Abstract
Dextran, a biocompatible, water-soluble polysaccharide, was modified at its hydroxyls with acetal moieties such that it became insoluble in water but freely soluble in common organic solvents enabling its use in the facile preparation of acid-sensitive microparticles. These particles degrade in a pH-dependent manner: FITC-dextran was released with a half-life at 37 ºC of 10 hours at pH 5.0 compared to a half-life of approximately 15 days at pH 7.4. Both hydrophobic and hydrophilic cargoes were successfully loaded into these particles using single and double emulsion techniques, respectively. When used in a model vaccine application, particles loaded with the protein ovalbumin (OVA) increased the presentation of OVA-derived peptides to CD8+ T-cells 16-fold relative to OVA alone. Additionally, this dextran derivative was found to be non-toxic in preliminary in vitro cytotoxicity assays. Due to its ease of preparation, processability, pH-sensitivity, and biocompatibility, this type of modified dextran should find use in numerous drug delivery applications.
Polyesters,1 polyorthoesters,2 and polyanhydrides3 are widely used materials for biomedical applications due to their biodegradability, biocompatibility and processability. Microparticles made from these polymers have been used as carriers for vaccine applications,4 gene delivery5 and chemotherapeutic agents.6 The encapsulated cargo is typically released over the course of several months via surface erosion and the slow degradation of the polymer.7
For many drug delivery applications, it is desirable to release therapeutic agents under mildly acidic conditions, as may be found in sites of inflammation, lysosomal compartments, or in tumor tissue.8 Acid-sensitive liposomes, micelles and hydrogels9 have been extensively explored, but few easily-prepared polymeric materials exist that combine acid-sensitivity and biodegradability. Poly(β-amino esters), which are protonated and thus become soluble at lower pH,10 constitute one such material. However, these polymers become polycationic under acidic conditions and must be blended with biocompatible polyesters to reduce their toxicity.11 We sought to create a system with the flexibility and biocompatibility of polyester materials, but with the additional benefit of a change in rate of payload release sensitive to physiologically relevant acidic conditions. Therefore, a solubility switching mechanism was envisioned in which a biocompatible, water-soluble polymer could be reversibly modified to make it insoluble in water, but soluble in organic solvents. Materials made from the modified polymer could then be degraded under the specific conditions that reverse the original modification. Dextran, a bacterially derived homopolysaccharide of glucose, was chosen because of its biocompatibility, biodegradability, wide availability, and ease of modification.12 Acetals were chosen to modify dextran due to their well understood and tunable pH-dependant hydrolysis rates.13
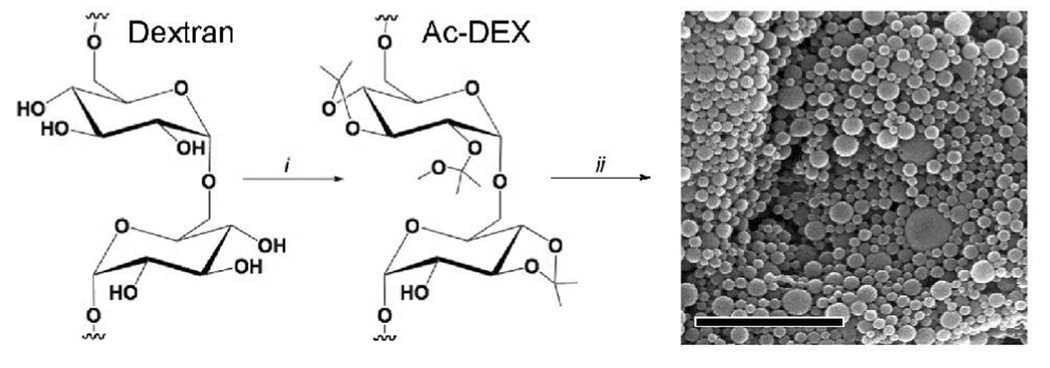
Dextran was rendered insoluble in water by modification of its hydroxyl groups through reaction with 2-methoxypropene under acid catalysis (Figure 1). The high density of pendant acetals makes the new “acetalated-dextran” (Ac-DEX) soluble in organic solvents such as dichloromethane, ethyl acetate or acetone. Based on multi-angle light scattering data, the molecular weight of the dextran increases upon modification from 13 kDa to 29 kDa while its polydispersity remains essentially constant (1.13 to 1.20), suggesting a high degree of coverage of the hydroxyl groups and minimal polymer crosslinking. Using a standard double emulsion protocol, a model hydrophilic payload, ovalbumin (OVA), was encapsulated with a protein loading of 3.7 ± 0.4 wt % (Figure 1). Using a single emulsion technique, we were able to encapsulate a model hydrophobic drug, pyrene, with a loading of 3.6 ± 0.5 wt %. The particles were imaged using scanning electron microscopy (Figure S1) and particle size was analyzed using dynamic light scattering. The double emulsion particles were found to have an average diameter of 230 ± 93 nm (Figure S2) and the single emulsion particles had similar shapes and sizes with an average diameter of 258 ± 70 nm.
Figure 1.
Synthesis of Ac-DEX and particle formation (i) 2-methoxypropene, pyridinium-p-toluenesulfonate, DMSO (ii) solvent-evaporation-based particle formation (scale bar is 2 µm).
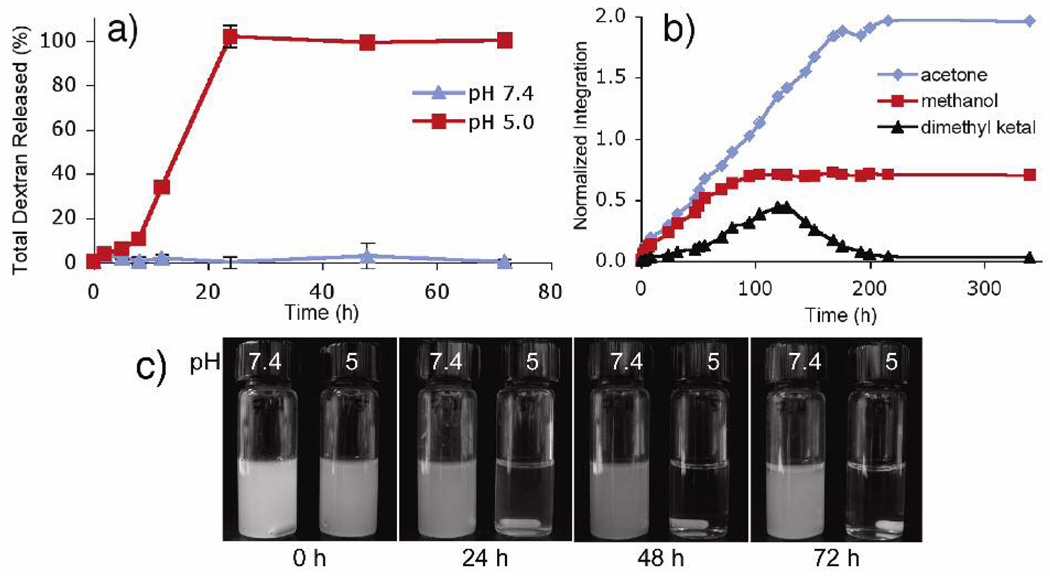
Masking the hydroxyl groups of dextran as acetals not only provides a hydrophobic material that is easily processable using various emulsion techniques, it also provides a mechanism for introducing pH-sensitivity. Under mildly acidic aqueous conditions, the pendant acetal groups are expected to hydrolyze, thus unmasking the parent hydroxyl groups of dextran. The complete hydrolysis of Ac-DEX should result in the release of acetone, methanol and water-soluble dextran. To study the degradation of Ac-DEX, empty particles were prepared and incubated under physiological (pH 7.4) or mildly acidic conditions (pH 5.0) at 37 °C. The supernatant was analyzed at various times for the presence of reducing polysaccharides using a bicinchoninic acid based assay.14 Ac-DEX particles incubated in pH 7.4 buffer remained as an opaque suspension for days and essentially no soluble dextran was detected after 72 hours (Figure 2a,c). In contrast, suspensions of Ac-DEX particles in pH 5.0 buffer showed continuous release of soluble reducing polysaccharides, becoming transparent after 24 hours, thus suggesting full dissolution of the particles. This pH-dependent degradation of Ac-DEX particles is further reflected in the release profile of a model fluorescently labeled hydrophilic payload (Figure S3). In this experiment fluorescein isothiocyanate (FITC) labeled dextran was released from Ac-DEX particles much faster under acidic conditions than in pH 7.4 buffer. Specifically, the half-life of the release of FITC-dextran at 37 °C and pH 5.0 was about 10 hours compared to approximately 15 days at pH 7.4. These release rates may be faster than bulk particle dissolution due to potential leaching of hydrophilic cargo.
Figure 2.
(a) Dissolution of dextran from Ac-DEX particles in either pH 5 or pH 7.4 buffer at 37 °C. (b) Normalized 1H-NMR data from the degradation of Ac-DEX particles at pH 5.5 and 37 °C showing integrations of signals corresponding to acetone, methanol and acetal groups. (c) Time-lapse photos of Ac-DEX particles under physiological or acidic conditions.
The degradation of empty Ac-DEX particles was also followed using 1H-NMR. A suspension of particles was incubated at 37 °C in deuterated PBS (pH 5.5) in a flame-sealed NMR tube. The release of acetone and methanol due to acetal hydrolysis was observed and the normalized integrals of these compounds were plotted as a function of time (Figure 2b and Figure S4). The particles first released a roughly equivalent amount of acetone and methanol, which is consistent with the rapid hydrolysis rate of pendant acyclic acetals.13 Following this phase, acetone, but not methanol continued to be released from the degrading particles. This second phase is presumably due to the slower hydrolysis rate of cyclic isopropylidene acetals, signals from which appear, then subsequently disappear as the acetals are hydrolyzed.15 Following complete hydrolysis, the 1H-NMR spectrum of the degraded particles showed signals corresponding only to unmodified dextran, acetone and methanol (Figure S5). Based on this final spectrum, it was calculated that 73% of the available hydroxyl groups were modified and the ratio of cyclic to acyclic acetals was estimated at 1.8:1.16
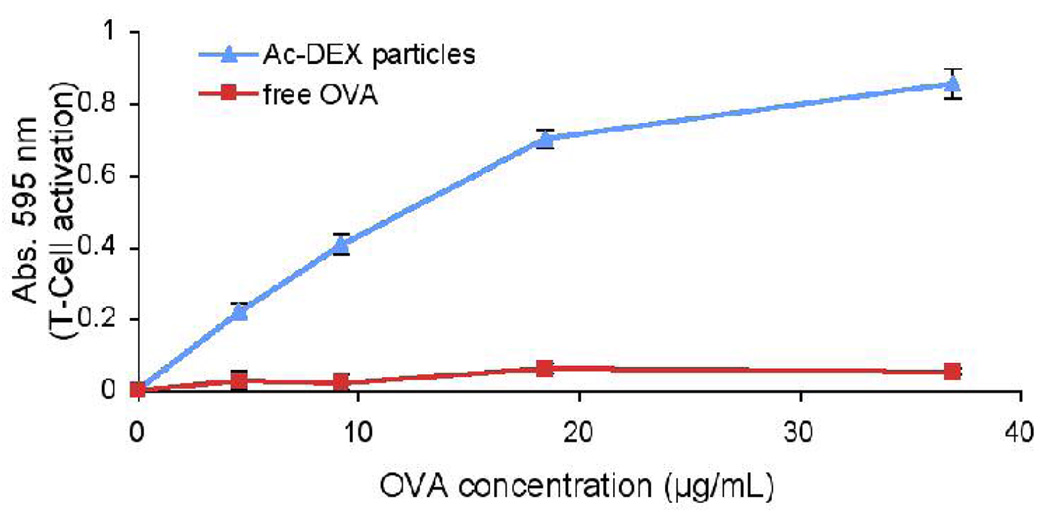
We have previously shown that acid-labile polyacrylamide particles enhance protein-based vaccine efficacy in cancer treatment by enhancing MHC class I presentation and CD8+ T cell activation.17 However, because the particles are prepared from acrylamide, toxicity and biocompatibility issues might limit their future clinical applications. Ac-DEX based particles are expected to be more biocompatible than our previous system since the byproducts are dextran (a clinically used plasma expander), acetone (a non-toxic, metabolic intermediate) and methanol (non-toxic in small quantities).18,19 To assess the biocompatibility of Ac-DEX particles, we compared them to particles prepared from an FDA approved polymer, poly (lactic-co-glycolic acid). We found no significant difference in toxicity between the two materials in both HeLa cells and RAW macrophages. To simulate their long-term toxicity, the degradation products of Ac-DEX particles were also tested and found to be non-toxic (Figure S6). In order to assess the feasibility of using Ac-DEX based materials for vaccine applications, OVA-loaded Ac-DEX particles were incubated with RAW macrophages. After six hours of incubation, Ac-DEX particles increased MHC class I presentation of the OVA-derived CD8+ T-cell epitope, SIINFEKL, by a factor of 16 relative to free OVA (Figure 3) as measured by the B3Z assay.20 This drastic increase in presentation indicates that these particles may be promising materials for vaccines against tumors and certain viruses, where MHC-I presentation is crucial for the activation and proliferation of CD8+ T-cells.
Figure 3.
B3Z assay measuring antigen presentation of RAW macrophages pulsed with free OVA or Ac-DEX particles encapsulating OVA.
In conclusion, we present a new method for the preparation of acid-sensitive, biocompatible dextran-based materials. Ac-DEX is synthesized in a single step from a natural polymer, possesses a favorable toxicity profile, and can be processed into materials encapsulating either hydrophobic or hydrophilic payloads. Due to these favorable attributes, Ac-DEX based materials may have significant advantages over other pH-sensitive or biocompatible materials currently used in biomedical applications. We are currently investigating the functionalization and use of these and other modified polysaccharides in vaccine and chemotherapeutic settings. In addition, we believe Ac-DEX has the potential to be used as scaffolds, sutures, and other bulk materials in vivo due to its physical properties, biodegradability, and biocompatibility.
Supplementary Material
Figures S1–S6, experimental methods, general procedures and materials. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank the National Institutes of Health (grant RO1 EB005824) for funding of this research and Ann Fisher in the UC Berkeley Cell Culture Facility for her expertise and help.
References
- 1.Yolles S, Leafe TD, Meyer FJ. J. Pharm. Sci. 1975;64:115–116. doi: 10.1002/jps.2600640124. [DOI] [PubMed] [Google Scholar]
- 2.Heller J. Ann. N. Y. Acad. Sci. 1985;446:51–66. doi: 10.1111/j.1749-6632.1985.tb18390.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen HB, Chang J, Wnek GE, Linhardt RJ, Langer R. Biomaterials. 1983;4:131–133. doi: 10.1016/0142-9612(83)90054-6. [DOI] [PubMed] [Google Scholar]
- 4.Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Mol. Pharm. 2007;4:47–57. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- 5.Gvili K, Benny O, Danino D, Machluf M. Biopolymers. 2007;85:379–391. doi: 10.1002/bip.20697. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, Eavarone D, Capila I, Zhao GL, Watson N, Kiziltepe T, Sasisekharan R. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto A, Matsukawa Y, Suzuki T, Yoshino H. J. Control Release. 2005;106:172–180. doi: 10.1016/j.jconrel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 8.(a) Sun-Wada GH, Wada Y, Futai M. Cell Struct. Funct. 2003;28:455–463. doi: 10.1247/csf.28.455. [DOI] [PubMed] [Google Scholar]; (b) Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Clin. Cancer Res. 2002;8:1284–1291. [PubMed] [Google Scholar]
- 9.(a) Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. Bioconjug Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mandracchia D, Pitarresi G, Palumbo FS, Carlisi B, Giammona G. Biomacromolecules. 2004;5:1973–1982. doi: 10.1021/bm0497567. [DOI] [PubMed] [Google Scholar]; (c) Murthy N, Thng YX, Schuck S, Xu MC, Fréchet JMJ. J. Am. Chem. Soc. 2002;124:12398–12399. doi: 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]
- 10.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Proc. Natl. Acad. Sci. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little SR, Lynn DM, Puram SV, Langer R. J. Control Release. 2005;107:449–462. doi: 10.1016/j.jconrel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 12.(a) Hermanson GT. Bioconjugate Techniques. San Diego: Academic Press; 1996. [Google Scholar]; (b) Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ. J. Chem. Technol. Biotechnol. 2005;80:845–860. [Google Scholar]
- 13.(a) Fife TH, Jao LK. J. Org. Chem. 1965;30:1492. [Google Scholar]; (b) Gillies ER, Goodwin AP, Fréchet JMJ. Bioconj. Chem. 2004;15:1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 14.Doner LW, Irwin PL. Anal. Biochem. 1992;202:50–53. doi: 10.1016/0003-2697(92)90204-k. [DOI] [PubMed] [Google Scholar]
- 15.(a) Cai JQ, Davison BE, Ganellin CR, Thaisrivongs S. Tetrahedron Lett. 1995;36:6535–6536. [Google Scholar]; (b) Debost JL, Gelas J, Horton D, Mols O. Carbohydr. Res. 1984;125:329–335. [Google Scholar]
- 16.These values were calculated using the integration of the acetone and methanol signals compared to the integration of the anomeric proton.
- 17.(a) Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Fréchet JM. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Fréchet JMJ. Bioconjugate Chem. 2004;15:1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 18.Paine A, Davan AD. Hum. Exp. Toxico. 2001;20:563–568. doi: 10.1191/096032701718620864. [DOI] [PubMed] [Google Scholar]
- 19.Although toxic in large quantities, the level of methanol released by Ac-DEX materials (~7 wt. %) would be below the EPA recommended limit of daily exposure as long as the dosage does not exceed 7 g/kg/day. If complete elimination of methanol becomes necessary, 2-ethoxypropene instead of 2-methoxypropene could be used.
- 20.Karttunen J, Shastri N. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S6, experimental methods, general procedures and materials. This material is available free of charge via the Internet at http://pubs.acs.org.