Abstract
Human and non-human animal studies reveal that early experiences with caregivers shape children's ability to regulate their responses to stress. To understand the effects of early deprivation on the regulation of the hypothalamic-pituitary-adrenal axis following social interactions, we examined urinary cortisol levels in a group of internationally adopted children who had experienced institutional care, and thus, species-atypical attachment relationships, early in life prior to adoption. Cortisol regulation was assessed both basally and following standardized interpersonal interactions between the child and his/her mother and the child and an unfamiliar adult. Post-institutionalized children showed prolonged elevations in cortisol levels following the mother, but not the stranger, interaction. More severe neglect was associated with the highest basal cortisol levels and the most impaired cortisol regulation following the mother interaction. These results suggest that early social deprivation may contribute to long-term regulatory problems of the stress-responsive system, and that these differences are most evident within the context of ongoing, close interpersonal relationships.
Keywords: Early experience, HPA axis, Social, Human
Much research activity in the biobehavioral sciences has been aimed at gaining a better understanding of how early caregiving experiences influence children's development and the role that later experiences can have in remediating the effects of early adversity. These issues have taken center stage in basic science approaches to understanding mechanisms of human development and the translation of such information is now viewed as crucial for the creation of interventions that can meaningfully improve children's health (Gunnar, Fisher, & The Early Experience, Stress, & Prevention Network, 2006). However, research investigating the biological mechanisms linking early adversity with the development of behavioral problems is still in a relatively early stage of development. The aim of this experiment is to apply basic research on the developmental neurobiology of stress to the understanding of how aberrations in early caregiving may create vulnerabilities to healthy emotional and social functioning in children. To do so, we examined the effects of socioemotional deprivation on the regulation of the hypothalamic-pituitary-adrenal (HPA) axis functioning in children following interactions between children and their primary caregivers. In humans, the HPA axis develops over the first several years of life and is highly sensitive to early adverse caregiving experiences (DeBellis et al., 1999; de Weerth, Zijl, Buitelaar, 2003; Watamura, Donzella, Kertes, & Gunnar, 2004). Thus, examining the ways in which early adversity may impact the regulation of the HPA axis is important for gaining a better understanding of the relationship between early environments and biobehavioral development.
Relatively little research has focused on the impact of early neglect on the functioning of the HPA system in humans. This is largely because it is difficult to identify appropriate samples of children. In contrast to other forms of child maltreatment, neglect is characterized by insufficient and inconsistent responsive caregiving and is thus difficult to objectively assess. In typical rearing situations, responsive caregiving helps infants modulate immature emotional systems (Cicchetti, Ganiban, & Barnett, 1991). Over time, appropriate caregiving helps infants develop increasingly sophisticated mechanisms for flexibly regulating arousal and stress (Dawson, Hessl, & Frey, 1994; Derryberry & Rothbart, 1984; Feldman, Greenbaum, Yirmiya, 1999; Kopp, 1982). The present study is motivated by the idea that failure to receive this type of external “scaffolding” from adults may have long-term effects on the physiological systems underlying children's emotion regulation (Kraemer, Ebert, Schmidt, & McKinney, 1989). Extant research on HPA system functioning among high-risk children is consistent with this view; however, these studies have focused upon disruptions to either the baseline or the circadian functioning of this system rather than regulation of the system in response to environmental events (e.g., Cicchetti & Rogosh, 2001; Dozier, et al., 2006).
We focus on the HPA axis because it is critically involved in the body's response to stress. Environmental stimuli perceived as physically or psychologically stressful, novel, or challenging trigger the HPA axis to release glucocorticoids—primarily in the form of cortisol in primates (Gunnar, Marvinney, Isensee, & Fisch, 1988). Social and emotional stimuli are especially important triggers of the HPA response. Circulating glucocorticoids modulate a wide range of biological responses such as energy release, cardiovascular function, immune activity, growth, emotion, and cognition (Diorio, Viau, & Meaney, 1993; Sapolsky, Romero, & Munck, 2000; Takahashi et al., 2004). Termination of, and recovery from, stress-induced HPA activity is accomplished via the negative feedback action of glucocorticoids on multiple brain regions (Dallman, 1993). These transient hormonal changes allow the organism to adapt and cope effectively with current stressors. However, chronic elevation of cortisol impairs behavioral adaptation and has been associated with emotion regulation difficulties and psychopathology (Goodyer, Park, Netherton, & Herbert, 2001; Gunnar & Vazquez, 2001; Heim, Owens, Plotsky, & Nemeroff, 1997; Sapolsky, 2000).
Neuroendocrine Dysregulation following Adverse Early Social Experiences: Non-human Animal Models
Experimental research utilizing nonhuman animal models has been extremely valuable in delimiting short- and long-term effects of early social experiences on the organization of the physiological response to stress (e.g., Fahlke, Lorenz, Long, Champoux, Suomi, & Higley, 2000; Francis & Meaney, 1999; Suomi, 1999). In rodents, negative feedback inhibition of the HPA axis develops post-natally and relies on parental behavior for its development (van Oers, de Kloet, Li, & Levine, 1998). Although basal glucocorticoid levels are often unaffected by early maternal deprivation, both heightened reactivity and an inability of the HPA axis to effectively terminate the stress response have been reported in rodents exposed to adverse rearing environments (Caldji, Francis, Liu, Plotsky, & Meaney, 2000; Ladd, Huot, Thrivikraman, Nemeroff, & Plotsky, 2004; van Oers et al., 1998). Most relevant to the present study, elevated glucocorticoid levels have been found up to 10 days following an acute stressor in rodents (Milde, Enger, & Murison, 2004).
Results from non-human primate studies have provided additional evidence that experimentally-induced parental deprivation during infancy leads to larger and more prolonged elevations in cortisol levels in response to social and nonsocial stressors and to novelty (Higley, Suomi, Linnoila, 1992; Sanchez et al., 2005; Stanton, Gutierrez, & Levine, 1988; Suomi, 1991). For example, maternally deprived rhesus monkeys show diminished negative feedback of the HPA axis following a pharmacological (dexamethasone) challenge (Capitanio, Mendoza, Mason, & Maninger, 2005). This finding has also emerged in squirrel monkeys following intermittent separations from their natal group during infancy (Lyons, Yang, Mobley, Nickerson, & Schatzberg, 2000). It does not appear that only extreme events can influence functioning of this system; naturally occurring variations in mother-infant relationships in primates differentially affect HPA regulation as well. For example, rhesus infants who experienced highly responsive mothering showed a faster decline in cortisol levels following an experimental separation from their mothers (Gunnar, Gonzalez, Goodline, & Levine, 1981). In contrast, those infants who had experienced less responsive mothering showed a prolonged cortisol response following a challenging event (Dettling, Pryce, Martin, & Dobeli, 1998). These studies suggest that responsive and consistent care is important in shaping neuroendocrine regulation (Rosenblum & Andrews, 1994).
Neuroendocrine Dysregulation following Adverse Early Experiences: Human Studies
The impact of adverse caregiving environments on subsequent HPA axis functioning in children is less clearly understood, in part because ethical considerations make it impossible to impose on humans the types of experimental manipulations that are possible with nonhuman animals. Thus, researchers must rely on naturally occurring variations in children's environments to study the impact of early experience on cortisol levels. Such “experiments in nature” are undoubtedly fraught with confounds that are easily controlled in the laboratory; yet, these types of situations have also yielded important insights into child development (Rutter, 1981). These studies suggest that the types of parental care that foster a secure attachment relationship between infant and parent (e.g., one marked by sensitive, contingent, and responsive caregiving) are critical for the healthy development of the HPA axis (Gunnar & Donzella, 2002). For example, in a study of 17-month old children, emotional unavailability/withdrawal on the part of the mother was associated with elevated basal cortisol and the use of physically harsh discipline was related to hyperresponsiveness of the HPA axis to stress (Bugental, Martorell, & Barraza, 2003). Differences in cortisol production have also been observed in groups of maltreated children (Hart, Gunnar, & Cicchetti, 1995). Additionally, it appears that maltreatment severity may be an important factor in shaping HPA axis development: children who experience multiple forms of abuse (e.g., neglect, physical abuse, and sexual abuse) evince higher morning cortisol values than either nonmaltreated children or children who experience a single form of abuse (Cicchetti & Rogosch, 2001). Although research on the regulation or termination of the HPA stress-response in maltreated children is scant, one study involving depressed adult women with histories of abuse during childhood found that these adults exhibit a prolonged elevation in cortisol following a standardized psychosocial laboratory stressor compared to those without an abuse history (Heim et al., 2000).
Neuroendocrine Dysregulation and Neglect in Children
The study of maltreated or other groups of high-risk children allows us to glimpse the ways in which caregiving variation affects child development. However, an interpretive limitation to these studies is that these samples of children typically experience cumulative life stressors, often in chronically disadvantaged environments. Therefore, it is difficult to ascertain the relative effects of early experience versus cumulative experience on the HPA axis (Essex, Klein, Cho, & Kalin, 2002). Yet, the central role of social relationships in modulating the stress response (Adam, Klimes-Dougan, & Gunnar, 2007) and the magnitude of the response of the HPA axis to social stressors (Dickerson & Kemeny, 2004) raises important issues about the developmental timing of social experience. To begin to address this issue, we examined stress regulation following a discrete interaction with a caregiver in a sample of post-institutionalized children. Unlike most populations of maltreated children, children adopted from institutions (i.e., orphanages) have experienced a circumscribed period of social and emotional neglect, including a prominent lack of emotionally and physically available caregivers and little responsiveness to their individual needs, followed by an abrupt transition into often enriched family environments in the US (Human Rights Watch, 1998; Johnson, 2000). Because their environments change so dramatically and quickly, this particular group of children allows researchers to begin to address questions regarding the relative importance of the developmental timing of early events on subsequent regulation of the stress response without the confounding influence of continued environmental adversity.
Prior descriptive research has indicated that institutional care dramatically increases risk for social attachment disturbances and difficulty with emotion regulation (Ames, 1997; Chisholm, 1998; Hodges & Tizard, 1989; Rutter & ERA Study Team, 1998; Rutter, Kreppner, & O'Connor, 2001; Tizard & Rees, 1974). These socioemotional regulation difficulties include higher rates of insecure or atypical patterns of attachment to primary caregivers, indiscriminate sociability, and inappropriate affection seeking toward strangers (Chisholm, 1998; Marcovitch, Goldberg, & Gold, 1997; O'Connor, Marvin, Rutter, Olrick, Britner, & ERA Study Team, 2003; O'Connor, Rutter, Beckett, Keaveney, Kreppner& ERA Study Team, 2000; Vorria, Rutter, & Pickles, Wolkind, & Hobsbaum, 1998). The kinds of emotional difficulties observed among many post-institutionalized children, such as insecure/disorganized attachment patterns, should relate to differences in cortisol production (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996; Hertsgaard, Gunnar, Farrell Erickson, & Nachmias, 1995). For example, in a community sample of children with an insecure pattern of attachment, cortisol levels were elevated in response to the reunion portion of the Strange Situation Task (Spangler & Grossmann, 1993). These data suggest that elements of the mother-infant relationship lead to HPA activation for this group of children and thus motivate the present experiment.
To date, there have been few published studies investigating HPA activity in a post-institutionalized sample. In one of these studies, data revealed elevated basal cortisol levels in post-institutionalized children, even after the children had resided in their adoptive homes for 6 to 7 years (Gunnar, Morison, Chisholm & Schuder, 2001). However, little is known about neuroendocrine regulation in these children, which may represent an important link between early experience and the socioemotional difficulties observed in many of these children (Chisholm, 1998; O'Connor et al., 2003). A previous investigation with the current sample of children (Wismer Fries, Ziegler, Jacoris, Kurian, & Pollak, 2005) tested whether caregiver interactions were associated with children's regulation of oxytocin, a hormone centrally important in establishing attachment (Carter, 1998; Insel, 1992, 2002). Post-institutionalized children in that study showed lower oxytocin levels than comparison children following an interaction with their (adoptive) mothers. Given that oxytocin and cortisol have been implicated in the regulation of opposing biological systems (Parker, Buckmaster, Schatzberg, & Lyons, 2005), this previous finding with post-institutionalized children further underscores the importance of investigating possible cortisol dysregulation within an interpersonal context.
The Present Study
Guided by the findings reviewed above, we tested a group of children who had experienced early caregiving neglect followed by placement in a normative family environment; we examined the children's HPA responses following physical contact with a primary caregiver. The main goal of the experiment was to determine whether this social interaction would be associated with a failure of the HPA axis to effectively contain a stress-response among children exposed to early social deprivation. To test the hypothesis that neuroendocrine dysregulation would be specific to interactions with the primary caregiver, a control condition involving a similar interaction between the child and an unfamiliar female adult was used. Furthermore, cortisol was measured on four additional mornings in which no novel events occurred to ascertain a stable baseline for each child.
Our hypotheses were as follows. First, we expected that children who had experienced early neglect would show a prolonged HPA response following the interaction with a putative attachment figure. This hypothesis was motivated by reports involving nonhuman animals (Caldji et al., 2000), maltreated children (Bugental et al., 2003), and children exhibiting attachment difficulties (Hertsgaard et al., 1995). We also examined a second, exploratory, hypothesis that individual differences in the degree of adversity children endured (operationalized as length and severity of pre-adoption neglect) would be associated with diminished negative feedback within the HPA system (as reflected in higher post-interaction cortisol levels). This hypothesis was motivated by reports involving maltreated children suggesting that variability in the severity and duration of early adverse caregiving may have important implications for developmental outcomes (Cicchetti & Rogosch, 2001; Gunnar et al., 2001; Wismer Fries & Pollak, 2004).
Materials and Methods
Participants
18 post-institutionalized children (12 females) residing with their adoptive parents were compared to 21 children (12 females) residing with their biological parents. The two groups of children were drawn from a database of approximately 500 children who had responded to community postings, flyers, mailings, or advertisements indicating their interest in participating in research. Children who met study criteria (i.e., age, region of the world, age at adoption, no known birth defects or developmental disabilities) were randomly selected and approached for participation. One adoptive family declined to participate due to a lack of time and an additional four control families declined to participate due to lack of interest or time. Post-institutionalized children had resided in orphanages for an average of 16.6 months (range: 7 months to 42 months) shortly after birth. Twelve of the post-institutionalized children had resided in Russian orphanages and 6 children were adopted from Romanian orphanages. To ensure that children had time to acclimate to their new home environments, we studied only children who had been residing in their adoptive homes for approximately three years (M = 35 months, SD = 11 months). The two groups of children were equivalent in age (post-institutionalized sample: M = 53.7 months, SD = 4.4 months, comparison sample: M = 54.3 months, SD = 7.1 months), and the families were drawn from similar high socioeconomic backgrounds (years of parent education (SD): post-institutionalized sample 15.8 (2.0), comparison sample 16.2 (2.0)). Children were excluded if birth defects or developmental disabilities were present.
Instruments
Prior to participation in the study, parents of all participants completed several questionnaire measures, including background data (e.g., parent education) and pre-adoption information (e.g., length of time in institution, conditions in the institution, age at adoption). Adoptive parents were asked to make a global rating of the extent of social or emotional neglect they believed their child had experienced based on their impressions of the institution in which their child had resided prior to adoption on a 4-point scale: 0 (none) to 3 (severe). This single item measure was utilized in an attempt to capture a broad picture of children's early living conditions.
Procedure
Parents were instructed to collect overnight urine samples from their child's first void of the day on six separate mornings. On average, collection occurred 20.9 minutes after awakening. Four samples of urine were collected on mornings following “typical” days for children, where nothing particularly unusual or stressful occurred. Two basal samples were collected prior to the first experimental session and the other two basal samples were collected on days that fell in between the experimental sessions. The other two collections took place on the mornings immediately following the two experimental sessions (described below). Urine samples were stored in sterile specimen containers, frozen immediately, and kept frozen until assayed. For the experimental sessions, researchers visited children on two separate days in their homes 7 to 14 days apart. During the visits, children engaged in a “Simon Says” type computer game that facilitated child-adult interactions while sitting on either their mother's or an unfamiliar female experimenter's lap. The unfamiliar female adult was the same individual for all experimental sessions. Order of these interactions was counterbalanced. Throughout the 30-minute interaction, the mother (or unfamiliar adult) and child engaged in regularly timed and standardized close physical contact (e.g., tickling, patting on the head, counting each other's fingers, whispering in each other's ears, hugging).
Cortisol Assay
Urinary measurement of cortisol, rather than saliva or serum measurement, was used for three reasons. First, serum measurement may be perceived as stressful for young children, potentially triggering a stress response in this age range. Second, saliva or serum reflects HPA activity over several minutes, but urine samples integrate cortisol levels over the much longer timeframe of interest for this experiment. Third, analyses of other hormones of potential interest required the use of urine samples.
Urine samples were thawed and a portion aliquotted directly for cortisol and creatinine measurement. Urinary cortisol was measured by an enzymeimmunoassay using methods described in Ziegler, Scheffler, & Snowdon (1995). All samples for cortisol measurement were assayed as 50 μl of 1:100 dilutions. Serially diluted child urine was parallel to the standard curve (t(9) = 0.17, P>0.05) and accuracy was 117.3% ± 2.7 SEM. Intra- and inter-assay coefficients of variation were 1.8% and 13.2%, respectively, for the low pool, and 1.2% and 8.4%, respectively, for the high pool. Cortisol is expressed per mg of creatinine to control for fluid variability (μg/dL Cr). The creatinine assay has been described previously (Ziegler et al., 1995).
Data Analytic Strategy
Basal cortisol and cortisol responses to the experimental tasks were then analyzed using Hierarchical Linear Modeling (HLM, 6.0, Bryk & Raudenbush, 1992, Snijders & Bosker, 1999). Hierarchical Linear Modeling of basal cortisol allows separate estimates of within- and between-individual variation (Hruschka, Kohrt, & Worthman, 2005). This strategy was chosen because: (1) individual observations of cortisol were not independent (Hox, 2002); (2) including basal and cortisol change in the same model controls for the law of initial values (Ramsay & Lewis, 2003); (3) simultaneous assessment of multiple cortisol measures allows us to take advantage of the intra-class correlation coefficient as a measure of basal cortisol; and (4) only the systematic portion of variability is predicted by individual difference predictors.
The dependent variable was the natural log of urinary cortisol measured across six mornings. First, we examined if ‘trait’ variation accounts for a significant proportion of the total variability in cortisol levels to establish that young children have stable, basal cortisol (Shirtcliff, Granger, Booth, & Johnson, 2005). Second, the basic model then assessed whether cortisol systematically deviates from its basal level after children interacted with their parent using a dummy variable (0 = basal, 1 = after parent) as a within individual predictor. Once intra-individual differences were characterized, individual difference predictors of basal cortisol at the between individual level were considered, including group status (0 = comparison, 1 = post-institutionalized), gender, severity of neglect, and length of institutionalization. Such cross-level interactions examine if deviations from basal levels after the tasks were related to individual differences in severity of neglect, length of institutionalization, gender or group. Because our sample size was small, we tested whether these predictors were individually associated with cortisol in a series of models (see Table 1). If significant, we then confirmed that each of these individual difference predictors were significant beyond the effect of group because group differences were primarily of interest. This allowed us to test whether the predictor was operating within the post-institutionalized group as well as between the groups. Third, parallel analyses using the After Stranger values as a within individual predictor and between individual outcome were computed. Finally, to aid interpretation, nonsignificant coefficients were removed so as to arrive at a parsimonious model that included all independent significant effects. As illustrated in mathematical form below, Basal levels (β0), After Parent (β1) and After Stranger (β2) slopes were modeled simultaneously (Snijders & Bosker, 1999):
Table I.
Gamma (γ) coefficients for a series of Hierarchical Linear Models in which each predictor variable (i.e., group, severity of neglect, gender, length of institutionalization, and age) was examined independently.
| Initial Level | Group | Severity of Neglect | Gender | Length of Institutionalization | Age | |
|---|---|---|---|---|---|---|
| Basal Cortisol LevelsA | 5.62** (.06) | .13 (.12) | .10a (.06) | -.13 (.11) | .06 (.04) | .008 (.009) |
| After Parent SlopeB | .12* (.06) | .23* (.11) | .22** (.06) | -.13 (.11) | .08 (.04) | .006 (.007) |
| After Stranger SlopeC | .02 (.07) | .11 (.15) | .03 (.07) | -.04 (.14) | .05 (.05) | .011 (.012 |
p<.05;
p<.001;
When both group and neglect were modeled together, neglect was a significant predictor of basal cortisol levels.
A, B, C denote rows; see text for further explanation.
| Level 1 (within individual): | Cortisol = β0 + β1Parent + β2Stranger + r |
| Level 2 (between individual) | β0 = γ00 + γ01neglect + U0 |
| β1Parent = γ10 + γ11group + γ12neglect + U1 | |
| β2Stranger = γ20 + U2 |
Results
Severity of Neglect and Height/Weight Measures
The global parent rating of orphanage conditions correlated with weight (r = -.33, p = .04) and height (r = -.40, p = .009) at first exam in the US for the post-institutionalized group. These correlations provide some evidence that the parents' ratings of orphanage conditions do, in fact, capture developmentally-relevant features of their adopted children's early experiences.
Basal Cortisol Levels
Trait (systematic) cortisol comprised a significant proportion (47.2%) of the total variability in basal cortisol, χ2(39)=248.32, p<0.0001, suggesting that young children have moderately stable urinary cortisol levels across several mornings. Day-to-day fluctuations in morning cortisol levels accounted for 52.8% of the total variance in morning cortisol, thereby providing some support for an analytical strategy, which removes this non-systematic variability from the basal cortisol estimates.
We then explored individual difference predictors of basal cortisol, including group (post-institutionalized vs. comparison), gender, age, severity of neglect, and length of institutionalization (See Table 1, Row A). The effect of group, severity of neglect, gender and length of institutionalization were examined independently first. We then confirmed whether a significant effect was independent of group status to ensure that the finding was not redundant with group status. Basal cortisol did not differ between post-institutionalized and comparison children (Cortisol (SEM): 342.66 μg/dL Cr (37.6) in post-institutionalized group and 285.5 μg/dL Cr (26.4) in comparison group), yet post-institutionalized children who had experienced severe neglect in the institution had higher basal cortisol levels, γ01=0.11, t (38)=2.68, p<0.01 than post-institutionalized children who had experienced less severe neglect. Severity of neglect explained 1.9% of the variance in basal cortisol. Gender, age, and length of institutionalization did not help explain variability in basal cortisol levels.
After Parent Cortisol Regulation
We then tested if some of the day-to-day variability in cortisol was due to children's response to the parent interaction task. Children's cortisol levels after interacting with their parent (Cortisol (SEM): 344.01 μg/dL Cr (48.22)), were significantly higher than on other mornings (Cortisol (SEM): 312.94 μg/dL Cr (12.79)), γ10=0.12, t (39)=2.11, p=0.04. This effect was driven by the post-institutionalized group (see Figure 1). Variability in the After Parent slope was not statistically significant, U1=.03, χ2(39)=47.90, p=0.16. Nevertheless, we allowed the magnitude of the After Parent slope to vary from person to person (i.e., as a random term) because variance tests are traditionally underpowered (since their true distribution is somewhat narrower than a χ2) and the chi-square was large enough to suggest that there was moderate variability in the After Parent slope.
Figure 1.
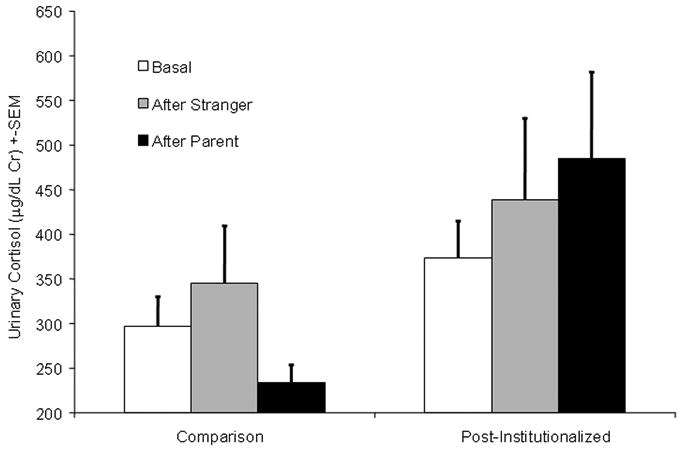
Cortisol levels (μg/dL Cr) for post-institutionalized children and comparison condition by condition.
We then examined individual difference predictors of children's response to the task with their parent. Post-institutionalized children's cortisol levels after interacting with their parent were higher than comparison children's After Parent cortisol values (Cortisol (SEM): 485.3 μg/dL Cr (96.5) in the post-institutionalized group and 234.1 μg/dL Cr (19.5) in the comparison group, γ11=0.23, t (38) = 1.99, p = 0.04). Indeed, comparison's children's cortisol levels actually decreased after the parent interaction (see Figure 1). Group status explained 5% of the total variance in After Parent slope. Further, within the post-institutionalized group, those who had experienced more severe neglect had higher cortisol levels after interacting with the parent, γ11=0.22, t (38) = 3.88, p<0.001, with neglect severity explaining 34.96% of the variance in After Parent slope. Both group and neglect severity were independent predictors of After Parent Slope when simultaneously modeled, together explaining 45.23% of the variance in After Parent Slope, p < 0.02. Figure 2 shows that post-institutionalized children who were more severely neglected had higher After Parent cortisol levels than less neglected children, γ12=0.35, t (37) = 4.22, p<0.001. Gender, age, and length of institutionalization were not predictors of After Parent Slope (see Table 1, Row B).
Figure 2.
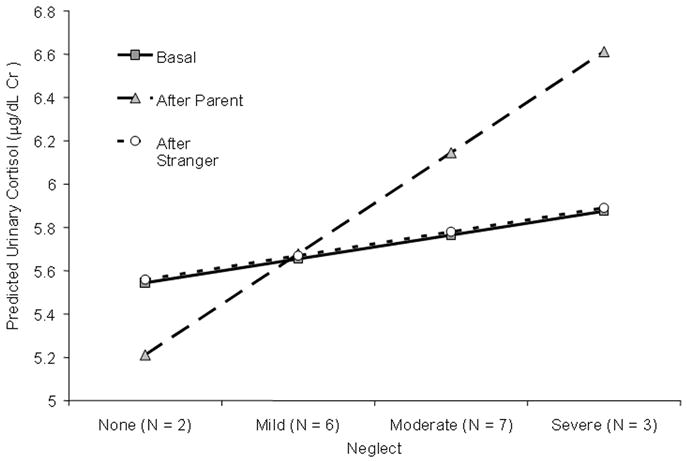
Log transformed Cortisol levels (μg/dL Cr) by severity of reported neglect for post-institutionalized children. Values represent the predicted levels based on the HLM analyses.
After Stranger Cortisol Regulation
Children's cortisol levels after interacting with the stranger (Cortisol (SEM): 388.38 μg/dL Cr (54.16)) were not different from the other mornings, p = 0.8. There was substantial variability in After Stranger cortisol levels, U2=0.10, χ2(39) = 73.89, p<0.001, suggesting that a subset of children may have been responding to the stranger's visit (see Figure 1). However, none of the variables that we assessed accounted for individual differences in the After Stranger slope (see Table 1, Row C).
Discussion
The present experiment tested the effects of early social deprivation on basal cortisol levels and regulation of the HPA axis following close physical and social contact with children's primary caregivers. Given the relatively small sample size of the current study, results should be viewed as a first step in understanding these relationships. The data generated from this study suggest three primary findings. First, basal cortisol levels were generally similar among comparison and post-institutionalized children; elevated basal cortisol levels were found only among the children who had experienced severe neglect. Second, previously neglected children's after-parent cortisol values were (a) higher than comparison children's after-parent interaction values and (b) higher than their own basal values. Finally, we sought to examine individual difference factors that might highlight which features of early experience are affecting development of the HPA regulatory system. Children who had reportedly experienced more severe socioemotional neglect showed greater elevations in cortisol levels following the interpersonal interaction with their mothers as compared to those children who also experienced institutionalized rearing but who experienced less severe or no neglect.
Although a lack of difference in basal cortisol levels between post-institutionalized and comparison children appears inconsistent with the Gunnar et al. (2001) findings with abused children, our data are consistent with non-human animal research, which often fails to find a relationship between early experience and basal HPA activity. In primates, abnormal stress responsivity has been reported to remain for years in socially deprived animals, even if basal behavioral and physiological indices of emotional arousal do not differ from control animals (Gilmer & McKinney, 2003). However, within the post-institutionalized group, the most severely neglected children demonstrated increased basal cortisol levels compared to children who experienced relatively mild neglect early in life. However, given that an extremely small number of severely neglected children drove the current results and that severity of neglect explained just 1.9% of the variance in basal cortisol levels, follow-up studies will be necessary to confirm this preliminary finding.
Contrary to findings in the cognitive and behavioral realms, which often report a negative relationship between length of institutionalization and performance on the outcome of interest (Ames, 1997; O'Connor et al., 2000; Wismer Fries & Pollak, 2004), children with longer orphanage stays were not more likely to show elevated levels of cortisol than children with shorter stays. The present findings suggest that the nature and quality of the social and emotional caregiving received, not simply length institutionalization itself, may be a critical factor for predicting chronic hyperactivation of the HPA axis. It is important to note that the children in the present sample were institutionalized on average for over 16 months, during a developmental epoch when interactions with primary caregivers appears to play a role in setting HPA reactivity thresholds (Gunnar et al., 2006). It would be useful for future research to examine the development of children with only a few months of institutional care, prior to the age when a consolidated attachment relationship with a primary caregiver would be expected.
To be effective, an HPA axis response must quickly activate to a challenging event but also, through negative feedback actions of glucocorticoids, return to baseline. The data presented here indicate that previously neglected children both responded to a social interaction with a caregiver as a challenging event and were unable to effectively contain or regulate their HPA response—up to several hours following the social interaction. This finding is consistent with non-human animal research, which also demonstrates protracted stress responses in animals exposed to low levels of maternal care or maternal deprivation early in life (Dettling et al., 1998). However, the prolonged increase in cortisol did not generalize to all social interactions, as post-institutionalized children responded to an interaction with an unfamiliar adult similarly to the comparison children.
One limitation of the present study is that it did not include a cortisol measurement immediately following the experimental session. Therefore, we cannot determine whether the post-institutionalized children had a hyperreactive response following the interaction with their parent, a prolonged response to the event, or both. However, it is certainly likely that both were the case, given that primates reared in conditions of isolation or disrupted infant-mother relationships show initial hyperresponsivity to stressful stimuli later in development (Kraemer et al., 1989). Regardless of children's cortisol levels immediately following the parent interaction, what is significant is the extended nature of the stress response, which demonstrates inefficiency or a breakdown of the negative feedback mechanisms of the HPA system. Furthermore, the differential response of the previously neglected children to the two social events (parent vs. stranger) suggests that having close physical contact with a caregiver was a particular challenge for these children, rather than a buffer against prolonged activation. This differential response to the parent vs. stranger suggests that the HPA axis is not globally dysregulated in post-institutionalized children, but rather, the dysregulation is meaningfully related to the nature of the early caregiving environment. Lacking a primary attachment figure, children residing in Eastern European orphanages are exposed to a large number of different caregivers, even before their first birthday. One study found that children in a Russian orphanage experienced 120 different caregivers during their first year of life (D. Johnson, unpublished data). Attachment difficulties following adoption may ensue if the child is not able to successfully adapt to such a drastic change in caregiving circumstances and effectively begin to navigate close interactions with the new primary caregiver. In fact, indiscriminate friendliness and lack of reticence with unfamiliar adults have been commonly reported behavioral differences in post-institutionalized samples of children that tend to persist following adoption (Chisholm, 1998; Hodges & Tizard, 1989; O'Connor et al., 2000). We can only speculate at this point that a close relationship with a primary caregiver continues to be experienced as a social challenge for these children, therefore evoking a cortisol response. This interpretation is consistent with research with post-institutionalized children, which found a decrease in cortisol levels while children participated in a short-term summer camp program as compared to their home cortisol values both before and after camp (Purvis & Cross, 2006). The authors suggest that some of the post-institutionalized children may remain in an “alarm state” at home with their parents, despite living in a safe, loving environment for several years.
In securely attached children, parents often serve as a buffer in novel or challenging situations; therefore, when an attachment figure is present, toddlers who have a secure relationship with a parent do not show elevations in cortisol (Gunnar et al., 1996; Nachmias, Gunnar, Manglesdorf, Hornik Parritz, & Buss, 1996; Spangler & Grossman, 1993). This may have been the case for the comparison children who showed no elevations in cortisol on the morning following the parent or stranger interaction, despite the fact that the situations were quite novel for the children (having several researchers in their home with video and computer equipment, etc). Given the general difficulty in eliciting a cortisol response in typically developing preschool aged children (see Gunnar & Donzella, 2002, for review), it is possible, but less likely, that the comparison children did mount a stress response during both the Parent and Stranger interactions, but subsequently returned to baseline levels via efficient negative feedback of the HPA system.
The finding that post-institutionalized children in the current study showed prolonged elevations only after the parent interaction is consistent with the idea that the early postnatal social environment plays an important role in HPA axis development. However, the post-institutionalized children in the current study were likely also exposed to a variety of prenatal risk factors, which may have included malnutrition, fetal alcohol exposure, and exposure to a variety of disease pathogens. All of these factors could also have played a role in the development of the HPA system. Therefore, it is difficult to make a causal argument for the role of postnatal social factors in their HPA axis dysregulation. In addition, important current factors, such as the amount of existing parenting stress and the parents own attachment style, may also play a significant role in children's cortisol regulation. Nevertheless, the specificity of these findings may help motivate future studies linking direct assessment of attachment-related disturbances with HPA axis functioning.
Although length of institutionalization was unrelated to cortisol regulation, children who had experienced the more severe socioemotional neglect while in the orphanage, according to parent report, showed more dysregulation than post-institutionalized children who had experienced less neglect. These findings suggest that institutionalization per se may not be the critical factor in disrupting HPA activity, but that the quality of interactions between the infant and caregiver is important early in development. For example, rat pups that received less tactile input from their mothers showed prolonged increases in coricosterone following a stressor, and this effect was present even into adulthood (Francis & Meaney, 1999; Liu et al., 1997; Meaney, Aitken, Viau, Sharma, & Sarrieau, 1989). It is possible that children who were handled less by caregivers within the orphanage, experienced a larger number of caregivers, or experienced more insensitive or noncontingent caregiving develop increased or prolonged stress reactivity later in life. Future studies that include additional comparison groups (such as children adopted from regions of the world where foster care or higher quality institutional care is more prevalent) may be helpful in further understanding the most salient aspects of the early environment for the development of HPA axis regulation.
Although we believe that the effects of severity of neglect reported here are potentially significant, these data must be interpreted with caution. We relied upon a global retrospective parent report of the orphanage conditions to measure “severity”. Because of this, it is impossible to know on what information parents were basing their ratings. Therefore, one possibility is that parents of children who are experiencing more developmental problems had a systematically negative bias in rating their child's pre-adoption conditions. On the other hand, parents often spend several weeks at the orphanage, giving them first hand knowledge about their child's living conditions immediately prior to adoption. In addition, a bias in rating pre-adoption conditions would not likely explain an association with cortisol because parents are unaware of the hormonal status of their children. Consistent with this view, height and weight at first exam in the US were correlated with the parent rating of severity of neglect. The difficulty in adequately assessing post-institutionalized children's pre-adoption living conditions remains a significant limitation of the work with this group of children. While more “objective” measures such as number of pre-adoption placements, length in the institution, or height/weight may be useful and specific, it is unlikely that these variables alone are broad enough to capture a complete understanding of the early living conditions that post-institutionalized children experienced. Development of more extensive interviews or questionnaires for parents may be useful in addressing this limitation, although this issue will likely always be problematic in research, which includes groups of post-institutionalized children.
Conclusion
The effects of early deprivation on subsequent behavioral and emotional functioning are likely to be attributable to changes in multiple regulatory neural systems, including the HPA axis. In this preliminary study, we have shown that early social and emotional neglect is associated with disruption in the normal activity of the HPA system and that this disruption is specifically related to salient social stimuli. Better understanding of the processes that are impacted by early caregiving experiences will inform both models of how developmental change occurs and the creation of effective interventions for children who have encountered early caregiving adversity.
Acknowledgments
The authors thank Toni Ziegler and Dan Wittwer for conducting hormonal assays, and Gabrielle Sowle, Marna Brown, and Justin Martin for their help with data collection. This experiment would not have been possible without the participation of many children and their families, for whose collaboration we are extremely appreciative. A preliminary version of these data was presented at the 2005 Biennial Meeting of the Society for Research in Child Development. Seth Pollak was supported by the National Institute of Mental Health (MH 068858 and MH61285), Alison Wismer Fries and Elizabeth Shirtcliff were supported by an NIMH Training Program in Emotion Research (MH18931).
References
- Adam EK, Klimes-Dougan B, Gunnar MR. Social regulation of the adrenocortical response to stress in infants, children and adolescents: Implications for psychopathology and education. In: Coch D, Dawson G, Fischer KW, editors. Human behavior, learning, and the developing brain: Atypical development. New York: Guilford Press; 2007. pp. 264–304. [Google Scholar]
- Ames E. Final report to the National Welfare Grants Program: Human Resources Development Canada. Burnaby, British Columbia: Simon Fraser University; 1997. The Development of Romanian Orphanage Children Adopted to Canada. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Sage Publications, Inc; 1992. [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–44. doi: 10.1016/s0018-506x(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Liu D, Plotsky PM, Meaney MJ. The role of early experience in the development of individual differences in behavioral and endocrine responses to stress. In: McEwen BS, Stellar E, editors. Handbook of Physiology: Coping with the Environment. New York: Oxford University Press; 2000. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Chisholm K. A three-year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Development. 1998;69:1092–1106. [PubMed] [Google Scholar]
- Cicchetti D, Ganiban J, Barnett D. Contributions from the study of high-risk populations to understanding the development of emotion regulation. In: Garber J, Dodge KA, editors. Development of Emotion Regulation and Dysregulation. New York: Cambridge University Press; 1991. pp. 15–48. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress update: Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends in Endocrinology Metabolism. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dawson G, Hessl D, Frey K. Social influences on early developing biological and behavioral systems related to risk for affective disorder. Development and Psychopathology. 1994;6:759–779. [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology: I. Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart M. Emotion, attention, and temperament. In: Izard CE, Kagan J, Zajonc R, editors. Emotions, Cognition, and Behavior. Cambridge: Cambridge University Press; 1984. pp. 132–166. [Google Scholar]
- Dettling A, Pryce CR, Martin RD, Doebeli M. Physiological responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi's monkeys. Developmental Psychobiology. 1998;33:21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Human Development. 2003;73:39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children's diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism: Clinical and Experimental Research. 2000;24:644–650. [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinions in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: Human and non-human primate studies. Journal of Affective Disorders. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The British Journal of Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher P, Early Experience, Stress & Prevention Network Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez CA, Goodlin BL, Levine S. Behavioral and pituitary--adrenal responses during a prolonged separation period in infant rhesus macaques. Psychoneuroendocrinology. 1981;6:65–75. doi: 10.1016/0306-4530(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Marvinney D, Isensee J, Fisch RO. Coping with uncertainty: New models of the relations between hormonal, behavioral, and cognitive processes. In: Palermo DS, editor. Coping with Uncertainty: Behavioral and Developmental Perspectives. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. pp. 101–129. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-Adrenal and Autonomic Responses to Stress in Women After Sexual and Physical Abuse in Childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacology Bulletin. 1997;33:185–192. [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar MR, Farrell Erickson M, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and family relationships of ex-institutional adolescents. Journal of Child Psychology and Psychiatry. 1989;30:77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Hox J. Multilevel analysis techniques and applications. Hillsdale, NJ: Erlbaum Associates; 2002. [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Human Rights Watch. Abandoned to the State: Cruelty and Neglect in Russian Orphanages. New York: 1998. [Google Scholar]
- Insel TR. Implications for the neurobiology of love. In: Pos SG, Underwood LG, Schloss JP, Hurlbut WB, editors. Altruism & Altruistic Love: Science, Philosophy, and Religion in Dialogue. New York: Oxford University Press; 2002. pp. 254–263. [Google Scholar]
- Insel TR. Oxytocin-A neuropeptide for affiliation: Evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Medical and developmental sequelae of early childhood institutionalization in Eastern European adoptees. In: Nelson CA, editor. The Minnesota Symposia on Child Psychology, Vol 31: The Effects of Early Adversity on Neurobehavioral Development. Mahwah, NJ: Erlbaum Associates; 2000. pp. 113–162. [Google Scholar]
- Kopp C. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid, norepinephrine, and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mrna and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky D, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. Early Environmental Regulation of Glucocorticoid Feedback Sensitivity in Young Adult Monkeys. Journal of Neuroendocrinology. 2000;12:723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Goldberg S, Gold A. Determinants of behavioural problems in Romanian children adopted in Ontario. International Journal of Behavioral Development. 1997;20:17–31. [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Milde AM, Enger O, Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiology and Behavior. 2004;81:71–84. doi: 10.1016/j.physbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Guannar MR, Mangelsdorf S, Hornik Parritz R, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- O'Connor TG, Marvin RS, Rutter M, Olrick JT, Britner PA, ERA study team Child-parent attachment following early institutional deprivation. Development and Psychopathology. 2003;15:19–38. doi: 10.1017/s0954579403000026. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Rutter MR, Beckett C, Keaveney L, Kreppner JM, ERA Study Team The effects of global severe privation on cognitive competence: Extension and longitudinal follow-up. Child Development. 2000;71:376–390. doi: 10.1111/1467-8624.00151. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Purvis KB, Cross DR. Improvements in salivary cortisol, depression, and representation of family relationships in at-risk adopted children utilizing a short-term therapeutic intervention. Adoption Quarterly. 2006;10:25–43. [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Development. 2003;74:456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Andrews MW. Influences of environmental demand on maternal behavior and infant development. Acta Paediatric. 1994;397:57–63. doi: 10.1111/j.1651-2227.1994.tb13266.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Maternal Deprivation Reassessed. New York: Penguin Books; 1981. [Google Scholar]
- Rutter M, ERA Study Team Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- Rutter M, Kreppner JM, O'Connor TG. Specificity and heterogeneity in children's responses to profound institutional privation. British Journal of Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Noble P, Lyon C, Plotsky P, Davis M, Nemeroff C, Winslow J. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative Actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks: Sage Publications; 1999. [Google Scholar]
- Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Attachment in rhesus monkeys. In: Cassidy J, Shaver P, editors. Handbook of Attachment: Theory, Research, and Clinical Applications. New York: Guilford Press; 1999. pp. 181–197. [Google Scholar]
- Suomi SJ. Uptight and laid-back monkeys: Individual differences in the response to social challenges. In: Brauth SE, Hall WS, editors. Plasticity of Development. Cambridge, MA: The MIT Press; 1991. pp. 27–56. [Google Scholar]
- Takahashi T, Ikeda K, Ishikawa M, Tsukasaki T, Nakama D, Tanida S, Kameda T. Social stress-induced cortisol elevation acutely impairs social memory in humans, Neuroscience Letters. 2004;363:125–130. doi: 10.1016/j.neulet.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Tizard B, Rees J. A comparison of the effects of adoption, restoration to the natural mother, and continued institutionalization on the cognitive development of four-year-old children. Child Development. 1974;45:92–99. [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Li C, Levine S. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 1998;139:2838–2846. doi: 10.1210/endo.139.6.6037. [DOI] [PubMed] [Google Scholar]
- Vorria P, Rutter M, Pickles A, Wolkind S, Habsbaum A. A comparative study of Greek children in long-term residential group care and in two-parent families: I. Social, emotional, and behavioural differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:225–236. [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–33. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Development and Psychopathology. 2004;16:355–369. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Science. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]


