Summary
Reduced function mutations in the insulin/IGF-I signaling pathway increase maximal lifespan and health span in many species. Calorie restriction (CR) decreases serum IGF-1 concentration by ~40%, protects against cancer and slows aging in rodents. However, the long-term effects of CR with adequate nutrition on circulating IGF-1 levels in humans are unknown. Here we report data from two long-term CR studies (1 and 6 years) showing that severe CR without malnutrition did not change IGF-1 and IGF-1 : IGFBP-3 ratio levels in humans. In contrast, total and free IGF-1 concentrations were significantly lower in moderately protein-restricted individuals. Reducing protein intake from an average of 1.67 g kg −1 of body weight per day to 0.95 g kg −1 of body weight per day for 3 weeks in six volunteers practicing CR resulted in a reduction in serum IGF-1 from 194 ng mL −1 to 152 ng mL −1 . These findings demonstrate that, unlike in rodents, long-term severe CR does not reduce serum IGF-1 concentration and IGF-1 : IGFBP-3 ratio in humans. In addition, our data provide evidence that protein intake is a key determinant of circulating IGF-1 levels in humans, and suggest that reduced protein intake may become an important component of anticancer and anti-aging dietary interventions.
Keywords: aging, calorie restriction, IGF-1, metabolism, protein restriction
Introduction
In the last few decades, large amounts of money and research effort have been, and continue to be, devoted to the study of the anti-aging and anticancer mechanisms underlying calorie restriction (CR) in yeast, worms, insects and rodents. Presumably this expenditure of funds and research effort is motivated by the belief that the data obtained in various short-lived species showing that CR improves health and slows aging has relevance to humans. To date, several studies have consistently shown that long-term CR without malnutrition and reduced function mutations in the insulin/IGF-1 signaling pathway are the most robust interventions known to increase maximal lifespan and health span in rodents (Fontana & Klein, 2007). CR decreases serum IGF-1 concentration by approximately 40% in rodents, and this CR-mediated reduction in IGF-1 levels is believed to play a key role in protecting against cancer and slowing aging (Dunn et al., 1997; Sonntag et al., 1999; Fontana & Klein, 2007). Growth hormone-deficient and growth hormone receptor-deficient mice have also low circulating IGF-1 levels and increased maximal lifespans (Flurkey et al., 2001; Ikeno et al., 2003; Bonkowski et al., 2006). In addition, decreased IGF-1 signaling is involved in the delayed aging phenotype of IGF-1 receptor-deficient mice and klotho transgenic mice (Holzenberger et al., 2003; Kurosu et al., 2005). IGF-1 plays an important role in metabolism, growth and development (Jones & Clemmons, 1995). Furthermore, IGF-1 promotes tumor development, the second leading cause of death in industrialized countries, by stimulating cell proliferation and differentiation, and inhibiting cell apoptosis of normal and cancer cells (Dunn et al., 1997; Samani et al., 2007).
Results and discussion
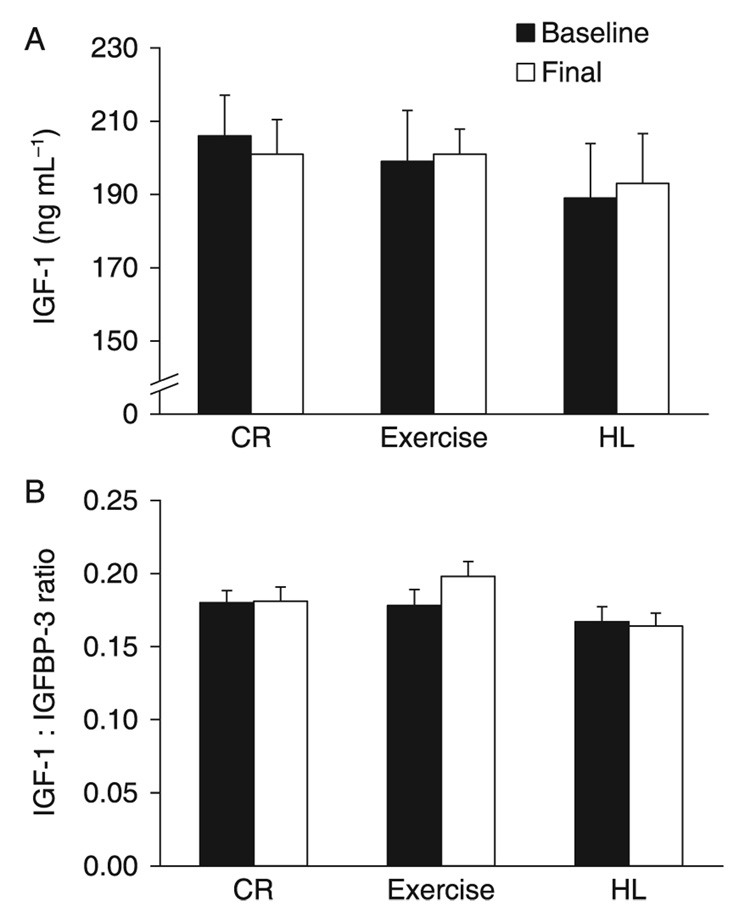
Fasting and short-term (6 days) CR or protein restriction acutely lower serum IGF-1 concentration in humans (Thissen et al., 1994; Smith et al., 1995). However, it is not known whether long-term CR without malnutrition reduces serum IGF-1 concentration or slows aging in humans. To determine whether the duration of CR or the metabolic and body composition changes associated with CR affect serum IGF-1 concentration in humans, we measured serum IGF-1 and IGFBP-3 in two studies that manipulated energy flux for different periods of time. In one study, 46 middle-aged non-obese individuals [29 women and 17 men, age 57 ± 3 years, body mass index (BMI) 27.3 ± 2.0 kg m −2] (Table 1) were randomized to 1 year of a 20% decrease in energy intake from baseline without a change in energy expenditure (CR, n = 18), a 20% increase in energy expenditure through daily exercise without a change in energy intake (EX, n = 18); or a healthy lifestyle control group (HL, n = 10) (Racette et al., 2006). Energy intake was reduced by 304 ± 408 kcal day − 1 ( p = 0.006) in the CR group, while in the EX and HL groups average daily energy intake was unchanged (Racette et al., 2006). Protein intake remained the same (~16% of total energy intake) in all groups throughout the study (Table 2). At 1 year of treatment, weight loss averaged 8.2 ± 4.8 kg in the CR group, 6.6 ± 5.5 kg in the EX group, and 1.2 ± 2.1 kg in the HL group. Body fat decreased by 6.3 ± 3.8 kg in CR, 5.6 ± 4.4 kg in EX and 0.4 ± 1.7 kg in HL, which corresponded to reductions of 24.9%, 22.3% and 1.2% of baseline body fat mass, respectively (Racette et al., 2006). Surprisingly, we found that, unlike in rodents, the decrease in energy intake in the CR group was not accompanied by a reduction in serum IGF-1 concentration or IGF-1/IGFBP-3 ratio (Fig. 1). Weight-loss induced by exercise training was also not coupled with a decrease in serum IGF-1 and IGFBP-3 levels (Fig. 1). In contrast, as previously reported, and in agreement with the metabolic adaptations that occur in CR mice, 1 year of CR in these volunteers resulted in a significant improvement in insulin sensitivity, and in significant reductions in serum leptin (a circulating hormone that reflects the amount of energy stored in fat tissue), C-reactive protein (a marker of systemic inflammation), insulin and triiodothyronine levels (Racette et al., 2006; Weiss et al., 2006, 2008; Fontana et al., 2007b; Villareal et al., 2007).
Table 1.
Baseline study subject characteristics
| CR (n = 18) | EX (n = 18) | HL (n = 10) | p-value* | |
|---|---|---|---|---|
| Sex (% female) | 61% | 67% | 60% | 0.92 |
| Race (% white, black, other) | 94/0/6 | 89/6/6 | 70/20/10 | 0.32 |
| Age (year) | 55.2 ± 3.4† | 58.9 ± 2.7 | 56.0 ± 2.7† | 0.002 |
| Height (m) | 1.71 ± 0.1 | 1.68 ± 0.1 | 1.71 ± 0.1 | 0.57 |
Age and height are mean ± standard deviation. EX, exercise group; CR, calorie restriction group; HL, healthy lifestyle group.
p-value compares groups using chi-squared test for sex, Fisher’s exact test for race, and analysis of variance for age and height.
p ≤ 0.05 vs. EX group using Tukey’s HSD test.
Table 2.
Energy intake and macronutrient intake before and after 12 months of calorie restriction, exercise training or no change in energy balance
| CR (n = 18) | Exercise (n = 18) | HL (n = 10) | Among-group p | |
|---|---|---|---|---|
| Energy intake, kcal day−1 | ||||
| Baseline | 2081 ± 551 | 2044 ± 440 | 2230 ± 492 | |
| 12 months | 1777 ± 378 | 2100 ± 364 | 2235 ± 529 | |
| Change | −304 ± 408 | 55.9 ± 245 | 4.82 ± 216 | 0.0004* |
| Within-group p | 0.006 | 0.36 | 0.95 | |
| Protein (g kg−1 per day) | ||||
| Baseline | 1.11 ± 0.22 | 1.10 ± 0.16 | 1.01 ± 0.27 | |
| 12 months | 1.16 ± 0.29 | 1.23 ± 0.31 | 1.06 ± 0.28 | |
| Change | 0.06 ± 0.25 | 0.13 ± 0.28 | 0.05 ± 0.12 | 0.55 |
| Within-group p | 0.33 | 0.07 | 0.24 | |
| Protein (% of energy) | ||||
| Baseline | 17.68 ± 4.8 | 16.72 ± 2.2 | 15.26 ± 2.5 | |
| 12 months | 18.75 ± 3.8 | 16.65 ± 2.9 | 15.83 ± 3.1 | |
| Change | 1.07 ± 4.6 | −0.07 ± 2.8 | 0.57 ± 2.2 | 0.19 |
| Within-group p | 0.33 | 0.92 | 0.46 | |
| Fat (% of energy) | ||||
| Baseline | 33.74 ± 5.8 | 36.74 ± 6.3 | 35.02 ± 5.6 | |
| 12 months | 31.63 ± 5.8 | 35.72 ± 5.2 | 38.54 ± 5.0 | |
| Change | −2.10 ± 7.2 | −1.02 ± 4.3 | 3.52 ± 5.7 | 0.01† |
| Within-group p | 0.23 | 0.34 | 0.10 | |
| Carbohydrate (% of energy) | ||||
| Baseline | 46.71 ± 11.3 | 45.84 ± 5.9 | 51.57 ± 7.5 | |
| 12 months | 49.11 ± 8.4 | 47.09 ± 5.8 | 46.74 ± 5.6 | |
| Change | 2.41 ± 8.0 | 1.25 ± 5.6 | −4.83 ± 7.2 | 0.13 |
| Within-group p | 0.22 | 0.37 | 0.08 |
Data are mean ± standard deviation. Among group p-values compare groups using analysis of covariance with the change as the dependent variable and baseline as the covariate. Tukey’s tests were used for post hoc comparisons between groups. Within-group p-values test for change between baseline and 12 months within group and are based on paired t-tests.
CR vs. EX p = 0.001; CR vs. HL p = 0.005 by Tukey adjusted post-hoc comparison.
CR vs. HL p = 0.007 by Tukey adjusted post-hoc comparison.
Fig. 1.
Effects of 1 year of calorie restriction (CR) on serum IGF-1 concentration and IGF-1 : IGFBP-3 ratio. Serum IGF-1 concentration (A) and the IGF-1 : IGFBP-3 ratio in response to 1 year of CR (n = 18), exercise-induced weight loss (Exercise; n = 18), and a healthy lifestyle control condition (HL; n = 10), as assessed during the randomized trail. Data are mean ± standard error. There were no significant differences within or among groups.
In the second study, we evaluated the possibility that longer periods of CR are necessary to reduce serum IGF-1 concentration in humans. Serum IGF-1 and IGFBP-3 concentrations, and IGF-1/IGFBP-3 ratio were assessed in a group of 28 weight-stable members of the Calorie Restriction Society (age 51.6 ± 12.7 year; BMI 19.7 ± 1.8 kg m −2), who had been practicing severe CR with adequate nutrition (at least 100% of the reference daily intake for each nutrient) for an average of 6 years, and in 28 age-matched controls (age 53.6 ± 8.5 year; BMI 25.6 ± 2.5 kg m−2, p = 0.0001 vs. CR) eating a typical Western diet. The Calorie Restriction Society members ate a balanced diet providing approximately 1800 kcal day−1 with 24% calories from protein and 28% calories from fat. The Western diet group ate a typical Western diet containing foods which provided approximately 2500 kcal day−1 with 16% calories from protein and 33.6% calories from fat (Table 3). Energy intake was significantly lower and protein intake was significantly higher in the CR group than in the Western diet group (Table 3). BMI and total body fat were significantly lower in the CR group than in the Western diet in both men and women (Table 4). As in our 1-year CR study, we found that there were no differences in serum IGF-1 and IGFBP-3 concentrations, and IGF-1 : IGFBP-3 ratio between the CR and Western diet groups (Fig. 2). In contrast, as reported previously in a smaller group (Fontana et al., 2004, 2006b; Meyer et al., 2006), fasting insulin, C-reactive protein and triiodothyronine concentration were significantly lower in the CR group than in the Western diet comparison group (Fig. 2). The findings from these two studies demonstrate that 1 year and 6 years of CR do not reduce total and free IGF-1 levels in humans.
Table 3.
Macronutrient intake of the three groups in the cross-sectional study
| Low-protein diet group (n = 28) | CR diet group (n = 28) | Western diet group (n = 28) | Among-group p | |
|---|---|---|---|---|
| Energy (kcal day–1) | 1980 ± 5351 | 1772 ± 3511 | 2505 ± 522 | 0.0001 |
| Protein (g kg–1 per day) | 0.76 ± 0.21,2 | 1.73 ± 0.41 | 1.24 ± 0.3 | 0.0001 |
| Protein (% of energy) | 9.6 ± 3.31,2 | 23.5 ± 5.71 | 15.9 ± 3.0 | 0.0001 |
| Fat (% of energy) | 41.3 ± 102,3 | 28.1 ± 9.2 | 33.6 ± 5.7 | 0.0001 |
| SFA (% of energy) | 7.8 ± 4.43 | 5.6 ± 2.71 | 10.9 ± 2.6 | 0.0001 |
| MUFA (% of energy) | 19.9 ± 5.61,2 | 12.3 ± 5.8 | 12.8 ± 2.4 | 0.0001 |
| PUFA (% of energy) | 10.5 ± 4.61,4 | 7.6 ± 2.3 | 6.8 ± 2.1 | 0.001 |
| Carbohydrate (% of energy) | 48.8 ± 8.6 | 47.3 ± 12.3 | 49.7 ± 13.0 | NS |
All values are mean ± standard deviation. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Significantly different from Western diet group: p ≤ 0.002
Significantly different from Western diet group: p ≤ 0.05.
Significantly different from the CR diet group: p ≤ 0.0001
Significantly different from the CR diet group: p ≤ 0.05.
Table 4.
Body composition of the three groups in the cross-sectional study
| Low-protein diet group (n= 28) | CR diet group (n= 28) | Western diet group (n= 28) | Among group p | |
|---|---|---|---|---|
| Height (m) | ||||
| Men | 1.75 ± 0.06 | 1.76 ± 0.07 | 1.79 ± 0.06 | NS |
| Women | 1.63 ± 0.07 | 1.62 ± 0.05 | 1.66 ± 0.05 | NS |
| Weight (kg) | ||||
| Men | 67.1 ± 8.41,2 | 60.9 ± 6.51 | 83.9 ± 9.6 | 0.0001 |
| Women | 56.6 ± 12.2 | 52.0 ± 2.75 | 66.9 ± 7.7 | 0.026 |
| Body mass index (kg m−2) | ||||
| Men | 21.9 ± 3.01,4 | 19.7 ± 1.81 | 26.3 ± 2.5 | 0.0001 |
| Women | 21.3 ± 3.4 | 19.8 ± 1.75 | 24.2 ± 1.8 | 0.041 |
| Body fat (% body weight) | ||||
| Men | 15.2 ± 5.41,6 | 7.1 ± 4.61 | 23.6 ± 6.5 | 0.0001 |
| Women | 25.8 ± 7.77 | 20.5 ± 9.97 | 36.9 ± 3.9 | 0.001 |
All values are mean ± standard deviation. Low-protein diet group and Western diet group (9 women and 21 men), low-calorie diet group (4 women and 26 men).
Significantly different from Western diet group: p ≤ 0.001
Significantly different from Western diet group: p = 0.07
Significantly different from Western diet group: p ≤ 0.05
Significantly different from Western diet group: p ≤ 0.007.
Significantly different from the CR diet group: p ≤ 0.05
Significantly different from the CR diet group: p = 0.013
Significantly different from the CR diet group: p ≤ 0.001.
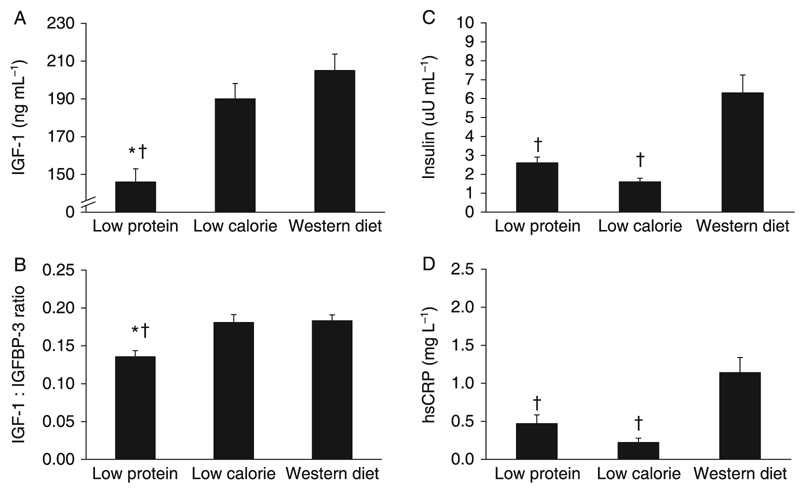
Fig. 2.
Long-term effects of calorie restriction (CR) and protein restriction (PR) on serum IGF-1 concentration. Serum IGF-1 concentration (A), IGF-1 : IGFBP-3 ratio (B), and the serum concentrations of insulin (C) and C-reactive protein (D) from the cross-sectional comparison of individuals who were habitually consuming a low protein diet, a low calorie diet, or a typical Western diet. Data are mean ± standard error. *p ≤ 0.01 vs. the low calorie group. †p ≤ 0.01 vs. the Western diet group.
These data provide evidence that, in contrast to the decrease in IGF-1 in rodents, a reduction of IGF-1 expression is not a component of the adaptive response to long-term CR in humans. On the other hand, fasting for 10 days markedly reduces serum IGF-1 concentration into the range observed for growth hormone-deficient patients (Thissen et al., 1994). Moreover, the changes of serum IGF-1 during fasting and refeeding are closely correlated with the rate of excretion of urinary urea, a marker of nitrogen balance and protein intake (Clemmons et al., 1981). Therefore, we conducted additional studies to evaluate the importance of long-term protein intake in modulating serum IGF-1 concentration in humans. In one study, we evaluated serum IGF-1 and IGFBP-3 concentrations, and IGF-1 : IGFBP-3 ratio in 28 vegans who had been consuming a moderately protein-restricted (PR) diet (0.76 g kg−1 per day; ~10% of intake from protein) for ~5 years age-matched with 28 members of the Calorie Restriction Society who consume a high-protein diet (1.73 g kg−1 per day; ~24% of energy intake from protein) (Table 3). Protein intake was significantly lower in the moderately PR group than in the CR group, while energy intake tended to be higher (Table 3). Both serum IGF-1 concentration and IGF-1 : IGFBP-3 ratio were significantly lower in the moderately PR diet group than in the severe CR diet group, whereas fasting insulin and C-reactive protein were similarly low in the moderately low-protein vegan and CR groups (Fig. 2), as previously reported in a smaller group of raw food vegans (Fontana et al., 2006a, 2007a). This effect of a moderate protein restriction is independent of body weight and body fat content, as serum total and free IGF-1 concentrations were lower in the moderately PR group than in the severe CR high-protein diet group, despite the PR groups’ higher body weight, BMI and body fat content (Table 4).
It also seemed possible that the CR groups’ rather high protein intake (~24% of the calories from protein; 1.73 g kg−1 per day of protein) may have prevented a reduction in IGF-1 level. As a first step in evaluating this possibility we were able to arrange for six of the CR volunteers to reduce their protein intake from 1.67 ± 0.1 g kg−1 of body weight per day to a protein intake of 0.95 ± 0.1 g kg−1 of body weight per day for 3 weeks. This short-term isocaloric reduction of protein intake resulted in a 25% reduction in serum IGF-1 concentration (from 194 ± 34 ng mL−1 to 152 ± 41 ng mL−1; p = 0.01) in the six CR individuals, suggesting that the high protein intake was preventing a reduction in IGF-1 levels in response to CR.
In conclusion, our findings demonstrate that, unlike in rodents, long-term severe CR does not reduce total and free IGF-1 levels in healthy humans if protein intake is high. In addition, our data suggest that chronic protein intake is more powerful than calorie intake in modulating circulating IGF-1 concentration in humans. This is important because the median protein requirement of the healthy adult population is 0.65 g kg−1 per day and the reference daily intake (97.5th percentile) is 0.83 g kg−1 of body weight per day (Rand et al., 2003) that is close to the protein intake of our vegan group in this study. In contrast, half of the US males are eating 40% or more protein (≥ 1.34 g kg−1 per day) than the reference daily intake (Moshfegh et al., 2005), which is presently considered to be harmless and, according to public opinion and advocators of ‘low-carb’ diets, may even be beneficial. More studies are necessary to understand the biological and clinical implications of a chronic high protein intake, especially in sedentary people with a positive family history for cancer. In addition, more studies are needed to understand the effects of PR and methionine restriction on metabolism, disease prevention and longevity in humans, because several studies in rodents have shown major beneficial effects (Richie et al., 1994; Miller et al., 2005; Pamplona & Barja, 2006; Sanz et al., 2006). Finally, these findings underscore the importance of dietary macronutrient intake in regulating metabolic events, and suggest that reduced protein intake may become an important component of anti-aging and anticancer dietary interventions, due to the importance of IGF-1 in the biology of aging (Sonntag et al., 1999; Flurkey et al., 2001; Holzenberger et al., 2003; Ikeno et al., 2003; Kenyon, 2005; Kurosu et al., 2005; Bonkowski et al., 2006; Russell & Kahn, 2007) and in the pathogenesis of many human tumors (Samani et al., 2007; Sachdev & Yee, 2007).
Experimental procedures
Participants
In the 1-year randomized clinical trial, sedentary 50- to 60-year-old non-obese men and women (BMI values of 23.5–29.9 kg m−2) were randomly assigned to 20% CR (n = 18), or 20% increase in calorie expenditure by means of exercise (EX; n = 18), or to a healthy lifestyle (HL) control group (n = 10) (http://www.ClinicalTrials.govidentifier:NCT00099138). Individualized diet and activity prescriptions for the CR and EX groups, respectively, were calculated from baseline total energy expenditure, which was determined by the doubly labeled water method as described previously (Racette et al., 2006). The CR and EX interventions were designed to result in the same energy deficit. The goal of the CR intervention was to decrease energy intake by 16% during the first 3 months and by 20% during the remaining 9 months. To achieve a decrease in energy intake, participants were encouraged to substitute foods with low energy density for those with high energy density and to reduce portion sizes. Participants randomized to the exercise intervention were instructed to maintain energy intake at baseline levels and to exercise in order to increase total energy expenditure by 16% for the first 3 months and by 20% for the subsequent 9 months. Participants were given exercise energy expenditure prescriptions on a weekly basis and exercised in our facility or on their own. Adherence to the energy expenditure goals was assessed by using heart rate monitors that estimate exercise energy expenditure (S610, Polar Electro Oy, Kempele, Finland). Exercise consisted of jogging, walking, elliptical machine exercise, cycling and/or rowing on an ergometer. Participants in the HL group were offered advice about consuming a healthful diet and were given free access to community-based yoga classes. Participation in dietary consultations and yoga classes was rare. Medical history and physical examination were used to identify and exclude volunteers with cardiovascular disease, diabetes, lung disease, uncontrolled hypertension, and evidence of malignancy. Informed written consent was obtained from all participants and the study was approved by the Human Studies Committee at Washington University School of Medicine. Additional details about the interventions and adherence to the interventions have been presented previously (Racette et al., 2006).
In the cross-sectional study, three age-matched groups were compared. Group 1 participants (52.2 ± 12 years old; 4 women and 24 men) were recruited through the Calorie Restriction Society. The reason for the small number of women is that nearly all of the Calorie Restriction Society members are men and only four women were available for testing. These individuals have been practicing severe CR with adequate nutrition for an average of 6 years (range 3–15 years). They came to Washington University Medical School (WUMS) in St. Louis to undergo a series of tests and measurements. Group 2 participants (53.4 ± 11 years old; 9 women and 19 men) were recruited by contacting the St. Louis Vegetarian Society and a Raw Food online magazine (Raw Food News, http://www.rawfoodsnewsmagazine.com). These individuals were consuming an ‘ad-lib’ low-protein diet, composed exclusively of plant-derived foods, for at least 2 years (mean 5.3 ± 4.1 years, range 2–17 years). Twenty-one of the volunteers in group 2 were eating only unprocessed and uncooked plant-derived foods. Group 3 participants (53.7 ± 8 years old; 9 women and 19 men) were recruited by local advertising. These individuals were healthy, non-obese (BMI < 30 kg m−2) subjects, eating typical Western diets. These volunteers served as a non-obese sedentary control group. All subjects underwent a comprehensive medical evaluation, including a medical history, physical examination, routine blood tests, and urinalysis. None of the volunteers had evidence of chronic disease, including cardiovascular, lung, gastrointestinal, autoimmune diseases, type 2 diabetes, or cancer, and none smoked tobacco. In addition, no individual was taking hormone replacement therapy, or other medications that could have affected the outcome variables. All participants were weight stable (i.e. less than 2 kg weight change) for at least 6 months before the study. This study was approved by the Human Studies Committee and the General Clinical Research Center Scientific Advisory Committee of Washington University School of Medicine, and all participants gave informed consent before their participation.
Study protocol
Dietary assessment
Subjects were instructed by a research dietitian to record all food and beverages consumed, including preparation methods and portion sizes, for 7 consecutive days. Measuring spoon and cup sets, and food diaries with a ruler imprinted on the back cover were provided to the participants to assist with portion size determinations. Food records were analyzed by using the NDS-R program (version 4.03_31), which is the Nutrition Data System for research from the Nutrition Coordinating Center at the University of Minnesota.
Clinical and metabolic assessment
Subjects were admitted to the outpatient facilities of Washington University School of Medicine General Clinical Research Center in the morning after they had fasted for 12 h overnight. Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale. Total body fat mass and lean body mass were determined by using dual-energy X-ray absorptiometry (QDR 1000/w, Hologic, Waltham, MA, USA). A venous blood sample was obtained to determine plasma IGF-1, IGFBP-3, insulin, and C-reactive protein concentrations.
Sample analyses
Serum IGF-1 and IGFBP-3 were measured by using Coated Tube Immunoradiometric Assays (Diagnostic Systems Laboratories, Webster, TX, USA). C-reactive protein was measured by using a highly sensitive ELISA kit (American Laboratory Products Company Diagnostics, Windham, NH, USA). Plasma insulin concentrations were measured by using commercially available RIA kits (Linco Research, St. Louis, MO, USA).
Statistical methods
In the randomized clinical trial, all participants who provided both baseline and 1-year data were included in the analyses. Baseline characteristics were compared between groups using chi-squared tests or Fisher’s exact test for categorical variables, and analysis of variance (ANOVA) for continuous variables. Analysis of covariance was used for between-group comparisons of data collected at baseline and 1-year with the 12-month value as the dependent variable and the baseline value as the covariate. When the overall model was significant, Tukey’s adjusted pairwise comparisons of groups were performed. Paired t-tests were used for within-group comparisons. All statistical tests were two-tailed, and significance was accepted at p ≤ 0.05. Data are presented as the mean ± standard deviation at each time point, and for the change between baseline and 12 months. All analyses in the randomized clinical trial were performed using SAS software, version 9.1.3 of the SAS System for Linux (SAS Institute Inc., Cary, NC, USA). In the cross-sectional study comparison for variables between groups were made by using ANOVA. Data for men and women were pooled, unless a significant interaction between group and sex was present. If the interaction term from the ANOVA was significant, then post-hoc comparisons were performed with Tukey’s test for normally distributed variables and with Games-Howell for distributions where equal variances could not be assumed. Statistical significance was set at p < 0.05 for all tests. All data were analyzed by using SPSS for Windows software, version 13.0 (SPSS Inc., Chicago, IL, USA).
Acknowledgments
We are grateful to the study participants for their cooperation and to the staff of the Applied Physiology Laboratory and Nurses of the General Clinical Research Center at Washington University Medical School (WUMS) for their skilled assistance. The study design was developed by J.O.H. and L.F.; data collection was performed and supervised by L.F., E.P.W., D.V., and J.O.H.; data analyses and interpretation were performed by L.F., D.V., E.P.W., S.K., and J.O.H.; writing was performed by L.F. and J.O.H. L.F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors declare that they participated in the study as mentioned above and that they reviewed and approved the manuscript in its final version.
This research was supported by NIH Cooperative Agreement AG20487, General Clinical Research Center Grant RR00036, Clinical Nutrition Research Unit Grant DK56351, a grant from the Longer Life Foundation (an RGA/Washington University Partnership) and a donation from the Scott and Annie Appleby Charitable Trust. The funding agency had no role in the analysis or interpretation of the data or in the decision to submit the report for publication. None of the authors had conflicts of interest.
References
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, Klibanski A, Underwood LE, McArthur JW, Ridgway EC, Beitins IZ, Van Wyk JJ. Reduction of plasma immunoreactive somatomedin C during fasting in humans. J. Clin. Endocrinol. Metab. 1981;53:1247–1250. doi: 10.1210/jcem-53-6-1247. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl Acad. Sci. USA. 2004;10:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am. J. Clin. Nutr. 2006a;84:1456–1462. doi: 10.1093/ajcn/84.6.1456. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 2006b;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Shew J, Holloszy JO. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007a;10:225–234. doi: 10.1089/rej.2006.0529. [DOI] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO the Washington University School of Medicine CALERIE Group. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab. 2007b;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;42:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro OM. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am. Coll. Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh A, Goldman J, Cleveland L. [accessed on 3 May 2008];What We Eat in America, NHANES 2001–2002: Usual Nutrient Intakes from Food Compared to Dietary Reference Intakes. 2005 [WWW document]. URL http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/usualintaketables2001-02.pdf.
- Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim. Biophys. Acta. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003;77:109–127. doi: 10.1093/ajcn/77.1.109. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat. Rev. Mol. Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol. Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 2007;2:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J. Clin. Endocrinol. Metabol. 1995;80:443–449. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci. 1999;5:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994;1:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch. Intern. Med. 2007;16:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO Washington University School of Medicine CALERIE, Group. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am. J Clin. Nutr. 2006;8:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Villareal DT, Racette SB, Steger-May K, Schechtman KB, Premachandra BN, Klein S, Holloszy JO, Fontana L. Caloric restriction but not exercise-induced reductions in fat mass decrease plasma triiodothyronine concentrations: a randomized controlled trial. Rejuven. Res. 2008;11:605–609. doi: 10.1089/rej.2007.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]