:401–429. doi: 10.1016/j.cbpb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Venturini G, Cataldi E, Marino G, et al. Serum ions concentration and ATPase activity in gills, kidney and oesophagus of European sea bass (Dicentrarchus labrax) during acclimatation trials to fresh water. Comp Biochem Physiol A Mol Integr Physiol. 1992;103:451–454. [Google Scholar]
- Wales W, Tytler P. Changes in chloride cell distribution during early larval stages of Clupea harengus. J Fish Biol. 1996;49:801–814. [Google Scholar]
- Wilson JM, Randall DJ, Donowitz M, Vogl AW, Ip AK. Immunolocalisation of ion-transport proteins to branchial epithelium mitochondria-rich cells in the Mudskipper (Periophthalmodon schlosseri. J Exp Biol. 2000;203:2297–2310. doi: 10.1242/jeb.203.15.2297. [DOI] [PubMed] [Google Scholar]