Abstract
Sutures, joints that allow one bone to articulate with another through intervening fibrous connective tissue, serve as major sites of bone expansion during postnatal craniofacial growth in the vertebrate skull and represent an aspect of cranial ontogeny which may exhibit functional and phylogenetic correlates. Suture evolution among hystricognath rodents, an ecologically diverse group represented here by 26 species, is examined using sequence heterochrony methods, i.e. event pairing and parsimov. Although minor nuances in suture closure sequence exist between species, the overall sequence was found to be conserved both across the hystricognath group and, to an increasing degree, within selected clades. At species level, suture closure pattern exhibited a significant positive correlation with patterns previously reported for hominoids. Patterns for most clades revealed the first sutures to close are those contacting the exoccipital, interparietal, and palatine bones. Heterochronic shifts were found along 19 of 35 branches within the hystricognath phylogeny. The number of shifts per node ranged from one to seven events and, overall, involved 21 of 34 suture sites. The topology generated by parsimony analyses of the event pair matrix yielded only one grouping that was congruent with the evolutionary relationships, compiled from morphological and molecular studies, taken as framework. Sutures contacting the exoccipital displayed the highest levels of most complete closure across all species. Level of suture closure is negatively correlated with cranial length (P < 0.05). Differing life history and locomotory strategies are coupled in part with differing suture closure patterns among several species.
Keywords: development, heterochrony, Hystricognathi, Rodentia, skull, suture
Introduction
The relation between ontogenetic and phylogenetic change has commonly been addressed using an analytical framework constructed from the quantification and exploration of size and shape change (Klingenberg, 1998), with a focus on heterochrony or changes in developmental rate or timing. Although organismal form is innately a multivariate concept and thus may be expressed by size and shape, some aspects of development are best studied with other approaches. The study of sequence heterochrony (Smith, 2001) provides a methodology to study changes in the timing of developmental events. Earlier studies of sequence heterochrony within mammals have concentrated upon differences between marsupial and placental mammal ossification sequences (Smith, 1997; Nunn & Smith, 1998; Sánchez-Villagra, 2002; Bininda-Emonds et al. 2003; Goswami, 2007; Sánchez-Villagra et al. 2008; Weisbecker et al. 2008). The present study considers the influence of heterochronic processes and developmental conservatism in the generation of diversity within a single clade of placentals and a later aspect of skeletal development: sutures in hystricognath rodents.
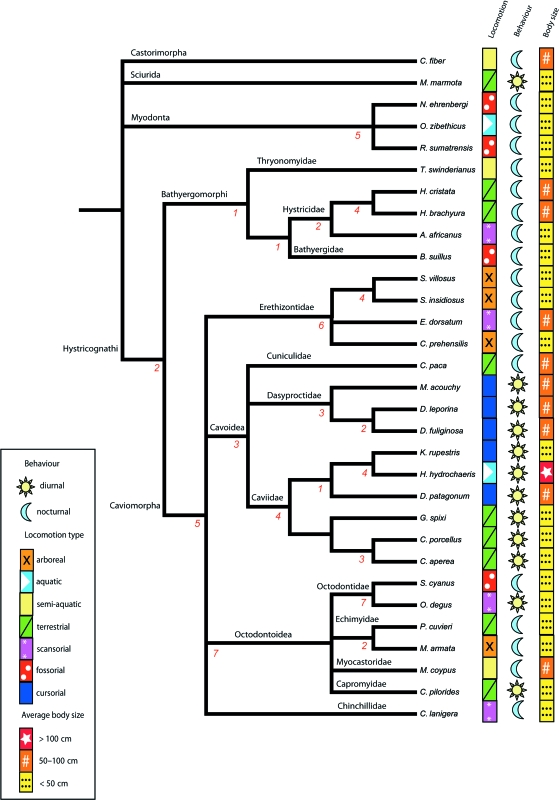
Rodents are the most abundant and taxonomically diverse order of living mammals, with 2277 members representing almost half of all living species (Wilson & Reeder, 2005). Living rodents inhabit all continents except Antarctica, and are found in almost every terrestrial habitat throughout their geographic range, playing integral roles in the ecosystems they inhabit. Within Rodentia a polarization exists which pivots upon Hystricognathi. This monophyletic clade, which includes Old World phiomorphs and New World caviomorphs (Adkins et al. 2001, 2003), contains less than 13% of all rodent species (Wilson & Reeder, 2005). Nevertheless, extant hystricognaths show ranges in body size and ecological diversity (Fig. 1, Table 1) that exceed those observed for any other group of rodents (Nowak, 1999; Sánchez-Villagra et al. 2003): members of the clade possess different life history strategies, locomotion styles and reproductive strategies, thereby providing a potentially relevant subject to investigate developmental mechanisms intrinsic to the evolution of diversity (e.g. Mess, 2003). From this precept, we concentrate upon the cranium and specifically on one aspect that mediates its growth: suture closure.
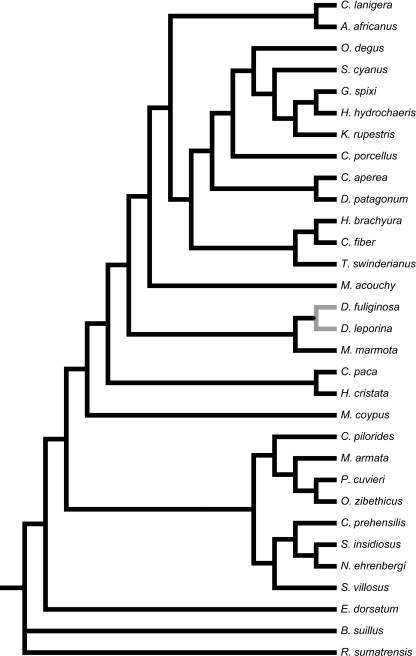
Fig. 1.
Compound phylogeny featuring species investigated: 26 Hystricognath taxa and five outgroup species selected from Myodonta, Sciurida and Castorimorpha. Number of recorded heterochronic shifts, or changes in timing of suture closure events following the consensus approach of parsimov, denoted under each branch.
Table 1.
Ecological and reproductive data for 26 investigated Hystricognath species and the five outgroups (marked with an asterisk *); number of specimens sampled (n). Data from Sherman et al. (1991), Nowak (1999), Lacey et al. (2000), Hutchins et al. (2003), and Mess (2007)
| Scientific name | n | Adult body length (cm) | Adult body mass (kg) | Age to maturity (months) |
|---|---|---|---|---|
| Marmota marmota* | 20 | 30–60 | 3–7.5 | 24 |
| Castor fiber* | 24 | 60–80 | 12–25 | 18–24 |
| Ondatra zibethicus* | 30 | 22.9–32.5 | 0.68–1.8 | 1.5–2 |
| Nannospalax ehrenbergi* | 21 | 28–65 | 0.1–0.22 | 24 |
| Rhizomys sumatrensis* | 13 | 23–48 | 1–4 | 5 |
| Thryonomys swinderianus | 31 | 35–61 | 4–7 | 12 |
| Hystrix cristata | 20 | 60–93 | 10–30 | 8–18 |
| Hystrix brachyura | 14 | 60–93 | 10–30 | 8–18 |
| Atherurus africanus | 10 | 36.5–57 | 1.5–4 | 24 |
| Bathyergus suillus | 10 | 8–33 | 0.64–0.93 | 12 |
| Sphiggurus villosus | 23 | 28–65 | 0.5–1.34 | 19 |
| Sphiggurus insidiosus | 11 | 28–65 | 0.5–1.34 | 19 |
| Erethizon dorsatum | 19 | 64.5–80 | 3.5–7 | 30 |
| Coendou prehensilis | 26 | 30–60 | 0.9–5 | 19 |
| Cuniculus paca | 30 | 50–77 | 7–12 | 12 |
| Myoprocta acouchy | 13 | 45–70 | 0.6–1.3 | 12 |
| Dasyprocta leporina | 31 | 41.5–62 | 1.3–4 | 6.5 |
| Dasyprocta fuliginosa | 9 | 41.5–62 | 1.3–4 | 6.5 |
| Kerodon rupestris | 16 | 38 | 1 | 5 |
| Hydrochoerus hydrochaeris | 35 | 106–134 | 35–66 | 15 |
| Dolichotis patagonum | 32 | 69–75 | 8–9 | 8 |
| Galea spixii | 17 | 15–20 | 0.3–0.6 | 2–3 |
| Cavia porcellus | 37 | 22–36 | 0.7–1.2 | 2–3 |
| Cavia aperea | 16 | 22–36 | 0.4–0.6 | 2–3 |
| Spalacopus cyanus | 8 | 11.5–16 | 0.06–0.12 | 8 |
| Octodon degus | 16 | 12.5–19 | 0.17–0.3 | 6 |
| Proechimys cuvieri | 24 | 26–30 | 0.3–0.38 | 5 |
| Makalata armata | 11 | 17–24 | 0.15–0.4 | ? |
| Myocastor coypus | 32 | 43–63.5 | 5–10 | 3–7 |
| Capromys pilorides | 9 | 33–45 | 1–2 | 10 |
| Chinchilla lanigera | 20 | 22.5–38 | 0.5–0.8 | 8 |
Sutures are joints in the vertebrate skull that have two bone fronts interposed with fibrous connective tissue (Rice, 1999; Depew et al. 2008). The vertebrate skull may be segmented into the bones surrounding the skull, the neurocranium, and the bones that form the face, the viscerocranium. The neurocranium has two subdivisions; the base, formed by endochondral ossification, and the calvaria, or cranial vault, formed from membrane bones and primarily composed of five elements: paired frontals, paired parietals and an interparietal (Rice, 1999). During early development an increase in intracranial volume is achieved primarily by sutural growth (Henderson et al. 2004). In response to signals transmitted through the dura mater, new bone is produced perpendicularly to the orientation of the suture, at the bone fronts of the sutural margins. Sutures maintain an approximately similar width, whereas the cranial vault expands to accommodate the developing brain (Opperman, 2000; Morriss-Kay & Wilkie, 2005). Sutures must remain patent to function; premature closure (craniosynostosis) results in growth constraint at the site of the affected suture and can lead to deformity. The genetic etiology of craniosynostosis has been studied extensively, revealing sutural biology to be intimately linked with transforming growth factors (TGFβs), fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs) (Rice, 1999; Ogle et al. 2004). Critical for the maintenance of normal cranial sutures, these factors must interact in the presence of dura mater (Opperman et al. 1993).
Topological correspondence is an important component of the invocation of homology between two elements (Hall, 1994; Depew et al. 2008). Cranial elements are bounded by sutures and thus in definition the suture becomes a prime unit in the identification of homology between elements. Nonetheless, past study has focussed upon elements: their cellular, molecular and functional characteristics, and not the sutural boundary that constrains their topological relation with other structures.
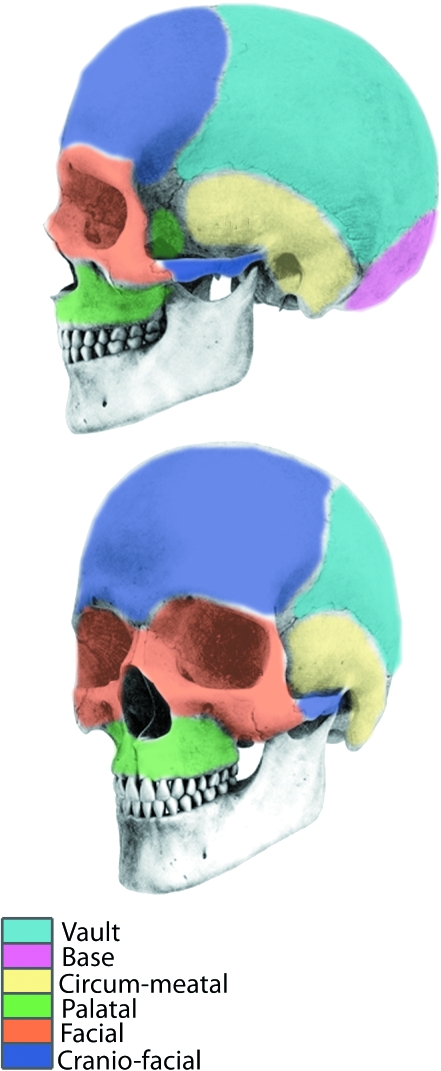
Early studies of cranial suture closure in humans focussed upon the forensic application of quantifying suture synostosis. An extensive study by Todd & Lyon (1924, 1925a,b,c) concluded that suture closure exhibited a definite periodicity and as such could be used to estimate skeletal age at death. Patterns of suture closure were investigated in hominoids by Krogman (1930) who described a reference pattern of suture closure that was considered to be of general application to mammals (Chopra, 1957; Herring, 1993). The sequence is commonly given as follows: vault, base, circum-meatal, palatal, facial, and cranio-facial (Fig. 2). Work by Schultz (1940, 1941, 1942) concerning apes and by Chopra (1957), who studied 10 genera of Old and New World monkeys, further corroborated this pattern.
Fig. 2.
Illustration of divisions within the pattern of suture closure proposed for hominoids (Krogman, 1930). The sequence is considered to be generally applicable to mammals and is as follows: vault, base, circum-meatal, palatal, facial, cranio-facial.
Herring (1972, 1974) attributed differences in endocranial suture fusion pattern among pigs and peccaries to cranial stresses. Sutures are more compliant than the bones they join and thus are able to absorb tensile and compressive forces. In particular, peccaries exhibited an early fusion of sutures associated with the palatal and facial regions, which Herring (1974) noted to be a marked contrast to Krogman's (1930) pattern and proposed to be directly related to stress in these regions of the skull. Several studies have suggested a functional relationship between suture complexity and forces associated with mastication (Sun et al. 2004; Wu et al. 2007). Indeed the work of Herring (1972) on pigs, Dolan (1971) on ceboid monkeys and Giannini et al. (2006) on a fruitbat indicated suture closure pattern to be species specific. Recently, Cray et al. (2008) reported that Gorilla gorilla exhibits a unique pattern of ectocranial vault suture closure, further substantiating this notion. Moreover, for a sample of Rhesus monkeys (Macaca mulatta) of known age and sex, Wang et al. (2006) detected familial groupings in suture patterns, suggesting variation may be heritable.
A comprehensive assay of heterochronies in cranial suture growth has not previously been conducted for any clade of mammals. The available methods to study sequence heterochrony (Smith, 1997; Jeffery et al. 2005) provide the opportunity to evaluate evolutionary changes in sutural timing patterns. In this report we address these issues by examining hystricognaths, an ecologically diverse clade of rodents. Several hypotheses are tested: (1) Hystricognathi is defined by a specific pattern of suture closure; (2) heterochronic shifts in suture closure events characterize monophyletic clades within Hystricognathi; (3) suture closure patterns for the species examined here are similar to patterns for hominoids (Krogman, 1930); (4) event pair data accurately reflects evolutionary relationships; (5) level of suture closure is correlated with cranial dimension.
Materials and methods
Data collection
The study sample comprised 628 crania from a total of 31 species. The sample included 26 species of Hystricognathi spanning a taxonomic and ecological breadth; additionally the outgroup consisted of three species of Myomorpha and one species each of Sciuromorpha and Castorimorpha (Table 1). Data were collected from specimens at the following Institutions: Palontologisches Institut und Museum der Universität Zürich, Naturhistorisches Museum Bern, Naturhistorisches Museum Basel, Museum für Naturkunde (Humboldt-Universität zu Berlin), Zoologische Staatssammlung München, National Museum of Natural History (Naturalis) Leiden, Muséum national d’Histoire naturelle Paris, and Naturhistorisches Museum Wien. A total of 34 suture sites were coded for each cranium (Fig. 3 and Table 2). Sutures were scored as either open (score 1), 1/4 closed (score 2), 1/2 closed (score 3) or completely closed (score 4) (see Fig. 4). Crania exhibiting gross pathology were excluded from the sample. The length of each cranium was measured and crania were specifically chosen such that the sample for each species resembled a growth series (Fig. 5). Species that exhibited fewer than five different suture closure events were considered to provide inadequate resolution and hence removed before final analyses. All specimens were coded by L.A.B.W.; to control for error a subset was re-coded at the end of the data collection period.
Fig. 3.
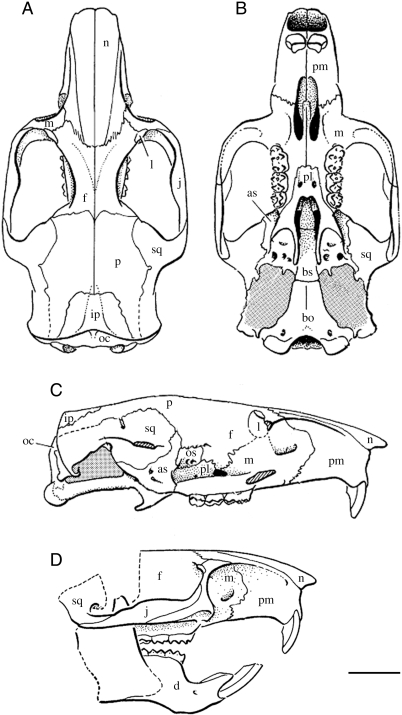
Sutural contact of cranial bones viewed in dorsal (A), ventral (B) and lateral (C,D) orientation. The zygomatic arch was removed from the lateral view (c) to illuminate the sutures surrounding the orbit: bones contacting the lacrimal or orbitosphenoid. Cranial bones: nasal (n), maxillary (m), jugal (j), lacrimal (l), frontal (f), parietal (p), squamosal (sq), interparietal (ip), occipital (o), premaxillary (pm), palatine (pl), alisphenoid (as), basisphenoid (bs), basioccipital (bo), dentary (d), (os) orbitosphenoid. Scale = 1 cm. Modified after Carrasco & Wahlert (1999).
Table 2.
Cranial sites representing sutural contact with each bone of the skull. Sutures denoted to be visible from orbital (o), frontal (f) and ventral (v) positions where applicable
| Cranial suture site |
| Mandibular symphysis |
| Interpremaxillary |
| Premaxillo-maxillary (v) |
| Intermaxillary |
| Maxillo-palatine (v) |
| Interpalatine |
| Pterygo-palate |
| Palate-alisphenoid |
| Basispheno-basioccipital |
| Basispheno-presphenoid |
| Alispheno-squamosal |
| Alispheno-orbitosphenoid |
| Orbitospheno-frontal |
| Palato-orbitosphenoid |
| Maxillo-lacrimal (o) |
| Jugo-squamosal |
| Lacrimo-frontal (o) |
| Lacrimo-frontal (f) |
| Jugo-frontal |
| Maxillo-jugal |
| Premaxillo-maxillary (f) |
| Premaxillo-nasal |
| Internasal |
| Naso-frontal |
| Interfrontal |
| Fronto-parietal |
| Interparietal |
| Fronto-squamosal |
| Parieto-squamosal |
| Supraoccipito-parietal |
| Supraoccipito-squamosal |
| Exoccipito-squamosal |
| Exoccipito-supraoccipital |
| Exoccipito-basioccipital |
Fig. 4.
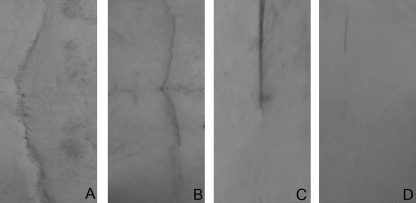
Illustration of suture sites displaying varying levels of closure: completely open – score 1 (A), 1/4 closed – score 2 (B), 1/2 closed – score 3 (C) and completely closed – score 4 (D).
Fig. 5.
Growth series of Dasyprocta leporina, from the Naturhistorisches Museum Basel (from left to right NHMBa-8409, 6955, 7647, 6645). Scale = 2 cm.
Phylogenetic framework
Evolutionary relationships among the hystricognaths were reconstructed from several literature sources, as there is no comprehensive phylogenetic study including all taxa studied. The clades used here are indicated in the phylogenetic framework depicted in Fig. 1. Relationships between Old World hystricognaths, Cavoidea, Erethizontoidae, Chinchillidae, and Octodontoidea follow Huchon & Douzery (2001). Within Octodontoidea, relationships follow the molecular work of Honeycutt et al. (2003). Relations among the Echimyidae follow Galewski et al. (2005), and the systematics of Hystricidae are based on the classification by McKenna & Bell (1997).
Analyses of suture pattern conservation
The pattern of suture closure was determined for Bathyergomorphi and Caviomorpha and also for Hystricognathi as a whole. The raw closure scores for each species were totalled for each suture site and the suture sites were then ranked by descending numerical value, reflecting the first to last incidence of suture closure. Kendall's coefficient of concordance (Kendall's W) was calculated, using the ranked raw closure scores for Bathyergomorphi and Caviomorpha, and then for Hystricognathi as a whole, to determine the relative conservation of suture closure pattern. Kendall's W is a non-parametric statistic that may be applied to ordinal data to evaluate the degree to which several judges (p) concur when ranking a given set of objects (n): here the judges are replaced by species and the objects are the suture sites. The null hypothesis of Kendall's W states that the judges produce rankings that are independent of one another, hence the different species do not share any identifiable pattern of suture closure. Kendall's W statistic initially calculates a sum-of-squares statistic (S) computed from row marginal sums of ranks (Ri) received by the suture sites (Equation 1.0). The S statistic is then utilized to obtain the coefficient value (Equation 1.1). Thus the variance present in the row sums of ranks is evaluated in the context of the maximum possible value the variance may have, which is equal to all species exhibiting identical suture closure patterns. Therefore 0 ≤ W ≤ 1 (Zar, 1999) where 0 reflects total independence of suture closure pattern between species and 1 represents identical patterns between species.
| (Eq. 1.0) |
| (Eq. 1.1) |
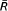 is the mean of Ri and Tis a correction factor to account for tied values between ranks.
is the mean of Ri and Tis a correction factor to account for tied values between ranks.
Kendall's W statistic is related to the Friedman chi-square 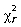 and thus W may be converted to its equivalent
and thus W may be converted to its equivalent 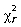 value (Equation 1.2) to determine whether the Kendall's W statistic produced from a sample represents an association different from zero within the sample population.
value (Equation 1.2) to determine whether the Kendall's W statistic produced from a sample represents an association different from zero within the sample population.
| (Eq. 1.2) |
A Kendall's tau (τ) rank correlation was constructed to assess the correspondence of suture closure pattern between each species and the general pattern proposed by Krogman (1930). Kendall's τ (Equation 1.3) measures cross-tabulation associations between two rankings. A rank was created to represent the order of suture closure that would be expected following the pattern suggested by Krogman (1930) such that a number was assigned to each suture site reflecting the spatial divisions and order of these divisions as follows: vault sutures (1), base sutures (2), circum-meatal sutures (3), palatal sutures (4), facial sutures (5), and cranio-facial sutures (6). The suture closure pattern for each species was re-ranked using this scheme to enable comparison with Krogman's (1930) sequence. Kendall's tau-b statistic was chosen to enable adjustments to be made for ties within the rankings and is defined as:
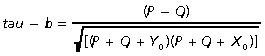 |
(Eq. 1.3) |
where the number of concordant (P) over discordant (Q) pairs is divided by the geometric mean of the number of tied events for the first suture sequence (Y0) and the number of tied events for the second suture sequence (X0).
All statistical analyses were performed using SPSS version16.0 (SPSS Inc, 2007).
Heterochrony analysis
Thirty-four events were identified, representing each site of sutural contact between two cranial bones. To identify heterochronies within the pattern of suture closure between the 31 different taxa, the timing of each of these events was assessed by comparing the relative timing of pairs of elements. An event pair matrix was constructed for each taxon in which the timing of each of the 34 events relative to each other event could be expressed: a given event was coded to have occurred earlier than (score 0), simultaneously with (score 1), or later than (score 2) each of the other events in turn (Smith, 1997). The resultant matrix contained 561 event pairs. Event pairs were mapped onto the composite phylogeny using paup*4.0b10 (Swofford, 2002) to reconstruct apomorphic character state changes. Parsimov (Jeffery et al. 2005), a parsimony-based computer program, was used to implement event pairing analysis. The Parsimov method derives a solution that accounts for all the possible event pair changes along a given branch that may be explained by the least number of event movements (Jeffery et al. 2005). Data were optimized using acctran and deltran criteria to consider ambiguous character-state reconstructions. For situations where a character state change offers equally parsimonious explanations, an acctran optimization will imply the character state originated early within the phylogenetic framework and subsequently a reversal occurred at a later stage. In reverse, a deltran optimization delays a change resulting in the occurrence of parallel origination at a later stage. Jeffery et al. (2005) proposed a conservative approach (Sánchez-Villagra et al. 2008), adopted here, using the consensus of acctran and deltran optimizations thus only unambiguous changes are inferred to be heterochronic.
Event pairs for phylogenetic reconstruction
Event pair data were analysed with paup*4.0b10 (Swofford, 2002) using maximum parsimony methods, notwithstanding the limitations of this approach (see Discussion). A heuristic search was performed with tree bisection-reconnection (TBR) branch-swapping on all most parsimonious trees (MPTs). The resulting topology was compared with the composite phylogeny (Fig. 1) to assess the utility of event pairs in the context of phylogenetic reconstruction.
Relationship between level of sutural closure and cranial dimension within and between species
To quantify the level of suture closure per species across the 34 examined sutural contact sites the raw scores for each site were averaged and the total number of completely open (score 1) and completely closed (score 4) sites were recorded as a percentage of total sites. To illuminate the typical closure trend for a species the overall amount of closure was defined as a percentage of complete closure: Σ site 1–34/(34*4). The same method was also applied to quantify the level of suture closure per site across the 31 species examined.
To examine the relationship between cranial dimension and level of suture closure, cranial length was recorded for each specimen using callipers. Cranial length measurements and maximum raw suture closure scores were averaged for each species and between species. Spearman's rank correlation coefficient was used to test the significance of the relationship between cranial length and level of suture closure for both within-species and between-species data.
Results
Suture closure quantification between sites and species
Suture sites remained completely open in 195 specimens (31%), whereas only 25 specimens (4%) displayed any completely closed sutures (Table 3a). Indeed 21 of 34 suture sites remained open in all species: the least amount of overall closure (26–28%) was observed for the mandibular symphysis, premaxillo-maxillary (v), maxillo-jugal, premaxillo-maxillary (f), and naso-frontal sutures (Fig. 6). Conversely, the interpalatine, interparietal, exoccipito-supraoccipital, and exoccipito-basioccipital sutures displayed the highest amount of closure (81–87%). Overall closure at a site averaged 43% (Table 5a).
Table 3a.
Quantification of closure per suture site for all crania studied. Percentage of crania scoring 1 (open) or 4 (closed) for each suture and evaluation of overall closure exhibited at a given site
| Cranial suture site | % open (score 1) | % closed (score 4) | % closure |
|---|---|---|---|
| Mandibular symphysis | 62 | 0 | 27 |
| Interpremaxillary | 48 | 0 | 32 |
| Premaxillo-maxillary (v) | 55 | 0 | 28 |
| Intermaxillary | 19 | 3 | 40 |
| Maxillo-palatine (v) | 19 | 0 | 41 |
| Interpalatine | 0 | 13 | 81 |
| Pterygo-palate | 45 | 0 | 34 |
| Palate-alisphenoid | 26 | 0 | 41 |
| Basispheno-basioccipital | 10 | 0 | 43 |
| Basispheno-presphenoid | 6 | 3 | 66 |
| Alispheno-squamosal | 22 | 0 | 37 |
| Alispheno-orbitosphenoid | 3 | 0 | 52 |
| Orbitospheno-frontal | 6 | 0 | 43 |
| Palato-orbitosphenoid | 0 | 13 | 68 |
| Maxillo-lacrimal (o) | 16 | 16 | 55 |
| Jugo-squamosal | 68 | 0 | 27 |
| Lacrimo-frontal (o) | 23 | 3 | 49 |
| Lacrimo-frontal (f) | 46 | 3 | 41 |
| Jugo-frontal | 42 | 3 | 42 |
| Maxillo-jugal | 74 | 0 | 28 |
| Premaxillo-maxillary (f) | 45 | 0 | 29 |
| Premaxillo-nasal | 81 | 0 | 26 |
| Internasal | 52 | 0 | 30 |
| Naso-frontal | 71 | 0 | 28 |
| Interfrontal | 3 | 3 | 46 |
| Fronto-parietal | 26 | 0 | 37 |
| Interparietal | 0 | 29 | 86 |
| Fronto-squamosal | 26 | 0 | 38 |
| Parieto-squamosal | 45 | 0 | 35 |
| Supraoccipito-parietal | 32 | 0 | 39 |
| Supraoccipito-squamosal | 45 | 0 | 34 |
| Exoccipito-squamosal | 49 | 0 | 34 |
| Exoccipito-supraoccipital | 0 | 19 | 84 |
| Exoccipito-basioccipital | 0 | 26 | 87 |
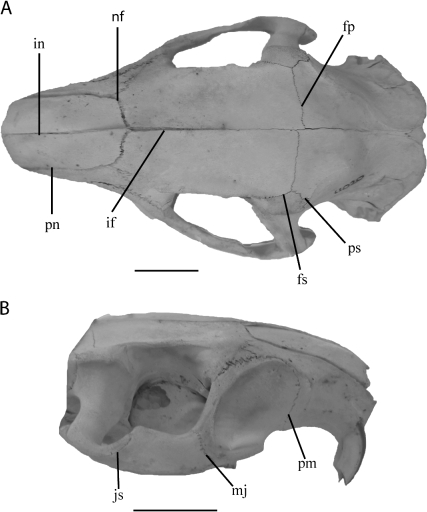
Fig. 6.
Dorsal (A) and lateral (B) view of Myocastor coypus. Open (score 1) cranial sutures indicated as follows: interfrontal (if), premaxillo-nasal (pn), internasal (in), fronto-parietal (fp), fronto-squamosal (fs), parieto-squamosal (ps), naso-frontal (nf), jugo-squamosal (js), maxillo-jugal (mj), premaxillo-maxillary (pm). Scale bar represents 2 cm.
Table 5a.
Correlation, using Kendall's tau (τ) rank, of suture pattern per species in relation to the classical paradigm (Krogman, 1930), outgroups marked with an asterisk (*)
| Species | τ | P | Average cranial length recorded (mm) |
|---|---|---|---|
| Marmota marmota* | 0.43 | < 0.01 | 56.7 |
| Castor fiber* | 0.33 | < 0.05 | 77.6 |
| Ondatra zibethicus* | −0.01 | < 0.75 | 66.7 |
| Nannospalax ehrenbergi* | −0.05 | < 0.75 | 59.4 |
| Rhizomys sumatrensis* | 0.19 | < 0.25 | 65.2 |
| Thryonomys swinderianus | 0.39 | < 0.01 | 80.1 |
| Hystrix cristata | 0.34 | < 0.05 | 125.4 |
| Hystrix brachyura | 0.39 | < 0.01 | 99.7 |
| Atherurus africanus | 0.26 | < 0.10 | 90.5 |
| Bathyergus suillus | 0.01 | < 0.75 | 72.2 |
| Sphiggurus villosus | 0.12 | < 0.50 | 66.1 |
| Sphiggurus insidiosus | −0.04 | < 0.75 | 65.5 |
| Erethizon dorsatum | 0.20 | < 0.25 | 82.9 |
| Coendou prehensilis | 0.15 | < 0.50 | 75.7 |
| Cuniculus paca | 0.49 | < 0.001 | 128.8 |
| Myoprocta acouchy | 0.40 | < 0.01 | 71.1 |
| Dasyprocta leporina | 0.37 | < 0.01 | 96.1 |
| Dasyprocta fuliginosa | 0.45 | < 0.01 | 74.7 |
| Kerodon rupestris | 0.18 | < 0.25 | 71.4 |
| Hydrochoerus hydrochaeris | 0.28 | < 0.05 | 117.3 |
| Dolichotis patagonum | 0.41 | < 0.10 | 95.4 |
| Galea spixii | 0.25 | < 0.10 | 56.0 |
| Cavia porcellus | 0.22 | < 0.25 | 59.8 |
| Cavia aperea | 0.20 | < 0.25 | 60.2 |
| Spalacopus cyanus | 0.21 | < 0.25 | 44.0 |
| Octodon degus | 0.19 | < 0.25 | 47.6 |
| Proechimys cuvieri | −0.08 | < 0.75 | 58.9 |
| Makalata armata | 0.01 | < 0.75 | 46.8 |
| Myocastor coypus | 0.03 | < 0.75 | 90.6 |
| Capromys pilorides | 0.29 | < 0.05 | 84.1 |
| Chinchilla lanigera | 0.28 | < 0.10 | 58.27 |
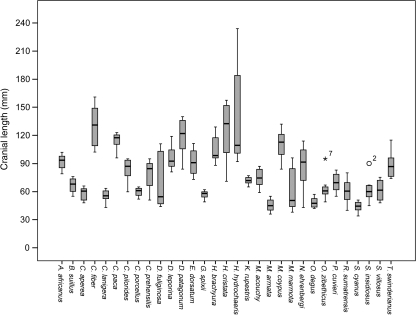
For all species studied, an average of 12 sites (35%) per cranium remained completely open and one site (4%) displayed complete closure (Table 3b). Bathyergus suillusand Spalacopus cyanusexhibited the highest number of completely closed sutures (29% and 13%, respectively) and the highest overall closure (52–75%) was observed in Nannospalax ehrenbergi, Sphiggurus villosus, Sphiggurus insidiosus, Coendou prehensilis, and Makalata armata. Several taxa did not exhibit any completely open sutures (C. prehensilis, S. insidiosus, Hystrix brachyura, and Marmota marmota), whereas 61–68% of suture sites remained open among specimens belonging to Dasyprocta fuliginosa and Hydrochoerus hydrochaeris: representatives for both species included individuals with cranial lengths above 100 mm; especially, some H. hydrochaeriscrania spanned 245 mm (Fig. 7).
Table 3b.
Quantification of closure per species, including outgroup taxa (*), for all crania studied. Percentage of suture sites scoring 1 (open) or 4 (closed) for each species and evaluation of overall closure exhibited by a given species
| Species | % open (score 1) | % closed (score 4) | % closure |
|---|---|---|---|
| Marmota marmota* | 0 | 0 | 43 |
| Castor fiber* | 42 | 0 | 34 |
| Ondatra zibethicus* | 23 | 10 | 54 |
| Nannospalax ehrenbergi* | 3 | 6 | 75 |
| Rhizomys sumatrensis* | 39 | 0 | 49 |
| Thryonomys swinderianus | 52 | 0 | 34 |
| Hystrix cristata | 26 | 0 | 40 |
| Hystrix brachyura | 0 | 3 | 44 |
| Atherurus africanus | 39 | 3 | 39 |
| Bathyergus suillus | 42 | 29 | 56 |
| Sphiggurus villosus | 13 | 0 | 52 |
| Sphiggurus insidiosus | 0 | 10 | 60 |
| Erethizon dorsatum | 29 | 3 | 49 |
| Coendou prehensilis | 0 | 10 | 55 |
| Cuniculus paca | 52 | 0 | 35 |
| Myoprocta acouchy | 52 | 10 | 38 |
| Dasyprocta leporina | 45 | 0 | 36 |
| Dasyprocta fuliginosa | 61 | 3 | 38 |
| Kerodon rupestris | 52 | 3 | 44 |
| Hydrochoerus hydrochaeris | 68 | 0 | 33 |
| Dolichotis patagonum | 42 | 0 | 37 |
| Galea spixii | 55 | 3 | 40 |
| Cavia porcellus | 42 | 0 | 40 |
| Cavia aperea | 39 | 6 | 43 |
| Spalacopus cyanus | 52 | 13 | 46 |
| Octodon degus | 42 | 0 | 43 |
| Proechimys cuvieri | 32 | 0 | 42 |
| Makalata armata | 32 | 10 | 54 |
| Myocastor coypus | 32 | 0 | 40 |
| Capromys pilorides | 35 | 3 | 40 |
| Chinchilla lanigera | 29 | 0 | 41 |
Fig. 7.
Box plot illustrating cranial size variation among specimens studied. Median line represents 122.5 mm.
Suture closure pattern for Hystricognathi
Kendall's coefficient of concordance and supporting Friedman's 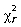 values indicate suture closure pattern is conserved across Hystricognathi (Table 4) with correlation between ranks varying from 0.57 to 0.90. Related critical values for Friedman's chi-square
values indicate suture closure pattern is conserved across Hystricognathi (Table 4) with correlation between ranks varying from 0.57 to 0.90. Related critical values for Friedman's chi-square 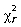 ranged between P < 0.025 and P < 0.001. The highest degree of correlation was displayed by clades comprising the least number of taxa. Nevertheless, W = 0.67 for the six taxa representing Octodontoidea, reflecting a marginal decrease compared to W = 0.71 for the 10 taxa incorporated within Cavoidea. Equally, Kendall's W for Hystricidae (0.70) is considerably lower than that for the members of Dasyprocta (0.86) despite each clade being represented by three different species. This discrepancy likely reflects the differing number of total specimens within the two clades (Table 1) and additionally the unequal distribution of specimens across the three species within the clade.
ranged between P < 0.025 and P < 0.001. The highest degree of correlation was displayed by clades comprising the least number of taxa. Nevertheless, W = 0.67 for the six taxa representing Octodontoidea, reflecting a marginal decrease compared to W = 0.71 for the 10 taxa incorporated within Cavoidea. Equally, Kendall's W for Hystricidae (0.70) is considerably lower than that for the members of Dasyprocta (0.86) despite each clade being represented by three different species. This discrepancy likely reflects the differing number of total specimens within the two clades (Table 1) and additionally the unequal distribution of specimens across the three species within the clade.
Table 4.
Kendall's coefficient of concordance (Kendall's W statistic) and Friedman's chi square for selected clades: number of taxa (N) and number of specimens (Ns) detailed for each clade. W = 1 indicates identical suture closure sequence among clade members. Critical values based on (n − 1) degrees of freedom
| Clade | N | Ns | Kendall's W (0–1) | Friedman 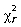
|
|---|---|---|---|---|
| Hystricognathi | 26 | 520 | 0.57 | P < 0.001 |
| Caviomorpha | 21 | 435 | 0.64 | P < 0.001 |
| Caviidae | 6 | 153 | 0.79 | P < 0.001 |
| Caviinae | 3 | 70 | 0.84 | P < 0.001 |
| Cavoidea | 10 | 236 | 0.71 | P < 0.001 |
| Dasyproctidae | 3 | 53 | 0.86 | P < 0.001 |
| Echimyidae | 2 | 35 | 0.89 | P < 0.025 |
| Erethizontidae | 4 | 79 | 0.83 | P < 0.001 |
| Hystricidae | 3 | 44 | 0.70 | P < 0.001 |
| Octodontidae | 2 | 24 | 0.90 | P < 0.025 |
| Octodontoidea | 6 | 100 | 0.67 | P < 0.001 |
Heterochronic shifts in suture closure pattern
Heterochronic shifts were found along 19 of 35 branches in the composite phylogeny (Fig. 1). The number of heterochronic shifts per node ranged from one to seven events and, overall, involved 21 of 34 investigated suture sites (62%). In total, 65 consensus heterochronic events were identified (Fig. 1); of these events, 50 shifts occurred within Caviomorpha, eight shifts occurred within Bathyergomorphi and two shifts were found to characterize Hystricognathi: early closure of the fronto-parietal and late closure of the alisphenoid-squamosal.
Heterochronies in the Caviomorpha
Caviomorpha was characterized by relatively early closure of vault sutures (supraoccipital-parietal and supraoccipital-squamosal) relative to palatal sutures and a relatively late closure of the internasal suture (Fig. 6A) in relation to suture contact between the nasal and premaxillary or frontal. Within Caviomorpha the octodontids exhibited the greatest number of shifts per branch; particularly Octodontidae, represented by S. cyanusand Octodon degus, showed seven shifts and was the only group to display closure of the jugo-squamosal (Fig. 6B), which was identified to be delayed relative to facial suture closure. Members of Echimyidae shared an early closure of cranial base sutures (basisphenoid-basioccipital and basisphenoid-presphenoid) compared to closure of the maxillo-palatine. Both Octodontoidea and Cavoidea exhibited early closure of the parieto-squamosal suture (Fig. 6A), whereas Cavoidea was distinguished from Octodontoidea by the late closure of the mandibular symphysis, shared by both species of Cavia(Cavia porcellusand Cavia aperea), in relation to sutures associated with the jugal. Additionally, five of 16 heterochronic shifts (31%) occurring within Octodontoidea were characterized by a relative late event shift compared to 14 of 27 instances (52%) in Cavoidea. Members of Dasyproctidae, within Cavoidea, exhibited early closure of the maxillo-palatine and palate-alisphenoid in relation to more than 10 other sutures associated with lacrimal, jugal and nasal elements. Differently, Caviidae was characterized by relatively early closure of the maxillo-lacrimal, shared by members of both Caviinae and Hydrochoerinae. All lacrimal contacting sutures closed late, relative to the sutural margins of the alisphenoid, within the New World porcupines (Erethizontidae), a marked contrast to the early closure of selected lacrimal sutures amongst other members of Caviomorpha, especially Octodontidae and Cavinae.
Heterochronies in the Bathyergomorphi
The maxillo-palatine suture closed relatively late amongst members of Bathyergomorphi, although this suture was also found to close late amongst Erethizontidae, specifically Sphiggurusand, in reverse, comparatively early within Dasyproctidae. Species belonging to Hystricidae were characterized by late closure of supraoccipital sutures relative to sutures joining the frontal and maxillary to the lacrimal. Similarly the late closure of the naso-frontal relative to sutures linked with the maxillary was also observed within Hystricidae.
Comparison of suture closure pattern with previous pattern suggested for hominoids
When compared with Krogman's (1930) pattern, the suture closure patterns for sampled species revealed τ correlations between −0.08 and 0.49 (Table 5a). A significant positive relationship (P < 0.05) with Krogman's (1930) pattern existed among ten of the 31 species analysed, ranging from τ = 0.28 to τ = 0.43. Particularly, all representatives of Hystrixand Dasyprocta exhibited a significant relationship to Krogman's (1930) pattern while the highest level of significance was displayed by Cuniculus paca (P < 0.001, τ = 0.49). Notably, the five largest average cranial lengths belonged to those species that exhibited a suture pattern which significantly correlated with Krogman's: ranging from 129 mm for C. pacato 96 mm for Dasyprocta leporina(Table 5a). With the exception of Bathyergomorphi (τ = 0.23, P < 0.05), the τ correlations for monophyletic clades were weakly positive. Examining the patterns closely (Table 5b) this may be evidenced by the relatively late closure of facial sutures (premaxillo-maxillary, naso-frontal, maxillo-jugal, jugo-squamosal and premaxillo-nasal) that characterizes each clade. Sutures within the facial division were penultimate to close within Krogman's (1930) pattern.
Table 5b.
Suture closure patterns for Hystricognathi (26 taxa), Caviomorpha (21 taxa) and Bathyergomorphi (5 taxa) beginning with the first closure event. Simultaneously occurring events are shaded grey
| Hystricognathi | Caviomorpha | Bathyergomorphi |
|---|---|---|
| Interparietal | Interparietal | Exoccipito-supraoccipital |
| Exoccipito-basioccipital | Interpalatine | Interparietal |
| Exoccipito-supraoccipital | Exoccipito-basioccipital | Exoccipito-basioccipital |
| Interpalatine | Exoccipito-supraoccipital | Interpalatine |
| Basisphenoid-presphenoid | Basisphenoid-presphenoid | Palato-orbitosphenoid |
| Palato-orbitosphenoid | Palato-orbitosphenoid | Basisphenoid-presphenoid |
| Maxillo-lacrimal (o) | Maxillo-lacrimal (o) | Intermaxillary |
| Alispheno-orbitosphenoid | Alispheno-orbitosphenoid | Maxillo-palatine (v) |
| Lacrimo-frontal (o) | Lacrimo-frontal (o) | Interfrontal |
| Basisphenoid-basioccipital | Basisphenoid-basioccipital | Maxillo-lacrimal (o) |
| Interfrontal | Orbitospheno-frontal | Alispheno-orbitosphenoid |
| Orbitospheno-frontal | Interfrontal | Supraoccipito-parietal |
| Maxillo-palatine (v) | Palate-alisphenoid | Lacrimo-frontal (f) |
| Maxillo-lacrimal (f) | Maxillo-palatine (v) | Maxillo-lacrimal (f) |
| Intermaxillary | Maxillo-lacrimal (f) | Basisphenoid-basioccipital |
| Palate-alisphenoid | Alispheno-squamosal | Lacrimo-frontal (o) |
| Lacrimo-frontal (f) | Lacrimo-frontal (f) | Fronto-parietal |
| Supraoccipito-parietal | Supraoccipito-parietal | Orbitospheno-frontal |
| Alispheno-squamosal | Intermaxillary | Fronto-squamosal |
| Fronto-squamosal | Pterygo-palatine (v) | Parieto-squamosal |
| Fronto-parietal | Fronto-squamosal | Alispheno-squamosal |
| Pterygo-palatine (v) | Fronto-parietal | Internasal |
| Parieto-squamosal | Supraoccipito-squamosal | Palate-alisphenoid |
| Exoccipito-squamosal | Exoccipito-squamosal | Interpremaxillary |
| Supraoccipito-squamosal | Parieto-squamosal | Exoccipito-squamosal |
| Interpremaxillary | Interpremaxillary | Naso-frontal |
| Premaxillo-maxillary (f) | Premaxillo-maxillary (f) | Premaxillo-maxillary (f) |
| Internasal | Premaxillo-maxillary (v) | Supraoccipito-squamosal |
| Premaxillo-maxillary (v) | Mandibular symphysis | Maxillo-jugal |
| Mandibular symphysis | Naso-frontal | Pterygo-palatine (v) |
| Naso-frontal | Internasal | Premaxillo-maxillary (v) |
| Maxillo-jugal | Maxillo-jugal | Mandibular symphysis |
| Jugo-squamosal | Jugo-squamosal | Jugo-squamosal |
| Premaxillo-nasal | Premaxillo-nasal | Premaxillo-nasal |
Event pairs for phylogenetic reconstruction
The topology generated from paup*4.0b10 (Swofford, 2002) analyses of the event pair matrix (Fig. 8) yielded only one grouping that was congruent with the evolutionary relationships taken from the literature (Fig. 1).
Fig. 8.
Most-parsimonious tree generated using paup*4.0b10 (Swofford, 2002) from parsimony analysis of event pair character matrix (3432 steps, CI = 0.26, RI = 0.39). The only sister group relationship preserved from the composite tree topology (see Fig. 1) is highlighted in grey.
Correlation of suture closure with cranial length
Level of suture closure displayed a significant negative correlation with cranial length within species for 11 of 31 species (Table 6). The most significant relationship between these two variables was exhibited by C. paca, and N. ehrenbergi (P < 0.01). Four of the remaining 20 species displayed a marginally insignificant (P = 0.6–0.7) correlation between level of suture closure and cranial length.
Table 6.
Spearman's rank correlation coefficient and associated significance values (P) for the relationship between cranial length and level of suture closure. Outgroups marked with an asterisk (*)
| Species | Correlation coefficient | P |
|---|---|---|
| Marmota marmota* | −0.38 | 0.07 |
| Castor fiber* | −0.46 | 0.05 |
| Ondatra zibethicus* | −0.19 | 0.31 |
| Nannospalax ehrenbergi* | −0.77 | < 0.01 |
| Rhizomys sumatrensis* | −0.57 | 0.07 |
| Thryonomys swinderianus | −0.41 | < 0.05 |
| Hystrix cristata | −0.54 | 0.05 |
| Hystrix brachyura | 0.17 | 0.56 |
| Atherurus africanus | 0.17 | 0.65 |
| Bathyergus suillus | −0.52 | 0.10 |
| Sphiggurus villosus | −0.42 | < 0.05 |
| Sphiggurus insidiosus | −0.57 | < 0.05 |
| Erethizon dorsatum | −0.03 | 0.91 |
| Coendou prehensilis | −0.50 | 0.01 |
| Cuniculus paca | −0.48 | < 0.01 |
| Myoprocta acouchy | 0.26 | 0.36 |
| Dasyprocta leporina | −0.54 | 0.01 |
| Dasyprocta fuliginosa | −0.73 | < 0.05 |
| Kerodon rupestris | 0.22 | 0.42 |
| Hydrochoerus hydrochaeris | −0.13 | 0.47 |
| Dolichotis patagonum | 0.16 | 0.35 |
| Galea spixii | 0.33 | 0.19 |
| Cavia porcellus | 0.31 | 0.06 |
| Cavia aperea | 0.22 | 0.42 |
| Spalacopus cyanus | 0.11 | 0.79 |
| Octodon degus | 0.25 | 0.34 |
| Proechimys cuvieri | −0.37 | 0.07 |
| Makalata armata | 0.24 | 0.48 |
| Myocastor coypus | 0.42 | 0.05 |
| Capromys pilorides | 0.43 | 0.24 |
| Chinchilla lanigera | 0.14 | 0.62 |
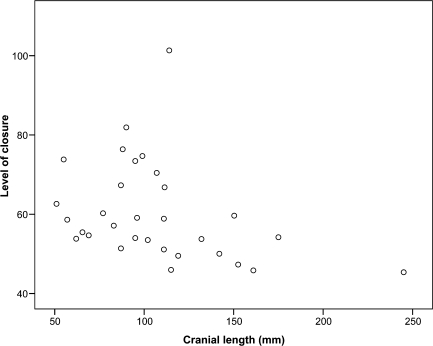
Level of suture closure also displayed a significant correlation with cranial length between species (Fig. 9). A trend to a decrease in level of closure is coupled with an increase in cranial length, evidenced by a correlation coefficient of −0.45 (P < 0.05).
Fig. 9.
Relationship between measured cranial length and level of suture closure, based upon raw closure scores, for each of the 31 sampled species (Spearman's correlation coefficient, −0.45, P < 0.05).
Discussion
Although minor differences in suture closure sequence existed between species, the overall sequence was conserved both across the group and, to an increasing degree, within selected clades (Table 3). For several species and selected clades (Table 4a) this pattern correlated significantly with that found in hominoids (Krogman, 1930) and hyenas (Schweikher, 1930). The application of sequence heterochrony methods to investigate suture closure revealed unique patterns occur among several clades but equally numerous closure events are characterized in part by parallelism.
Suture closure pattern quantification between sites and species
The capybara (H. hydrochaeris) had the greatest percentage of sutures remaining fully open (68%). The pattern of suture closure exhibited by this species also correlated significantly with Krogman's (1930) pattern (P < 0.05). In contrast none of the species that displayed the highest levels of overall suture closure (54–75%) exhibited a significant correlation to the pattern proposed by Krogman (1930); these species actually displayed the lowest levels of significance (P < 0.75) and included two subterranean rodents (B. suillusand N. ehrenbergi) in addition to three of four members of New World porcupines (Erethizontidae). The anomalous species within Erethizontidae was Erethizon dorsatum: specimens belonging to this species exhibited marginally less overall suture closure (49%) and an increased amount of complete patency (29%) compared to other group members. Contrasting with the other representative members of Erethizontidae, E. dorsatumadopts a scansorial, not arboreal, mode of life and is a larger animal: the lowest average body mass for E. dorsatumis more than twice the highest value for either Sphiggurusor Coendou whilst, similarly, ranges in body length between the latter two genera and the former species barely overlap (Table 1).
Sutures displaying the highest levels of closure across all sampled species were the exoccipito-basioccipital (87%), interparietal (86%), exoccipito-supraoccipital (84%), and the interpalatine (81%) (Table 3a). Herring (1972) proposed the early fusion of palatal sutures in peccaries was directly related to high levels of palatal stress. A similar pattern was not demonstrated here: the interpalatine was the only suture within the palatal division (Krogman, 1930) to exhibit a high degree of overall closure, other sutures within this division include the intermaxillary (40% overall closure) and interpremaxillary (32%). Expectedly the palatal sutures did not cluster together within the closure pattern sequence (Table 5b), whereas the facial sutures, previously implicated in cranial stress mitigation, all exhibit an analogous degree of overall closure (26–30%).
Chopra (1957) found high intraspecific and intrageneric variability in suture closure of monkeys but noted that each genus had a regular sequence. The number of specimens per species within our study ranged from nine to 37 (Table 1) and within species the range in cranial length varied from 13 mm for Galea spixiito 2 mm for H. hydrochaeris (Fig. 7), hence differing degrees of intraspecific variation are not completely comparable across the studied sample. Intraspecific variation was low for Capromys pilorides, C. paca, M. marmota, N. ehrenbergi, and S. cyanus, whereas C. prehensilis and Myocastor coypus exhibited a comparatively higher level of intraspecific variability. The species with the highest range of measured cranial length and the greatest overall cranial size (H. hydrochaeris) did not exhibit a high level of intraspecific variability, perhaps indicating the limited effect of differing cranial size.
Heterochronic shifts in suture pattern
Heterochronies recorded within Hystricognathi exhibit considerable parallelism, evidenced in part by the reconstruction of evolutionary relationships using event pairs (Fig. 8). A late closure of the lacrimal associated sutures relative to alisphenoid sutural boundaries was found to distinguish Erethizontidae. This shift also occurred within Muroidea, although only three of four lacrimal sutures closed late. Nonetheless within the reconstructed tree (Fig. 8) N. ehrenbergiclusters amongst the erethizontids and when evaluating the position of the lacrimal sutures within the suture sequence for each of the three representatives of Muriodea, N. ehrenbergi was found to display the latest occurrence of lacrimal suture closure. The correct reconstruction of sister species D. leporinaand D. fuliginosa reflects the early closure of the maxillo-palatine and palate-alisphenoid shared by these two species: the palate-alisphenoid closes later for all other taxa.
Octodontids are characterized by the greatest amount of heterochronic shifts per branch. This group was represented by six taxa and 100 specimens, the increased number of heterochronic shifts likely reflects the divergent nature of the closure sequences for each of the species within the group: the Kendall's W for Octodontoidea was 0.67, which, in the context of the observed trend to an increased level of correspondence coupled with a decreased number of inclusive taxa, is comparatively low.
Ecological correlates with suture closure pattern
Although cranial sutures form in the absence of muscle activity, research directed to understanding the biomechanical role of sutures (e.g. Jaslow, 1990; Rafferty et al. 2003) has supported experimental evidence that the fine details of suture morphology are secondary responses to extrinsic forces (Moss, 1957). Sutures associated with the facial region of the skull in pigs have been implicated in a protective role for the facial bones they join (Rafferty et al. 2003). Specifically the premaxillary and internasal bones of pigs are subject to a high degree of strain during mastication, and deformation within these sutures is considered to circumvent high levels of strain in the delicate facial bones (Rafferty et al. 2003; Sun et al. 2004). From their study of hominoid ectocranial sutures, Cray et al. (2008) noted that sutures subject to the influence of masticatory forces exhibit the least amount of interspecific variation in degree of closure. For all species studied here, the highest level of patency was exhibited by the seven, representative, facial division sutures: 45–81% of specimens displayed completely open sutures at these sites (Table 3a). Moreover, inspection of suture patterns for individual species indicated no fewer than four of seven facial sites were the last to close, whereas sutures that belonged to other divisions did not share repeated associations between species, perhaps indicating suture closure pattern conservatism reflects the occurrence of stress-related constraint within the facial region. Nevertheless, in mammals the muscles of mastication are complex and although the same terminology is used for each muscle, these references relate not to function but to location of bone attachment. Hence rodents have an anteriorly directed, fascially subdivided masseter muscle, modified to strengthen propalinal jaw movement, which has little resemblance in function to the vertical, unitary masseter muscle in humans (Ball & Roth, 1995; Herring, 2007), making difficult a direct comparison of the strain-loading environment that may influence suture patency within these two groups.
Interestingly the facial sutures also closed last among B. suillus, Rhizomys sumatrensisand N. ehrenbergi; these rodents exhibit osteological modifications for their fossorial mode of life (Fig. 1), several of which impact upon jaw musculature (Topachevskii, 1976; Stein, 2000). Among hystricognaths the anterior masseter medialis muscle, originating largely on the rostrum, passes through an enlarged infraorbital foramen before descending to insert on the lower jaw (Moore, 1981). Subterranean rodents typically have reduced, degenerate eyes; this is coupled with a migration in site of origin of the jaw musculature; moreover, most bathyergids (B. suillus) possess a small infraorbital foramen that is not penetrated by the masseter muscle. Instead the zygomatic arches are posteriorly widened to accommodate the enlarged jaw muscles, making the skull appear wedge-shaped, a feature especially prominent in Nannospalax(Stein, 2000). The aforementioned cranial features of subterranean rodents, compared with species that adopt a non-fossorial habit, likely create differing levels of strain on analogous cranial elements which may in turn be realized by heterochronies in suture closure pattern, as sutures have been shown to exhibit varying degrees of tension in response to local muscle contractions (Herring & Teng, 2000).
Nevertheless, an exact causal relationship between a specific heterochronic event (e.g. relative late closure of the naso-frontal suture) and an ecological factor cannot be established without a considerable degree of speculation. Further elucidation is beyond the scope of this work, and could be achieved in part by a comparative study, directly extending the methods applied herein. Indeed, morphofunctional analyses of postcranial elements have indicated ecomorphological factors are correlated with, though not a major determinant of, variation in the scapular shape of caviomorph rodents (Morgan, in press).
Comparison of suture closure pattern with previous pattern suggested for hominoids
Examination of the suture closure pattern across Hystricognathi revealed sutures located at the cranial base and vault, including the exoccipito-basioccipital, exoccipito-supraoccipital, and basisphenoid-presphenoid, were the first to close (Table 5b). Indeed, sutures contacting the exoccipital displayed the highest levels of closure across all species (Table 3a). This finding compares positively with the order Krogman (1930) identified, and is supported, though not to a significant level, by a positive τ value of 0.19. Suture closure was similarly conserved towards the latter stages of the pattern reported for Hystricognathi (Table 5b). The last seven sutures to close were all located in the facial region of the skull. In contrast, Krogman (1930), and also Schweikher (1930), found the facial division closed before the cranio-facial division (alisphenoid-orbitosphenoid, lacrimal-frontal, orbitosphenoid-frontal). Towards the earlier, compared with the latter, stages of the closure pattern, hystricognaths were found to exhibit a relatively random closure of the cranio-facial sutures (Table 5b).
When considering suture closure pattern at a specific level, Kendall's τ values supported a positive correlation with Krogman's (1930) pattern; 11 of 31 species displayed a significant correlation and five of these species exhibited the largest average cranial lengths from the total sampled species (Table 5a). All members of Dasyproctidae (Fig. 1, Table 5a) were found to display a significant relationship to the closure pattern previously proposed for hominoids (Krogman, 1930); members of this group exhibited average cranial lengths between 71 and 96 mm (Fig. 6). Sister group Cuniculidae, represented by C. paca(Fig. 1), also exhibited a similar, significant relationship (Table 5a) and had the largest average cranial length (129 mm) of all species studied. This shared similarity in suture pattern supports a close association between these two families, recently sustained by molecular analyses (Rowe & Honeycutt, 2002).
Utility of event pair data for phylogenetic reconstruction
The non-independence of event pair data makes their application to phylogenetic analyses potentially inappropriate (Bininda-Emonds et al. 2002; Schulmeister & Wheeler, 2004; Harrison & Larsson, 2008). Similar to recent investigations of mammalian cranial (Sánchez-Villagra et al. 2008) and postcranial ossification sequences (Weisbecker et al. 2008), the topology reconstructed from event pair data here retains few groups congruent with the phylogenetic framework taken from morphological and molecular analyses of these taxa.
Correlation of suture closure with cranial length
Cranial size was found to display a significant relationship with level of suture closure within species, for 11 of the 31 species sampled (Table 6). The use of suture closure as a guide to skeletal age for humans has been unequivocally revoked by several authors, who report the erratic nature makes accurate age estimations difficult (Stewart, 1934; Singer, 1953; Powers, 1962; Sahni et al. 2005).
Although cranial size was found to correlate weakly with level of suture closure for many species, an increased percentage of completely patent sutures (score 1, Table 3b) appears to couple with large body (> 1 kg mass) measurements, evidenced for H. hydrochaerisand C. pacaand moreover within Dasyproctidae and Hystricidae, excepting H. brachyura, for which the size range of representative specimens was diminished compared to sister Hystrix cristata(Fig. 7). In correspondence, when examined between species, cranial length and level of suture closure are negatively correlated (P > 0.05) (Fig. 9).
Conclusions
Numerous heterochronies in ectocranial suture closure have occurred in the evolution of a diverse clade of mammals, the Hystricognathi. The overall pattern is similar but not equal to the scheme previously suggested to be characteristic for mammals (Krogman, 1930). Differing life history and locomotory strategies appear, in part, to be coupled with differing degrees of suture closure and pattern among several species; some heterochronic transformations in suture patterns do provide diagnostic features for clades of hystricognaths at different taxonomic levels.
The study of late growth using newly developed techniques to analyse sequence heterochrony is potentially a rich avenue of research to address the question ‘is sequence heterochrony an important evolutionary mechanism in mammals?’ (Bininda-Emonds et al. 2003, p. 335). As exemplified here, this question has different answers depending on the taxonomic level and the organ system or window of developmental timing examined.
Acknowledgments
This work was supported by the Swiss National Fond (3100A0-116013) to M.R.S.-V. and by a Forschungskredit of the Universität Zürich (Nr 3771) to L.A.B.W. We thank A. Ziems, W. Etter, M. Haffner, F. Mayer, S. Hertwig, H. van-Grouw, M. Hiermeier, R. Kraft, J. Cuisin, and B. Herzig for providing access to the collections in their charge. We also thank Anjali Goswami and Ingmar Werneburg for helpful discussion during the preparation of this manuscript, Janine Ziermann for guidance with analyses, and two anonymous reviewers for their useful comments on an earlier version of this paper.
References
- Adkins RM, Gelke EL, Rowe D, et al. Molecular phylogeny and divergence time estimates for major rodent groups: Evidence from multiple genes. Mol Biol Evol. 2001;18:777–791. doi: 10.1093/oxfordjournals.molbev.a003860. [DOI] [PubMed] [Google Scholar]
- Adkins RM, Walton AH, Honeycutt RL. Higher level systematics of rodents and divergence time estimates based on two highly congruent nuclear genes. Mol Phylogenet Evol. 2003;26:409–420. doi: 10.1016/s1055-7903(02)00304-4. [DOI] [PubMed] [Google Scholar]
- Ball SS, Roth VL. Jaw muscles of New World squirrels. J Morphol. 1995;224:265–291. doi: 10.1002/jmor.1052240303. [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds ORP, Jeffery JE, Richardson MK. Is sequence heterochrony an important evolutionary mechanism in mammals? J Mammal Evol. 2003;10:335–361. [Google Scholar]
- Carrasco MA, Wahlert JH. The cranial anatomy of Cricetops dormitor, an Oligocene fossil rodent from Mongolia. Am Mus Novitat. 1999;3275:1–14. [Google Scholar]
- Chopra SRK. The cranial suture closure in monkeys. Proc Zool Soc Lond. 1957;128:67–112. [Google Scholar]
- Cray J, Jr, Meindl RS, Sherwood CC, et al. Ectocranial suture closure in Pan troglodytesand Gorilla gorilla: Pattern and phylogeny. Am J Phys Anthropol. 2008 doi: 10.1002/ajpa.20821. (DOI: 10.1002/ajpa.20821) [DOI] [PubMed] [Google Scholar]
- Depew MJ, Compagnucci C, Griffin J. Suture neontology and paleontology: the bases for where, when and how boundaries between bones have been established and have evolved. In: Rice D, editor. Craniofacial Sutures: Development, Disease and Treatment. Basel: Karger Press; 2008. pp. 57–78. [DOI] [PubMed] [Google Scholar]
- Dolan KJ. Cranial suture closure in two species of South American monkeys. Am J Phys Anthropol. 1971;35:109–118. doi: 10.1002/ajpa.1330350113. [DOI] [PubMed] [Google Scholar]
- Galewski T, Mauffrey J-F, Leite YLR, et al. Ecomorphological diversification among South American spiny rats (Rodentia; Echimyidae): a phylogenetic and chronological approach. Mol Phylogenet Evol. 2005;34:601–615. doi: 10.1016/j.ympev.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Giannini NP, Wible JR, Simmons NB. On the cranial osteology of Chiroptera. I. Pteropus(Megachiroptera: Pteropodidae) Bull Am Mus Nat Hist. 2006;295:1–134. [Google Scholar]
- Goswami A. Modularity and sequence heterochrony in the mammalian skull. Evol Dev. 2007;9:291–299. doi: 10.1111/j.1525-142X.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- Hall BK. Homology: the Hierarchical Basis of Comparative Biology. San Diego: Academic Press; 1994. [Google Scholar]
- Harrison LB, Larsson HCE. Estimating evolution of temporal sequence changes: A practical approach to inferring ancestral developmental sequences and sequence heterochrony. Syst Biol. 2008;57:378–387. doi: 10.1080/10635150802164421. [DOI] [PubMed] [Google Scholar]
- Henderson JH, Longaker MT, Carter DR. Sutural bone deposition rate and strain magnitude during cranial development. Bone. 2004;34:271–280. doi: 10.1016/j.bone.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Herring SW. Sutures – a tool in functional cranial analysis. Acta Anat. 1972;83:222–247. doi: 10.1159/000143860. [DOI] [PubMed] [Google Scholar]
- Herring SW. A biometric study of suture fusion and skull growth in peccaries. Anat Embryol. 1974;146:167–180. doi: 10.1007/BF00315593. [DOI] [PubMed] [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. In: Hanken J, Hall BK, editors. The Skull. Vol. 1. Chicago: University of Chicago Press; 1993. pp. 153–206. [Google Scholar]
- Herring SW. Masticatory muscles and the skull: a comparative perspective. Arch Oral Biol. 2007;52:296–299. doi: 10.1016/j.archoralbio.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt RL, Rowe DL, Gallardo MH. Molecular systematics of South American caviomorph rodents: relationships among species and genera in the family Octodontidae. Mol Phylogenet Evol. 2003;26:476–489. doi: 10.1016/s1055-7903(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Huchon D, Douzery EPJ. From the Old World to the New World: a molecular chronicle of the phylogeny and biogeography of hystricognath rodents. Mol Phylogenet Evol. 2001;20:238–251. doi: 10.1006/mpev.2001.0961. [DOI] [PubMed] [Google Scholar]
- Jaslow CR. Mechanical properties of cranial sutures. J Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- Jeffery JE, Bininda-Emonds ORP, Coates MI, et al. A new technique for identifying sequence heterochrony. Syst Biol. 2005;54:230–240. doi: 10.1080/10635150590923227. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol Rev. 1998;73:79–123. doi: 10.1017/s000632319800512x. [DOI] [PubMed] [Google Scholar]
- Krogman WM. Studies in growth changes in the skull and face of anthropoids: Ectocranial and endocranial suture closure in anthropoids and Old World apes. Am J Phys Anthropol. 1930;46:315–353. [Google Scholar]
- McKenna MC, Bell SK. Classification of Mammals above the Species Level. New York: Columbia University Press; 1997. [Google Scholar]
- Mess A. Evolutionary transformations of chorioallantoic placental characters in Rodentia with special reference to hystricognath species. J Exp Zool Comp Exp Biol. 2003;299A:78–98. doi: 10.1002/jez.a.10292. [DOI] [PubMed] [Google Scholar]
- Mess A. Development of the chorioallantoic placenta in Octodon degus– a model for growth processes in caviomorph rodents? J Exp Zool Mol Devel Evol. 2007;308B:371–383. doi: 10.1002/jez.b.21160. [DOI] [PubMed] [Google Scholar]
- Moore WJ. The Mammalian Skull (Biological structure and function 8) Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Morgan CC. Geometric morphometrics of the scapula of South American caviomorph rodents (Rodentia: Hystricognathi): form, function and phylogeny. Mamm Biol. 2008 in press. [Google Scholar]
- Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML. Experimental alteration of sutural area morphology. Anat Rec. 1957;127:569–590. doi: 10.1002/ar.1091270307. [DOI] [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 6th edn. Baltimore: The Johns Hopkins University Press; 1999. [Google Scholar]
- Nunn CL, Smith KK. Statistical analyses of developmental sequences: The craniofacial region in marsupial and placental mammals. Am Nat. 1998;152:82–101. doi: 10.1086/286151. [DOI] [PubMed] [Google Scholar]
- Ogle RC, Tholpady SS, McGlynn KA, et al. Regulation of cranial suture morphogenesis. Cells Tissues Organs. 2004;176:54–66. doi: 10.1159/000075027. [DOI] [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Sweeney TM, Redmon J, et al. Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn. 1993;198:312–322. doi: 10.1002/aja.1001980408. [DOI] [PubMed] [Google Scholar]
- Powers R. The disparity between known age and sex as estimated by cranial suture closure. Man. 1962;84:52–54. [Google Scholar]
- Rafferty KL, Herring SW, Marshall CD. Biomechanics of the rostrum and the role of facial sutures. J Morphol. 2003;257:33–44. doi: 10.1002/jmor.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. Molecular mechanisms in calvarial bone and suture development. Helsinki: Helsinki University Press; 1999. PhD thesis. [Google Scholar]
- Rowe DL, Honeycutt RL. Phylogenetic relationships, ecological correlates, and molecular evolution within the Caviodea (Mammalia, Rodentia) Mol Biol Evol. 2002;19:263–277. doi: 10.1093/oxfordjournals.molbev.a004080. [DOI] [PubMed] [Google Scholar]
- Sahni D, Jit I, Sanjeev N. Time of closure of cranial sutures in northwest Indian adults. Forensic Sci Int. 2005;148:199–205. doi: 10.1016/j.forsciint.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR. Comparative patterns of postcranial ontogeny in therian mammals: an analysis of relative timing of ossification events. J Exp Zool Mol Dev Evol. 2002;294B:264–273. doi: 10.1002/jez.10147. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Aguilera OA, Horovitz I. The anatomy of the world's largest extinct rodent. Science. 2003;301:1708–1710. doi: 10.1126/science.1089332. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Goswami A, Weisbecker V, et al. Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evol Dev. 2008;10:519–530. doi: 10.1111/j.1525-142X.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- Schulmeister S, Wheeler WC. Comparative and phylogenetic analysis of developmental sequences. Evol Dev. 2004;6:50–57. doi: 10.1111/j.1525-142x.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- Schultz AH. Growth and development of the chimpanzee. Contr Embryol Carnegie Inst. 1940;170:1–63. [Google Scholar]
- Schultz AH. Growth and development of the orang-utan. Contr Embryol Carnegie Inst. 1941;182:57–110. [Google Scholar]
- Schultz AH. Growth and development of the proboscis monkey. Bull Mus Comp Zool Harv. 1942;89:277–314. [Google Scholar]
- Schweikher FP. Ectocranial suture closure in hyaenas. Am J Anat. 1930;45:443–460. [Google Scholar]
- Singer R. Estimation of age from cranial suture closure: a report on its unreliability. J Foren Med. 1953;1:52–59. [Google Scholar]
- Smith KK. Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution. 1997;51:1663–1678. doi: 10.1111/j.1558-5646.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Smith KK. Heterochrony revisited: the evolution of developmental sequences. Biol J Linn Soc. 2001;73:169–186. [Google Scholar]
- SPSS Inc. SPSS 16.0 for Windows Release 16.0.1. Chicago: SPSS Inc; 2007. [Google Scholar]
- Stein BR. Morphology of subterranean rodents. In: Lacey EA, Patton JL, Cameron GN, editors. Life Underground: the Biology of Subterranean Rodents. Chicago: Chicago University Press; 2000. pp. 19–61. [Google Scholar]
- Stewart TD. Sequence of epiphyseal union suture closure in Eskimo and American Indians. Am J Phys Anthropol. 1934;19:433–452. [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: Growth and fusion in relation to masticatory strain. Anat Rec. 2004;276A:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony. Sunderland, MA: Sinauer Associates; 2002. Version 4.0b10. [Google Scholar]
- Todd TW, Lyon D. Endocranial cranial suture closure: its progress and age relationship. I. Adult males of white stock. Am J Phys Anthropol. 1924;7:325–384. [Google Scholar]
- Todd TW, Lyon D. Cranial suture closure: its progress and age relationship. II. Ectocranial closure of males of white stock. Am J Phys Anthropol. 1925a;8:23–43. [Google Scholar]
- Todd TW, Lyon D. Cranial suture closure: its progress and age relationship. III. Endocranial suture closure of adult males of Negro stock. Am J Phys Anthropol. 1925b;8:47–71. [Google Scholar]
- Todd TW, Lyon D. Cranial suture closure: its progress and age relationship. IV. Ectocranial suture closure of adult males of Negro stock. Am J Phys Anthropol. 1925c;8:149–168. [Google Scholar]
- Topachevskii VA. Fauna of the USSR. Vol 3. Mammals. No 3. Mole Rats, Spalacidae. Leningrad: Nauka Publishers, Leningrad Section; 1976. [Google Scholar]
- Wang Q, Strait DS, Dechow PC. Fusion patterns of craniofacial sutures in rhesus monkey skulls of known age and sex from Cayo Santiago. Am J Phys Anthropol. 2006;131:461–485. doi: 10.1002/ajpa.20481. [DOI] [PubMed] [Google Scholar]
- Weisbecker V, Goswami A, Wroe S, Sánchez-Villagra MR. Ossification heterochrony in the therian postcranial skeleton and the marsupial-placental dichotomy. Evolution. 2008;62:2027–2041. doi: 10.1111/j.1558-5646.2008.00424.x. (DOI: 10.1111/j.1558-5646.2008.00424) [DOI] [PubMed] [Google Scholar]
- Wilson DE, Reeder DM. Mammal Species of the World: a Taxonomic and Geographic Reference. Washington DC: Smithsonian Institution Press; 2005. [Google Scholar]
- Wu YD, Chien CH, Chao YJ, et al. Fourier analysis of human sagittal sutures. Cleft Palate-Cran J. 2007;44:482–493. doi: 10.1597/06-122.1. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 4th edn. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]