Abstract
Despite the fact that the retina is a fairly accessible portion of the central nervous system, there are virtually no treatments for early age-related macular degeneration (AMD). AMD is a degenerative retinal disease that causes progressive loss of central vision and is the leading cause of irreversible vision loss and legal blindness in individuals over the age of 50. Both environmental and genetic components play a role in its development. AMD is a multifactorial disease with characteristics that include drusen, hyperpigmentation and/or hypopigmentation of the retinal pigment epithelium (RPE), geographic atrophy and, in a subset of patients, late-stage choroidal neovascularization (CNV). Drugs that inhibit vascular endothelial growth factor (VEGF) have proven effective in treating late-stage CNV, but optimal means of drug delivery remains to be determined. Microscopic particles, whose size is on the nanometer scale, show considerable promise for drug delivery to the retina, for gene therapy, and for powering prosthetic “artificial retinas.” This article summarizes the pathophysiology of AMD stressing potential applications from nanotechnology.
Keywords: macular, nanotechnology, AMD, retinal degeneration, gene therapy
Introduction
Age-related macular degeneration (AMD) is a degenerative retinal disease that causes progressive loss of central vision. AMD is the leading cause of irreversible vision loss and legal blindness in individuals over the age of 50; according to the National Eye Institute (NEI), it is most common in people in their 70s. The risk of developing macular degeneration increases with age. The acronyms ARMD, ARM, and AMD are often used interchangeably. Early disease is sometimes referred to as age-related maculopathy “ARM” and late disease (geographic atrophy or choroidal neovascularization) “AMD,” but use of this terminology is not universal. Here, for simplicity, we will use “AMD” to refer to the spectrum of disease. Although some treatments to slow progression are available for AMD, there is currently no cure for this irreversible disease. Recently, nanotechnology has emerged as an approach with great potential for unique medical applications. Nanotechnology involves using nanoparticles (NPs) which are microscopic particles whose size is on the nanometer scale. These NPs can be synthesized with organic, inorganic polymers or a combination of both polymers in various molecular sizes and conformations allowing for encapsulation of specifically adapted formulations. Also, they can be made biodegradable. It is these properties of NPs that have encouraged research into their possible application in the prevention of ARM and the treatment of AMD.
The available data on the prevalence of AMD are estimates obtained by extrapolation from independent studies involving a few thousand individuals. The non-uniform classification of AMD in these studies makes it even more difficult to make accurate estimates. According to the NEI and Prevent Blindness America’s (PBA’s) Vision Problems in the US report, 1.65 million Americans age 50 and older have advanced stages of AMD, and this number is expected to double by 2020 (Friedman et al 2004). Worldwide, as many as 30 million people have AMD of various stages. About 11 million Americans are potentially at risk for AMD. It is estimated that about 15–17 million people in the US may have symptoms of AMD and that 2 million have functional blindness; 200 000–500 000 new cases are diagnosed each year (Massof 2002). Some studies suggest more women than men will get macular degeneration, but this may be a consequence of a greater longevity for women. As many 20% of people over age 60 develop AMD and the risk jumps dramatically to as many as 40% over age 75 (VanNewkirk et al 2000). Recently, in a large study with three racially similar populations of 14 752 participants from North America, Europe, and Australia, the prevalence of AMD was found to be 0.2% of the combined population aged 55–64 years, rising to 13% of the population older than 85 years. There is also a striking racial difference. AMD is very uncommon in native African people and Aboriginal Australians. It is uncommon in the US in African Americans, slightly more common in Hispanic Americans, and quite common in European Americans.
Etiology, risk factors, and prevention
AMD is a multi-factorial disease; both environmental and genetic components play a role in its development. The exact causes and the underlying pathomechanisms are not known but several risk factors have been linked to AMD. These include age, gender, social class, race, ethnicity, family history of AMD, smoking, cardiovascular disease, high blood pressure, dietary fat intake, cholesterol levels, alcohol consumption, estrogen levels, light exposure (UV-A and UV-B rays), and low dietary intake of antioxidants (Evans 2002). Current evidence suggests that progression may be slowed with a diet rich in leafy green vegetables, antioxidants, zinc, avoidance of excessive sunlight, and smoking. As for the family history, it has been shown that siblings of a person with AMD have four times greater chance of developing the disease than those who had no relatives with AMD. In the case of identical twins, there is a 40%–100% chance that the other twin develops AMD. For non-identical twins there is a 20%–40% probability (Hammond et al 2002).
Types, classification, signs, and symptoms
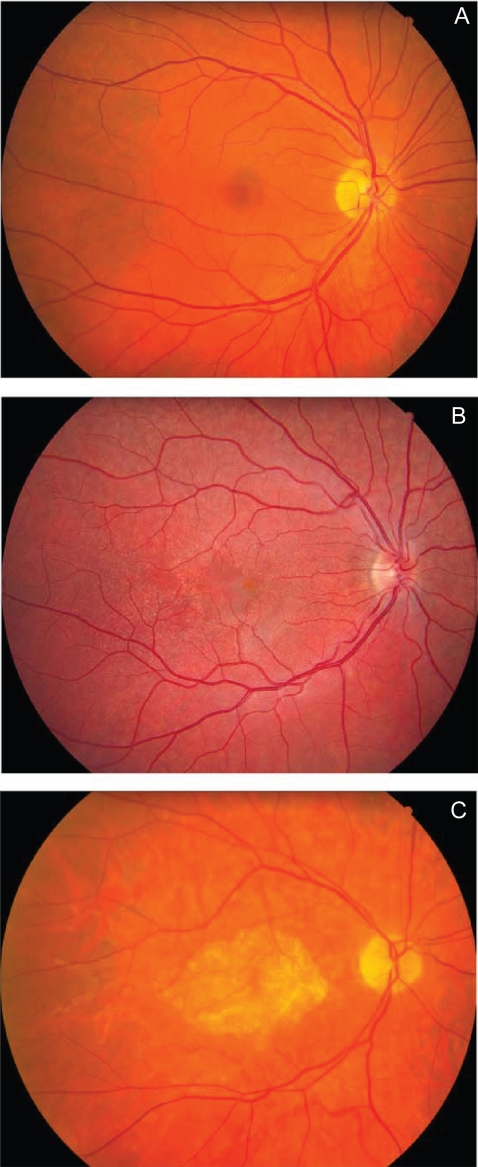
AMD is classified into two types: “dry” (atrophic or nonexudative) and “wet” (neovascular or exudative) forms. The “dry” form is the most common form (90%) and usually progresses slowly. The “wet” form is rare (10%), more severe, and may progress rapidly and cause the most severe vision loss. The characteristic lesions of dry AMD are soft drusen (63 micron or larger) and changes in pigmentation (hypo-, and/or hyperpigmentation) of the retinal pigment epithelium (RPE) (Figure 1). Drusen are multiple, usually discrete, round, slightly elevated, variable-sized, sub-RPE deposits in the macula and elsewhere in the fundus of both eyes. They can be seen as yellow or white spots in the fundus. The overlying RPE shows thinning, whereas the RPE between drusen shows thickening (Bressler and Rosbergerm 1999; Gass et al 2003). In its earliest stages, AMD can be difficult to diagnose. The dry form can advance and cause vision loss without turning into the wet form. It is possible to experience both forms at the same time, in one or both eyes. The onset and progression of either type does not follow any particular pattern. Sometimes the dry form progresses so slowly that a patient may not notice a change in vision. However, it can also suddenly turn into the wet form, even during early stage AMD. There is presently no way to tell if or when the dry form will turn into the wet form. Those with dry AMD have a 4%–12% chance per year of developing choroidal neovascularization (CNV). Patients with extremely large drusen (more than 500 microns in size) have an ever higher (30%) chance that their AMD will convert to the wet form within 5 years. Pigment clumping within the RPE may also be a risk factor for the wet form. All patients start with the dry form of macular degeneration. Atrophy of the RPE becomes more extensive with time. The end stage of the dry form involves the whole macula becoming affected as the geographic atrophy evolves.
Figure 1.
Fundus photographs showing the macula and optic nerve. A. Normal subject; B. Patient with multiple drusen within the posterior pole; C. Patient with advanced AMD showing geographic atrophy GA within the macula.
In the wet type of AMD, new blood vessels grow and leak blood and fluid under the macula. This can lead to retinal detachment, scarring, and irreversible vision loss. Blood vessels grow in from the choriocapillaris under the retina (CNV). The vessels grow through Bruch’s membrane and spread under or above the retinal pigment epithelium or both. These new blood vessels are very fragile, and easily leak and bleed. No blood vessels grow under the macula at first. Instead, they start off to the side of the retina, and grow towards the center. For some, it can take only days to grow under the macula, for others it could take weeks. Abnormal blood vessels can reoccur, sometimes many years later. The consequences of abnormal vessel growth are hemorrhage and scar formation (disciform scarring). Although AMD tends to occur in one eye at a time, approximately 50% of patients who have wet AMD in one eye will also develop this condition in their second eye within 5 years. The progression of the disease varies from a few months to 3 years. Untreated, the majority of eyes affected with wet AMD will become functionally blind within approximately 2 years.
Biomarkers
Inflammation has been implicated in diseases of aging like Alzheimer’s disease, stroke, and cardiovascular disease. Several chronic systemic infections can result in subretinal neovascularization in younger individuals and could play a role in AMD. Therefore, attempts have been made to identify biomarkers for AMD. Carboxyethylpyrrole (CEP) is a biomarker that has been found recently to be associated with susceptibility to AMD. CEP protein adducts are free radical-induced oxidative protein modifications generated from docosahexaenoate (DHA)-containing lipids. DHA is easily oxidizable and abundant in ocular tissues where it is exposed to high photooxidative stress. Immunocytochemistry localized CEP to photoreceptor rod outer segments and retinal pigment epithelium in mouse retina. In a recent report, the mean level of CEP adducts was 1.5 fold higher (p = 0.004) and that of antibody titers in plasma was 2.3-fold higher (p = 0.02) in patients with AMD compared with normal age-matched controls (Gu et al 2003). Of individuals (n = 13) exhibiting both antigen and autoantibody levels above the mean for non-AMD controls, 92% had AMD. Based on the results, CEP immunoreactivity and autoantibody titer may have diagnostic utility in predicting AMD susceptibility. In the future, serum mass spectrometric proteomic pattern analysis will be used to identify additional AMD biomarkers. Being a complex disease, it is possible that multiple biomarkers will be needed to identify those at risk for AMD. The use of signature ion clusters may provide better accuracy and reliability in the process.
C-reactive protein (CRP) is produced by the liver and, similar to CEP, can be detected in blood serum by using an immunological test. It is present during episodes of acute systemic inflammation, and higher levels of CRP have been associated with higher risk of developing cardiovascular disease. Since many factors associated with AMD are also related to cardiovascular disease, the relationship between CRP levels and AMD was examined in a study of 930 individuals from the multi-center Age-Related Eye Disease Study (AREDS) (Cho et al 2004). After adjustment for age, sex, and other variables, including smoking and body mass index, CRP levels were found to be significantly higher among individuals with intermediate and advanced stages of AMD as compared to controls. A two-fold increased risk of AMD was associated with the highest levels of CRP for both smokers and non-smokers. These results suggest that inflammation may play a role in the pathophysiology of AMD and therefore anti-inflammatory agents might be useful for delaying the disease.
General concepts in the pathogenesis of AMD
AMD is a multifactorial disease with characteristics that include drusen, hyperpigmentation, and/or hypopigmentation of the RPE, geographic atrophy and, in a subset of patients, late-stage CNV. To account for these characteristics, the major approaches to understanding the pathogenesis of AMD involve lipofuscin build-up in the RPE; oxidative stress; inflammatory components; abnormal extracellular matrix; atrophy, apoptosis, and CNV.
Lipofuscin
During aging, many postmitotic cells accumulate autofluorescent lysosomal storage bodies known as lipofuscin. Lipofuscin is the generic name given to a heterogeneous group of lipid/protein aggregates that have characteristic yellowish-blue-green fluorescence emission when excited with ultraviolet light. These lipophilic inclusions are located within lysosomes and accumulate with age in a variety of postmitotic metabolically active cells throughout the body and constitute non-degradable end products. In most cell types the primary origin of this material is from the autophagy (degradation of intracellular organelles) of exhausted or damaged organelles (eg, mitochondria, Golgi, endoplasmic reticulum). However, for some cell types lipofuscin can be derived from the incomplete degradation of extracellular material taken into the cell by phagocytosis as in RPE cells (Mullen and LaVail 1976). Lipofuscin is often considered a marker for senescence and referred to as “age-pigment.” Oxidative modification of cellular components through lipid peroxidation is thought to play a role in the mechanism of lipofuscin formation (Kennedy et al 1995). For example, lipid hydroperoxides and cyclic peroxides degrade to form aldehydes such as malondialdehyde and 4-hydroxynonenal that are precursors of fluorescent products. Lipofuscin accumulation has been correlated with a variety of non-ocular diseases, including ceroid-lipofuscinosis, cardiac hypertrophy, cirrhosis of the liver as well as with ocular diseases such as AMD, Best’s disease, and Stargardt disease (Boulton et al 1984; Holz et al 1999; Mata et al 2000). Although lipofuscin has been studied for over a century and remains a focus of much research in AMD, there is still no definitive evidence that the accumulation of lipofuscin results in the impairment of cell function. The main difficulty in assessing the effects of lipofuscin accumulation on cell function is that the same factors that influence lipofuscin accumulation may have effects on cell function independent of their effects on the rate at which lipofuscin builds up in cells (Katz 2002).
RPE lipofuscin is unique in that its formation is dependent, in large part, on the ingestion of photoreceptor outer segments that provide a unique group of proteins, high concentrations of DHA-lipids, and retinoids. Notably, lipofuscin granules have also been observed in human photoreceptors, particularly cones (Iwasaki and Inomata 1988). Formation of RPE lipofuscin appears to be closely associated with retinoids and a normally functioning visual cycle for the regeneration of visual pigments. For example, RPE65 knockout mice do not regenerate 11-cis-retinal and also do not accumulate significant amounts of RPE lipofuscin (Katz and Redmond 2001). Animals deprived of dietary retinol exhibit very little age-related accumulation of lipofuscin in the RPE (Robison et al 1980). There are at least 10 fluorophores in the chloroform fraction of RPE lipofuscin (Eldred and Katz 1988). Two of these fluorophores have now been identified, namely, retinyl palmitate and A2E (Eldred and Laskey 1993; Ben-Shabat et al 2001). Carotenoids may also contribute to RPE lipofuscin since they are present in significant amounts in macular photoreceptors and appear to contribute to lipofuscin formation in brain and heart.
Much has been learned recently about the relationship between lipofuscin and photoreceptor death by studying animal models of Stargardt disease. Stargardt disease is an inherited form of macular degeneration. The disease causing gene (ABCA4) encodes a photoreceptor membrane transport protein (rim protein) (Allikmets et al 1997; Weng et al 1999). Patients show a substantial acceleration in lipofuscin accumulation in the RPE and because this pigment contains A2E, it has been proposed that rim protein is involved in transporting a compound formed in the first step in synthesis of A2E (Weng et al 1999; Molday et al 2000). This is a reaction product of vitamin A aldehyde with ethanolamine, probably derived from phosphatidyl ethanolamine head groups of the photoreceptor outer segment membranes. One molecule of vitamin A aldehyde can react with the ethanolamine group of a phospholipid. It is proposed that transport proteins are involved in moving this intermediate across the outer segment membrane where presumably it is hydrolyzed and the retinal released is reduced to retinol which is delivered back to the RPE (Weng et al 1999). A defect in rim protein may interfere with this transport process resulting in retinal and phosphatidylethanolamine buildup in the outer segments. This allows a second molecule of vitamin A to react, and results in A2E in the shed disks and eventually to accelerated formation of lipofuscin fluorophores in the RPE. Whether defects in ABCA4 are associated with AMD is controversial. Following the initial report indicating a much higher incidence of certain alleles of that gene in familial macular degeneration (Allikmets et al 1997), a subsequent report in a different population showed comparable numbers of polymorphisms in AMD and normal subjects (Stone et al 1998).
Although a cause-and-effect relationship between RPE lipofuscin accumulation and altered cell function has not been established, there is a clear correlation between RPE lipofuscin content, impaired RPE cell function, and photoreceptor cell damage and loss. In particular, the accumulation of extracellular material on the basal side of the RPE has been associated with the development of AMD (Sarks et al 1999; Spraul et al 1999; Delori et al 2000). Experimental manipulations that cause an increase in RPE lipofuscin accumulation have been shown to result in photoreceptor cell losses. For example, animals fed diets lacking certain antioxidant nutrients demonstrate a greatly accelerated rate of RPE lipofuscin-like pigment accumulation (Hayes 1974; Katz et al 1978). Accompanying the build-up of autofluorescent storage bodies in the RPE are significant decreases in photoreceptor cell densities. Thus, if lipofuscin accumulation interferes with normal RPE cell functions, the build-up of this pigment during aging is likely to play a major role in the development of AMD. Indeed, monitoring the fundus by scanning laser ophthalmoscopy has shown that zones of RPE exhibiting intense autofluorescence are prone to atrophy (Scholl et al 2004).
Oxidative stress
Strong indirect evidence that oxidative damage plays a role in AMD comes from studies showing that smoking significantly increases the risk of AMD (Seddon et al 1996) and that antioxidant vitamins and zinc can slow the progression of the disease for select individuals (Age-related eye disease study group 2001). Recent proteomic characterization of drusen established a direct molecular link between oxidative damage and AMD. Oxidative protein modifications uniquely generated from docosahexaenoate (DHA)-containing lipids, namely carboxyethylpyrrole (CEP) adducts, are more abundant in AMD than in normal Bruch’s’s membrane/RPE/choroid tissues. In addition, CEP adducts and CEP autoantibodies were found to be elevated in the plasma of AMD donors (Crabb et al 2002). The photo-oxidative environment in the retina and the lipid- and retinoid-rich photoreceptor outer segments provide an excellent source of reactive oxygen species and oxidation products. Accordingly, it has been proposed that oxidative protein modifications serve as the primary catalysts for macular degeneration associated with age. It has been further hypothesized that lipofuscin plays a major role in generating oxidative protein modifications and in the pathogenesis of AMD. For greater detail, the readers are referred to recent reviews of the role of oxidative stress in AMD (Beatty et al 2000; Liang and Godley 2003).
Inflammatory components
There has been considerable attention recently to the inflammation hypothesis due to the recent discovery of a polymorphism in the gene for complement factor H that occurs more frequently in AMD patients than in controls (Edwards et al 2005; Hageman et al 2005; Haines et al 2005; Klein et al 2005). Anatomical studies provided initial evidence for the role of inflammation in CNV formation in AMD (Penfold et al 1987; Green and Enger 1993). Subsequently, molecular evidence for the role of inflammation in AMD pathogenesis has been developed and summarized (Hageman et al 2001; Johnson et al 2001; Anderson et al 2002). Protein components of drusen include immunoglobulin and components of the complement pathway associated with immune complex deposition (eg, C5b-9 complex), molecules involved in the acute-phase response to inflammation (eg, amyloid P component and 1-antitrypsin), proteins that modulate the immune response (eg, vitronectin, clusterin, apolipoprotein E, membrane cofactor protein, and complement receptor 1), major histocompatibility complex class II antigens, and HLA-DR and cluster differentiation antigens. Cellular components of drusen include RPE blebs, lipofuscin, and melanin, as well as choroidal dendritic cells. It has been suggested that choroidal dendritic cells are activated and recruited by injured RPE (eg, via monocyte chemotactic protein) and oxidized proteins and lipids in the Bruch’s membrane (Hageman et al 2001). A similar process occurs in atherosclerosis. The RPE cells respond to control dendritic cell activation by secreting proteins that modulate the immune response, including vitronectin, apolipoprotein E, and membrane cofactor protein. Cytoplasmic accumulation of vitronectin, apolipoprotein E, and other drusen-associated molecules suggests that the cells are subjected to a chronic sublethal complement attack (Johnson et al 2001). Complement attack can result in the elimination of surface-associated membrane attack complexes (by shedding or endocytosis of cell membrane) and in the formation of extracellular deposits of immune complexes and complement intermediates. An increase in major histocompatibility complex class II immunoreactivity on retinal vascular elements and morphologic changes in microglia has been reported in eyes with incipient AMD (Penfold et al 1997). These immunological changes seemed to be related to early pathological changes in RPE pigmentation and drusen formation. Evidence of inflammatory cell involvement in the later stages of AMD includes the presence of multinucleated giant cells and leukocytes in the choroid of AMD eyes (Dastgheib and Green 1994) and in excised CNVs (Seregard et al 1994). Macrophages and foreign body giant cells near Bruch’s membrane become more common when basal linear deposit is present (Killingsworth et al 1990). Activated macrophages and other inflammatory cells secrete enzymes that can damage cells and degrade the Bruch’s membrane, and, by releasing cytokines, inflammatory cells might foster CNV growth into the sub-RPE space. Thus, in AMD eyes, breaks in the Bruch’s membrane probably are the result and not the cause of CNVs. In some systems, degradation of the extracellular matrix (ECM) is associated with free radical release (Faury et al 1995).
Poorly degradable RPE debris and Bruch’s membrane components (eg, wide-spaced collagen) might stimulate chronic inflammation (Burns and Feeney-Burns 1980; Loeffler and Lee 1992; van der Schaft et al 1993). Hageman and co-workers suggested that activation of choroidal dendritic cells might initiate an autoimmune response to retinal and/or RPE antigens or to neoantigens created within the Bruch’s membrane (Hageman et al 2001). Despite the RPE and retina being immune-privileged tissues, antiretinal and anti-RPE antibodies have been detected in the serum of patients with AMD (Penfold et al 1990; Hageman et al 2001). Johnson and co-workers pointed out that complement activation and associated inflammatory events occur in diseases exhibiting cellular degeneration and accumulation of abnormal tissue deposits, for example, atherosclerosis and Alzheimer’s disease (Johnson et al 2001). In these diseases, damaged cells and highly insoluble protein deposits and extracellular debris activate the classical and alternative complement pathways, resulting in chronic direct and bystander cellular damage with attendant cell surface blebbing, endocytosis, and upregulation of defense proteins.
Abnormal extracellular matrix (ECM)
The RPE deposits cytoplasmic material into the Bruch’s membrane throughout life, possibly to eliminate cytoplasmic debris or as a response to chronic inflammation (Burns and Feeney-Burns 1980; Ishibashi et al 1986; Feeney-Burns et al 1990). Histologically, AMD eyes exhibit abnormal extracellular material in two locations: (1) between the RPE plasmalemma and the RPE basement membrane (basal laminar deposit), and (2) external to the RPE basement membrane within the collagenous layers of the Bruch’s membrane basal linear deposit (Green and Enger 1993). Although basal laminar deposit persists in areas of geographic atrophy, basal linear deposit disappears, which is consistent with the notion that basal linear deposit arises mostly from the RPE-photoreceptor complex (Sarks et al 1994). Basal linear deposit may be more specific to AMD than basal laminar deposit (Curcio and Millican 1999). Soft drusen can represent focal accentuations of basal linear deposit in the presence or absence of diffuse basal linear deposit–associated thickening of the inner aspects of the Bruch’s membrane. Soft drusen can also represent a localized accumulation of basal laminar deposit in an eye with diffuse basal laminar deposit. Thus, the abnormal ECM of AMD eyes includes basal laminar deposit, basal linear deposit, and their clinically evident manifestation, soft drusen.
Drusen represent the earliest clinical finding in AMD. Drusen composition and origin have been analyzed extensively. Small (ie, <63-μm-diameter) drusen generally do not signify the presence of AMD (Klein et al 1992; Green and Enger 1993; Sarks et al 1999). Excessive numbers of small hard drusen, however, can predispose to RPE atrophy at a relatively young age (Sarks et al 1999). Soft drusen are usually pale yellow and large (>63 μm in diameter), with poorly demarcated boundaries. Many different molecules have been identified in drusen, including glycoconjugates containing mannose, sialic acid, N-acetylglucosamine, and β-galactose. Abnormal constituents of the ECM probably underlie the increased blue-green autofluorescence of the Bruch’s membrane in AMD eyes (Marmorstein et al 2002).
A variety of drusen constituents (eg, vitronectin, apolipoproteins B and E, complement, and lipid) are present in atherosclerotic plaques, which may reflect the association of some atherosclerosis risk factors with the development of AMD. Amyloid P component, C5, and 1-antitrypsin are acute-phase reactants (ie, upregulated expression in response to inflammation), and vitronectin, C5, and apolipoprotein E have roles in mediating immune responses. These findings have led to the suggestion that immune complex-mediated damage to RPE cells plays a role in the initiating events of drusen formation. It may be that terminal complement activation promotes drusen breakdown by enzymatic digestion and phagocytosis (Johnson et al 2000). An immune response directed against RPE-derived antigens might be the trigger for drusen formation. In patients with AMD, delayed choroidal perfusion (as visualized with fluorescein and indocyanine green angiography) and psychophysical retinal functional abnormalities may result from the diffusion barrier created by a thickened, lipid-laden Bruch’s membrane.
Atrophy, apoptosis, and CNV
The accumulation of extracellular debris alters Bruch’s membrane composition (ie, increased lipid and protein content) and permeability (eg, decreased permeability to water-soluble constituents in plasma, decreased amino acid transport, and possibly decreased bulk flow of extruded RPE-derived cytoplasmic debris across the Bruch’s membrane). These changes may lead to impaired diffusion of waste products from and of hormones and nutrients to the RPE, including oxygen and vitamin A. In response to this metabolic distress, the RPE probably produces substances that stimulate CNV growth. Several investigators (Kvanta et al 1996; Lopez et al 1996; Frank 1997) have shown that RPE cells associated with CNVs produce VEGF and basic fibroblast growth factor, which may act synergistically to stimulate new blood vessel growth.
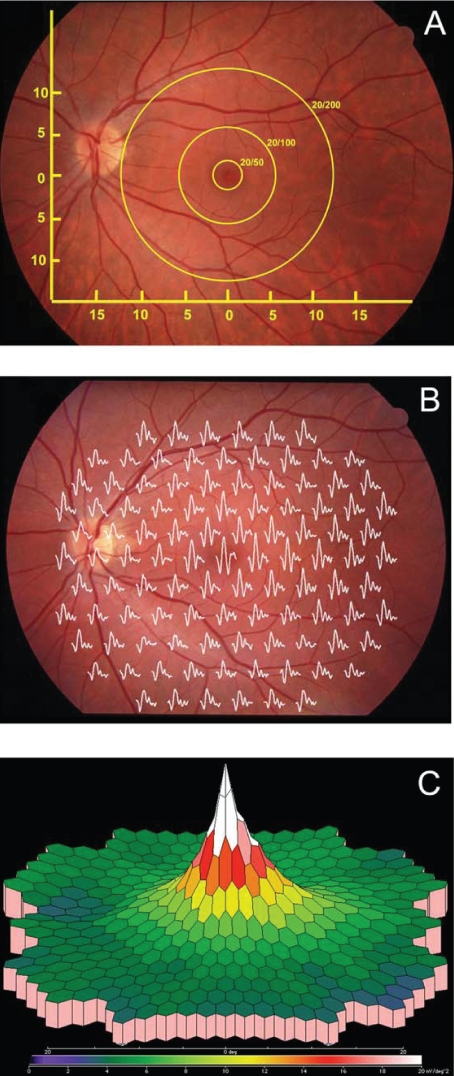
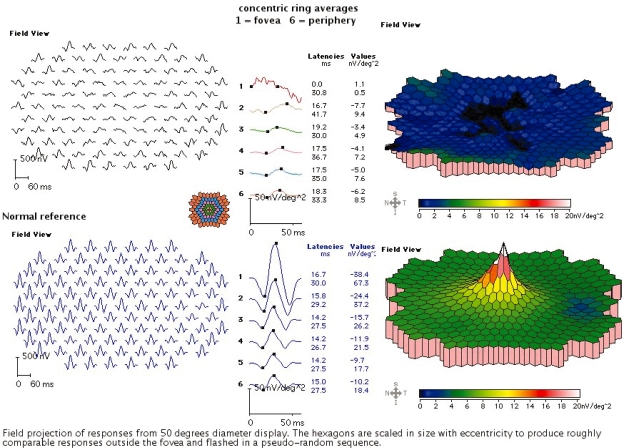
A decrease in visual acuity (VA) is often an early symptom of AMD and is the most commonly used outcome measure in clinical trials. As shown in Figure 2A, a functional fovea is required for normal (20/15–20/20) VA. In the normal macula, regions increasingly eccentric to the fovea have progressively lower resolution. Thus potential VA drops precipitously as geographic atrophy or CNV grows within the macula. An alternative potential outcome measure is shown in Figure 2B. The multifocal electroretinograms (mfERG) (Sutter and Tran 1992) elicit sub-microvolt electrical signals from focal regions within the posterior pole. With suitable scaling (Figure 2C), the complex topography of the macula can be visualized. MfERGs from patients with advanced geographic atrophy reflect the extensive damage to cone photoreceptors within the macula (Figure 3).
Figure 2.
Functional test of the macula. A. Visual acuity tests the resolution limit of the retina. 20/20 vision requires an intact fovea. When the macula is damaged in AMD, the best acuity possible depends on the eccentricity of healthy retina. B. Multi-focal electroretinograms mfERGs can be obtained simultaneously from multiple locations within the macula. C. Three-dimensional false color representations are useful for visualizing the pattern of macula loss. The responses in B and C are from a normal subject.
Figure 3.
mfERGs in AMD. The responses above are from a patient with 20/100 VA. The bottom traces show a representative normal subject. For each patient, the left panels show individual responses, the center panels show summed responses from concentric rings of increasing eccentricity, and the right panels show three-dimensional representations.
Recent functional studies have also shown that the rod system is preferentially affected in aging and ARM. The topography of sensitivity loss at the posterior pole varies considerably among individual patients with AMD. However, in almost all patients (87%) who exhibit reduced light sensitivity, the magnitude of mean rod-mediated sensitivity loss exceeds the magnitude of cone-mediated sensitivity loss (Owsley et al 2000). In areas of increased fundus autofluorescence in patients with AMD, rod-mediated sensitivity loss considerably exceeds cone-mediated sensitivity loss (Scholl et al 2004). Preferential vulnerability of the rods in AMD has also been shown by histological studies. In eyes with late AMD virtually all surviving photoreceptors in the macula are cones, a reversal of the normal predominance of rods (Curcio et al 1996, 2000). In order to account for these findings, Curcio and colleagues introduced the retinoid-deficiency hypothesis for the pathogenesis of AMD (Curcio et al 2000; Curcio 2001; Jackson et al 2002). According to this hypothesis, diffuse deposits of abnormal debris might account for both decreased choroidal perfusion and reduced rod sensitivity by acting as a diffusion barrier between the choroid and the RPE (Chen et al 1992). This interferes with the visual cycle, which includes transport of vitamin A derivatives such as 11-cis-retinal and all-trans-derivatives required by the photoreceptors. Lack of vitamin A affects primarily rods but eventually cones as well (Kemp et al 1989). Relative retinoid deficiency caused by AMD could explain the topographic correspondence between rod-mediated sensitivity loss and RPE dysfunction as indexed by increased lipofuscin (Scholl et al 2004).
Implications for therapy
The basic characteristics outlined previously lead to five general concepts regarding the cell biology of AMD (Zarbin 2004). First, AMD involves aging changes plus additional pathological events. Second, in aging and AMD, oxidative stress causes RPE and, possibly, choriocapillaris injury. Third, in AMD (and perhaps in aging), RPE and, possibly, choriocapillaris injury results in a chronic inflammatory response in Bruch’s membrane and the choroid. Fourth, RPE and, possibly, choriocapillaris injury and inflammation lead to formation of an abnormal extra-cellular matrix (ECM). This abnormal ECM causes altered diffusion of nutrients to the retina and RPE, which may precipitate further RPE and retinal damage. Fifth, the abnormal ECM results in altered RPE choriocapillaris function, leading ultimately to atrophy of the retina, RPE, and choriocapillaris and/or to CNV growth. In this sequence of events, environment and genetics can alter any given patient’s susceptibility to the disease. Manipulation of environmental variables (eg, antioxidant levels) provides an opportunity for early therapeutic intervention. Gene and/or cellular therapy provide an opportunity for later, sight-restoring treatment.
Currently, most pharmacotherapies for AMD require periocular or intraocular administration. Although these approaches generally achieve improved therapeutic concentrations at the target tissues over systemically or topically applied drugs, the rapid clearance of these agents is still an issue. Repeated intraocular injection is not desirable due to the risk of surgery. Moreover, AMD, particularly CNV, probably require sustained release of drugs in order to achieve a successful outcome. To this end, continuous intraocular drug release has been developed through coupling desired drugs to liposomes, microparticles (1–1000 μm) or nanoparticles (1–1000 nm, generally 20–300 nm). These microscopic-size spheres are made from artificial or natural polymers. The commonly used polymers are polylactide (PLA), polylactide-co-glycolide (PLGA), and acrylic polymers, which can be degraded in vivo to form natural metabolites. The degradation rates can be regulated by changing their chemical composition and molecular weight to achieve long-term delivery ranging from months to years (Kranz et al 2000). Thus, this nanotechnology may offer several advantages for administration of drugs in vivo, such as controlled release, injectable and sterilizable formulation, and long shelf-life after lyophilization. In addition, drugs and genetic materials embedded or encapsulated into the NPs can be protected from immediate dilution and degradation and also overcome drug solubility issues. NPs can be synthesized to carry any number of molecules, including proteins, DNA, lipids and organic and inorganic substances (Prow et al 2006). Therefore the flexibility of the target system of NPs is much greater than viral vectors. Some NPs can be made so small, such as dendrimer, less than 5 nm in diameter, which can easily enter the cells through tiny pores in cell membrane. For greater detail, the readers are referred excellent reviews of nanoparticles (PintoMartin et al 1996; Moghimi et al 2005; Moshfeghi and Peyman 2005).
While the majority of studies concerning biological application of nanotechnology have been focused primarily on delivery of chemotherapeutic agents for treating cancers in vivo, using NPs has also recently emerged as a promising tool for ocular research. Among many ocular diseases, AMD may be particularly suitable for usage of this technology as a future treatment option. As described earlier in this article, CNV is a major pathological condition in wet AMD, and VEGF is known to play a major role in the development of CNV. Thus, inhibition of VEGF expression in the retina by various methods has become a central focus for the treatment of neovascular (wet) AMD. Pegaptanib, an anti-VEGF aptamer, can selectively bind with VEGF and inhibit both the growth of blood vessels and vascular leakage, and was approved by the Food and Drug Administration (FDA) as the therapy for the treatment of neovascular AMD in December 2004 (Chakravarthy et al 2006; D’Amico et al 2006). Most recently, ranibizumab (Lucentis™), another VEGF inhibitor, has also been approved by the FDA for treatment of this disease (Heier et al 2006; Rosenfeld et al 2006). Although both drugs showed some efficacy in slowing disease progress and improving vision, they require multiple intravitreal injections, which cause serious adverse reactions, such as endophthalmitis, retinal detachment, and iatrogenic traumatic cataract, iridocyclitis and injection-site reactions (Chapman and Beckey 2006; D’Amico et al 2006; Rosenfeld et al 2006; Singh and Sears 2006). To reduce these side-effects resulting from multiple intravitreal injections, NPs may be used to deliver these drugs for sustained release in a longer time period, which may reduce the frequency of intravitreal injection. In fact, NPs loaded with ganciclovir was administered into the vitreous and was found to prolong the presence of drugs in the eye without toxicity (Merodio et al 2002).
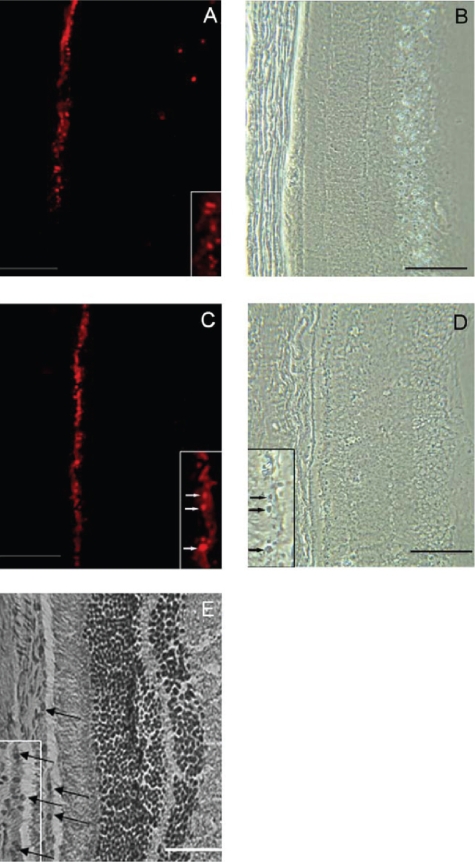
In addition to delivering chemical drugs, nanoparticle-mediated gene delivery has also emerged as a promising tool for gene therapy strategies (Vijayanathan et al 2002; Panyam and Labhasetwar 2003). Compared with viral vector-mediated gene transfer, NPs-mediated delivery of therapeutic genes may reduce concerns about immnunogenecity and reversion of the engineered virus to the wild type (Vasir et al 2006), while in the meantime providing biodegradability and safe pharmaceutical processing. As the pathogenesis of AMD involves RPE and/or phototoreceptor cells, therapeutic genes may be delivered to these cells by NPs. Indeed, NPs were shown to be taken up by RPE cells both in vitro and in vivo (Aukunuru et al 2003; Bourges et al 2003; Bejjani et al 2005; Moshfeghi and Peyman 2005). Compared with other non-viral gene delivery systems, NPs yield higher transfection efficiency and no cell toxicity (Bejjani et al 2005). Recent studies using NPs to deliver a marker gene encoding green fluorescent protein (GFP) into the subretinal space or vitreous of adult mice showed significant levels of GFP expression in photoreceptors and RPE cells (Liu et al 2006; Naash 2006). Transgene expression can be observed within 2–4 days after injection and remain detectable for the following several weeks (Bourges et al 2003; Liu et al 2006; Naash 2006). Interestingly, nanoparticles, due to their small size, injected in the vitreous can migrate through the retinal layers and tend to accumulate in the RPE cells (Figure 4) (Bourges et al 2003; Bejjani et al 2005). The transretinal movement of NPs following intravitreal injection may greatly facilitate intraocular drug/vector delivery to RPE/choroids since intravitreal injection is technically much easier than subretinal injection. In addition, NPs can be used to deliver other types of nucleic acid fragments, such as oligonucleotides and neurotrophic factors. VEGF antisense oligonucleotides encapsulated by NPs were successfully delivered to ARPE-19 cells and inhibit VEGF secretion and mRNA expression (Aukunuru et al 2003). bFGF-loaded NPs showed significant protection against photoreceptor degeneration in RCS rats due to sustained release of bFGF following intravitreal injection (Sakai et al 2006). Hence, the intracellular delivery of molecules by NPs to RPE or photoreceptor cells may open a wide range of therapeutic avenues for AMD. Although intraocularly injected NPs seem to primarily target photoreceptor and RPE cells, other retinal cells, although fewer, were also transduced (Bourges et al 2003; Liu et al 2006; Naash 2006). To facilitate cell-specific binding and transduction into RPE or receptors and reduce unwanted side-effects, cell adhesion molecules or antibody may be conjugated onto NPs. This approach has been successfully tested in other tissues/cells and holds promise as a selective drug delivery system for specific cell types (Fishbein et al 2000; Thomas et al 2004; Dinauer et al 2005; Gullberg et al 2006).
Figure 4.
Phase contrast and fluorescent microscopy of rat retina sections after intravitreous injection of nanoparticles loaded with RNFP plasmid: 7 days A, B and 14 days C, D after the initial intravitreal injection of nanoparticles. Inserts demonstrate the localization of the red fluorescence expression of RNFP within the RPE nuclei. E. Hematoxylin-eosin stained rat retina section. Arrows show nuclei. Bars represent 20 μm. Modified from Bejjani et al (2005) with permission.
One of the commonly used treatments for wet AMD (choroidal neovascularization) is photodynamic therapy (PDT), which is based on the delivery of a photosensitizer to the CNV site via a liposomal formulation of verteporfin (Renno and Miller 2001). For effective PDT against AMD, the selective delivery of photosensitizer to the CNV lesions is necessary. Although low-density lipoproteins and antibodies against endothelial cell markers have been used as carrier molecules (Schmidt-Erfurth et al 1994), photosensitizer also distributes to normal blood vessels as they also express such markers. To circumvent this side effect to the normal retinal/choroidal vessels, nanotechnology-based PDT has been recently tested in laser-induced CNV animal models. Use of a dendritic photosensitizer (dendrimer porphyrin encapsulated by a polymeric micelle) led to a highly selective accumulation of photosensitizer in the CNV lesions, and significantly enhanced the efficacy of PDT, as indicated by the lower power energy of light that was required to occlude the CNV (Ideta et al 2005). These data provide a novel paradigm for treatment of AMD through dendrimer-based nanomedicine.
Patients with advanced AMD suffer severe vision loss due to RPE and photoreceptor cell degeneration. One of the logical treatment approaches is to replace dead RPE cells with normal healthy RPE, ie, RPE transplantation. Although extensive studies have been conducted in animals, RPE transplantation in human has failed so far to provide effective treatments. In a recent study, magnetite nanoparticles were used to construct and deliver RPE cell sheets in vitro (Ito et al 2005). The magnetically labeled RPE cells were seeded into an ultra-low-attachment plate and a magnet was placed under the well. After a 24 hours of culture the magnetically labeled RPE formed an approximately 15-layered cell sheet. When the magnet was removed, the sheets were detached from the bottom of the plate and then harvested and transferred to a tissue culture dish. Subsequently, the cell sheets were attached onto the dish, and the cells growing on the sheets were observed. This nanotechnology-based methodology may provide a new approach for RPE transplantation. Another treatment approach for late-stage AMD patients is to implant an artificial retina (Loewenstein et al 2004). Artificial retinas require electric power. Recently NEI has established the National Center for Design of Biomimetic Nanoconductors. The first task for the center is to design a class of devices for generating electric power—nanobatteries—for a wide array of implantable devices, including artificial retinas. With the artificial retina and accompanying nanobattery, it may be possible to substantially improve vision in patients with certain types of macular degeneration.
Conclusion
The first proven “early” treatment for AMD is oral therapy with antioxidant vitamins and minerals. Other approaches may also be effective. Lipofuscin accumulation in RPE cells can be reduced by treatment with lutein, zeaxanthin, lycopene, or -tocopherol or reversed by centrophenoxine treatment. The role of chronic inflammation in AMD pathogenesis has led to the consideration of anti-inflammatory therapy as treatment for the early stages of the disease (Hageman et al 2001; Anderson et al 2002). NPs represent an excellent potential delivery approach. Clearly, better knowledge of AMD pathogenesis is facilitating the design of more effective therapy for earlier stages of the disease. Nanotechnology will increasingly be useful for delivery of therapeutics. Better knowledge of the biological changes underlying AMD will also foster the development of sight restoring treatments for the late stages of AMD.
Acknowledgments
Supported by the National Institutes of Health (EY05235, EY09076), the Foundation Fighting Blindness, and a grant from the Mary Silverthorne family.
Abbreviations
- AMD
age-related macular degeneration;
- AREDS
Age-Related Eye Disease Study;
- ARM
age-related maculopathy;
- ARMD
age-related macular degeneration;
- CEP
carboxyethylpyrrole;
- CNV
choroidal neovascularization;
- CRP
C-reactive protein;
- DHA
docosahexaenoic acid;
- ECM
extracellular matrix;
- ERG
electroretinogram;
- NPs
nanoparticles;
- RPE
retinal pigment epithelium;
- mfERG
multi-focal ERG;
- NEI
National Eye Institute;
- PDT
photodynamic therapy;
- VA
visual acuity;
- VEGF
vascular endothelial growth factor.
References
- Age-related eye disease study group A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and visual loss. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, et al. Mutation of the Stargardt disease gene ABCR in age-related macular degeneration. Science. 1997;277:1805–7. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, et al. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Aukunuru JV, Ayalasomayajula SP, et al. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3562–9. doi: 10.1211/0022357021701. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh H, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Bejjani RA, BenEzra D, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–32. [PubMed] [Google Scholar]
- Ben-Shabat S, Parish CA, et al. Fluorescent pigments of the retinal pigment epithelium and age-related macular degeneration. Bioorg Med Chem Lett. 2001;11:1533–40. doi: 10.1016/s0960-894x(01)00314-6. [DOI] [PubMed] [Google Scholar]
- Boulton M, Marshall J, et al. Retinitis pigmentosa: a quantitative study of the apical membrane of normal and dystrophic human retinal pigment epithelial cells in tissue culture in relation to phagocytosis. Graefes Arch Clin Exp Ophthalmol. 1984;221:214–29. doi: 10.1007/BF02134143. [DOI] [PubMed] [Google Scholar]
- Bourges JL, Gautier SE, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–9. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- Bressler S, Rosbergerm DF. Nonneovascular nonexudative age-related macular degeneration. In: Guyer DR, Yanuzzi LA, Chang S, et al., editors. Retina-Vitreous-Macula. W. B. Saunders Company; 1999. pp. 79–93. [Google Scholar]
- Burns RP, Feeney-Burns L. Clinicomorphologic correlations of drusen and Bruch’s membrane. Trans Am Ophthalmol Soc. 1980;78:206–23. [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Adamis AP, et al. 2006Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration Ophthalmology 1131508 e1–25. [DOI] [PubMed] [Google Scholar]
- Chapman JA, Beckey C. Pegaptanib: a novel approach to ocular neovascularization. Ann Pharmacother. 2006;40:1322–6. doi: 10.1345/aph.1G604. [DOI] [PubMed] [Google Scholar]
- Chen JC, Fitzke FW, et al. Functional loss in age-related Bruch’s membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–40. [PubMed] [Google Scholar]
- Cho E, Seddon JM, et al. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004;122:883–92. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–83. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, et al. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Visual Sci. 1996;377:1236–49. [PubMed] [Google Scholar]
- Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–39. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- Curcio C, Owsley C, et al. Spare the rods, save the cones in aging and are-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–18. [PubMed] [Google Scholar]
- D’Amico DJ, Patel M, et al. 2006Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials Ophthalmology 1131001 e1–6. [DOI] [PubMed] [Google Scholar]
- Dastgheib K, Green WR. Granulomatous reaction to Bruch’s membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–8. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- Delori FC, Fleckner MR, et al. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:496–504. [PubMed] [Google Scholar]
- Dinauer N, Balthasar S, et al. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26:5898–906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Edwards A O, Ritter R, 3rd, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Katz ML. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp Eye Res. 1988;47:71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Laskey MR. Retinal age pigments generated by self-assembling lysosomotrophic detergents. Nature. 1993;361:724–6. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Evans JR. Antioxidant vitamin and mineral supplements for age-related macular degeneration. Cochrane Database Syst Rev. 2002;2:CD000254. doi: 10.1002/14651858.CD000254. [DOI] [PubMed] [Google Scholar]
- Faury G, Ristori MT, et al. Effect of elastin peptides on vascular tone. J Vasc Res. 1995;32:112–19. doi: 10.1159/000159084. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Burns RP, et al. Age-related macular changes in humans over 90 years old. Am J Ophthalmol. 1990;109:265–78. doi: 10.1016/s0002-9394(14)74549-0. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Chorny M, et al. Nanoparticulate delivery system of a tyr-phostin for the treatment of restenosis. J Control Release. 2000;65:221–9. doi: 10.1016/s0168-3659(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Frank RN. Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Ophthalmic Res. 1997;29:341–53. doi: 10.1159/000268032. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Gass JD, Agarwal A, et al. Focal inner retinal hemorrhages in patients with drusen: an early sign of occult choroidal neovascularization and chorioretinal anastomosis. Retina. 2003;23:741–51. doi: 10.1097/00006982-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–35. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Gu X, Meer SG, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Gullberg E, Keita AV, et al. Identification of cell adhesion molecules in the human follicle associated epithelium FAE that improves nanoparticle uptake into the Peyer’s patches. J Pharmacol Exp Ther. 2006;319:632–9. doi: 10.1124/jpet.106.107847. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, et al. A common haplotype in the complement regulatory gene factor H HF1/CFH predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;206:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Webster AR, et al. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–6. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- Hayes KC. Retinal degeneration in monkeys induced by deficiencies of vitamin E or A. Invest Ophthalmol. 1974;13:499–510. [PubMed] [Google Scholar]
- Heier JS, Antoszyk AN, et al. 2006Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study Ophthalmology 113642 e1–4. [DOI] [PubMed] [Google Scholar]
- Holz FG, Schutt F, et al. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–43. [PubMed] [Google Scholar]
- Ideta R, Tasaka F, et al. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Letters. 2005;5:2426–31. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Patterson R, et al. Formation of drusen in the human eye. Am J Ophthalmol. 1986;101:342–353. doi: 10.1016/0002-9394(86)90830-5. [DOI] [PubMed] [Google Scholar]
- Ito A, Hibino E, et al. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;11:489–96. doi: 10.1089/ten.2005.11.489. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Inomata H. Lipofuscin granules in human photoreceptor cells. Invest Ophthalmol Vis Sci. 1988;29:671–9. [PubMed] [Google Scholar]
- Jackson GR, Owsley C, et al. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–96. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–9. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- Katz ML. Potential role of retinal pigment epithelial lipofuscin accumulation in age-related macular degeneration. Arch Gerontol Geriatr. 2002;34:359–70. doi: 10.1016/s0167-4943(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001;42:3023–30. [PubMed] [Google Scholar]
- Katz ML, Stone WL, et al. Fluorescent pigment accumulation in retinal pigment epithelium of antioxidant-deficient rats. Invest Ophthalmol Vis Sci. 1978;17:1049–58. [PubMed] [Google Scholar]
- Kemp CM, Jacobson SG, et al. Rhodopsin levels and retinal function in cats during recovery from vitamin A deficiency. Exp Eye Res. 1989;49:49–65. doi: 10.1016/0014-4835(89)90075-4. [DOI] [PubMed] [Google Scholar]
- Kennedy CJ, Rakoczy PE, et al. Lipofuscin of the retinal pigment epithelium: a review. Eye. 1995;9:763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- Killingsworth MC, Sarks JP, et al. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4:613–21. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- Klein R, Meuer SM, et al. Detection of drusen and early signs of age-related maculopathy using a nonmydriatic camera and a standard fundus camera. Ophthalmology. 1992;99:1686–92. doi: 10.1016/s0161-6420(92)31745-2. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz H, Ubrich N, et al. Physicomechanical properties of biodegradable polyD,L-lactide and polyD,L-lactide-co-glycolide films in the dry and wet states. J Pharm Sci. 2000;89:1558–66. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, et al. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liu J, Min SH, et al. 2006Linear polyethylenimine-based DNA nanoparticle delivery into mouse retinas Invest Ophthalmol Vis Sci, S:39 [Google Scholar]
- Loeffler KU, Lee WR. Is basal laminar deposit unique for age-related macular degeneration? Arch Ophthalmol. 1992;110:15–6. doi: 10.1001/archopht.1992.01080130017009. [DOI] [PubMed] [Google Scholar]
- Loewenstein JI, Montezuma SR, et al. Outer retinal degeneration: an electronic retinal prosthesis as a treatment strategy. Arch Ophthalmol. 2004;122:587–96. doi: 10.1001/archopht.122.4.587. [DOI] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–68. [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, et al. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci. 2002;43:2435–41. [PubMed] [Google Scholar]
- Massof RW. A model of the prevalence and incidence of low vision and blindness among adults in the U.S. Optom Vis Sci. 2002;79:31–8. doi: 10.1097/00006324-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Mata NL, Weng J, et al. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–9. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merodio M, Irache JM, et al. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials. 2002;23:1587–94. doi: 10.1016/s0142-9612(01)00284-8. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, et al. Nanomedicine: current status and future prospects. Faseb J. 2005;19:311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- Molday LL, Rabin A, et al. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–8. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- Moshfeghi AA, Peyman GA. Micro- and nano-particulates. Adv Drug Deliv Rev. 2005;57:2047–52. doi: 10.1016/j.addr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Naash M.2006Applications of nanotechnology to gene delivery in ophthalmology Invest Ophthalmol Vis Sci, S:53 [Google Scholar]
- Owsley C, Jackson GR, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–73. [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Liew SC, et al. Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:2125–33. [PubMed] [Google Scholar]
- Penfold PL, Provis JM, et al. Age-related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1987;225:70–6. doi: 10.1007/BF02155808. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, et al. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–4. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- PintoMartin JA, Dobson V, et al. Vision outcome at age 2 years in a low birth weight population. Pediat Neurol. 1996;14:281–7. doi: 10.1016/0887-8994(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Prow T, Smith JN, et al. Construction, gene delivery, and expression of DNA tethered nanoparticles. Mol Vis. 2006;12:606–15. [PubMed] [Google Scholar]
- Renno RZ, Miller JW. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv Drug Deliv Rev. 2001;52:63–78. doi: 10.1016/s0169-409x(01)00195-8. [DOI] [PubMed] [Google Scholar]
- Robison WG, Kuwabara T, et al. Deficiencies of vitamin E and A in the rat: retinal damage and lipofuscin accumulation. Invest Ophthalmol Vis Sci. 1980;19:1030–7. [PubMed] [Google Scholar]
- Rosenfeld PJ, Heier JS, et al. 2006Tolerability and efficacy of multiple escalating doses of ranibizumab Lucentis for neovascular age-related macular degeneration Ophthalmology 113632 e1. [DOI] [PubMed] [Google Scholar]
- Sakai T, Kohno H, et al. 2006Prolonged protective effect of basic fibroblast growth factor-loaded nanoparticles in Royal College of Surgeons RCS rats Invest Ophthalmol Vis Sci, S48. [DOI] [PubMed] [Google Scholar]
- Sarks SH, Arnold JJ, et al. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 1999;83:358–68. doi: 10.1136/bjo.83.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks JP, Sarks SH, et al. Evolution of soft drusen in age-related macular degeneration. Eye. 1994;8:269–83. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Hasan T, et al. Vascular targeting in photodynamic occlusion of subretinal vessels. Ophthalmology. 1994;101:1953–61. doi: 10.1016/s0161-6420(13)31079-3. [DOI] [PubMed] [Google Scholar]
- Scholl HP, Bellmann C, et al. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–83. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, et al. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–6. [PubMed] [Google Scholar]
- Seregard S, Algvere PV, et al. Immunohistochemical characterization of surgically removed subfoveal fibrovascular membranes. Graefes Arch Clin Exp Ophthalmol. 1994;232:325–9. doi: 10.1007/BF00175983. [DOI] [PubMed] [Google Scholar]
- Singh RP, Sears JE. Retinal pigment epithelial tears after pegaptanib injection for exudative age-related macular degeneration. Am J Ophthalmol. 2006;142:160–2. doi: 10.1016/j.ajo.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, et al. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(Suppl 1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Stone EM, Webster AR, et al. Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration. Nat Genet. 1998;20:328–9. doi: 10.1038/3798. [DOI] [PubMed] [Google Scholar]
- Sutter EE, Tran D. The field topography of ERG ccomponents in man–I. The photopic luminance response. Vision Res. 1992;32:433–46. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- Thomas TP, Patri AK, et al. In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules. 2004;5:2269–74. doi: 10.1021/bm049704h. [DOI] [PubMed] [Google Scholar]
- Van der Schaft TL, Mooy CM, et al. Early stages of age-related macular degeneration: an immunofluorescence and electron microscopy study. Br J Ophthalmol. 1993;77:657–61. doi: 10.1136/bjo.77.10.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNewkirk MR, Nanjan MB, et al. The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology. 2000;107:1593–600. doi: 10.1016/s0161-6420(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Vijayanathan V, Thomas T, et al. DNA nanoparticles and development of DNA delivery vehicles for gene therapy. Biochemistry. 2002;41:14085–94. doi: 10.1021/bi0203987. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]