Abstract
Untimely rupture of the fetal membranes (FMs) is a major precipitant of preterm birth. Although the mechanism of FM weakening leading to rupture is not completely understood, proinflammatory cytokines, including tumor necrosis factor (TNF) and interleukin 1 beta (IL1B), have been shown to weaken FMs concomitant with the induction of reactive oxygen species, collagen remodeling, and prostaglandin release. We hypothesized that alpha-lipoic acid, a dietary antioxidant, may block the effect of inflammatory mediators and thereby inhibit FM weakening. Full-thickness FM fragments were incubated with control media or TNF, with or without alpha-lipoic acid pretreatment. Fetal membrane rupture strength and the release of matrix metalloproteinase 9 (MMP9) and prostaglandin E2 (PGE2) from the full-thickness FM fragments were determined. The two constituent cell populations in amnion, the mechanically strongest FM component, were similarly examined. Amnion epithelial and mesenchymal cells were treated with TNF or IL1B, with or without alpha-lipoic acid pretreatment. MMP9 and PGE2 were analyzed by ELISA, Western blot, and zymography. TNF decreased FM rupture strength 50% while increasing MMP9 and PGE2 release. Lipoic acid inhibited these TNF-induced effects. Lipoic acid pretreatment also inhibited TNF- and IL1B-induced increases in MMP9 protein activity and release in amnion epithelial cells, as well as PGE2 increases in both amnion epithelial and mesenchymal cells. In summary, lipoic acid pretreatment inhibited TNF-induced weakening of FM and cytokine-induced MMP9 and PGE2 in both intact FM and amnion cells. We speculate that dietary supplementation with alpha-lipoic acid might prove clinically useful in prevention of preterm premature rupture of fetal membranes.
Keywords: amnion, fetal membrane rupture, fetal membranes, lipoic acid, matrix metalloproteinase, MMP9, parturition, PGE2, placenta
Physiologically relevant concentrations of alpha-lipoic acid inhibit TNF-induced weakening and remodeling of full thickness fetal membrane explants.
INTRODUCTION
Preterm premature rupture of the fetal membranes (PPROM) is the initiating event in approximately one third of preterm births, resulting in significant infant mortality and morbidity [1]. The exact mechanisms by which fetal membranes (FMs) weaken and rupture in term and preterm gestations are unknown. Preventative measures for ill-timed or aberrant FM rupture are thus unavailable.
Fetal membrane rupture was previously thought to be caused entirely by tearing due to stresses resulting from the contractions of labor. This is clearly inconsistent with reports that 10% of term and nearly one third of preterm births are preceded by rupture of membranes prior to the initiation of labor [2]. In addition, it has recently been shown that cyclical stretching paradoxically strengthens FM by a strain hardening process [3]. Fetal membranes are now hypothesized to weaken and ultimately rupture as a result of collagen remodeling and apoptosis [4, 5]. In previous reports we described a localized region of the FM overlying the cervix that is mechanically weak relative to other regions of the FM, and exhibits biochemical and histological characteristics suggestive of increased collagen remodeling. This paracervical weak zone is present in the FM of both vaginally delivered infants and infants delivered by cesarean delivery with no labor [6, 7]. Other investigators have also reported distinctive biochemical and histological differences suggestive of increased tissue remodeling in the paracervical region of the FM. Specifically, numerous reports have described increased matrix metalloproteinase 9 (MMP9), increased apoptosis, and differences in the cellular density of the FM component membranes (amnion and choriodecidua) in the paracervical region [8–10]. Because the rupture tear line generally passes through the remodeled, paracervical weak zone, we have presumed that FM rupture likely initiates within this region [4].
Generation of reactive oxygen species (ROS) has been strongly associated with tissue remodeling, particularly with inflammatory processes initiated by tumor necrosis factor (TNF) and interleukin 1 beta (IL1B) [11–14]. Furthermore, ROS have been associated with the induction of MMP9 and prostaglandins [15]. Antioxidants have therefore been proposed as potential inhibitors of premature FM remodeling and preterm rupture. Vitamin C has been the initial prime candidate used in a number of in vitro studies and some preliminary clinical trials, but with only limited success [16, 17]. Concerns have also been raised by some investigators about the possible adverse effects of vitamin C [18, 19]. We have reported that vitamin C can increase apoptosis in intact amnion, amnion cells, and amnion-derived WISH cells, and thus may exacerbate the weakening process [20, 21].
Alpha-lipoic acid (6,8-dithio-octanoic acid; LA), another antioxidant, was initially isolated from bovine liver in 1950 [22]. Alpha-lipoic acid is found in the human diet and is also available as a dietary supplement in its natural R-form or racemic R/S mixture. It is rapidly absorbed and reduced intracellularly by NAD(P)H-dependent enzymes to the dithiol compound dihydrolipoic acid (DHLA) [23]. In addition to playing an important role in mitochondrial energy metabolism, the LA/DHLA system can scavenge ROS, regenerate physiological antioxidants (vitamins C/E and glutathione), chelate metal ions, and stimulate insulin signaling [24–27]. For these reasons, LA is also a candidate inhibitor of cytokine-mediated remodeling and weakening of human FM.
Recently, we reported that FM weakening, concomitant with changes in biochemical markers of remodeling mimicking the natural weak zone at term, can be reproduced in vitro with either TNF or IL1B [28]. This finding made available an in vitro model system for the investigation of the FM weakening process. The initial objective of this study was to use this in vitro model system to determine whether the antioxidant LA could prevent TNF-induced weakening and concomitant MMP9 and prostaglandin E2 (PGE2) release from intact FM. PGE2 release was measured in addition to MMP9 because it has been shown to regulate MMP9 [29, 30]. After this proved successful, a second objective was added: initiation of the investigation of the cellular basis of both cytokine-induced FM weakening and the LA inhibition of this weakening process. Because the amnion is the strongest component of the fetal membranes [31], we elected to focus initially upon its two constituent cell populations, amnion epithelial and mesenchymal cells.
MATERIALS AND METHODS
Materials
Reagents were from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise.
Biological Samples
The study protocol was approved by the Institutional Review Board of the MetroHealth Medical Center, Case Western Reserve University (Cleveland, OH). Fetal membranes were collected from uncomplicated patients undergoing prelabor, elective, repeat cesarean delivery at term (all were 37–39 wk) following written informed consent. Membranes were discarded if they were meconium stained, if infection was suspected from the clinical history, or if chorioamnionitis was detected in pathological review. Eleven FMs were used for all of the studies reported here. Fetal membranes were cut away from the placental disc and washed extensively in cold PBS. Regions within 1 cm of the placental disc and near the paracervical weak zone were not used.
FM Explant Culture
Fetal membrane fragments were cultured per previously described protocols [28, 32]. Fetal membranes from five patients were used for experiments on full-thickness, intact FMs. One patient FM was used for each experiment. In each experiment, three FM fragments were used for each of the four treatment groups (12 fragments per experiment). Fetal membrane fragments were bluntly dissected (8 × 8 cm) and placed in 150-mm2 culture dishes containing 20 ml of Earle minimum essential medium, alpha modification (MEM), antibiotic antimycotic solution, 50 μg/ml gentamicin sulfate, and 0.2% lactalbumin hydrosylate (EMEML). Culture dishes were rocked gently in an atmosphere containing 5% CO2, air and 100% relative humidity at 37°C. After 24 h of equilibration in EMEML, medium was removed and replaced. Cultures were pretreated with 0.1% DMSO (vehicle) or 0.5 mM LA for 6 h, and then with or without addition of 50 ng/ml TNF for an additional 72 h. Four study conditions were used: control, LA pretreatment alone, TNF alone, and LA pretreatment followed by TNF. Following incubation, FM samples were subjected to biomechanical testing as outlined below. Medium from each culture was clarified by centrifugation (15 000 × g for 15 min), frozen at −70°C, and then thawed later and assayed for the MMP9 and PGE2 release.
FM Biomechanical Testing
Treated FM fragments were removed from culture, washed twice in 20 ml of MEM, maintained in MEM, and kept moist for the entirety of biomechanical testing. Fetal membrane physical properties were determined using our previously reported methodology [6]. Briefly, biomechanical testing was performed using modified industrial rupture testing equipment (Com-Ten Industries, St Petersburg, FL) by the American Society for Testing and Materials standards. A mechanically driven, 1-cm diameter, rounded plunger was forced at a speed of 8.4 cm/min through membrane pieces supported on a fixture with a 2.5-cm diameter orifice. Force (applied to the FM) and resultant FM displacement data were collected continuously and analyzed by data reduction software. Rupture strength was determined from generated force/displacement curves.
Amnion Cell Culture Studies
Reflected amnion was peeled from choriodecidua, washed three times in cold PBS, and minced. Amnion epithelial and mesenchymal cells were isolated by sequential digestion with trypsin and collagenase as previously reported [20, 33, 34].
Amnion epithelial cell isolation.
Amnion fragments were incubated in 100 ml of TMEM (MEM/antibiotic-antimycotic/10 mM Hepes, pH 7.2, containing 0.2% trypsin [T8128; Sigma]) for 10 min at 37°C with intermittent agitation. After straining through 4-mm2 stainless mesh, the initial eluate was discarded.
Fragments were then incubated with 150 ml of TMEM for 30 min at 37°C and strained, and the eluate was maintained on ice. Digestion with TMEM was repeated three times. Following digestion, fragments were washed in 500 ml of cold PBS and strained, and this eluate was pooled along with the previous digests.
Epithelial cells were pelleted by centrifugation at 300 × g for 8 min at 10°C, resuspended in MEM containing 10% fetal bovine serum, antibiotic-antimycotic, and 50 μg/ml gentamycin sulfate (EMEMC), and plated in 150-mm2 tissue culture dishes containing 30 ml of EMEMC. Dishes were incubated at 37°C, 5% CO2 with 48-h medium changes until confluent (5–7 days).
Amnion mesenchymal cell isolation.
Following trypsinization and washing, de-epithelialized amnion fragments were incubated in 100 ml of MEM/0.1% collagenase (C2139; Sigma), 25 mg of DNAase, and 10 mM Hepes, pH 7.2, for 30 min at 37°C, strained through 1-mm2 stainless mesh, and the eluate was centrifuged 400 × g for 10 min at 10°C. Pelleted mesenchymal cells were plated in EMEMC on 6 × 150 mm2 tissue culture dishes for 6 h, and then medium was removed and replaced.
Upon confluency (5–7 days), epithelial and mesenchymal cells were trypsinized (0.25% trypsin/0.02% EDTA/PBS) and plated on 24-well plates in EMEMC. At 90%–95% confluency (48–72 h), medium was replaced with antibiotic-antimycotic-free EMEML for 24 h prior to experimentation. Purity and viability were routinely monitored by cytokeratin/vimentin differential immunohistochemistry and trypan blue exclusion, as described previously [20]. The purity and viability of isolated cell types were greater than 97% and 99%, respectively, following passage 2–3 days prior to experimentation.
Cell culture incubations.
Cells were preincubated in EMEML for 6 h with increasing doses of LA prior to addition of increasing doses of TNF or IL1B. After 24 h of incubation, medium was removed and clarified by centrifugation for 15 min at 15 000 × g at 10°C. Supernatants were stored at −70°C until assay.
Monolayers were washed with 2 ml of cold PBS then lysed by sonication (30 sec on ice, setting 40; Artek Industries) in 200 μl of Zymogram Buffer (ZB; Bio-Rad, Hercules, CA) and frozen at −70°C.
Protein content was determined by Bio-Rad DC Protein Assay.
Immunoassays
Prostaglandin E2 (Cayman Chemical Co., Ann Arbor, MI) and MMP9 (EMD Biosciences, San Diego, CA) levels in spent medium were determined using commercial ELISA kits and following the manufacturers' protocols. Sensitivity measurements were 93 pg/ml and 0.1 ng/ml, and interassay and intra-assay coefficients of variation were 2.1%–4.5% and 2.9%–6.5%, respectively.
Gel Zymography
In-gel zymographic analysis was performed following PAGE using 40-μl aliquots of medium:ZB (1:1) or 40 μg of cell lysate/ZB on 10% gels/Tris-HCl containing gelatin, according to the Bio-Rad protocol. Active, human, recombinant, 66-kDa MMP2 and/or 83-kDa MMP9 (90% purity by SDS-PAGE; EMD Biosciences) were used as relative markers in some zymograms and Western blots (below).
Western Blotting
β-Mercaptoethanol, to 5% v/v, was added to ZB cell lysates (20 μg of protein), and samples were incubated in a boiling waterbath for 5 min. Denatured samples were electrophoresed on 4%–15% gels/Tris-HCl, and resolved proteins were electrophoretically transferred to polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ) according to Bio-Rad protocols. Membranes were blocked in 5% nonfat dry milk/Tris-buffered saline, 0.5% Tween-20 (TBST) for 30 min and then incubated overnight at 10°C with MMP9 antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed three times in TBST, incubated with horseradish peroxidase-conjugated secondary antibody (1:10 000) in 5% nonfat milk/TBST for 30 min, and then again washed three times in TBST.
Chemiluminescent detection was performed using Lumigen-PS according to the manufacturer's protocol (GE Healthcare), and blots were exposed against Super RX film (Fuji, Tokyo, Japan) for equivalent time periods.
Quantitation and Statistical Analysis
Developed films and stained gels were scanned using an Epson Perfection 1200U scanner. Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD). The value for an equivalent scanned blank region within a “dye-only” lane of each blot was subtracted from the value for each lane containing protein. Data points for cumulative Western blots and zymograms represent the mean ± SD for amnion cell lysates obtained from three different patients. Immunoassay data points for PGE2 and MMP9 represent the mean ± SD of medium from cultures treated in triplicate and assayed in duplicate. All experiments were performed at least three times.
All intact FM data (biomechanical, released MMP9, and released PGE2) and cell data (densitometry, released MMP9, and released PGE2) were analyzed by ANOVA followed by posthoc pairwise comparisons using Statview software. Data and results are termed significant when P < 0.05.
RESULTS
Studies Using Full-Thickness FM
Full-thickness FM fragments were incubated with control media, LA (0.5 mM) alone, TNF (50 ng/ml) alone, or both (TNF added after 6 h of pretreatment with LA) as described in Materials and Methods. Tumor necrosis factor decreased the rupture strength of the FM fragments by more than 50% (10.0 ± 3.5 N versus 4.5 ± 1.4 N; P = 0.001), consistent with our previous report [28]. The LA alone had no effect on the basal FM rupture strength, but it inhibited TNF-induced weakening (4.5 ± 1.4 N [TNF] versus 15.3 ± 7.03 N [LA pretreatment and then TNF]; P < 0.0001; Fig. 1A). The TNF increased MMP9 release from intact FM into the medium. The LA alone only modestly inhibited basal MMP9 release but completely abolished the TNF-induced MMP9 increase (Fig. 1B). In a similar fashion, PGE2 release into the medium from intact FM was increased with TNF, and this TNF-induced effect was completely inhibited by LA pretreatment (Fig. 1C).
FIG. 1.
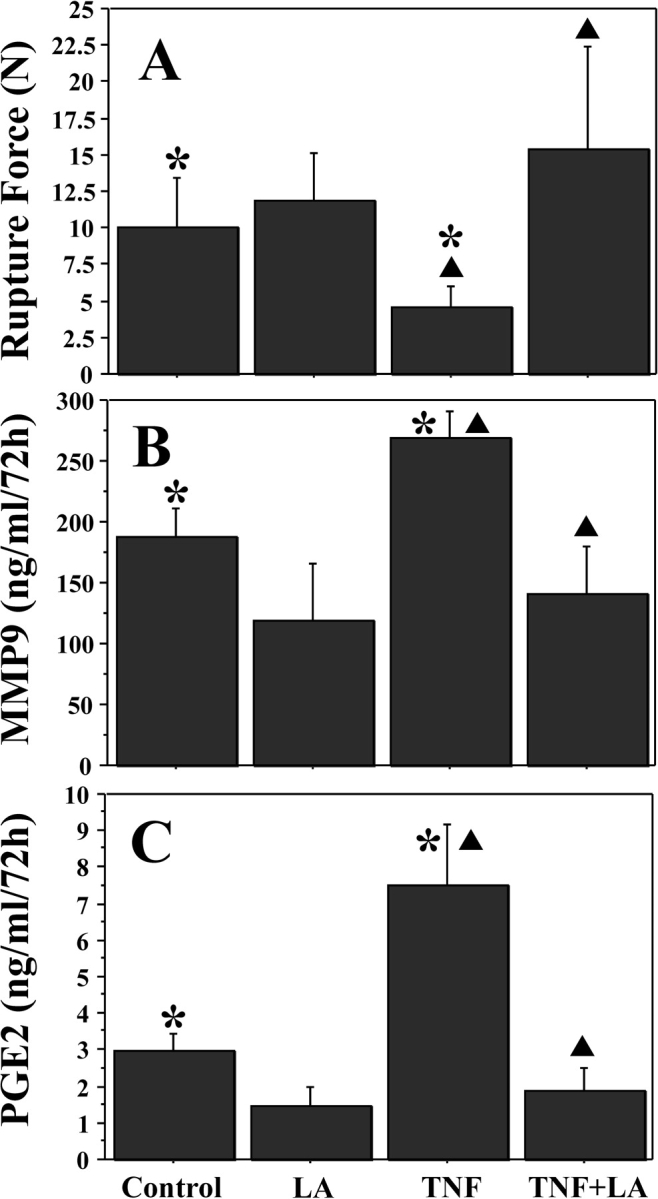
Alpha-lipoic acid inhibits TNF-induced weakening, decreasing MMP9 and PGE2 release in cultured FM. Fetal membrane fragments were preincubated with or without LA (0.5 mM) for 6 h, and then incubated with or without the addition of TNF (50 ng/ml) for 72 h. Four groups are displayed: control, LA pretreatment, TNF, and LA pretreatment plus TNF. Rupture force (A) was determined as outlined in Materials and Methods. MMP9 release (B) and PGE2 release (C) in media were measured by ELISA. Data points represent pooled results of five experiments using three FM fragments per treatment group in each experiment (n = 15). Data are presented as mean ± SD. TNF incubation resulted in significant FM weakening that was inhibited by pretreatment with LA (A). TNF also induced increases in MMP9 (B) and PGE2 (C) release that were inhibited by LA pretreatment (B and C, respectively). Columns with symbols (* or triangle) indicate data are significantly different; P < 0.001.
Studies Using Amnion Epithelial Cells
MMP9 release into medium.
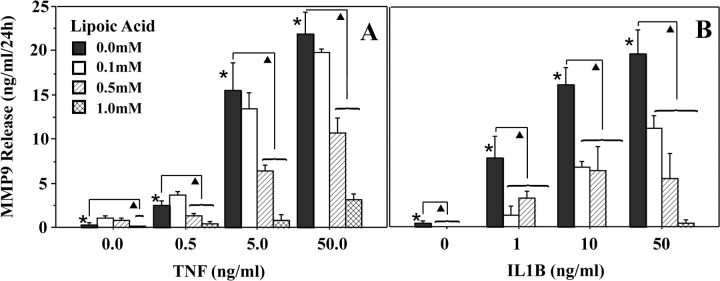
Treatment of amnion epithelial cells for 24 h with TNF (0–50 ng/ml) resulted in a dose-dependent increase in MMP9 protein released into the media (to 25-fold). Pretreatment with increasing doses of LA for 6 h resulted in a stepwise inhibition in the TNF-induced MMP9 release to near-basal levels (Fig. 2A). Treatment of amnion epithelial cells for 24 h with IL1B (0–50 ng/ml) also resulted in a dose-dependent increase in MMP9 release (to 14-fold). Pretreatment with LA inhibited the IL1B-induced MMP9 release to basal levels (Fig. 2B).
FIG. 2.
Alpha-lipoic acid inhibits TNF- and IL1B-induced MMP9 release by cultured amnion epithelial cells. Amnion epithelial cells were pretreated for 6 h with increasing doses of LA and then treated with increasing doses of TNF (A) or IL1B (B) for 24 h. Tumor necrosis factor (A) or IL1B (B) induced a dose-dependent increase in MMP9 release. The cytokine-induced increase was inhibited by pretreatment with increasing doses of LA. Data points represent the mean ± SD for triplicate cultures, assayed by ELISA in duplicate. Experiments were repeated with cells isolated from amnions of three different patients. Columns with symbols indicate data are significantly different; P < 0.001 (*, due to cytokine dose; triangle, due to inhibition of the cytokine-induced effect by LA).
MMP9 cellular protein and activity.
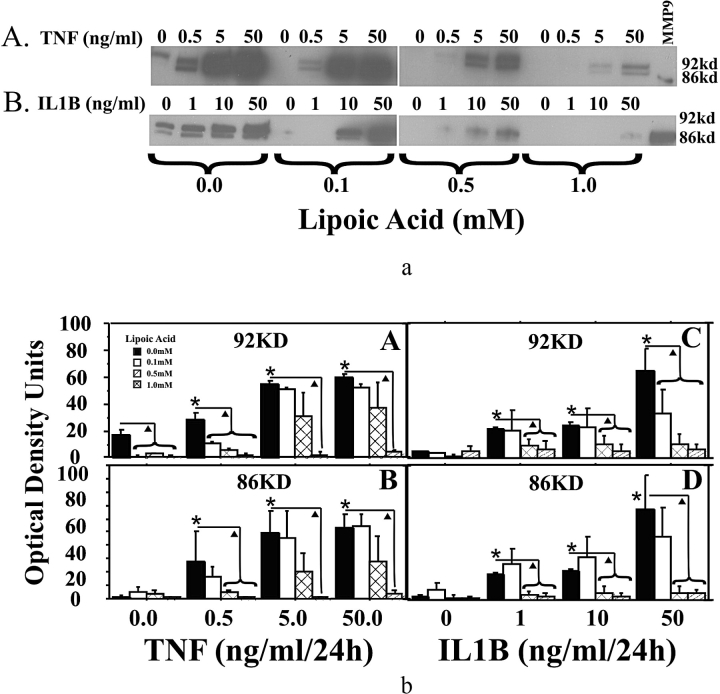
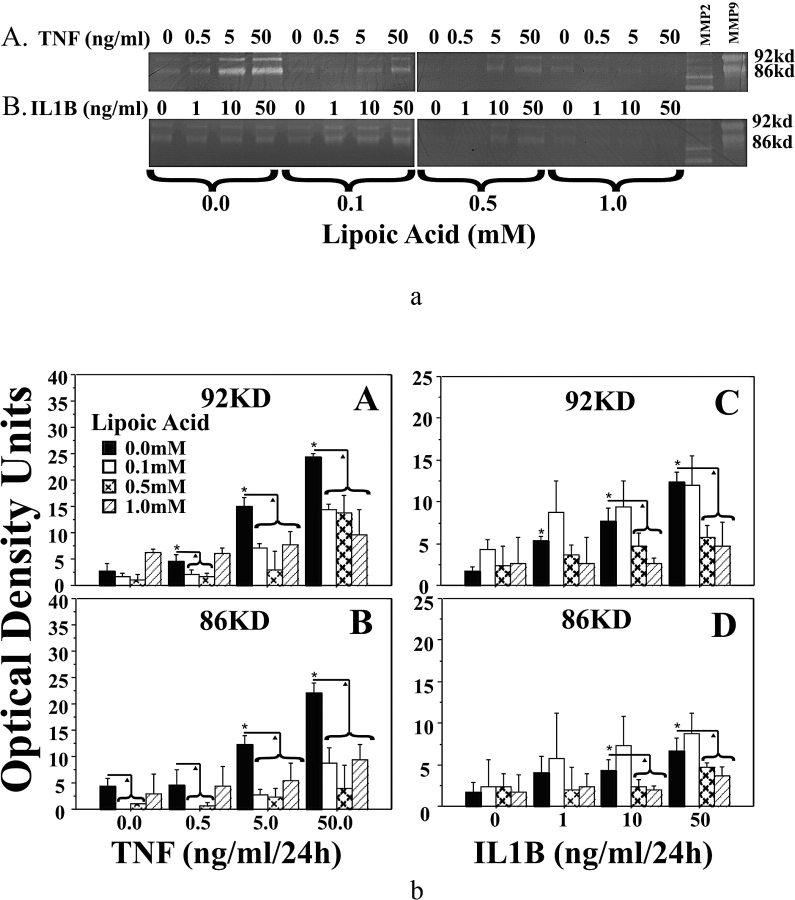
Western blot analysis of MMP9 protein in amnion epithelial cells demonstrated that both TNF (0–50 ng/ml) and IL1B (0–50 ng/ml) increased 92-kDa and 86-kDa MMP9 proteins in a dose-dependent manner. The LA pretreatment reduced TNF-induced and IL1B-induced increases in 92-kDa and 86-kDa MMP9 proteins to basal levels (Fig. 3). Zymogram analysis indicated that both TNF and IL1B increased 92-kDa and 86-kDa MMP9 enzyme activities. Pretreatment with LA reduced TNF-induced increases in 92-kDa and 86-kDa MMP9 activities, as well as IL1B-induced increases in 92-kDa and 86-kDa MMP9 activities (Fig. 4).
FIG. 3.
Alpha-lipoic acid inhibits TNF- and IL1B-induced MMP9 protein in cultured amnion epithelial cells. a) Western blot of MMP9 cellular protein from amnion epithelial cells. Cells were pretreated for 6 h with increasing doses of LA followed by treatment with increasing doses of TNF (A) or IL1B (B) for 24 h. Representative Western blots are shown. b) Densitometric analysis of Western blots for MMP9 from three experiments using epithelial cell isolates from amnions of three different patients (n = 3). Tumor necrosis factor (A and B) or IL1B (C and D) induced MMP9 proteins (92 and 86 kDa) by amnion epithelial cells. Pretreatment with increasing doses of LA inhibited these cytokine-induced increases. Data are presented as mean ± SD. Columns with symbols indicate data are significantly different; P < 0.001 (*, due to cytokine dose; triangle, due to inhibition of the cytokine-induced effect by LA). KD and kd, kilodalton.
FIG. 4.
Alpha-lipoic acid inhibits TNF- and IL1B-induced MMP9 activities in amnion epithelial cells. a) Representative zymogram of TNF-induced (A) and IL1B-induced (B) MMP9 activities and inhibition by pretreatment with LA in amnion epithelial cells treated as in Figure 3. b) Densitometric analysis of zymograms detecting MMP9 activities from three experiments using epithelial cell isolates from the amnions of three different patients (n = 3). Tumor necrosis factor-induced (A and B) or IL1B-induced (C and D) MMP9 enzyme activities by amnion epithelial cells. Pretreatment with increasing doses of LA inhibited these cytokine-induced increases. Data are presented as mean ± SD. Columns with symbols indicate data are significantly different; P < 0.001 (*, due to cytokine dose; triangle, due to inhibition of the cytokine-induced effect by LA). KD and kd, kilodalton.
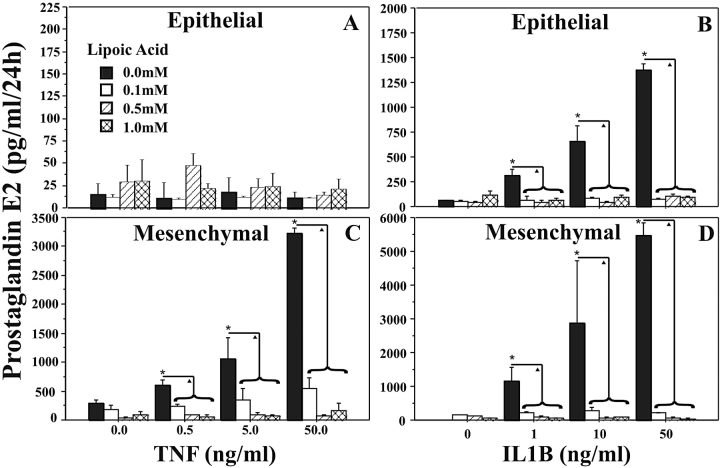
PGE2 release.
Amnion epithelial cells did not release significant amounts of PGE2 in response to TNF (Fig. 5A). However, treatment of amnion epithelial cells with increasing doses of IL1B (0–50 ng/ml) resulted in a dose-dependent increase in PGE2 release (Fig. 5B). The LA pretreatment inhibited IL1B-induced PGE2 release to basal levels (Fig. 5B).
FIG. 5.
Alpha-lipinoic acid inhibits TNF- and IL1B-induced PGE2 release in amnion cells. Amnion epithelial (A and B) or amnion mesenchymal (C and D) cells were pretreated for 6 h with increasing doses of LA, followed by increasing doses of TNF (A and C) or IL1B (B and D) for 24 h. The PGE2 release into medium from triplicate cultures, assayed by ELISA in duplicate, is shown. Experiments were repeated three times using cells isolated from the amnions of three different patients. Data are presented as mean ± SD. Columns with symbols indicate data are significantly different; P < 0.001 (*, due to cytokine dose; triangle, due to inhibition of the cytokine-induced effect by LA).
Studies Using Amnion Mesenchymal Cells
Isolated amnion mesenchymal cells did not release detectable levels of MMP9 over 24 h of culture in response to TNF, IL1B, or LA. Furthermore, no MMP9 proteins or enzymatic activities were detectable on Western blots or in zymograms (data not shown). Treatment of amnion mesenchymal cells with TNF (0.5–50 ng/ml; Fig. 5C) or IL1B (1–50 ng/ml; Fig. 5D) induced 10- or 20-fold increases in PGE2 release, respectively. Pretreatment with LA reduced PGE2 release to basal levels (Fig. 5, C and D).
DISCUSSION
Our data demonstrate that TNF-induced FM weakening and remodeling can be inhibited by the dietary antioxidant LA. Because the amnion is the major strength-bearing component of the FM [31], we also examined the effect of LA on cytokine stimulation of primary cultures of its constituent cell populations, amnion epithelial cells and amnion mesenchymal cells. Both TNF and IL1B increased MMP9 release, cellular protein, and enzymatic activity in amnion epithelial cells. Alpha-lipoic acid completely inhibited this TNF-induced increase. MMP9 was not detectable in mesenchymal cells. Both TNF and IL1B increased PGE2 release in amnion mesenchymal cells, whereas only IL1B increased PGE2 production in amnion epithelial cells. Alpha-lipoic acid also completely inhibited these increases. Therefore, LA inhibited all investigated effects of TNF and IL1B in amnion cells.
In a previous study we reported that TNF and IL1B, proinflammatory cytokines that normally increase toward the end of pregnancy, can cause significant FM weakening in vitro. A clear, dose-dependent, relationship between both cytokines and observed FM rupture strength and work to rupture was demonstrated in those studies. Furthermore, both cytokines produced weakness through a process involving collagen remodeling and apoptosis [28]. This previous study is consistent with numerous clinical studies that support a role for these cytokines in FM remodeling and rupture. Amniotic fluid levels of TNF and IL1B normally increase with gestation and the onset of labor [35]. These cytokines also are markedly elevated in amniotic fluid, maternal serum, and fetal tissue in preterm birth, especially in the context of infection [36]. In this regard, Menon and Fortunato [5] have postulated that PPROM is an autotoxic disease involving activation of extracellular MMPs by the host inflammatory response.
Numerous studies also have reported that MMP9 is upregulated following labor and delivery at term or preterm gestation, whereas the other major gelatinase, MMP2, is not [37, 38]. We have shown previously that MMP9 protein levels are elevated in a mechanically weak zone within the FM found in the region overlying the cervix in unlabored cesarean deliveries [6]. MMP9 is also elevated in a weak zone consistently present along the rupture tear line in term-labored, vaginally delivered FM [7]. Furthermore, our previous in vitro studies on cultured FM indicate that TNF and IL1B both induce increased MMP9 cellular protein concomitant with mechanical weakening [28]. The data presented here are consistent with these previous studies. Tumor necrosis factor weakened full-thickness FM fragments by more than 50% and markedly increased release of both MMP9 and PGE2. Alpha-lipoic acid inhibited these TNF-induced effects, both biomechanical as well as biochemical. Alpha-lipoic acid may therefore serve as a useful tool for probing the cellular mechanism of TNF-induced FM weakening.
We chose to investigate the effects of TNF and LA on the cells of the amnion initially, because amnion is the strongest component of the FM [31]. Amnion epithelial cells, but not amnion mesenchymal cells, clearly participate in the TNF-induced MMP9 release. Both amnion cell types secrete increased PGE2 in response to cytokines, which may in turn secondarily affect MMP9 production, release, and activity. Alpha-lipoic acid inhibits all of these cytokine-induced effects, which directly correlates with its effects on intact FM.
Choriodecidua, the remaining component of the FMs, is clearly also important for FM structural integrity. There are at least three fetal cell populations in the chorion, fibroblasts, and macrophages in the reticular layer, and cytotrophoblasts in the trophoblast layer [39]. Numerous maternal, marrow-derived cell types, in addition to modified uterine endometrial stromal cells, populate the decidua [40]. Many of these cells possess TNF and IL1B receptors and have been reported to synthesize and secrete MMP9 in response to cytokine treatment [41, 42]. Thus, they may also participate directly or indirectly in the cytokine-induced FM weakening process. Finally, interactions between different FM cell populations and/or the extracellular matrix may also be important in the cellular mechanism and biomechanics of rupture. Teasing out the roles played by these diverse cell populations in cytokine-induced FM weakening, and the mechanism of LA inhibition, are objectives for future investigation.
Although the mechanism by which LA inhibits TNF and IL1B effects in full-thickness FM and amnion cells is not certain, TNF and IL1 are known to generate ROS, with subsequent upregulation of nuclear transcription factor nuclear factor-κB (official symbol RELA). Induction and DNA binding of RELA are known inducers of both PGE2 production and MMP9 [43]. Alpha-lipoic acid has previously been shown to inhibit TNF-induced RELA transcriptional activity and MMP9 expression in vascular smooth muscle cells [44], inhibit bone resorption by suppressing PGE2 synthesis [45], and attenuate LPS-induced RELA-DNA binding in monocytes by activating the PI3K/AKT pathway [46].
Alpha-lipoic acid is an antioxidant with multiple reported mechanisms of action [24, 25]. Exogenous LA is reduced intracellularly by at least two and possibly three enzymes, and through the actions of its reduced form it influences a number of cell processes. These include direct radical scavenging, recycling of other antioxidants, accelerating glutathione synthesis, and modulating transcription factor activity, especially that of RELA [23, 44, 46]. The specific mechanisms of action resulting in each of the effects reported here remain to be investigated.
Alpha-lipoic acid inhibits TNF-induced weakening, MMP9 release, and PGE2 release in full-thickness FM fragments. A parallel, LA-induced inhibition of cytokine-stimulated MMP9 release, protein production and activity, and PGE2 release is seen in the two constituent cell populations in amnion, the mechanically strongest component of FM. We speculate that LA could be useful as part of a clinical regimen in the prevention of PPROM in the context of cytokine-induced inflammation.
Footnotes
1Supported by National Institutes of Health grant HD48476 to J.J.M.
REFERENCES
- Menon R.Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand 2008; 87: 590–600. [DOI] [PubMed] [Google Scholar]
- Parry S, Strauss JF.Premature rupture of the fetal membranes. N Engl J Med 1998; 338: 663–670. [DOI] [PubMed] [Google Scholar]
- Pandey V, Jaremko K, Moore RM, Mercer BM, Stetzer B, Kumar D, Fox JM, Mansour JM, Moore JJ.The force required to rupture fetal membranes paradoxically increases with acute in vitro repeated stretching. Am J Obstet Gynecol 2007; 196: 165.e1–165.e7. [DOI] [PubMed] [Google Scholar]
- Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ.The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta 2006; 27: 1037–1051. [DOI] [PubMed] [Google Scholar]
- Menon R, Fortunato SJ.The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig 2004; 11: 427–437. [DOI] [PubMed] [Google Scholar]
- El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, Redline RW, Mansour JM, Moore JJ.Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod 2005; 72: 720–726. [DOI] [PubMed] [Google Scholar]
- El Khwad M, Pandey V, Stetzer B, Mercer BM, Kumar D, Moore RM, Fox J, Redline RW, Mansour JM, Moore JJ.Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig 2006; 13: 191–195. [DOI] [PubMed] [Google Scholar]
- McLaren J, Malak TM, Bell SC.Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod 1999; 14: 237–241. [DOI] [PubMed] [Google Scholar]
- Xu P, Alfaidy N, Challis JR.Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab 2002; 87: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Tsatas D, Baker MS, Rice GE.Differential expression of proteases in human gestational tissues before, during and after spontaneous-onset labour at term. J Reprod Fertil 1999; 116: 43–49. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T.Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J 1996; 318: 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulciner DJ, Irani K, Yu ZX, Ferrans VJ, Goldschmidt-Clermont P, Finkel T.Rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol 1996; 16: 7115–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A, O'Neill LA.Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000; 59: 13–23. [DOI] [PubMed] [Google Scholar]
- Liu ZG.Molecular mechanism of TNF signaling and beyond. Cell Res 2005; 15: 24–27. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE.N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab 2003; 88: 1723–1729. [DOI] [PubMed] [Google Scholar]
- Steyn PS, Odentaal HJ, Schoeman J, Stander C, Fanie N, Grové D.A randomized, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labor. J Obstet Gynaecol 2003; 23: 150–155. [DOI] [PubMed] [Google Scholar]
- Rumbold A, Crowther CA.Vitamin C supplementation in pregnancy. Cochrane Database of Syst Rev 2005; 2: CD004072 [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Meyer C, Duncan JS, Redman P, Collins AR, Duthie GG, Speakman JR.Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech Ageing Dev 2006; 127: 897–904. [DOI] [PubMed] [Google Scholar]
- Lachili B, Hininger I, Faure H, Arnaud J, Richard MJ, Favier A, Roussel AM.Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol Trace Elem Res 2001; 83: 103–110. [DOI] [PubMed] [Google Scholar]
- Kumar D, Moore RM, Elkhwad M, Silver RJ, Moore JJ.Vitamin C exacerbates hydrogen peroxide induced apoptosis and concomitant PGE2 release in amnion epithelial and mesenchymal cells, and in intact amnion. Placenta 2004; 25: 573–579. [DOI] [PubMed] [Google Scholar]
- Kumar D, Lundgren DW, Moore RM, Silver RJ, Moore JJ.Hydrogen peroxide induced apoptosis in amnion-derived WISH cells is not inhibited by vitamin C. Placenta 2004; 25: 266–272. [DOI] [PubMed] [Google Scholar]
- Reed LJ, DeBusk BG, Gansalus IC, Hornberger CS., JrCrystalline α-lipoic acid: a catalytic agent associated with pyruvate dehydrogenase. Science 1951; 114: 93–94. [DOI] [PubMed] [Google Scholar]
- Biewenga GP, Haenen GR, Bast A.The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 1997; 29: 315–331. [DOI] [PubMed] [Google Scholar]
- Packer L, Witt EH, Tritschler HJ.α-Lipoic acid as a biological antioxidant. Free Radic Biol Med 1995; 19: 227–250. [DOI] [PubMed] [Google Scholar]
- Bilska A, Wlodek L.Lipoic Acid-drug of the future? Pharmacol Rep 2005; 57: 570–577. [PubMed] [Google Scholar]
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM.Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem 2004; 11: 1135–1146. [DOI] [PubMed] [Google Scholar]
- Hu HL, Forsey RJ, Blades TJ, Barratt ME, Parmar P, Powell JR.Antioxidants may contribute in the fight against ageing: an in vitro model. Mech Ageing Dev 2000; 121: 217–230. [DOI] [PubMed] [Google Scholar]
- Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, Mansour JM, Mercer BM, Redline RW, Moore JJ.Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod 2006; 74: 29–34. [DOI] [PubMed] [Google Scholar]
- McLaren J, Taylor DJ, Bell SC.Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod 2000; 6: 1033–1040. [DOI] [PubMed] [Google Scholar]
- Li W, Unlugedik E, Bocking AD, Challis JR.The role of prostaglandins in the mechanism of lipopolysaccharide-induced proMMP9 secretion from human placenta and fetal membrane cells. Biol Reprod 2007; 76: 654–659. [DOI] [PubMed] [Google Scholar]
- Arikat S, Novince RW, Mercer BM, Kumar D, Fox JM, Mansour JM, Moore JJ.Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol 2006; 194: 211–217. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Swan KF, Lyden TW.Organ culture of amniochorionic membrane in vitro. Am J Reprod Immunol 1994; 32: 184–187. [DOI] [PubMed] [Google Scholar]
- Okita JR, Sagawa N, Casey ML, Snyder JM.A comparison of human amnion tissue and human amnion cells in primary culture by morphological and biochemical criteria. In Vitro 1983; 19: 117–126. [DOI] [PubMed] [Google Scholar]
- Casey ML, MacDonald PC.Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod 1996; 55: 1253–1260. [DOI] [PubMed] [Google Scholar]
- Chow SS, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, Rawlinson WD.Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine 2008; 4: 78–84. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Witin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA.The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81: 941–948. [PubMed] [Google Scholar]
- Xu P, Alfaidy N, Challis JG.Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab 2002; 87: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Weiss A, Goldman S, Shalev E.The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci 2007; 12: 649–659. [DOI] [PubMed] [Google Scholar]
- Kim SS, Romero R, Kim JS, Abbas A, Espinoza J, Kusanovic JP, Hassan S, Yoon BH, Kim CJ.Coexpression of myofibroblast and macrophage markers: novel evidence for an in vivo plasticity of chorioamniotic mesodermal cells of the human placenta. Lab Invest 2008; 88: 365–374. [DOI] [PubMed] [Google Scholar]
- Audus KL, Soares MJ, Hunt JS.Characteristics of the fetal/maternal interface with potential usefulness in the development of future immunological and pharmacological strategies. J Pharmacol Exp Ther 2002; 301: 402–409. [DOI] [PubMed] [Google Scholar]
- Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knöfler M.Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first trimester villous explant cultures. J Clin Endocrinol Metab 2004; 89: 812–822. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, Murk W, Funai EF, Schatz F.Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod 2008; 78: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström TM, Bennett PR.The role of nuclear factor kappa B in human labour. Reproduction 2005; 130: 569–581. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK.α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp Mol Med 2007; 39: 106–113. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee JH, Kim HN, Kim HM, Kwak HB, Lee S, Kim HH, Lee ZH.α-Lipoic acid inhibits inflammatory bone resorption by suppressing prostaglandin E2 synthesis. J Immunol 2005; 176: 111–117. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Hagen T, Frei B.α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A 2007; 104: 4077–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]