Abstract
The Reelin signaling pathway controls radial neuronal migration and maturation in the developing brain. The platelet activating factor (PAF) acetyl hydrolase 1b (Pafah1b) complex is also involved in multiple aspects of brain development. We previously showed that the Reelin pathway and the Pafah1b complex interact genetically and biochemically. Lis1, the regulatory subunit of Pafah1b interacts with phosphoDab1, an essential mediator of Reelin signaling. Compound mutants carrying mutations in both, the Reelin pathway and Lis1 exhibit hydrocephalus, a phenotype that is suppressed by mutations in the gene encoding the Alpha2 subunit of Pafah1b. This subunit, like to other Pafah1b catalytic subunit Alpha1, also binds the Reelin receptor VLDLR. Here we investigated the molecular interactions of the Pafah1b catalytic subunits with Dab1. We found that Alpha2 coprecipitates with Dab1 from brain extracts of normal and reeler mutant mice lacking Reelin, and from cell-free extracts containing normal or a phosphorylation mutant form of Dab1, suggesting that Dab1 phosphorylation is not necessary for binding to Alpha2. This interaction is specific for Alpha2 and not Alpha1, and depends on a unique tyrosine residue of Alpha2. Biochemical assays using mutant mice lacking Alpha2 further demonstrated that this subunit is not required for Reelin-induced Dab1 phosphorylation. However, increasing amounts of Alpha2 in a cell free system disrupted the formation of Dab1-Lis1 complexes without affecting the association of Dab1 with VLDLR. Our data suggest that the Alpha2 subunit may play a modulatory role in the formation of protein complexes that affect brain development and hydrocephalus.
Keywords: reeler, neuronal migration, Disabled-1, lipoprotein receptor, platelet activating factor acetylhydrolase, neocortex
1. INTRODUCTION
The platelet activating factor (PAF) acetyl hydrolase 1b (Pafah1b) is a protein complex that can function as an intracellular phospholipase and catalyze PAF hydrolysis by the activity of two catalytic Alpha subunits (Hattori et al., 1993). These two highly similar subunits are differentially expressed during brain development, where Alpha1 levels are high at prenatal ages and Alpha2 levels predominate at postnatal ages (Manya et al., 1998). The complex also contains a non-catalytic Beta regulatory subunit (Lis1) (encoded by the Pafah1b1 gene). This gene is important for neuronal migration as heterozygous mutations in humans are responsible for lissencephaly in the Miller-Dieker syndrome (Hattori et al., 1994; Reiner et al., 1993). In the mouse mutations in the Pafah1b1 gene also cause neuronal migration defects in compound hypomorph/null mutants (Hirotsune et al., 1998). The role of the Alpha subunits in brain development is not well understood. Null mutations in the mouse Pafah1b2 or Pafah1b3 genes, alone or in combination, do not result in any overt neurological phenotype (Assadi et al., 2008; Koizumi et al., 2003), thus they are not essential for brain development but they may modulate the activity of interacting proteins. Lis1 is known to interact with the dynein motor complex (Faulkner et al., 2000; Smith et al., 2000), however it is not clear whether the Alpha subunits of Pafah1b affect this activity. We previously demonstrated that Lis1 genetically interacts with the Reelin pathway (Assadi et al., 2003), a signaling machinery that is crucially involved in the control of neuronal migration and maturation (reviewed in (D’Arcangelo, 2005)). Double mutant mice carrying disruptions in genes encoding Lis1 plus components of the Reelin pathway exhibit cortical layering defects and progressive hydrocephalus. Furthermore we found that Lis1 directly interacts with Dab1 in response to Reelin (Assadi et al., 2003), whereas Alpha1 and Alpha2 bind the Reelin receptor VLDLR (Zhang et al., 2007). These findings suggested an extensive interaction between the Pafah1b complex and the Reelin signaling pathway.
Reelin (D’Arcangelo et al., 1995) is secreted protein that promotes cortical layer formation through the activation of a well-characterized signaling machinery. Reelin binds to two receptors, VLDLR and ApoER2, which are members of the lipoprotein receptor superfamily (D’Arcangelo et al., 1999; Hiesberger et al., 1999). Reelin binding to these receptors causes the activation of Fyn and Src, two src-family kinases (SFKs) that phosphorylate the adapter protein Dab1 on specific tyrosine residues (Arnaud et al., 2003; Bock and Herz, 2003; Howell et al., 1999; Keshvara et al., 2001). PhosphoDab1 then binds a variety of intracellular proteins involved in cytoskeletal dynamics (Ballif et al., 2004; Bock et al., 2003; Pramatarova et al., 2003), including Lis1 (Assadi et al., 2003), and is then targeted for degradation by an E3 ubiquitin ligase containing Cullin5 (Feng et al., 2007). Dab1 can also bind proteins that can modulate cell motility, such as the actin-filament binding protein N-WASP, independently of its phosphorylation (Suetsugu et al., 2004).
We recently demonstrated that the Pafah1b Alpha subunits genetically interact very differently with Lis1 and the Reelin pathway. Mutations in the Pafah1b2 gene, but not Pafah1b3, specifically suppress the hydrocephalus phenotype of compound Lis1/Reelin and Lis1/Dab1 mutant mice (Assadi et al., 2008). Mutations in Pafah1b3, on the other hand, exacerbate the layering defects of compound mutants. To better understand the molecular bases of these different activities in this study we examined the biochemical interactions of Alpha1 and Alpha2 with the Reelin signaling machinery and demonstrated thatAlpha2, but not Alpha1, binds Dab1 in a phosphorylation-independent manner.
2. RESULTS
Alpha2 binds Dab1 in a phosphorylation-independent manner
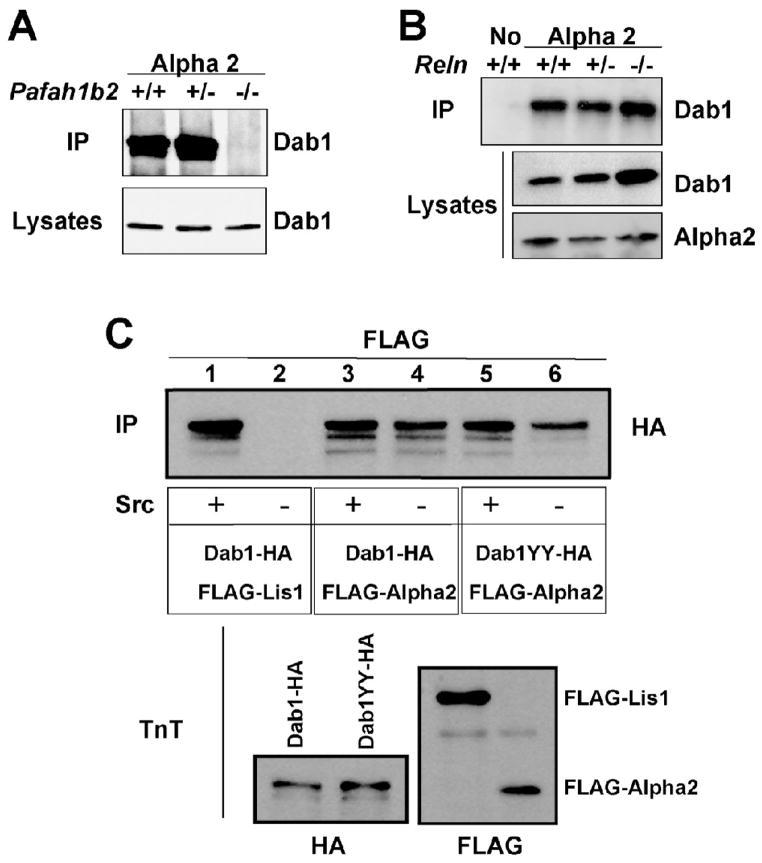
We have previously shown that Lis1, the regulatory subunit of the Pafah1b complex binds the Reelin transducer Dab1 (Assadi et al., 2003). This binding is dependent on Reelin-induced Dab1 phosphorylation and it does not occur in the reeler mutant brain. To determine whether Alpha2 is also capable of binding Dab1 we conducted co-immunoprecipitation experiments using embryonic brain extracts of normal and Pafah1b2 mutant mice. Antibodies against the Alpha2 subunit specifically co-precipitated Dab1 from the brain of wild type and heterozygous Pafah1b2 mice, but not from homozygous mutants (Fig. 1A). To investigate whether the binding of Alpha2 to Dab1 is affected by Reelin we conducted co-immunoprecipitation experiments using brain extracts obtained from wild type or Reln mutants. The data revealed that Alpha2, unlike Lis1, binds Dab1 even in the complete absence of Reelin expression in homozygous reeler brain (Fig. 1B). Since Reelin promotes the phosphorylation of Dab1, these results suggested that Dab1 interact with Alpha2 regardless of its phosphorylation status. To confirm these findings, we also conducted co-immunoprecipitation experiments using proteins translated in vitro in a cell-free system, in the presence or absence of recombinant Src kinase, which can phosphorylate Dab1 on specific tyrosine residues. In vitro translated Dab1 was tagged with the HA epitope and co-incubated with Flag-tagged Lis1 or Alpha2. Consistent with our previous report (Assadi et al., 2003), we found that Lis1 binds Dab1 in vitro and a SFK activity such as Src is required for this interaction (Fig. 1C, lanes 1 and 2). On the other hand we found that Alpha2 directly binds Dab1 in vitro even in the absence of Src, although the levels of immunoprecipitated Dab1 were slightly lower in the absence of this kinase (Fig. 1C, lanes 3 and 4). To further investigate the role of phosphorylation, we employed a mutant Dab1 protein in which two of the main Reelin-induced target sites, tyrosines 198 and 220 (Keshvara et al., 2001), were mutated to alanine (Dab1YY). Reelin-dependent phosphorylation of these residues is carried out in vivo by Src-family kinases Src and Fyn (Kuo et al., 2005), and is required for Lis1 binding to Dab1 (Assadi et al., 2003). The expression levels of the mutant protein were similar to native Dab1 (Fig. 1C, lower left panel). We found that mutant Dab1YY also co-precipitated with Alpha2 in the presence or absence of Src (Fig. 1C, lanes 5 and 6). Again, the levels of co-precipitated Dab1YY were slightly lower in the absence of the kinase, indicating that the phosphorylation of sites other than tyrosines 198 and 220 may contribute to the stability of the interaction, but it is not absolutely necessary for binding to Alpha2.
Figure 1. Alpha2 binds phosphorylated and unphosphorylated Dab1.
A, Alpha 2 binds Dab1 in the embryonic brain. Proteins were extracted from the brain of littermates that were wild type, heterozygous or homozygous null for the Pafah1b2 gene, immunoprecipitated with Alpha2 antibodies and probed with Dab1 antibodies (IP, upper panel). The brain lysates were also directly probed with Dab1 antibodies (lower panel) to ensure that similar amounts of proteins were present in each sample. B, Alpha 2 binds Dab1 in normal and reeler brain. Proteins were extracted from the brain of littermates that were wild type, heterozygous or homozygous null for the Reln gene, immunoprecipitated with no antibodies or with Alpha2 antibodies and probed with Dab1 antibodies (IP, upper panel). The brain lysates were also probed with Dab1 (middle panel) or Alpha2 (lower panel) antibodies. Elevated levels of Dab1 protein are present in the homozygous Reln mutant. C, FLAG-tagged subunits of Pafah1b and HA-tagged Dab1 proteins were expressed in vitro using a cell-free-system. Proteins were incubated with or without recombinant Src kinase and immunoprecipitated with FLAG antibodies. The blot (IP) was probed with HA antibodies to detect Dab1 proteins (upper panel). Normal Dab1 interacted with Lis1 only in the presence of Src, whereas normal and mutated Dab1 interacted with Alpha2 with or without the kinase. In vitro translated proteins (TnT) were probed with HA or FLAG antibodies to ensure similar levels of expression (lower panels).
Tyrosine 56 of Alpha2 is necessary for binding to Dab1
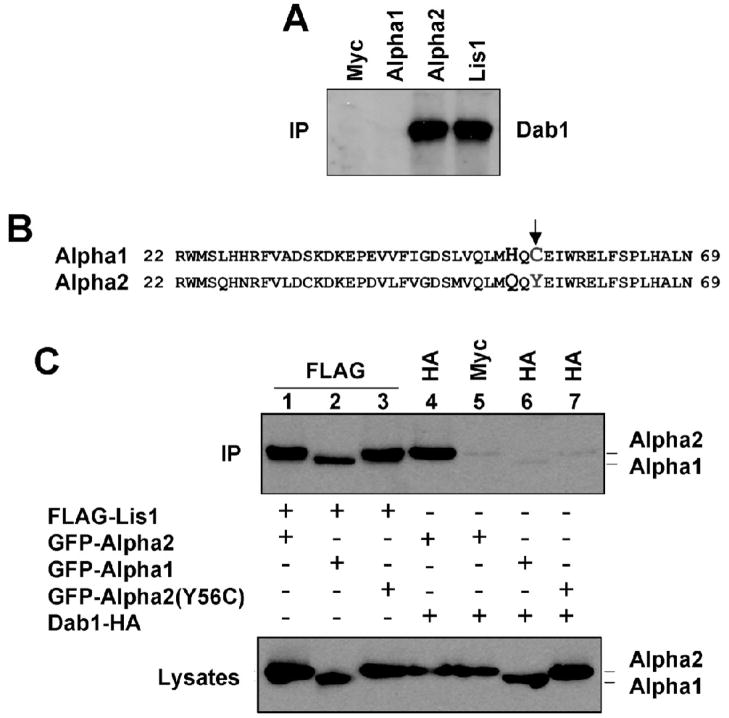
To investigate whether the Alpha1 subunit of the Pafah1b complex, like Alpha2 and Lis1, binds to Dab1 we conducted co-immunoprecipitation experiments using brain extracts of wild type mice. When antibodies against Alpha1 or a negative control antibody were used, no Dab1 was detected in the immunoprecipitated, whereas Alpha2 or Lis1 antibodies efficiently co-precipitated the protein (Fig. 2A). To gain insights into the differential binding activity of the two catalytic subunits, we examined their amino acid sequence. The two proteins share a high degree of similarity (60%) across the entire sequence, particularly in the N terminal region containing the catalytic serine (residues 48). One of the few non-conserved differences in this region consists of amino acid number 56, which is a tyrosine in Alpha2 and a cysteine in Alpha1 (Fig. 2B, arrow). To understand whether this amino acid substitution is responsible for the specific ability of Alpha2 to bind Dab1, we mutated the tyrosine residue 56 of Alpha2 to a cysteine (Y56C), as in Alpha1. GFP-tagged constructs encoding intact Alpha1 and Alpha2 subunits and the mutant Alpha2(Y56C) were co-transfected in COS7 cells along with HA-tagged Dab1 or FLAG-tagged Lis1. The data show that only intact Alpha2 co-immunoprecipitated with Dab1, whereas Alpha1 and Alpha2(Y56C) were unable to do so (Fig. 2C, lanes 4–7) despite comparable expression levels (lower panel) and similar interaction with Lis1 (Fig. 2C, lanes 1–3). These data suggest that tyrosine residue 56 of Alpha2 is not required for binding to Lis1, but it is specifically required for binding to Dab1.
Figure 2. The unique Y56 residue of Alpha2 is required for binding to Dab1.
A, Alpha1 does not bind Dab1 in the embryonic brain. Proteins were extracted from wild type embryonic mouse brain and immunoprecipitated with Alpha1, Alpha2, Lis1 or control Myc antibodies and probed with Dab1 antibodies (IP). Dab1 coprecipitated only with Alpha2 or Lis1. B, Amino acid sequence alignment of a highly conserved N terminal region of mouse Alpha1 and Alpha2 proteins containing residue 56 (arrow). C, FLAG-tagged Lis1, GFP-tagged Alpha1 or Alpha2 and HA-tagged Dab1 were coexpressed in COS7 cells. Proteins were immunoprecipitated with the indicated antibodies and probed with GFP antibodies to detect Alpha1 or Alpha2 (IP). The lysates were also directly probed with GFP antibodies to ensure that Alpha1 or Alpha2 proteins were expressed at similar levels. Mutant Alpha2(Y56C), like Alpha1, cannot bind Dab1.
Loss of Pafah1b2 does not prevent Dab1 phosphorylation by Reelin
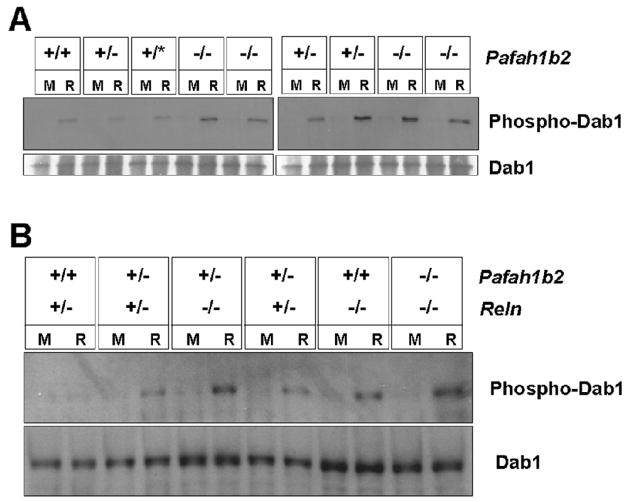
Propagation of the Reelin signaling and interaction with Lis1 requires SFK-dependent Dab1 phosphorylation in tyrosine residues (Assadi et al., 2003; Howell et al., 2000). To determine whether loss of Alpha2 affects the induction of Dab1 phosphorylation by Reelin, cortical neurons were isolated from littermate mice that were either wild type, heterozygous or homozygous Pafah1b2 null. The cultures were exposed to Reelin or to mock conditioned medium, Dab1 was immunoprecipitated from the cell lysates and subjected to Western blot analysis using anti-phosphotyrosine antibodies. We found that Reelin treatment induced an increase in the levels of phosphoDab1 compared to mock treatment in all samples analyzed, including those obtained from Pafah1b2 homozygous mutants (Fig. 3A). We also prepared cortical neuron cultures from double mutants carrying Pafah1b2 and Reln mutations (Fig. 3B). Treatment with recombinant Reelin again resulted in an increase of Dab1 phosphorylation in all samples analyzed, particularly in those prepared from reeler mice, regardless of compound Pafah1b2 mutations (Fig. 3B). Consistent with the lack of layer phenotype in Pafah1b2 nullmice, these data suggest that Alpha2 is not required for Reelin -induced Dab1 phosphorylation and signal transduction.
Figure 3. Pafah1b2 mutations do not inhibit Reelin-induced Dab1 phosphorylation.
A. Cortical neuron cultures obtained from mice of the indicated Pafah1b2 genotype were treated with Reelin (R) or a mock control medium (M). B. Cortical neuron cultures obtained from mice of the indicated Pafah1b2 and Reln genotypes were treated with Reelin (R) or a mock medium (M). Phospho-Dab1 levels are shown in the top panels. Total Dab1 protein levels are shown in the bottom panels. Reelin induced Dab1 phosphorylation in all samples analyzed.
Alpha2 expression interferes with the formation of the Lis1-Dab1 complex
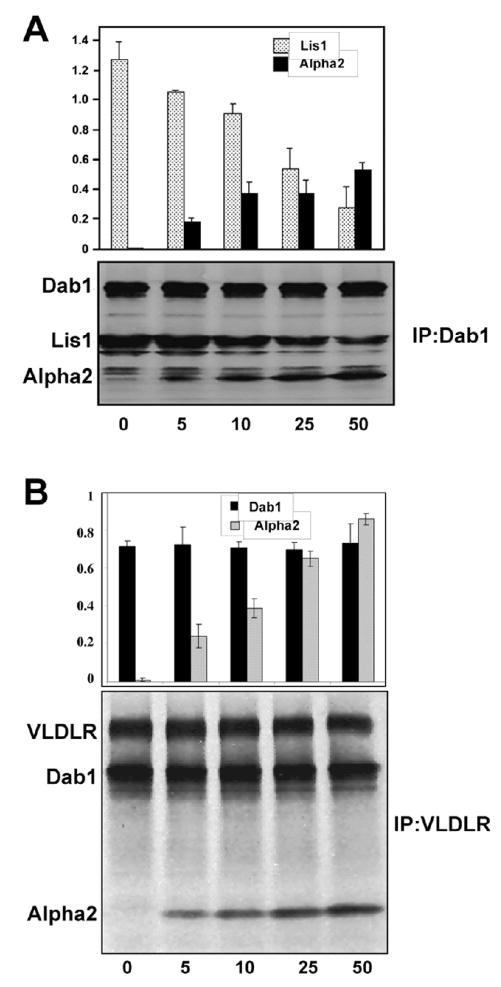
Since Alpha2 can bind both, Lis1 and Dab1, and these two latter proteins can also bind to each other in the presence of an SFK activity, we investigated the effect of Alpha2 on the Dab1-Lis1 complex in vitro. Dab1 and Lis1 were synthesized in a cell-free system and incubated in the presence of recombinant Src kinase. Under these conditions, Lis1 co-immunoprecipitated with Dab1 using Dab1-specific antibodies (Fig. 4A), as we previously reported (Assadi et al., 2003). When increasing concentrations of in vitro synthesized Alpha2 were added to the mixture, corresponding lower amounts of Lis1 were co-precipitated with Dab1 (Fig. 4A). These results suggest that Alpha2 interferes with the formation of the Lis1-Dab1 complex by binding to either Lis1 of Dab1 individually, rather than forming a trimeric complex. We also investigated the effect of Alpha2 on Vldlr-Dab1 interactions in vitro. This time we found that increasing concentrations of Alpha2 had no effect on this Dab1 complex suggesting that a trimeric complex containing VLDLR, Dab1 and Alpha2 may be able to form (Fig. 4B). Together, these data indicate that Alpha2 specifically downregulates the interaction of Dab1 with Lis1 and promotes a stable interaction with the VLDLR.
Figure 4. Alpha2 competes with Lis1 for binding to Dab1 but does not competes with Dab1 for binding to VLDLR.
Competition assays in cell-free extracts containing increasing amounts of Alpha2. A. 50μl of in vitro produced Dab1 were incubated with 50 μl of in vitro produced Lis1 and different amount (0, 5, 10, 25, 50 μl) of in vitro produced Alpha2. Proteins were immunoprecipitated with Dab1 antibodies, detected by autoradiography, and normalized to the amount of precipitated Dab1. Histograms show the ratio of Lis1/Dab1 (Grey) and Alpha2/Dab1 (black). B. 50μl of in vitro produced VLDLR were incubated with 50 μl of Dab1 and different amount (0, 5, 10, 25, 50 μl) of Alpha2. Proteins were immunoprecipitated with VLDLR antibodies and normalized to the amount of precipitated receptor. Histograms show the ratio of Dab1/VLDLR (black) and Alpha2/VLDLR (grey).
3. DISCUSSION
The Reelin transducer Dab1 and the regulatory subunit of the Pafah1b complex Lis1 are important for neuronal migration and physically interact to coordinate molecular events underlying the formation of cellular layers. Simultaneous disruption of the Reelin signaling pathway and Lis1 leads to layer formation defects and a high frequency of progressive hydrocephalus. We previously demonstrated that the Alpha2 catalytic subunit of the Pafah1b complex does not affect cortical layer formation but is critically involved in the development of hydrocephalus in double mutants defective in Reelin and Lis1 signaling. Loss of Alpha2 strongly suppressed the hydrocephalus phenotype, without affecting layer formation (Assadi et al., 2008). Loss of Alpha1, on the other hand, did not affect the hydrocephalus but exacerbated the layering defects of compound mice (Assadi et al., 2008). These studies suggested that the two Alpha subunits, despite their similarity and shared catalytic activity towards PAF, have distinct functions and interactions during brain development.
Here we showed that Alpha2, in addition to its well-known interaction with Lis1 in the formation of the Pafah1b complex and to its recently reported interaction with the Reelin receptor VLDLR (Zhang et al., 2007) also directly binds to Dab1, regardless of the phosphorylation status of this protein. This latter interaction, unlike those with Lis1 and VLDLR, is unique to Alpha2 and it is not shared with Alpha1. This finding was surprising because the Alpha1 and Alpha2 are remarkably similar both, in amino acid composition and crystal structure (Sheffield et al., 2001). Only a short region extending from residue 53 to 61 is significantly different between the two proteins, although its biological significance was unknown. The structure of this region is stable in Alpha2 but disordered in Alpha1. We found that only Alpha2, but not Alpha1, is capable of binding to Dab1. Interestingly, this binding activity requires the presence of a tyrosine at position 56, an amino acid contained within the residue 53–61 region that is structurally distinct between Alpha1 and Alpha2. We showed that conversion of tyrosine 56 of Alpha2 to a cysteine results in the complete loss of interaction with Dab1, without affecting binding to Lis1. These results are entirely consistent with structural data indicating that residue 56 is not involved in the binding of Alpha subunits to Lis1 (Sheffield et al., 2001; Tarricone et al., 2004) and suggest that the stable residue 53–61 stretch of Alpha2 is important for Dab1 interaction. Future studies will be required to determine whether conversion of cysteine to tyrosine at residue 56 of Alpha1 would be sufficient to confer Dab1 binding competence, and to identify the kinase involved in the presumed phosphorylation of this tyrosine residue.
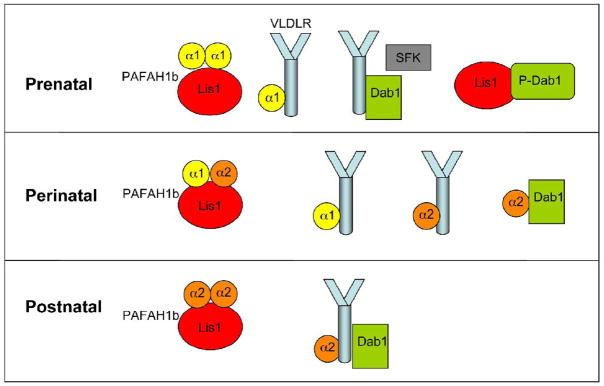
Our in vitro competition experiments demonstrated that the addition of Alpha2 interferes with the binding of Dab1 with Lis1, but not with that of Dab1 with VLDLR. Together with previous experiments, demonstrating that Alpha1 and Alpha2 compete with each other for binding to VLDLR and that Alpha1 interferes with the binding of VLDLR with Dab1 (Zhang et al., 2007), the data indicate that Alpha1 and Alpha2 affects the formation of Dab1-containing complexes that may thus play important roles in the control of neuronal migration and in the development of hydrocephalus. Developmental studies showed that Alpha1 expression is prominent during embryonic brain development, when migration takes place, but declines rapidly in the postnatal brain (Manya et al., 1998). Alpha2 expression, on the other hand, is low during embryonic development but it becomes elevated at postnatal ages. Thus, at prenatal ages in the absence of Alpha2 Dab1, upon Reelin-induced phosphorylation, may be free to interact extensively with Lis1 (Fig. 5). This interaction may be important for the control of neuronal migration and cortical layer formation. At perinatal ages, Alpha1 decreases whereas Alpha2 expression increases and new complexes may begin to appear. Finally at postnatal ages the abundance of Alpha2 may facilitate the disruption of the Dab1-Lis1 complex, possibly by creating a stable complex involving VLDLR (Fig. 5). Alpha2 binding partners such as Dab1, VLDLR and Lis1 may also be important to keep the activity of Alpha2 in the postnatal brain in check. An excess of free Alpha2 may in fact lead to hydrocephalus in compound mice lacking at least two of these binding partners, as it is rescued specifically by loss of Alpha2 (Assadi et al., 2008). Even though the exact mechanisms leading to the development of hydrocephalus have not yet been elucidated, our present observations may provide a molecular framework to better understand the origin of this phenotype.
Figure 5. Putative Lis1- and Dab1-containing protein complexes at different developmental ages.
At prenatal agesthe Pafah1b complex consists mostly of Alpha1 dimers and Lis1. Dab1 binds VLDLR, and is heavily phosphorylated by SFKs in response to Reelin. Dab1 also binds ApoER2 (not shown). PhosphoDab1 binds Lis1 in addition to other signaling molecules (not shown for simplicity). VLDLR, but not ApoER2, also binds Alpha1. At perinatal ages, as Alpha1 levels decrease and Alpha2 levels increase new complexes form. The Pafah1b complex becomes heterotrimeric, and Alpha2 competes and displaced Alpha1 from VLDLR. Levels of Dab1 phosphorylation drop and Alpha2 binds Dab1 instead of Lis1. At postnatal ages the Pafah1b complex contains mostly Alpha2 and Lis1. A multiprotein VLDLR-Alpha2-Dab1 complex may form.
4. EXPERIMENTAL PROCEDURES
Mouse colonies
All mice were maintained according to protocols approved by an Institutional committee at Baylor College of Medicine. Reeler mutantmice were obtained from The Jackson Laboratories on a C57BL/6 x C3H hybrid background and genotyped by PCR as described previously (Niu et al., 2004). Pafah1b2 knock out mice were obtained and genotyped by Southern blot as previously described (Yan et al., 2003).
Generation of expression plasmids
To generate FLAG-tagged fusion proteins, mouse Pafah1b1 cDNA (GenBank accession #NP_038653) and mouse Pafah1b2 cDNA (GenBank accession # Q61206) were cloned in the pCMV-tag vector (Stratagene). To generate GFP-tagged constructs Pafah1b1, Pafah1b2 and mouse Pafah1b3 cDNA (GenBank accession #Q61205) were cloned into pEGFP-C1 (Clontech). Mouse Dab1 cDNA was tagged with the HA epitope by PCR and subcloned into the pcDNA3.1 vector (Invitrogen). GFP-Alpha2(Y56C) and Dab1-YY mutations (Y198A and Y220A) were generated using the Stratagene QuikChange Site Directed Mutagenesis Kit. Human VLDLR cDNA (GenBank accession # NP_003374) was cloned into the pcDNA3.1 vector.
Fusion protein expression
COS-7 cells (ATCC) were plated in 100mm dishes in the presence of DMEM supplemented with 10% fetal bovine serum, penicillin/streptomycin, and glutamine (Life Technologies) and incubated overnight to 50–70% confluency. On the next day, the cells were transfected with mammalian expression vectors utilizing Fugene 6 reagent (Roche) according to the manufacturer’s instructions and incubated at 37°C for 30–40 hours. The cells were then examined by fluorescence microscopy, or prepared for immunoprecipitation and Western blot analysis as described below.
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blotting were performed as previously described using brain lysates or transfected cell lysates (Zhang et al., 2007). Antibodies used were: anti-FLAG M2 (Invitrogen), anti-Lis1 (Santa Cruz), anti-Myc (Millipore) anti-HA (Sigma), anti-GFP (Clontech), anti-VLDLR (Santa-Cruz) and anti-Dab1 (Rockland or Novus). Dab1 was phosphorylated in vitro using a Src assay kit (Upstate).
Reelin-induced Dab1 phosphorylation assay
To assay Reelin signaling, primary cortical neurons were cultured from embryonic mice and treated with Reelin-containing conditioned medium (Niu et al., 2004) for 20 min. Cells were lysed and Dab1 was immunoprecipitated using commercial antibodies (Novus). Immunoprecipitated proteins were subjected to Western blot analysis using anti-phosphotyrosine antibody 4G10 (Upstate) and Dab1 antibodies as previously described (Beffert et al., 2002).
In vitro binding assay
In vitro translations of Pafah1b1, Pafah1b2, VLDLR and Dab1 cDNAs were performed using the TnT Quick Couple Transcription/Translation System (Promega), according to the manufacturer instructions using 35S-labeled methionine. Antibodies were added for immunoprecipitation (IP) for 1–2 hours at 4°C followed by addition of protein A/G agarose beads (Pierce) as described previously (Zhang et al., 2007). Beads were washed three times with PBS. Co-IP proteins were separated by SDS-PAGE and treated with fixation buffer (10% glacial acetic acid, 50% methanol) for 30 min at room temperature, and dried the gel at 80°C for 1 hour. Finally, autoradiography was performed on dried gels and the bands intensity were measured using ImageJ (NIH).
Acknowledgments
This work was supported in part by NIH/NINDS R01 NS042616 (G.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Assadi A, Zhang G, McNeil R, Clark GD, D’Arcangelo G. Pafah1b2 mutations suppress the development of hydrocephalus in compound Pafah1b1; Reln and Pafah1b1; Dab1 mutant mice. Neurosci Lett. 2008;439:100–105. doi: 10.1016/j.neulet.2008.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–610. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J. PI3-Kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy and Behavior. 2005;8:81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O’Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–91. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Feng L, Allen NS, Simo S, Cooper JA. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 2007;21:2717–2730. doi: 10.1101/gad.1604207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Arai H, Inoue K. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1993;268:18748–53. [PubMed] [Google Scholar]
- Hattori M, Adachi H, Tsujimoto M, Arai N, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet AM, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates Tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet. 1998;19:333–9. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of Disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Keshvara L, Benhayon D, Magdaleno S, Curran T. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J Biol Chem. 2001;276:16008–16014. doi: 10.1074/jbc.M101422200. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Yamaguchi N, Hattori M, Ishikawa TO, Aoki J, Taketo MM, Inoue K, Arai H. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J Biol Chem. 2003;278:12489–94. doi: 10.1074/jbc.M211836200. [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–86. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manya H, Aoki J, Watanabe M, Adachi T, Asou H, Inoue Y, Arai H, Inoue K. Switching of platelet-activating factor acetylhydrolase catalytic subunits in developing rat brain. J Biol Chem. 1998;273:18567–72. doi: 10.1074/jbc.273.29.18567. [DOI] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Ochalski PG, Chen K, Gropman A, Myers S, Min KT, Howell BW. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol. 2003;23:7210–7221. doi: 10.1128/MCB.23.20.7210-7221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Sheffield PJ, McMullen TW, Li J, Ho YS, Garrard SM, Derewenda U, Derewenda ZS. Preparation and crystal structure of the recombinant alpha(1)/alpha(2) catalytic heterodimer of bovine brain platelet-activating factor acetylhydrolase Ib. Protein Eng. 2001;14:513–9. doi: 10.1093/protein/14.7.513. [DOI] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–75. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Tezuka T, Morimura T, Hattori M, Mikoshiba K, Yamamoto T, Takenawa T. Regulation of actin cytoskeleton by mDab1 through N-WASP and ubiquitination of mDab1. Biochem J. 2004;384:1–8. doi: 10.1042/BJ20041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarricone C, Perrina F, Monzani S, Massimiliano L, Kim MH, Derewenda ZS, Knapp S, Tsai LH, Musacchio A. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–21. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:7189–94. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, Herz J, Clark GD, D’Arcangelo G. The Pafah1b Complex Interacts with the Reelin Receptor VLDLR. PLoSONE. 2007;2:e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]