Abstract
The role of interleukin (IL)-12 (p40:p35) and IL-23 (p40:p19) is becoming clear in immune response and inflammation. However, biological functions of IL-12 p40 homodimer (p402) and monomer (p40) remain poorly understood due to the lack of specific monoclonal antibodies (MAb). Earlier we have demonstrated that both p402 and p40 activate microglia and macrophages to induce the expression of iNOS and TNF-α. To facilitate the studies on p402 and p40 further, we here describe the production of neutralizing MAb against mouse p402 and p40 for the first time after immunization of Armenian hamsters with recombinant p402. Antibodies produced from clones a3-1d and d7-12c specifically recognized p402 but not p40, IL-12, and IL-23. These MAbs also inhibited p402- but not p40-, IL-12-, and IL-23-induced production of inflammatory molecules and activation of NF-κB. On the other hand, antibodies produced from clones a3-3a and a3-7g specifically recognized p40 and inhibited p40- but not p402-, IL-12-, and IL-23-induced production of inflammatory molecules and activation of NF-κB. While MAbs a3-1d and d7-12c were used to establish p402-specific ELISA, we utilized MAbs a3-3a and a3-7g to develop p40-specific ELISA. Interestingly, the production of p402 and p40 but not IL-12 in mouse peritoneal macrophages and primary microglia was an immediate early response to bacterial lipopolysaccharides. Furthermore, double-stranded RNA, the active component of a viral infection, induced the production of p402 and p40 but not IL-12 in macrophages and microglia. These results indicate the presence of different regulatory mechanisms for the production of IL-12p402/p40 and IL-12p70.
Introduction
Interleukin-12 (IL-12) plays a critical role in the early inflammatory response to infection and in the generation of T helper type 1 Th-1 cells, which favor cell-mediated immunity.(1) IL-12 consists of a heavy chain (p40) and a light chain (p35) linked covalently by disulfide bonds to give rise to the so-called bioactive heterodimeric (p70) molecule.(2,3) It is produced mainly by antigen-presenting cells (APC) upon activation through Toll-like receptors and by interactions with CD4+ T cells.(4–6) Recently, p40 has been shown to pair with p19 to form a newly discovered cytokine, IL-23. IL-23 has biological functions that are similar to as well as distinct from IL-12; for example, similar to IL-12, IL-23 enhances proliferation of Th1 cells and increases their IFN-γ production.(4–6) However, in contrast to IL-12, IL-23 aids in the proliferation of memory T cells.(4–6) Apart from forming heterodimers (IL-12 and IL-23), the p40 subunit is also secreted as monomer (p40) and homodimer (p402).(2) Because all these cytokines (IL-12, IL-23, p40, and p402) contain the common p40 subunit, these cytokines can better be grouped into the p40 family of cytokines.
It has been found that overproduction of IL-12 and IL-23 can be dangerous to the host as these molecules are involved in the pathogenesis of a number of autoimmune inflammatory diseases (e.g., multiple sclerosis, arthritis, insulin-dependent diabetes).(2,7,8) On the other hand, much less is known about the biological functions of p402 and p40. Recently, we have shown that both p402 and p40 are capable of activating microglia and macrophages to induce the production of iNOS and TNF-α.(9,10) Either p19 or p35 is constitutively expressed in many cell types.(2,4–6) However, dendritic cells and macrophages, cells that are able to secrete heterodimeric IL-12 or IL-23, always produce an excess of p40 as monomer or homodimer.(2) These observations suggest that p40 may have a key function as monomer (p40) or homodimer (p402), not just as part of the p40:p35 heterodimer forming IL-12 or the p40:p19 heterodimer forming IL-23. Apart from autoimmune disorders, the production of p40 family of cytokines (heterodimers, monomer, and homodimer) is induced during any infection or immune challenge. In most cases, all p40 present in heterodimers, homodimer, and monomer are collectively quantified due to the lack of a proper detection system. Therefore, understanding the role of the p40 family of cytokines in infection and autoimmunity has been impeded by the lack of monoclonal antibodies (MAbs) that can specifically recognize and neutralize p402 or p40.
We have therefore raised functional blocking MAbs against mouse p402 and p40 capable of neutralizing functions of mouse p402 and p40. We have also developed ELISAs to quantify mouse p402 and p40 separately. After stimulation with bacterial LPS, mouse peritoneal macrophages and primary microglia produced both p402 and p40 within as early as 30 min. In contrast, the production of IL-12 in LPS-stimulated macrophages and microglia was evident at around 12 h, suggesting that the production of p402 and p40 but not IL-12 is an immediate early response to LPS challenge. Interestingly, viral double-stranded RNA induced the production of p402 and p40 but not IL-12 in macrophages and microglia. This is the first development of MAbs against p402 and p40, which may be of immense importance for delineating undiscovered functions of p402 and p40.
Materials and Methods
Reagents
Fetal bovine serum, Hank's balanced salt solution (HBSS), and DMEM/F-12 were purchased from Mediatech (Manassas, VA). HAT, HT, PEG, hybridoma enhancing factor, and polyinosinic-polycytidylic acid (poly IC) were from Sigma (St. Louis, MO). L-glutamine, penicillin (100 U/mL), and streptomycin (100 mg/mL) were purchased from Invitrogen (Carlsbad, CA). Recombinant mouse IL-12 p70, p402 (the p40 homodimer), and p40 (the monomer) were obtained from R&D (Minneapolis, MN).
Antigen
Carrier-free p402 (expressed in sf-21 insect cells; lipopolysaccharides [LPS] < 0.1 ng/μg p402) was obtained from R&D. Electrophoresis data obtained from R&D Tech Support also showed that p402 migrated at ∼96 kDa position in non-denaturing gels and at 40 kDa position in denaturing gels.
Immunization
Three-month-old male Armenian hamsters (Harlan, Indianapolis, IN) were immunized i.p. with 35 μg carrier-free p402 emulsified in complete Freund's adjuvant (CFA) and boosted twice with the same quantity of antigens in IFA at 14-day intervals. A final boost of 50 μg p402 in saline was injected i.v. 3 days before harvesting of splenocytes for hybridoma production.
Cell fusion
Hamster splenocytes from immunized animals were harvested and fused to the HAT-sensitive murine myeloma cell line P3X63-Ag8.653 (ATCC) with polyethylene glycol 1500 at a splenocyte:myeloma cell ratio of 5:1, as described elsewhere.(11) The fused cells were resuspended in RPMI 1640 supplemented with 4mM L-glutamine, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, HAT, 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and distributed in each well of a 12-well plate containing hybridoma enhancing factor. Following 10 days of incubation with occasional replacement of one-half of the culture medium from each well by fresh HAT containing RPMI-1640 medium, the wells containing growth-positive culture were selected and the medium in these wells were replaced by fresh RPMI 1640 supplemented with HT instead of HAT. Fused cells were plated in 12-well plates. Supernatants of fast-growing mother hybridomas were screened for antibodies against p402, p40, IL-12, and IL-23 by direct ELISA.
Cloning by limiting dilution
Mother hybridomas positive for both p402 and p40 but negative for either IL-12 or IL-23 were cloned twice in two 96-well plates by limiting dilution (calculated as 0.75 cell/well to ensure single cell in most of the wells) in RPMI 1640 containing 10% FBS. Supernatants of growth-positive wells were randomly screened for antibodies against p402, p40, IL-12, and IL-23 by direct ELISA.
Direct ELISA
Briefly, 100 ng of purified antigen (p402, p40, IL-12, or IL-23) were bound to the surface of 96-well ELISA plates. Plates were washed with PBS-Tween, and then incubated with 100 μL of diluted (1:200) hybridoma culture supernatant. After 1 h at room temperature (25°C), plates were washed and then treated with an appropriately diluted biotin-conjugated goat anti-hamster IgG (Pierce, Rockford, IL). After a final wash, biotin-conjugated antibodies were detected by streptavidin-HRP as described.(10,12)
Biotinylation
Monoclonal antibodies (MAbs) against p402 and p40 were concentrated from hybridoma supernatants by centrifuging in Amicon Ultra-15 (50,000 molecular weight compound) centrifugal filter device (Millipore, Bedford, MA) and purified by protein A sepharose (Bio-Rad, Hercules, CA). The concentrated antibody sample (2 mg/mL) was subjected to biotinylation using Sulfo-NHS-LC-Biotin as described in instructions in EZ-link Sulfo_NHS_LC Biotinylation Kit (Pierce, Rockford, IL). The biotinylated antibodies were used as conjugate for sandwich ELISA.
Sandwich ELISA for p402 and p40
Antibodies were purified by protein A sepharose (Bio-Rad). A sandwich ELISA was developed for p402 using p402 MAb a3-1d as the coating antibody and p402 MAb d7-12c as the detection antibody. For coating, a3-1d MAb (1.3 mg/mL) was diluted 1:3000 and added to each well (100 μL/well) of a 96-well ELISA plate. The biotinylated p402 MAb d7-12c (2 mg/mL) was diluted 1:3000 and used as detection antibody. Similarly p40 MAb a3-3a (1.3 mg/mL) and biotinylated p40 MAb a3-7g (2 mg/mL) were also diluted 1:3000 and used as coating and detection antibodies, respectively, to develop a sandwich ELISA for p40.
Isolation of mouse peritoneal macrophages
Resident macrophages were obtained from mouse by peritoneal lavage with sterile RPMI 1640 medium containing 1% fetal bovine serum and 100 μg/mL gentamicin.(10,13) Cells were washed three times with RPMI 1640 at 4°C and were maintained at 37°C in a humidified incubator containing 5% CO2 in air. Macrophages at a concentration of 106/mL in RPMI 1640 medium containing L-glutamine and gentamicin were added in volumes of 0.5 mL to each well of 12-well plates. After 1 h, nonadherent cells were removed by washing and 0.5 mL of serum-free RPMI 1640 medium with various stimuli were added to the adherent cells.
Isolation of primary mouse microglia
Microglia were isolated from mixed glial cultures, according to the procedure of Giulian and Baker.(14) Briefly, on days 7 to 9, the mixed glial cultures were washed three times with DMEM/F-12 and subjected to a shake at 240 rpm for 2 h at 37°C on a rotary shaker. The floating cells were washed and seeded onto plastic tissue culture flasks and incubated at 37°C for 2 h. The attached cells were removed by trypsinization and seeded onto new plates for further studies. Ninety to 95% of this preparation was found to be positive for Mac-1 surface antigen. For the induction of cytokine and NO production, cells were treated with different stimuli in serum-free DMEM/F-12.
Mouse BV-2 microglial cells (a kind gift from Virginia Bocchini, University of Perugia, Italy) were also maintained and induced as indicated above.
Assay for NO synthesis
Synthesis of NO was determined by assay of culture supernatants for nitrite, a stable reaction product of NO with molecular oxygen, using Griess reagent as described.(15,16)
Assay for TNF-α synthesis
Concentration of TNF-α was measured in culture supernatants by a high-sensitivity, enzyme-linked immunosorbent assay (BD Biosciences, San Jose, CA), according to the manufacturer's instruction as described earlier.(10,12)
Assay of transcriptional activities of NF-κB
To assay the transcriptional activity of NF-κB, cells at 50–60% confluence were transfected with either pBIIX-Luc, an NF-κB-dependent reporter construct, using the Lipofect-AMINE Plus (Invitrogen).(15,16) All transfections included 50 ng/μg total DNA of pRL-TK (a plasmid encoding Renilla luciferase used as transfection efficiency control). After 24 h of transfection, cells were treated with p40 for 6 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract according to standard instructions provided in the Dual Luciferase Kit (Promega, Madison, WI) in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA). Relative luciferase activity of cell extracts was typically represented as (firefly luciferase value/Renilla luciferase value) × 10−3.
Results
Generation of p402- and p40-specific MAbs
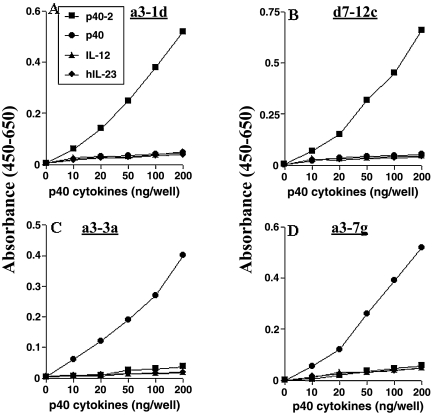
Male Armenian hamsters were immunized with sf-21 cell-derived p402, and the presence of antibodies in the sera of the immune hamsters was detected by direct ELISA. Fusion of immune hamster splenocytes to the HAT-sensitive mouse myeloma cell line resulted in the generation of 62% fast-growing wells and 38% slow-growing wells. Fast-growing mother wells were randomly analyzed by direct ELISA for the production of antibodies against p40 family of cytokines. As expected, all the mother wells tested were strongly positive for p402. Although we immunized hamsters with only p402, mother wells were weakly to fairly positive for p40, weakly positive for IL-12, and very weakly positive to negative for IL-23. Several p402- and p40-positive cultures were cloned twice by limiting dilution. The specificities of monoclonals against p40 family of cytokines were examined by direct ELISA. Supernatants of clone a3-1d and d7-12c specifically recognized p402 but not p40, IL-12, and IL-23 (Fig. 1A and B). On the other hand, supernatants of clone a3-3a and a3-7g specifically recognized p40 but not p402, IL-12, and IL-23 (Fig. 1C and D). However, supernatants of any of these clones also did not recognize TNF-α and IFN-γ, unrelated cytokines (data not shown). After isotyping, it was found that p402 MAb a3-1d and p40 MAb a3-3a were of IgG2a type whereas p402 MAb d7-12c and p40 MAb a3-7g were of IgG2b type.
FIG. 1.
Reactivity of hamster MAbs against p40 family of cytokines. Hybridoma supernatant of hamster MAb clone a3-1d (A), clone d7-12c (B), clone a3-3a (C), and clone a3-7g (D) were diluted (1:200) and added to each well of a 96-well microtiter plate coated with different amounts of p402, p40, IL-12, and IL-23. After 1 h of incubation at room temperature (25°C), plates were washed and then treated with biotin-conjugated goat anti-hamster IgG (Pierce) followed by detection with streptavidin-HRP. Data are mean ± SD of three different experiments.
Functional blocking activities of MAbs a3-1d and d7-12c
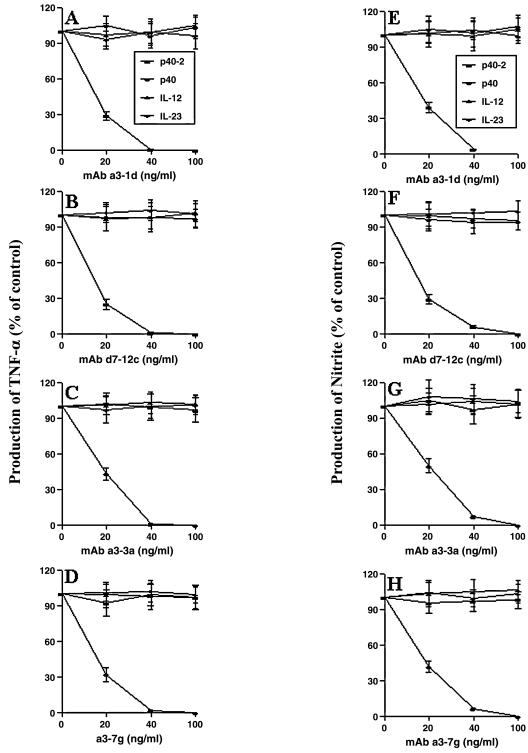
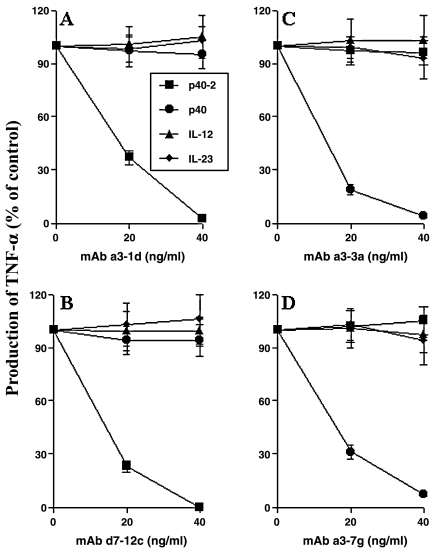
Earlier we have shown that p402 and p40 are capable of inducing the expression of iNOS and TNF-α in mouse microglia and macrophages.(9,10) Therefore, we examined the effect of these MAbs on p402- and p40-induced production of NO and TNF-α in macrophages and microglia. All four members of p40 family were capable of inducing the production of NO and TNF-α in mouse peritoneal macrophages (Fig. 2). However, purified p402 MAbs a3-1d and d7-12c markedly inhibited p402-induced production of both NO (Fig. 2A and C) and TNF-α (Fig. 2B and D). Dose-dependent studies showed that MAbs a3-1d and d7-12c were able to inhibit p402-induced production of NO and TNF-α significantly even at 20 ng/mL concentration (Fig. 2A–D). However, these MAbs at a dose of 100 ng/mL or higher markedly inhibited p402-induced production of NO and TNF-α (Fig. 2A–D). On the other hand, under similar treatment condition, these MAbs a3-1d and d7-12c did not inhibit p40-, IL-12-, and IL-23-induced production of NO and TNF-α (Fig. 2A–D). Similarly IL-12, IL-23, p402, and p40 induced the production of TNF-α (Fig. 3) and NO (data not shown) in primary mouse microglia. As expected, MAbs a3-1d and d7-12c specifically inhibited p402- but not p40-, IL-12-, and IL-23-induced production of TNF-α (Fig. 3A and B) and NO (data not shown) in primary microglia. Similarly, MAbs a3-1d and d7-12c also inhibited p402- but not p40-, IL-12-, and IL-23-induced production of NO and TNF-α in BV-2 microglial cells (data not shown).
FIG. 2.
Effect of MAbs a3-1d, d7-12c, a3-3a, and a3-7g on the induction of NO and TNF-α production by p40 family of cytokines in mouse peritoneal macrophages. Cells were stimulated with 10 ng/mL of p402, p40, IL-12, and IL-23 in the presence of different concentrations of purified MAbs a3-1d (A and E), d7-12c (B and F), a3-3a (C and G), and a3-7g (D and H) under serum-free condition. After 12 h of stimulation, supernatants were used for TNF-α assay (A–D), as described in the section on Materials and Methods. However, after 24 h of stimulation, supernatants were used for nitrite assay (E–H). Data are mean ± SD of three different experiments.
FIG. 3.
Effect of MAbs a3-1d, d7-12c, a3-3a, and a3-7g on the induction of TNF-α production by p40 family of cytokines in mouse primary microglia. Cells were stimulated with 10 ng/mL of p402, p40, IL-12, and IL-23 in the presence of different concentrations of purified MAbs a3-1d (A), d7-12c (B), a3-3a (C), and a3-7g (D) under serum-free condition. After 12 h of stimulation, supernatants were used for TNF-α assay. Data are mean ± SD of three different experiments.
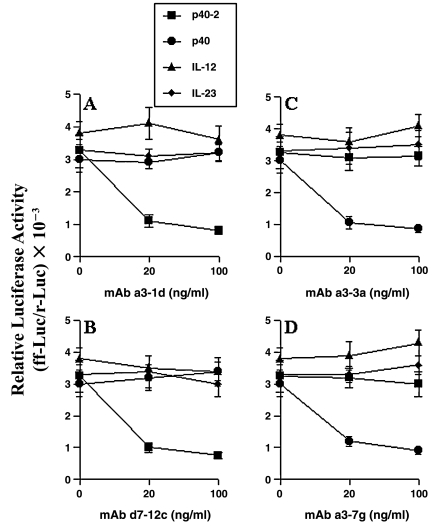
To further confirm that MAbs a3-1d and d7-12c specifically inhibit functions of p402, we examined the effect of these MAbs on p402-, p40-, IL-12-, and IL-23-induced activation of NF-κB in BV-2 microglial cells. Earlier we have shown that p402 and p40 are capable of inducing the activation of NF-κB in BV-2 microglial cells.(9,10) Activation of NF-κB was monitored by transcriptional activity using the expression of luciferase from pBIIX-Luc, an NF-κB-dependent reporter construct. All the four stimuli (p402, p40, IL-12, and IL-23) induced the transcriptional activity of NF-κB, with p402 being the most potent inducer (Fig. 4). However, MAbs a3-1d and d7-12c dose-dependently inhibited p402, but not p40-, IL-12-, and IL-23-induced transcriptional activity of NF-κB (Fig. 4A and B). Taken together, these studies suggest that MAbs a3-1d and d7-12c specifically neutralize functions of p402.
FIG. 4.
Effect of MAbs a3-1d, d7-12c, a3-3a, and a3-7g on the induction of NF-κB activation in BV-2 microglial cells. Cells plated at 50–60% confluence in 12-well plates were cotransfected with 0.5 μg of pBIIX-Luc and 25 ng of pRL-TK. After 24 h of transfection, cells were stimulated with 10 ng/mL of p402, p40, IL-12, and IL-23 in the presence of different concentrations of purified MAbs a3-1d (A), d7-12c (B), a3-3a (C), and a3-7g (D) under serum-free condition. After 6 h of stimulation, firefly and Renilla luciferase activities were obtained by analyzing total cell extract, as described in the Materials and Methods section. Results are expressed as relative to control 1. Data are mean ± SD of three different experiments.
Functional blocking activities of MAbs a3-3a and a3-7g
Because MAbs a3-3a and a3-7g specifically recognized p40 but not p402, IL-12, and IL-23 (Fig. 1C and D), we also examined the effect of these two MAbs on p40-, p402-, IL-12-, and IL-23-induced production of NO and TNF-α in mouse peritoneal macrophages and primary microglia. As is evident in Figure 2E–H, MAbs a3-3a and a3-7g dose dependently inhibited p40- but not p402-, IL-12-, and IL-23-induced production of NO and TNF-α in peritoneal macrophages. Similarly, MAbs a3-3a and a3-7g also inhibited p40- but not p402-, IL-12-, and IL-23-induced production of TNF-α (Fig. 3C and D) and NO (data not shown) in primary mouse microglia. All four stimuli also induced the production of NO and TNF-α in BV-2 microglial cells (data not shown). However, MAbs a3-3a and a3-7g specifically inhibited p40- but not p402-, IL-12-, and IL-23-induced production of NO and TNF-α in BV-2 microglial cells (data not shown). As is evident from Figure 4C and D, MAbs a3-3a and a3-7g markedly blocked p40- but not p402-, IL-12-, and IL-23-induced transcriptional activity of NF-κB. These studies suggest that MAbs a3-3a and a3-7g specifically block functions of p40.
Detection of p402 and p40 in LPS-stimulated mouse peritoneal macrophages and primary microglia
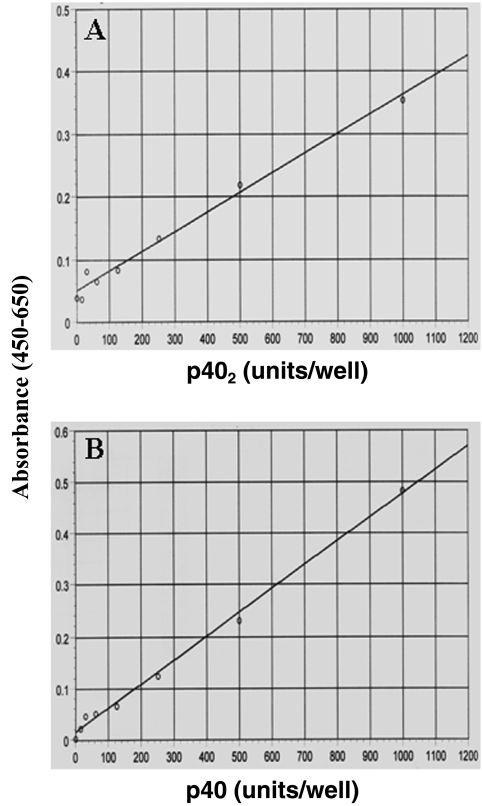
The generation of p402- and p40-specific MAbs allowed us to establish ELISAs to facilitate the quantitation of these two cytokines in biological samples. To date, no such assays/kits are available to quantify p402 and p40. To quantitate mouse p402, we used MAb a3-1d as the capture antibody and biotinylated MAb d7-12c as the detection antibody. The ELISA for p402 (Fig. 5A) was highly specific and capable of quantifying mouse p402 in a range between 5 and 500 pg (data not shown). No cross reactivity was observed with recombinant mouse p40 and IL-12 and human IL-23. Similarly, by using MAb a3-3a as the capture antibody and biotinylated MAb a3-7g as the detection antibody, we have developed a highly sensitive ELISA for mouse p40 (Fig. 5B) capable of quantifying mouse p40 in a range between 5 and 500 pg (data not shown). This ELISA did not recognize p402, IL-12, and IL-23.
FIG. 5.
Development of sandwich ELISA for mouse p402 and p40. (A) Standard curve for the quantification of p402 using MAb a3-1d as the coating antibody and biotinylated MAb d7-12c as the detection antibody. (B) Standard curve for the quantification of p40 using MAb a3-3a as the coating antibody and biotinylated MAb a3-7g as the detection antibody.
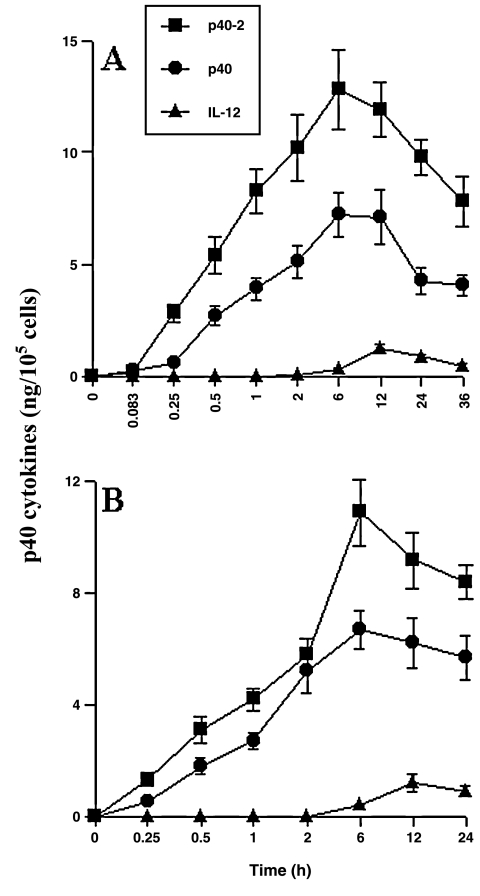
To understand the status of p402, p40, and IL-12 after LPS stimulation, mouse peritoneal macrophages and primary microglia were stimulated with LPS, and the levels of p402, p40, and IL-12 were quantified at different time periods by ELISA. It is clear from Figure 6A that LPS was capable of inducing the production of p402 and p40 at an astronomical level in peritoneal macrophages. The induction of p402 and p40 production, evident as early as 15 min of stimulation, increased markedly at later hours of stimulation with a peak at 6 h (Fig. 6A). On the other hand, the induction of IL-12 began at 6 h and peaked at 12 h of stimulation (Fig. 6A). However, the level of p402 was about 10-fold higher than IL-12, and the level of p40 was about 5-fold higher than IL-12. Similarly marked induction of p402 and p40 production was also observed in mouse primary microglia after LPS stimulation (Fig. 6B). However, the level of IL-12 was much lower than p402 and p40. Although the induction of p402 and p40 production was observed as early as 30 min of stimulation with a peak at 6 h, the production of IL-12 peaked at 18 h of stimulation. Taken together, these results suggest that the production of p402 and p40 but not IL-12 is an immediate early response to LPS challenge and that induction of p402 and p40 is much greater than IL-12.
FIG. 6.
Bacterial lipopolysaccharides induce the production of p402, p40, and IL-12 in mouse peritoneal macrophages (A) and primary microglia (B). Cells were treated with 1 −g/mL of LPS under serum-free condition. At different h of stimulation, supernatants were used for quantification of p402, p40, and IL-12 by sandwich ELISA. Data are mean ± SD of three different experiments.
Induction of p402 and p40 but not IL-12 by dsRNA in mouse peritoneal macrophages and primary microglia
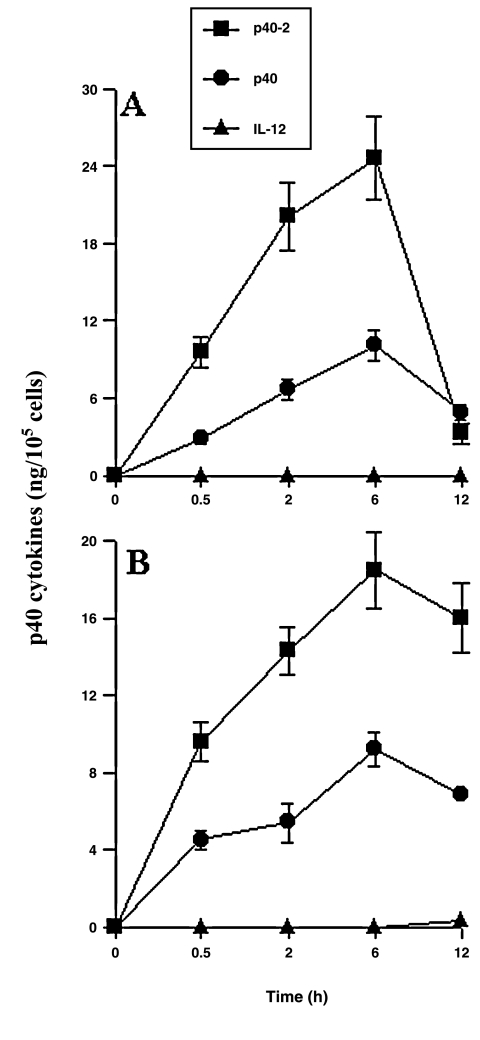
Poly IC, a synthetic double-stranded (ds) RNA copolymer of inosinic and cytidilic acids, has been often used as a tool to mimic the effects of dsRNA intermediates produced during viral infection of cells.(17) We examined if poly IC was capable of inducing the production of p402 and p40 in macrophages and microglia. Interestingly, poly IC strongly induced the production of p402 and p40 in both macrophages (Fig. 7A) and microglia (Fig. 7B) at different times of stimulation with the peak at 6 h of stimulation. In contrast to dsRNA, treatment of either macrophages or microglia with single-stranded (ss) RNA (either poly I or poly C) resulted in very little induction of p402 and p40 production (data not shown), suggesting that the presence of RNA with ds structures is required for the induction of p402 and p40. Furthermore, these results also suggested that the induction of p402 and p40 production was not due to LPS contamination in the RNA preparation, which is a potent inducer of p402 and p40 production in macrophages and microglia. Although the level of induction of p402 and p40 by poly IC (Fig. 7) was higher than LPS (Fig. 6), in contrast to the LPS, poly IC was unable to induce the production of IL-12 in these cell types (Fig. 7).
FIG. 7.
Poly IC induces the production of p402 and p40 but not IL-12 in mouse peritoneal macrophages (A) and primary microglia (B). Cells were treated with IL-1β (20 ng/mL), IFN-γ (12.5 U/mL), and poly IC (50 μg/mL) under serum-free condition. At different times of stimulation, supernatants were used for quantification of p402, p40, and IL-12 by sandwich ELISA. Data are mean ± SD of three different experiments.
Discussion
Among different p40 family of cytokines (IL-12, IL-23, p402, and p40), IL-12 and IL-23 have been widely studied for their role in coordinating innate and adaptive immunity. Recent studies are also delineating central role of these two cytokines in the pathogenesis of several autoimmune disorders. The biological activities of IL-12 are mediated through the high-affinity IL-12R, which is composed of IL-12Rβ1 and IL-12Rβ2 chains.(2) On the other hand, the receptor of IL-23 is a heterodimer composed of IL-12Rβ1 and a newly discovered IL-23R.(18) Although during any infection and immune challenge, p402 and p40 are also produced along with other cytokines, their biological role remains unknown. However, p402 reportedly binds to the IL-12Rβ1,(2) the receptor that is common for both IL-12 and IL-23. Because secretion of IL-12 and IL-23 is associated with excessive production of p402, it has been proposed that excess p402 may down-regulate IL-12- and IL-23-mediated immune responses through competition for the IL-12Rβ1.(2,19) On the other hand, p40 reportedly does not have any IL-12-antagonizing activity and binds IL-12Rβ1 very weakly (10–20 times less potent compared to p402).(2,19) Despite IL-12 antagonism in many systems, endogenous and exogenous p402 and p40 have been reported to act as IL-12 agonists in certain situations.(20,21) For example, according to Hölscher et al.,(21) administration of p402 restores the impaired delayed-type hypersensitivity responses in Mycobacterium bovis BCG-infected IL-12 p35 (− / −) p40 (− / −) mice and reverts them to a more resistant phenotype, suggesting that p402 may have some agonistic properties. Earlier we have consistently unearthed novel biological activities of p402 and p40, in which both the cytokines have been demonstrated to activate microglia and macrophages for the expression of iNOS and TNF-α.(9,10) Although these studies suggest that p402 and p40 may play a role in infectious and inflammatory disorders, at present, it is not possible to study the involvement of endogenous p402 and p40 in the pathogenesis of any such diseases. It is often quite straightforward to consider a knock out mouse model to investigate the role of a candidate molecule in any disease process. Although p40 (− / −) mice are available, these mice cannot be used for such studies because knocking out the p40 gene disables each and every member of the p40 family (IL-12, IL-23, p402, and p40). Therefore, to investigate the role of p402 and p40 in any disease model, the only feasible approach is to use neutralizing monoclonal antibodies against these molecules. Such MAbs are, however, not available.
Here we demonstrate successful generation of neutralizing MAbs against mouse p402 and p40 in Armenian hamsters. Both MAbs a3-1d and d7-12c specifically recognized p402 but not p40, IL-12, and IL-23. These MAbs also inhibited p402- but not p40-, IL-12-, and IL-23-induced production of NO and TNF-α in macrophages and microglia. On the other hand, both MAbs a3-3a and a3-7g specifically recognized p40 but not p402, IL-12, and IL-23, and inhibited p40-but not p402-, IL-12-, and IL-23-induced production of NO and TNF-α. To our knowledge, these are the first neutralizing monoclonal anti-mouse p402 and anti-mouse p40 reagents to be produced that could be of immense importance in elucidating the role of p402 and p40 in mouse models for infection and autoimmune disorders.
Although non-denaturing electrophoresis followed by immunoblot analysis with pan p40 MAb is capable of detecting p402 and p40,(22) until now it was not possible to quantify each of these molecules separately. With the anti-p402 and anti-p40 reagents produced in this study, we have established sensitive and reproducible ELISAs to quantify p402 and p40 separately. For example, using MAb a3-1d as the capture antibody and MAb d7-12c as the detection antibody, we have developed sandwich ELISA that specifically quantifies picogram levels of p402. Again utilizing MAb a3-3a as the capture antibody and MAb a3-7g as the detection antibody, we have established another sensitive ELISA to quantify picogram levels of p40. Therefore, our p402- and p40-specific ELISAs may be used as important tools in delineating signaling pathways for the production of homodimeric and monomeric forms of p40. Because ELISAs for quantification of IL-12 and IL-23 are available, our MAbs will serve as invaluable reagents to dissect out the role of each of these molecules in infection and immunity.
For example, bacterial lipopolysaccharides (LPS) is a prototype inducer of p40 family of cytokines and the level of p40 is much higher than that of the heterodimeric p70 in IL-12-producing cells(2); however, the induction status of p402 and p40 after LPS stimulation is poorly understood. We observed that the induction of p402 and p40 production in primary macrophages and microglia started within 30 min of LPS stimulation and peaked at 6 h while the production of IL-12 started at 6 h and peaked at 12 h of stimulation. It appears that the induction of p402 and p40 but not IL-12 is an immediate early response to LPS. It will be interesting to find out whether the immediate induction of huge amounts of p402 and p40 in response to LPS is actually protective or destructive. Therefore, further work is currently underway to delineate the exact role of p402 and p40 in LPS-induced toxicities in mice. It is widely believed that the induction of IL-12 is often associated with the induction of p402 and p40 and vice versa. However, contrary to the notion, dsRNA (the active component of a viral infection) induced the production of only p402 and p40 but not IL-12 in peritoneal macrophages and primary microglia at different times of stimulation. These results predict the possible presence of different signaling mechanisms for the production of p402/p40 and IL-12. Recent evidence suggests that the intracellular levels of cyclic AMP (cAMP) play an important role in re-directing cytokine synthesis in activated macrophages.(23,24) Thus, PGE2, a potent and physiological inducer of adenylate cyclase, as well as cAMP derivatives, have been shown to inhibit IL-12 synthesis in LPS-activated macrophages.(23,24) However, interestingly, PGE2 and cAMP stimulate the formation of p40.(24) Although the study by Karinski et al.(24) did not analyze p402 and p40 due to the lack of a proper detection system, cAMP may favor the generation of p402 and p40. Thus, it is tempting to speculate that dsRNA enhances the level of cAMP in macrophages and microglia that, in turn, up-regulates the production of p402 and p40 but not IL-12. In this connection, it is important to note that the cAMP/PKA pathway is overactive and the production of IL-12 is suppressed in a number of virus-induced pathological conditions such as HIV infection.(25,26)
Several human demyelinating disorders have a known viral etiology: subacute sclerosing panencephalitis as a late complication of measles virus infection of childhood,(27) progressive multifocal leukoencephalopathy caused by the JC papovavirus,(28) encephalopathy and myelopathy (neuro-AIDS) caused by human immunodeficiency virus,(29) and human T-lymphotropic virus type 1 associated myelopathy/tropical spastic paraparesis.(30) Although the association of a virus with multiple sclerosis (MS) has not been confirmed, based on detection of virions, viral nucleic acids, or viral proteins in CNS or the presence of antiviral antibodies in serum and/or CSF, several viruses have been suggested to play a role in MS.(31–33) A common viral structure that is almost universally recognized by eukaryotic cells is dsRNA (>100 bp). dsRNA is not present during the normal life cycle of eukaryotic cells; however, it is present in virally infected cells. dsRNA accumulates during the replication of many viruses.(17) However, the biological function of dsRNA within the CNS is poorly understood. Here we show that microglia, resident macrophages of the CNS, respond to dsRNA immediately to produce large amounts of p402 and p40. Due to the facts that p402 and p40 induce the expression of different proinflammatory molecules in microglia(9,10) and that viral infection is associated to several neuroinflammatory and neurodegenerative diseases, it is possible that dsRNA may induce the expression of different proinflammatory molecules in microglia via p402 and p40 to induce/potentiate the neural injury in the CNS.
Although the release of p40 monomer/homodimer has not been directly demonstrated in human cells, Fassbender et al.(34) have demonstrated an increased (up to 1,000-fold) compartmentalized release of the p40 subunit but not of the heterodimer p70 in MS patients. Release of p40 correlates with classic markers of CNS inflammation (CSF cell counts, immunoglobulin G index) and is significantly increased in patients with gadolinium-enhancing plaques on MRI. It is apparent that this huge pool of p40 contains not only the newly described heterodimer IL-23 but also p40 monomer and dimer. In addition, we have recently found that p402 and p40 stimulate the expression of iNOS in human primary astrocytes (unpublished observation), suggesting that these two cytokines may also play an important immunomodulatory role in human. Therefore, novel results obtained from our MAbs against mouse p402 and p40 may be replicated in the human as well.
Acknowledgment
This study was supported by a grant from NIH (NS39940).
References
- 1.Hsieh CS. Macatonia SE. Tripp CS. Wolf SF. O'Garra A. Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 2.Gately MK. Renzetti LM. Magram J. Stern AS. Adorini L. Gubler U. Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M. Kweon MN. Kuwata H. Schreiber RD. Kiyono H. Takeda K. Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppmann B. Lesley R. Blom B. Timans JC. Xu Y. Hunte B. Vega F. Yu N. Wang J. Singh K. Zonin F. Vaisberg E. Churakova T. Liu M. Gorman D. Wagner J. Zurawski S. Liu Y. Abrams JS. Moore KW. Rennick D. de Waal-Malefyt R. Hannum C. Bazan JF. Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 5.Cua DJ. Sherlock J. Chen Y. Murphy CA. Joyce B. Seymour B. Lucian L. To W. Kwan S. Churakova T. Zurawski S. Wiekowski M. Lira SA. Gorman D. Kastelein RA. Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 6.Lankford CS. Frucht DM. A unique role for IL-23 in promoting cellular immunity. J Leukoc Biol. 2003;73:49–56. doi: 10.1189/jlb.0602326. [DOI] [PubMed] [Google Scholar]
- 7.Zipris D. Greiner DL. Malkani S. Whalen B. Mordes JP. Rossini AA. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156:1315–1321. [PubMed] [Google Scholar]
- 8.Bright JJ. Musuro BF. Du C. Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 9.Pahan K. Sheikh FG. Liu X. Hilger S. McKinney M. Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jana M. Dasgupta S. Saha RN. Liu X. Pahan K. Induction of tumor necrosis factor-alpha by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–528. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schreiber RD. Hicks LJ. Celada A. Buchmeier NA. Gray PW. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- 12.Dasgupta S. Jana M. Liu X. Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pahan K. Sheikh FG. Namboodiri AMS. Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giulian D. Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jana M. Liu X. Koka S. Ghosh S. Petro TM. Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X. Jana M. Dasgupta S. Koka S. He J. Wood C. Pahan K. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–39319. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNair ANB. Kerr IM. Viral inhibition of the interferon system. Pharmacol Ther. 1992;56:79–95. doi: 10.1016/0163-7258(92)90038-2. [DOI] [PubMed] [Google Scholar]
- 18.Parham C. Chirica M. Timans J. Vaisberg E. Travis M. Cheung J. Pflanz S. Zhang R. Singh KP. Vega F. To W. Wagner J. O'Farrell AM. McClanahan T. Zurawski S. Hannum C. Gorman D. Rennick DM. Kastelein RA. de Waal Malefyt R. Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 19.Wang X. Wilkinson VL. Podlaski FJ. Wu C. Stern AS. Presky DH. Magram J. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur J Immunol. 1999;29:2007–2013. doi: 10.1002/(SICI)1521-4141(199906)29:06<2007::AID-IMMU2007>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Piccotti JR. Li K. Chan SY. Ferrante J. Magram J. Eichwald EJ. Bishop DK. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 21.Hölscher C. Atkinson RA. Arendse B. Brown N. Myburgh E. Alber G. Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 22.Heinzel FP. Hujer AM. Ahmed FN. Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 23.Wilkin F. Stordeur P. Goldman M. Boeynaems JM. Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Kalinski P. Vieira PL. Schuitemaker JH. de Jong EC. Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 25.Delemarre FGA. Stevenhagen A. Kroon FP. van Eer MY. Meenhorst PL. van Furth R. Reduced toxoplasmastatic activity of monocytes and monocyte-derived macrophages from AIDS patients is mediated via prostaglandin E2. AIDS. 1995;9:441–445. [PubMed] [Google Scholar]
- 26.Hofmann B. Nishanian P. Nguyen T. Liu M. Fahey JL. Restoration of T-cell function in HIV infection by reduction of intracellular cAMP levels with adenosine analogues. AIDS. 1993;7:659–664. doi: 10.1097/00002030-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Liebert UG. Measles virus infections of the central nervous system. Intervirology. 1997;40:176–184. doi: 10.1159/000150544. [DOI] [PubMed] [Google Scholar]
- 28.Weber T. Major EO. Progressive multifocal leukoencephalopathy: molecular biology, pathogenesis and clinical impact. Intervirology. 1997;40:98–111. doi: 10.1159/000150537. [DOI] [PubMed] [Google Scholar]
- 29.Power C. McArthur JC. Nath A. Wehrly K. Mayne M. Nishio J. Langelier T. Johnson RT. Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and non-demented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izumo S. Umehara F. Kashio N. Kubota R. Sato E. Osame M. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP) Leukemia. 1997;11:82–84. [PubMed] [Google Scholar]
- 31.Stewart JN. Mounir S. Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cristallo A. Gambaro F. Biamonti G. Ferrante P. Battaglia M. Cereda PM. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. Microbiologica. 1997;2:105–114. [PubMed] [Google Scholar]
- 33.Murray RS. Brown B. Brian D. Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fassbender K. Ragoschke A. Rossol S. Schwartz A. Mielke O. Paulig A. Hennerici M. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51:753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]