Abstract
Background
Despite the importance of face processing for normal social development, no fMRI studies of face processing in autism have focused exclusively on the childhood years. In order to fill that gap, forty-five children between the ages of 6-12 participated in practice scans, and after exclusion due to motion, 11 children with an ASD and 11 age-matched normal controls were included in final analyses.
Methods
Stimuli consisted of pictures of a familiar adult, familiar child, stranger adult, stranger child, and objects. During the scan, children pressed a button in response to an identical face shown on two consecutive trials. Based on our prior research, masks of four anatomical ROIs including the fusiform gyrus, amygdala, anterior and posterior cingulate were created and manually edited for anatomical precision for each subject. Following deconvolution analyses, the number of voxels significantly active and % signal change values that fell within each ROI mask were calculated for each subject.
Results
Analyses revealed normal fusiform activity in children with autism when viewing a face of their mother or other children. In contrast, looking at stranger adult faces initiated profound deficits in that the mean number of significantly active voxels in the fusiform bilaterally was approximately 25% of that shown in typically developing children.
Conclusions
A selective fusiform deficit in response only to the faces of adult strangers may be the result of reduced attention and interest during those conditions. Face processing abnormalities found in autism likely exists beyond the fusiform.
Keywords: Autism, face processing, fMRI, pediatric imaging, children, fusiform face area
Introduction
While there are many factors that influence neurofunctional responding to faces, two may be particularly important when studying autism: the age of the participants and the type of faces used in the experiment.
Although face processing is one of the most widely studied aspects of autism using fMRI, virtually every experiment has used adults or adolescents as the mean age of study (1-16). This period of life, however, represents a relative endpoint on a neurodevelopmental continuum. New neurobiological research has revealed a striking profile of deviant brain growth that changes considerably across the lifespan of the disorder. This growth pattern can be generally summarized by three phases: early brain overgrowth during the first years of life; arrest of growth during late childhood and preadolescence; and finally decline during adolescence and adulthood see (17, 18) for reviews. The dramatically changing landscape of neural development across ages in autism raises the caveat that results from functional brain imaging studies in autism should be placed in a developmental context.
In the three fMRI studies of face processing in autism that did include younger ages (4, 7, 9) the age range in those samples extended up to 25, 17 and 23 years respectively and age related effects were not specifically analyzed. As such, there is a large gap in knowledge regarding the brain response to faces in autism prior to the onset of the purported neural decline.
Although there have been exceptions (8, 10, 12), the vast majority of research on face processing leads to a general conclusion: the middle lateral aspect of the fusiform gyrus, the brain region highly involved in face processing in normals, is hypoactive in adults with autism (1-5, 7, 9, 14, 19-21). If there is developmental continuity in autism and hypoactivity of the fusiform is a fundamental and biologically defining feature of the disorder, then fusiform defects should be similar or perhaps even stronger at younger ages.
On the other hand, considering the tri-phasic brain growth trajectory in autism described above it is equally reasonable to predict the opposite: namely, that dysfunction in the fusiform may not be as severe in children with autism because they have not yet undergone the phase of cell loss or volume reduction typical of the adult phase.
While there are no fMRI studies of face processing exclusively in children with autism, a few studies using other imaging modalities have been conducted. Using ERP technology, Webb and colleagues (22) found a 10msec delay, but no amplitude differences, in the neural response to faces between 3-4 year old children with autism and controls. A magnetoencephalograpy study with 7-12 year olds found no differences from normal in the N140 response thought to be similar to the adult N170 over extrastriate areas in children with autism (23). The authors concluded that face processing in children with autism follows a similar trajectory to that which is seen in normal development with minor deviances. Taken together, these two studies raise the possibility that defects in the fusiform may be less severe, or at least have a different profile, than previously reported with adults with the disorder.
Because autism is fundamentally a disorder of sociability, it is important to consider the type of faces that are used to test social perception. With three exceptions (4, 8, 15) virtually every fMRI/face study of autism has used the faces of strangers (1-3, 5-14, 16). It has long been known that contact with strangers often induces distress and reduces social interaction in people with autism. Thus, while how the brain responds to stranger faces is essential to study in autism, it is but one aspect of face processing. The inclusion of faces that might hold more interest for people with autism, such as faces that are personally meaningful, may have a powerful impact on functional brain responding in this population (8).
Given that the present study’s focus was on the childhood years, another face-type that may influence fusiform function is child faces. Indeed, there is a strong developmental drive for infants and children to prefer to attend to the faces of other children (24). For example, when given the choice, the mean number of seconds an infant spends looking at child faces is significantly higher than the mean number of seconds spent looking at adult faces (25).
The fusiform, however, is but one structure within a larger “social brain” network that plays a role in evaluating faces in normal individuals, particularly when emotional or personally meaningful faces are used. Other structures such as the amygdala, anterior and posterior cingulate play key roles in evaluating the social and emotional significance of faces.
Overall the present study aimed to investigate face processing in a younger and narrower age sample than previous studies and to systematically vary the type of face on two important dimensions: whether or not a face was familiar or stranger, and whether or not the face was of a child or an adult. Functional imaging data collected during the early and middle childhood years are much closer to the time of symptom onset and as such may provide a clearer picture of basic phenomenon that are related to abnormal social development.
SUBJECTS AND METHODS
The study was approved by the University of California San Diego Human Research Protection Program. All parents of participants gave informed consent.
Subjects
Forty-five autism spectrum disorder (ASD) and typical children between the ages of 6-12 years participated in a series of pre-fMRI training procedures prior to the final experiment. (Supplemental Methods).
Final ASDS Group
Eighteen children with an ASD passed through all phases of training and participated in the final experiment. Children with movement exceeding motion criteria were not included in final analyses, leaving a final sample size of 11 children with an ASD (9 autistic disorder, 1 PDD-NOS and 1 Aspergers’s Disorder). (Supplemental Figure 1; Table 1).
Table 1.
Subject Characteristics
| AUTISM | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 6.1 | 6.2 | 8.1 | 10.3 | 10.5 | 11 | 11.5 | 11.6 | 11.5 | 11.6 | 11.2 | 9.9 |
| Sex | F | M | M | M | M | M | F | M | M | M | M | |
| ADI-R | ||||||||||||
| Social | 19 | 22 | 23 | 15 | 27 | 16 | 16 | 21 | 10 | 30 | 17 | 19.6 |
| Verbal | 16 | 24 | 17 | 20 | 18 | 9 | 10 | 23 | 15 | 21 | 20 | 17.5 |
| Non-Verb | 10 | 14 | 10 | 13 | 10 | 2 | 5 | 14 | 8 | 14 | - | 10.0 |
| Restr & Rep | 3 | 12 | 6 | 7 | 7 | 5 | 7 | 5 | 10 | 6 | 7 | 6.8 |
| ADOS | ||||||||||||
| Comm | 3 | 5 | 4 | 4 | 4 | 4 | 9 | 5 | 3 | 3 | 4 | 4.36 |
| Social | 5 | 8 | 10 | 6 | 8 | 9 | 14 | 11 | 4 | 11 | 5 | 8.27 |
| Stereo | 0 | 4 | 0 | 2 | 0 | 2 | 2 | 3 | 2 | 14 | - | 2.9 |
| IQ | ||||||||||||
| Non Verb | 89 | 71 | 79 | 86 | 91 | 82 | 92 | 72 | 83 | 92 | 126 | 87.55 |
| Verbal | 103 | 100 | 104 | 104 | 81 | 89 | 74 | 74 | 125 | 98 | 100 | 95.63 |
| Full S. IQ | 93 | 82 | 91 | 95 | 84 | 84 | 83 | 73 | 106 | 95 | 115 | 91 |
| TYPICAL | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 6.8 | 7.5 | 8.3 | 10.2 | 10.3 | 8.7 | 12.6 | 11.7 | 11.1 | 9.9 | 10.8 | 9.8 |
| Sex | F | M | M | M | M | M | F | M | M | M | M | |
| IQ | ||||||||||||
| Non Verb | 126 | 115 | 68 | 92 | 110 | 107 | 100 | 111 | 111 | 99 | 106 | 104.1 |
| Verbal | 129 | 128 | 117 | 100 | 104 | 125 | 93 | 95 | 118 | 99 | 117 | 111.4 |
| Full S. IQ | 130 | 124 | 92 | 95 | 107 | 118 | 96 | 104 | 116 | 99 | 112 | 108.5 |
Final Normal Control Group
When possible, typical children were matched on a one-to-one basis to each autistic child based on sex, chronological age and handedness. The mean age difference between each pair was 9 months. Autistic and typical children were not matched based on IQ, and the typical group had a significantly greater IQ score (mean 91 versus mean 109, t 20 = -3.4, p<.05). After elimination due to motion etc., 11 typical children were included in final analyses (Supplemental Methods).
Stimuli
Three stimulus sets, “familiar,” “stranger,” and “object” were used for each participant and contained pictures of their mother, friends as well as unknown adults and children and objects. Overall, a grand total of 130 non-repeating pictures were used. (Supplemental Methods and Supplemental Figure 2).
Behavioral Testing
During Scan - N-1 Back Task
To facilitate continuous attention to the stimuli during the scan, subjects pressed a button when the identical image was presented consecutively, also known as the N-1 back task (26).
Post-Scan - Face Processing Behavioral Tasks
A behavioral task was designed to evaluate relationships between face recognition ability and neurofunctional activation to familiar and unfamiliar faces. The task was based on previous studies showing shorter reaction times to familiar faces (27, 28). The test sheet contained a ‘target face’ at the top, followed by rows of faces, totaling 48 faces. The task was to scan the array and cross out the target face wherever it appeared (Supplemental Methods).
Post Scan — Face Identity Task
To verify that subjects could identify each photograph as a familiar person, subjects were asked to verbally name each familiar photograph shown on a printed page immediately following the scan.
Experimental Procedure and Image Processing
Procedures were similar to our previously published report that utilized a rapid event related fMRI design (8). Children viewed photographs of faces of and objects interspersed amongst trials that presented a fixation cross. The experimental run contained 188 trials. In 130 of the trials, photographs were presented for 2000 ms followed by 500 ms of a white screen. The remaining 58 trials presented the fixation cross for 2500 ms (null trials).
MRI Data Acquisition
Imaging data was collected on a 1.5 T Siemens Symphony MR scanner. All of the image registration and functional analyses were conducted using Analysis of Functional Neuroimages (AFNI) software (29). (Supplemental Methods).
Motion analysis
Images were corrected for motion by using the AFNI program 3DVolreg. An independent samples t- test was used to compare the motion indices between the final group of autistic and typical children. No significant between group differences were found. (Supplemental Methods).
fMRI Data and Whole Brain Analysis
After motion correction, the functional image time series were smoothed with a Gaussian filter (6 mm) and resampled into Talairach coordinates using AFNI. Individual subject analyses were performed using a deconvolution approach (3dDeconvolve program). (Supplemental Methods).
For group analyses, linear contrast scores for each participant obtained from the deconvolution analysis were included in a three-way analysis of variance (ANOVA) using face and object conditions as factors. Separate analyses were conducted for ASD and normal control children. Correction for multiple comparisons was established using a voxel-cluster threshold technique (34) for an overall corrected level of significance (alpha) of 0.05 (individual voxel p < 0.01, two-tailed; minimum cluster threshold required = 800 mm3). General linear tests (glt) were conducted to compare the BOLD activation from the first to the fourth acquisitions following stimulus presentation (2.5 to 10 s) for conditions of interest.
Region of Interest (ROI) Analyses
The fusiform gyrus, amygdala, anterior and posterior cingulate were regions of interest (ROI) identified a priori for specific analyses. Each ROI has been shown to be functionally active in response to personally meaningful faces in our previous work (8). Briefly, ROIs were traced using a combination of automated and manual procedures, and only voxels within the mask that exceeded a significance threshold of p<.01, two-tailed, were included in analyses (Supplemental Methods).
Correlation Analyses
Fusiform, Amygdala and Face Processing Task
Pearson correlation coefficients were computed to examine associations between # of voxels active in the fusiform and amygdala and behavioral performance on the face processing task.
Fusiform and other ROIs
Pearson correlation coefficients were computed to examine the relationship between the # of voxels active in the fusiform in relation to the remaining 3 ROIs in each group.
RESULTS
Behavioral Testing
During Scan - N-1 Back Task
Although all 22 subjects performed the N-1 back task during the scan, technical issues prevented a computer generated logfile for 4 subjects (2 ASD, 2 normal). Of the remaining 18 subjects, no differences in reaction time (normal 907 msec versus ASD 984 msec, t(16)= -.69 p>.05) or accuracy (normal correctly identified 13.6 targets versus ASD 12.5 targets, t(16) =.93, p>.05) between groups was found.
Post-Scan - Face Processing Behavioral Task
There were no significant differences between groups in reaction time, number of false alarms or misses in response to mother’s face. Children with ASD were significantly slower than typical children to identify the stranger female face (mean 31.9 seconds versus 45.8 seconds t = -1.9, p< .05) and had more misses (mean 1.36 misses versus 4.37 misses, t=-2.34, p<.05) in this condition. Figure 1.
Figure 1.
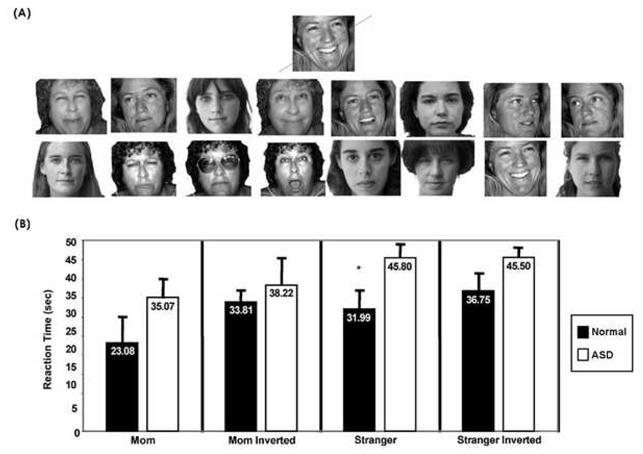
(A). Sample stimuli used during the mother condition of the face processing task for one subject. The target face (mother) is shown on top, followed by other faces. Note that while a total 48 face images were used in each of the 4 test conditions (mother, mother inverted, stranger adult, stranger inverted), only 16 faces are shown for illustration purposes. (B). Mean reaction time from each of the processing tasks for autism and normal groups. Error bars represent SEM. While children with an ASD were slower to identify target faces in all conditions, they were significantly slower in only the stranger adult condition.
Post Scan — Face Identity Task
Following the scan all children were able to identify the familiar faces used during the experiment.
ROI: # of Voxels Active
There were no statistically significant between group differences in the amygdala or anterior cingulate. Statistically significant fusiform and posterior cingulate findings are reported below.
Fusiform
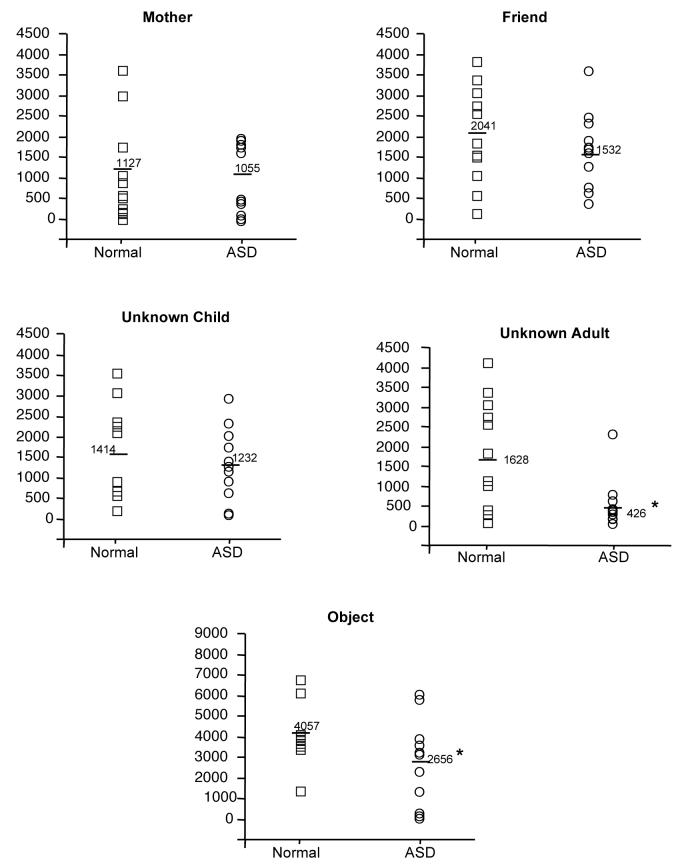
A repeated measures ANOVA for the right fusiform revealed a significant main effect of group [F (1, 20) = 3.158, p<.05], condition [F (4, 80) = 20.979, p<.05] and group x condition interaction [F (4, 80) =2.1, p<.05]. Follow up t-tests showed that only the stranger adult [t (1, 20) = 2.70, p<.05] and object [t (1, 20) = 1.8, p<.05] conditions differed between groups. Figure 2.
Figure 2.
Number of significantly active voxels in the right fusiform in each experimental condition for children with an ASD (circles) and normal control children (squares). Note the significant reduction in the number of active voxels in response to adult stranger faces for children with an ASD in comparison to typically developing children. Results are similar for the left fusiform (data not shown here). Each circle or square represents an individual subject.
A repeated measures ANOVA for the left fusiform revealed a trend effect of group [F (1, 20) = 1.1, p<.15] and a significant main effect of condition [F (4, 80) = 40.6, p<.05]. Because of our a priori interest in the fusiform, follow up t-tests were conducted and showed that only the stranger adult condition [t (1, 20) = 3, p<.05] differed between groups.
Posterior Cingulate
A repeated measures ANOVA revealed a significant condition x group interaction [F (4, 80) =2.55]. Follow up t-tests revealed reduced posterior cingulate activity in response to the faces of familiar (friend) children [t (1, 20) = 2.084].
ROI: % Signal Change
Comparing only voxels that were significantly active for each ROI, there were no % signal change differences between groups in any condition in any ROI, with the exception of the left fusiform in response to the stranger adult condition [mean percent signal change 0.78 normal versus 0.49 ASD; t (1, 20) = 1.9, p<.05]. Figure 3.
Figure 3.
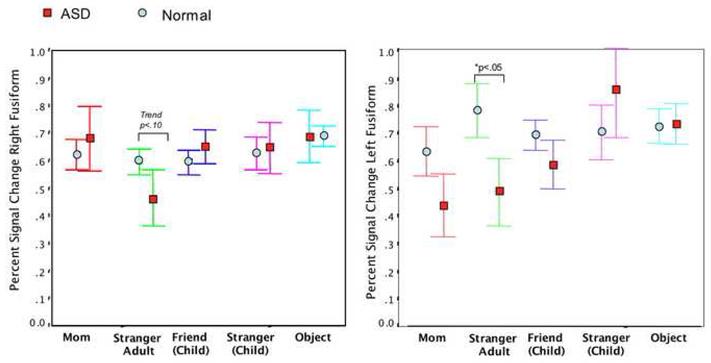
Illustration of the average percent signal change in the right and left fusiform across all experimental conditions. As shown, percent signal change in response to adult stranger faces was significantly reduced in children with an ASD in the left fusiform in comparison to typically developing children. Overall, average percent signal change values were more variable in the left fusiform than the right for children with autism. Error bars represent SEM. Only voxels that were significantly active at p<.01 were included in this analysis.
Whole Brain Analysis
Whole brain functional activity in response to all faces combined as well as familiar and stranger faces separately was examined in each group. After the cluster volume correction, there was significant bilateral fusiform activation in response to all face types in typically developing children, but predominantly right hemisphere activation in children with ASD. Furthermore, there was a weak bilateral fusiform response to stranger faces in children with ASD in comparison to typical children. Figure 4.
Figure 4.
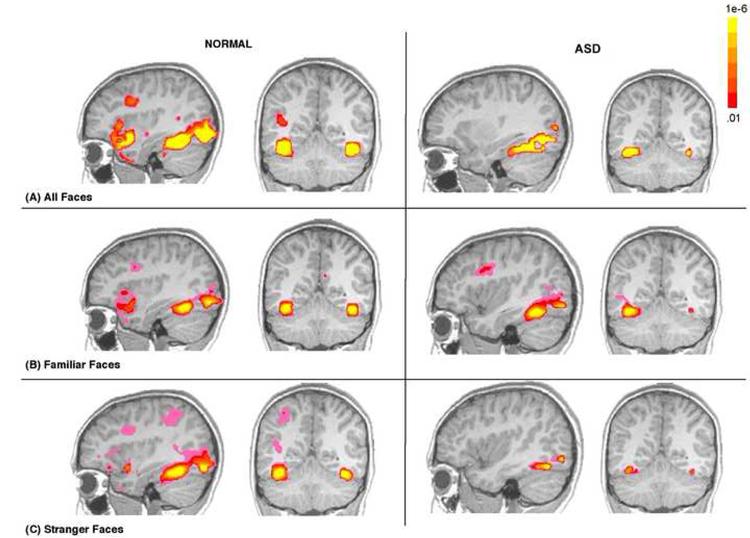
Functional activation maps illustrating the presence of significant functional activity in the fusiform in ASD and normal children in response to: (A) all faces combined (B), familiar faces only and (C) stranger faces only. While this figure highlights defects in fusiform function in response to stranger faces, it also illustrates that the fusiform is capable of functional responding in children with an ASD as depicted by robust functional activity in response to all faces combined and familiar faces. Data are shown at a voxel level of p<.01, overall alpha p<.05, whole brain corrected. The colors used in the functional maps represent p values associated with a t-statistic.
In response to familiar faces, the predicted social network of ROIs (fusiform, amygdala, anterior and posterior cingulate) were significantly active in the normal group. Within this social network, only the fusiform and amygdala were significantly active in the ASD group. Figure 5.
Figure 5.
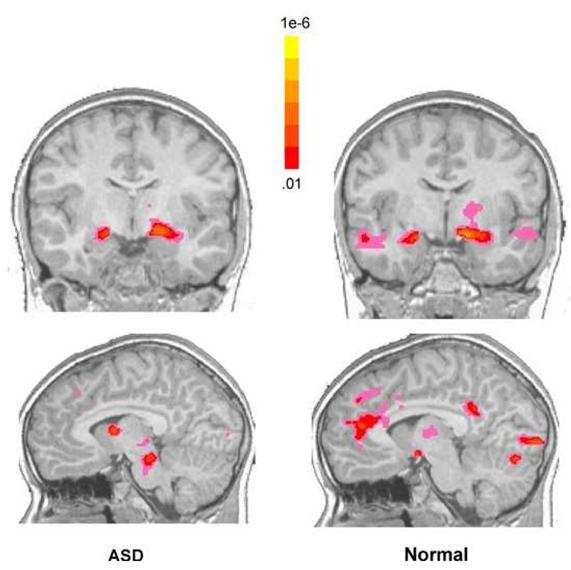
Functional activation maps illustrating the presence of significant functional activity in response to familiar faces for children with an ASD (left) and normal control children (right). While children with an ASD displayed significant amygdala activity in response to familiar faces, there was a reduction in functional activity in midline structures such as anterior and posterior cingulate in comparison to normal. Data are shown at a voxel level of p<.01, overall alpha p<.05, whole brain corrected. The colors used in the functional maps represent p values associated with a t-statistic.
Correlations
Fusiform, Amygdala, and Face Processing Task
No significant correlations between time during the face processing task and # of voxels active or percent signal change in the fusiform were found for either group. A significant relationship was found indicating that a reduced number of voxels in the left amygdala (r= -66, p<.04) and a trend for reduced left amygdala percent signal (r= -53, p<.09) was associated with a slower reaction time to identify stranger faces.
Fusiform and Other ROIs
To further evaluate the selective fusiform abnormality in response to the faces of stranger adults, correlations were performed between the # of active voxels in the right fusiform and the 3 remaining ROIs during this condition. Interestingly, there was a strong positive correlation between right fusiform and amygdala activity (r=.62, p<.05) and right fusiform and right posterior cingulate activity (r=.82, p<.05) in children with ASD, but no significant correlations in response to stranger faces in the normal group.
DISCUSSION
Our study revealed a striking selective deficit in fusiform function in children with an ASD when they viewed only one type of face: the face of an adult stranger. Because fusiform hypoactivity to stranger faces is consistent with the majority of previous research studies on adults with autism (30), we conclude that a selective fusiform abnormality in response to stranger adult faces may well be persistent across ages from middle childhood to adulthood. In the present study, the number of active voxels in response to stranger adult faces was approximately only 25% that of controls in both the right and left fusiform, and percent signal change values were significantly reduced in the left fusiform. In contrast, the fusiform response to other face types such as mother, friend or unknown child were similar between children with an ASD and typical children. Behavioral results echoed the notion of a selective deficit in response to the faces of strangers in that children with an ASD were slower to perform the face task and made more errors when the face presented was an adult stranger. In contrast, reaction time and accuracy in response to mother’s face were not statistically different from normal. As such our findings provide direct evidence of what has been clinically obvious in autism for decades: individuals with this disorder have considerable abnormality, both on the behavioral as well as neurological level, in response to strangers (31-33).
While the sample size was relatively modest and thus results should be interpreted with caution, the specificity of these findings raises an important question: What are the neurofunctional mechanisms that could be responsible for such a selective deficit in the fusiform in response to adult stranger faces?
While there are many possibilities to account for this finding, abnormal signaling from interconnected and face-relevant structures such as the amygdala may play a role (34-38). Connectivity between the two structures has been demonstrated in both humans (40) and non-human primates (39). Feedback loops between the fusiform and amygdala have been hypothesized to play a role in evaluating emotion in faces, particularly those that appear threatening (41). Exaggerated amygdala activation has been reported in response to emotional human faces in a range of social anxiety disorders (42-44). Children with autism often display anxiety, and a recent study found a positive correlation between amygdala volume and symptoms of anxiety in children with the disorder (45). While it may be plausible to speculate that the adult stranger faces shown in this experiment induced anxiety for children (and perhaps they did), hyperactivity of the amygdala was not observed. Instead, volumes of functional activity in the amygdala as well as percent signal change did not differ between groups. However, there was a significant positive correlation between the number of active voxels in the fusiform and amygdala during the stranger adult condition. Thus, those who showed a weak or absent fusiform activity in response to stranger faces also showed a weak or absent amygdala response. Furthermore, there was a trend showing that those children who were slowest to identify stranger faces were also those who showed the smallest percent signal change in the amygdala. Taken together, results suggest amygdala involvement in the abnormal fusiform response to adult strangers.
Another possibility is that enhanced attention or motivation to attend to the mother and child faces selectively influenced fusiform activity particular to these conditions. Conversely, reduced attention during the stranger adult condition, particularly to the eye region of the face, may have directly influenced fusiform responding. Although the present study did not use an eye tracker, enhanced face scanning particularly in the eye region is has been shown to correlate with fusiform activity in autism (4). To date, six studies have reported normal levels of fusiform activity in adolescents and adults with autism and all studies contained a feature that may have been particularly attention enhancing. The Pierce study (8) and Kleinhans study (15) used personally meaningful faces such as mother. Hadjikahni and colleagues (10, 16) and Bird and colleagues (12) directed attention to the eye region of the face by the use of a red dot placed between the eyes, and Wang and colleagues (9) instructed subjects to label the face. Consistent with ours and others’ previous hypotheses (4, 8, 10, 12) the present findings suggest abnormality in systems that modulate fusiform activity, rather than a defect in the fusiform per se.
The only other condition that showed reduced fusiform activity in children with autism was in response to common objects such as a hat or cup. While children with autism are often preoccupied with objects, it is usually only those of unique interest to a specific child (e.g., maps). Indeed, children with autism do not show an interest in novel objects and often display reduced exploration of their environment (46). A reduction in fusiform activity in the object condition further suggests that reduced attention and interest may be responsible when findings of hypoactivity of the fusiform are observed.
While the present study found no abnormalities in the fusiform in response to familiar faces in children with an ASD, it did reveal a general failure to recruit an extensive network in midline structures during the viewing of these personally meaningful faces. Whole brain analyses showed a reduction in both anterior and posterior cingulate cortex activity in children with an ASD, while ROI analyses showed a reduction in posterior cingulate when children with autism looked at the faces of their friends. Although trends were found, a failure to detect statistically significant between group differences in the anterior cingulate via the ROI analyses may have been due the relatively small final sample size used in this study.
The anterior and posterior cingulate are part of a newly defined system know as the “default network” which consists of brain areas that are involved during internally focused tasks such as autobiographical memory and perceiving the mental states of others (47, 48). A negative correlation between activity in the fusiform and posterior cingulate in a face matching study by Bokde (2006) been interpreted as a failure of particular control tasks to attenuate the default network (49). While the default network is presumably always “on” observed as deactivation during rest, Buckner and colleagues (2008) point out that the default network is observed as positive activation during tasks of autobiographical memory retrieval, theory of mind and the like. Theoretical discussions of the default network suggest that the development of this system may lie at the core of human ability to engage in socially complex interactions (47) and may not be fully mature until after the childhood period (50). Consistent with cingulate abnormalities detected in this study, abnormalities in the default network have recently been identified during rest in autism, suggesting a possible neural basis for observed abnormalities in introspective and social processing in the disorder (51).
Although face processing is right hemisphere dominant, the cortex responds to faces bilaterally (52). Until recently, the role of the left fusiform in face processing in autism has not been highlighted. Bird and colleagues (12) showed that attention did not modulate fusiform activity in the left hemisphere in subjects with autism. Additionally, Webb and colleagues (22) found a slower ERP response to faces in the left hemisphere in children with autism but no latency differences from controls in the right hemisphere. In the present study, percent signal change values were considerably lower in children with autism in the left hemisphere in three of the four face conditions, although statistical significance was only reached in the adult stranger condition. Whole brain analyses also revealed weak left fusiform activity in the children with autism in all conditions. In normal development many functions that show hemispheric dominance in adulthood exhibit a more bilateral and distributed pattern during childhood. The failure of children with autism to show strong patterns of bilateral fusiform activity raises the possibility that abnormal interhemispheric communication early in development may contribute to atypical patterns of functional activity, particularly between brain regions that are involved in continued processing of face stimuli. Defects in white matter are a consistent finding in autism (53, 54) including a thinning of the posterior region of the corpus callosum (55). Several research groups have theorized that autism is a disorder that results in increased local, but reduced long distance connectivity (17, 56-59).
Although precursors to the adult face processing system have been observed as early as 3 months in normal infants (60) a fully mature system may not be present until late childhood or preadolescence (61-63). For example, young children often do not show a bias for faces over objects within the classical fusiform face region (62-64). This less specialized response in typical children may allow for experience to play a greater role in the neural substrate underlying face processing in adulthood (64). Reduced experience with faces during the course of development in autism may also be a contributing factor as to why patterns of functional activity in the fusiform were inconsistent (e.g., stronger in response to some face types) and not fully elaborated as evidenced by a reduced extended network. Future pediatric imaging studies that utilize functional connectivity analyses will be pivotal for understanding such system development.
What makes interactions with strangers particularly challenging for individuals with autism remains a mystery. Here we show that not only do children with autism have defects at the neurofunctional level in response to adult stranger faces in the fusiform, but that this very same structure is capable of responding to more preferred faces such as mother or other children. As such, it eliminates the fusiform as the primary site of face-processing defect in autism, and instead suggests dysfunction in systems that modulate fusiform activity.
Supplementary Material
Acknowledgements
We would like to acknowledge the dedication of all of the families that participated in this research. A special thanks to Eric Courchesne for his helpful comments on the manuscript. This research was funded by NIMH grant K01 MH01814 awarded to Karen Pierce.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Biomedical Financial Interests and Potential Conflict of Interest
Dr. Pierce and Ms. Redcay reported no biomedical financial interest or potential conflict of interest.
References
- 1.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome [see comments] Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 2.Critchley HD, Daly EM, Bullmore ET, Williams SCR, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behavior: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- 3.Pierce K, Müller R-A, Ambrose J, Allen G, Courchesne E. People with autism process faces outside the "fusiform face area." : Evidence from fMRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 4.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- 6.Ogai M, Matsumoto H, Suzuki K, Ozawa F, Fukuda R, Uchiyama I, et al. fMRI study of recognition of facial expressions in high-functioning autistic patients. Neuroreport. 2003;14:559–563. doi: 10.1097/00001756-200303240-00006. [DOI] [PubMed] [Google Scholar]
- 7.Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43:473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 9.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 12.Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Bolte S, Hubl D, Feineis-Matthews S, Prvulovic D, Dierks T, Poustka F. Facial affect recognition training in autism: can we animate the fusiform gyrus? Behav Neurosci. 2006;120:211–216. doi: 10.1037/0735-7044.120.1.211. [DOI] [PubMed] [Google Scholar]
- 15.Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008 doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 16.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- 22.Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. J Autism Dev Disord. 2006;36:881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kylliainen A, Braeutigam S, Hietanen JK, Swithenby SJ, Bailey AJ. Face- and gaze-sensitive neural responses in children with autism: a magnetoencephalographic study. Eur J Neurosci. 2006;24:2679–2690. doi: 10.1111/j.1460-9568.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanefuji W, Ohgami H, Hashiya K. Preference for peers in infancy. Infant Behav Dev. 2006;29:584–593. doi: 10.1016/j.infbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bahrick LE, Netto D, Hernandez-Reif M. Intermodal perception of adult and child faces and voices by infants. Child Dev. 1998;69:1263–1275. [PubMed] [Google Scholar]
- 26.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 27.Tong F, Nakayama K. Robust representations for faces: evidence from visual search. J Exp Psychol Hum Percept Perform. 1999;25:1016–1035. doi: 10.1037//0096-1523.25.4.1016. [DOI] [PubMed] [Google Scholar]
- 28.Young AW, McWeeny KH, Hay DC, Ellis AW. Matching familiar and unfamiliar faces on identity and expression. Psychol Res. 1986;48:63–68. doi: 10.1007/BF00309318. [DOI] [PubMed] [Google Scholar]
- 29.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams E, Costall A, Reddy V. Children with autism experience problems with both objects and people. J Autism Dev Disord. 1999;29:367–378. doi: 10.1023/a:1023026810619. [DOI] [PubMed] [Google Scholar]
- 32.Corona R, Dissanayake C, Arbelle S, Wellington P, Sigman M. Is affect aversive to young children with autism? Behavioral and cardiac responses to experimenter distress. Child Dev. 1998;69:1494–1502. [PubMed] [Google Scholar]
- 33.Macintosh K, Dissanayake C. A comparative study of the spontaneous social interactions of children with high-functioning autism and children with Asperger’s disorder. Autism. 2006;10:199–220. doi: 10.1177/1362361306062026. [DOI] [PubMed] [Google Scholar]
- 34.Schulkin J. Autism and the amygdala: An endocrine hypothesis. Brain Cogn. 2007 doi: 10.1016/j.bandc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 37.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 39.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comp Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 40.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 41.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 42.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 43.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 45.Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: magnetic resonance imaging (MRI) study in autistic children. J Child Neurol. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- 46.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 48.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- 50.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbeau EJ, Taylor MJ, Regis J, Marquis P, Chauvel P, Liegeois-Chauvel C. Spatio temporal Dynamics of Face Recognition. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm140. [DOI] [PubMed] [Google Scholar]
- 53.Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 54.Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 55.Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Archives of Neurology. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- 56.Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- 58.Just M. International Meeting for Autism Research. Sacramento; California: 2004. Cortical underconnectivity in high-functioning autism: Brain activation and brain synchronization in cognitive tasks. [Google Scholar]
- 59.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. J Cogn Neurosci. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- 61.Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gathers AD, Bhatt R, Corbly CR, Farley AB, Joseph JE. Developmental shifts in cortical loci for face and object recognition. Neuroreport. 2004;15:1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, et al. Brain activation during face perception: evidence of a developmental change. J Cogn Neurosci. 2005;17:308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- 64.Passarotti AM, Smith J, DeLano M, Huang J. Developmental differences in the neural bases of the face inversion effect show progressive tuning of face-selective regions to the upright orientation. Neuroimage. 2007;34:1708–1722. doi: 10.1016/j.neuroimage.2006.07.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.