Abstract
Nanomedicine, known as the application of nanotechnology in medicine, has been applied to overcome the problems of poor bioavailability, in vitro and in vivo stability, and targeted delivery in the preparation of pharmaceutical products. Sirolimus, a water-insoluble immunosuppressant, has been formulated into an oral solid dosage form by using NanoCrystal® technology to increase the water solubility and thereby the bioavailability. The efficacy, safety, and pharmacokinetic properties are not significantly different between liquid and solid formulations except that less fluctuation of sirolimus blood concentration was observed in solid dosage form. The tablet formulation offers the advantages of better palatability and more convenience for long-term use. Sirolimus tablets are not only a successful example of nanomedicine, but also a more cost-effective treatment in renal transplantation than cyclosporine and tacrolimus.
Keywords: nanomedicine, sirolimus, kidney transplantation, transplantation
Introduction to nanomedicine and issues of transplant rejection
It has been more than 51 years since the first successful human kidney transplantation at the Peter Ben Brigham Hospital in Boston (Merrill et al 1956). Graft rejection is one of the greatest challenges to transplantation. It is the outcome of the natural response of the immune system to a foreign substance, or antigen. This complex process is mainly T-lymphocyte mediated, although it involves serial interactions between foreign antigens, T lymphocytes, macrophages, cytokines (also known as lymphokines or interleukins), adhesion molecules (ie, co-stimulatory molecules), and membrane proteins that enhance binding of T lymphocytes and B lymphocytes (Halloran and Miller 1996; Valente and Alexander 1998). The goal of immunosuppressive therapy is to prevent and treat organ rejection as well as prolong graft and patient survival. With the development of immunosuppressive agents (Table 1), kidney transplantation has become one of the most important renal replacement therapies nowadays (Morris 2004).
Table 1.
Immunosuppressive agents commonly used in transplantation
| Drugs | Indication in transplantation |
|---|---|
| Protein drugs | |
| Monoclonal antibody | |
| Basiliximab (Simulect®) | Induction |
| Daclilzumab (Zenapax®) | Induction |
| Muromonab-CD3 (Orthoclone OKT3®) | Induction, treatment of rejection |
| Polyclonal antibody | |
| Antithymocyte immunoglobulin rabbit (Thymoglobulin®) | Induction, treatment of rejection |
| Antithymocyte immunoglobulin equine (ATGAM®, Lymphoglobulin®) | Induction, treatment of rejection |
| Corticosteroids | |
| Methylprednisolone | Treatment of rejection |
| Prednisone, prednisolone | Maintenance therapy |
| Immunophilin-binding drugs | |
| Calcineurin inhibitors (CNI) | |
| Cyclosporine (Neoral®, Sandimmune®) | Maintenance therapy |
| Tacrolimus (Prograf®) | Maintenance therapy |
| Mammalian target of rapamycin inhibitors (m-TOR inhibitor) | |
| Sirolimus (Rapamune®) | Maintenance therapy |
| Everolimus (Certican®) | Maintenance therapy |
| Antiproliferative agents | |
| Antimetabolites | |
| Azathioprine (Imuran®) | Maintenance therapy |
| Purine synthesis inhibitors | |
| Mycophenolate mofetil (CellCept®) | Maintenance therapy |
| Mycophenolate sodium (Myfortic®) | Maintenance therapy |
During the “azathioprine era” from the 1960s to the early 1980s, 1-year cadaveric graft survival rate was around 60% (Morris 2004; Sayegh and Carpenter 2004). With the introduction in 1978 of cyclosporine, a calcineurin inhibitor (CNI), the 1-year graft survival rate increased to more than 80% (Sayegh and Carpenter 2004). In the 1990s, the availability of several other immunosuppressive agents with different mechanisms of action (tacrolimus, mycophenolate mofetil, and sirolimus) made combination immunosuppressive therapy a general modality in organ transplantation. However, due to the potency of these agents and inter- and intra-individual variability in pharmacokinetics, dose individualization is required to maintain adequate immunosuppression while minimizing adverse reactions.
Poor water solubility and bioavailability contribute to the complexity of dosing cyclosporine and sirolimus. Pharmaceutical companies have been formulating insoluble drugs into commercially available products and minimizing the problem of large intra- and inter-individual variability in pharmacokinetic profiles. A limited number of methods such as generation of ionized drugs, co-solvents, surfactants, soft-gel technology, micronization, and nanotechnology have been utilized in formulating water-insoluble drugs. In addition, other particular drug carriers, such as emulsion, liposome, and polymeric micelle, are considered as alternative methods to deliver water insoluble drugs (Yokoyama 2005). For example, cyclosporine has been emulsified by bile salts to form micelles and further modified into microemulsion from the olive oil-based solution to enhance bioavailability (Thomas et al 2005). The liposomal formulation of tacrolimus also has been demonstrated as an effective strategy to increase efficacy and decrease toxicity (McAlister et al 1999; Alemdar et al 2004). The formulation of amphiphilic block co-polymer micelles has been used to prepare injectable formulations of the highly lipophilic sirolimus (Ashok et al 2004; Forrest et al 2006).
After the introduction of tacrolimus (a CNI with a potency 32–100 times that of cyclosporine), less variation in bioavailability and not requiring bile salts for oral absorption made tacrolimus a better choice than cyclosporine (Sandimmune®, Novartis) in liver transplantation (Scott et al 2003). This forced a reformulation of cyclosporine to retain its market share. The new formulation of cyclosporine (Neoral®, Novartis) is a microemulsion utilizing 9.5% w/v alcohol, corn oil-mono-di-triglyceride, polyoxyl 40 hydrogenated castor oil, and polyethylene glycol as vehicle (PDR 2006). This is an application of microtechnology to drug delivery, a breakthrough in the 1990s.
In the 2000s, a more advanced technology called nanotechnology was applied to medicine, which led to the emergence of nanomedicine (Weber 1999). Nanomedicine is the application of nanotechnology to medicine (Freitas 2005). More broadly, it is the process of applying molecular tools and molecular knowledge of the human body in diagnosis, treatment, and prophylaxis of disease, as well as preserving and improving human health (Freitas 2005). Many nanomedicine technologies have been developed and are close to fruition. Among them, nanostructured materials such as fullerenes have been used in pharmaceuticals (Freitas 2005). Sirolimus (Rapamune®; Wyeth-Ayerst, Philadelphia, PA, USA) tablet is another example of the successful application of nanotechnology in pharmaceuticals to overcome the problems of formulation, poor bioavailability, and erratic absorption.
Chemistry, nanocrystal technology used, and formulation of sirolimus
Sirolimus is a triene marcolide antibiotic isolated from Streptomyces hygroscopicus (Vezina et al 1975; Sehgal 1998). It is challenging to formulate sirolimus into either an intravenous or oral dosage form due to its water insolubility and a logP (log of the octanol-water partition coefficient, a measure of a drug’s lipophilicity) of greater than 5. The solubility of sirolimus is 2.6 μg/mL, which is far below the target solution concentration of 1 mg/mL (Simamora et al 2001). It is impossible to enhance the solubility of sirolimus by the generation of a salt form because of the lack of an ionizable group of sirolimus in the pH range of 1–10 (Simamora et al 2001). The solubility of sirolimus in a single organic solvent, such as ethanol, γ-butyrolactone, dimethyl isosobide, and glycerol formal (a mixture of 5-hydroxy-1,3-dioxane and 4-hydroxymethyl-1,3-dioxolane in a ratio of 60:40), increases thousands-fold to greater than 90 mg/mL. Although a thousand-fold decrease in the solubility of sirolimus was observed in single cosolvent–water mixtures, the solubility could be increased to more than 10 mg/mL when it was prepared in multiple cosolvent and hydrotrope mixtures, such as 10% ethanol, 40% propylene glycol, 5% benzyl alcohol, and 3%–5% benzoate buffer (Simamora et al 2001). In 1999, the first commercially available product of sirolimus was thus an oral solution with a concentration of 1 mg/mL in Phosal 50 PG (a dispersion of 50% phosphatidylcholine in a propylene glycol/ethanol carrier) and polysorbate 80 (PDR 2003). The taste, and the requirement for refrigerator storage, protection from light, and disposal of the oral syringe after a single use make the oily solution an inconvenient dosage form (Vasquez 2000).
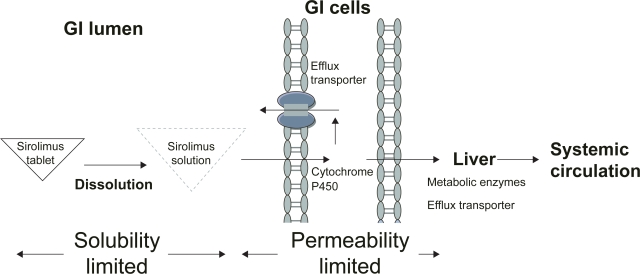
To deliver sirolimus orally in a solid dosage form, the major problem of the dissolution of sirolimus from the dosage form had to be overcome, as well as its permeability across the biological barrier, such as first-pass effect via intestinal and hepatic metabolism and efflux transporter P-glycoprotein (Figure 1). It took one more year to explore the oral tablet formulation of sirolimus using NanoCrystal® technology acquired by Elan Corporation (Rosen and Abribat 2005). This product, launched in 2002, offers greater palatability and convenience of administration and storage (at 20–25°C).
Figure 1.
Factors influencing the bioavailability of oral tablet sirolimus. Generally, the bioavailability of oral tablet sirolimus is determined by the solubility and permeability of sirolimus. The nanocrystalline sirolimus improves sirolimus dissolution, saturation solubility, and stability in gastrointestinal (GI) lumen and thereby improves the aborption. However, sirolimus is the substrate for the metabolic enzyme (cytochrome P450 3A) and efflux transporter (P-glycoprtoein) in intestinal and hepatic cells. Therefore, less than 20% of sirolimus can reach systemic circulation.
Sirolimus is formulated as nanometer-sized drug crystals by NanoCrystal technology using high-shear media mills. According to the Noyes-Whitney equation of dissolution (Noyes and Whitney 1897), the rate of dissolution is proportional to the effective surface area (A) and the difference (Cs–C)/h:
Where D is the diffusion coefficient of the drug, h is the thickness of diffusion boundary layer, Cs is the saturation solubility of the drug, and C is the concentration of drug in the medium. Nanocrystalline sirolimus not only increases the effective surface area (A), but also increases the saturation solubility (Cs) and decreases the thickness of the diffusion boundary layer (h) (Muller et al 2001; Merisko-Liversidge et al 2003; Muller and Keck 2004). The saturation solubility can be increased only when the particle size is reduced in a submicron range (Muller and Keck 2004). In addition, it possesses long-term physical stability without Ostwald ripening (the tendency for larger particles to grow in diameter over time, while the smaller particles dissolve in a highly dispersed systems) due to the uniform nanometer-sized particles (Muller and Keck 2004). High adhesiveness on biological surfaces and high endocytosis of nanocrystalline drugs were also reported (Kayser 2000; Muller and Keck 2004). Therefore, nanocrystalline sirolimus may provide a range of improvements in bioavailability, dose proportionality, absorption variability, and absorption rate.
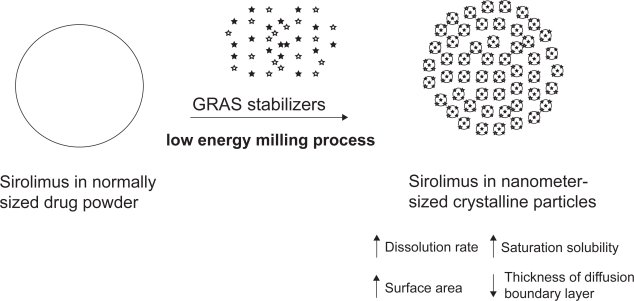
In the process of sirolimus nanocystallization, generally recognized as safe (GRAS) stabilizers were added to avoid the agglomeration/aggregation of the drug crystals due to surface energy of nanocrystalline particles (Figure 2) (Merisko-Liversidge et al 2003). A mixture of non-ionic and ionic stabilizers, such as cellulosics, poloxamer, polysorbates, lecithin, sodium glycocholate and polyvinylpyrrolidones, is required to create a steric barrier and an electrostatic repulsion among particles, respectively (Patravale et al 2004). The inactive ingredients of sirolimus tablet include microcrystalline cellulose, polyethylene glycol 8000, polyethylene glycol 20000, poloxamer 188, sucrose, lactose, calcium sulfate, pharmaceutical glaze, talc, titanium dioxide, magnesium stearate, povidone, glyceryl monooleate, carnauba wax, and other ingredients (PDR 2003). Some of the disclosed and concealed inactive GRAS ingredients were the essential components in the process of sirolimus nanocrystallization.
Figure 2.
The nanocrystalline sirolimus system. Generally recognized as safe (GRAS) stabilizers were milled with sirolimus into nanocrystal particles by NanoCrystal® technology.
Immunopharmacology and pharmcokinetics of sirolimus
Sirolimus, with a chemical structure related to tacrolimus, is one of the most potent immunosuppressive agents for prevention of rejection (Sehgal 1998; Vasquez 2000). When combined with cyclosporine- or tacrolimus-based regimen in kidney transplantation, sirolimus increases immunosuppressive activity through a sequential synergistic mechanism.
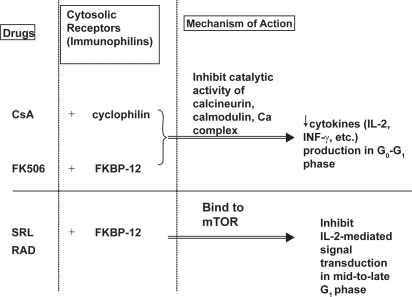
Like cyclosporine and tacrolimus, sirolimus binds to cytosolic receptors, an intracellular protein, known as immunophilins (Figure 3). Cyclosporine binds to cyclophilin of the immunophilin family, forming a complex that then engages calcinueurin (Clipstone and Crabtree 1992). Both sirolimus and tacrolimus engage another immunophilin called FK-binding protein-12 (FKBP-12). Tacrolimus-FKBP complex, like cyclosporine-cyclophilin complex, inhibits the catalytic activity of calcineurin-calcium-calmodulin complex. The inhibition of calcineurin activity decreases the production of IL-2 and other cytokines, thereby blocking their activation of T-lymphocytes in the G0 and G1 phases of cell cycle.
Figure 3.
Mechanism of action of cyclosporine, tacrolimus, and sirolimus. Adapted with permission from Wu FL, Tsai MK, et al. 2005. Effects of conversion from sirolimus oral solution to tablets in stable Taiwanese renal transplant recipients. J Formos Med Assoc, 104:22–8. © The Formosan Medical Association.
Abbreviations: CsA, cyclosporine; Ca, calcium; FK506, tacrolimus; KBP, FK binding protein; INF-γ, γ-interferon; IL, interleukin; mTOR; mammalian target of rapamycin; RAD; everolimus; SRL, sirolimus.
In contrast, sirolimus-FKBP complex binds to the mammalian target of rapamycin (mTOR), thereby inhibiting T-lymphocyte proliferation through inhibition of interleukin-2-mediated (IL-2-mediated) signal transduction in mid to late G1 phase and preventing cell-cycle progression from the G1 phase to the S phase (Vathsala et al 1990; Sehgal 1998). It also inhibits IL-2 dependent and independent B-lymphocyte proliferation and antibody production (Aagaard-Tillery and Jelinek 1994).
Sirolimus oral solution has an oral bioavailability of about 15% and distributes widely in tissue (volume of distribution 19 L/kg; Yatscoff 1996; Mahalati and Kahan 2001). The mean oral bioavailability of sirolimus tablets is not significantly different from that of oral solution (Kelly et al 1999; Hariharan and Zimmerman 2000; Kelly et al 2000; PDR 2003). The tablets are not bioequivalent to the oral solution. However, clinical equivalence has been demonstrated at the 2-mg dose level (VanBuren 2000; PDR 2003). At doses between 3 and 12 mg/m2, there is a linear correlation between the whole-blood concentration and dosage of sirolimus solution within the same transplant individuals (PDR 2003). The mean time-to-peak concentration (tmax) is about 1 hour after a single dose in healthy volunteers and 2 hours after multiple oral doses in renal transplant recipients (PDR 2003). A study by Kelly et al (1999) indicated that there were no differences between the liquid and tablet in trough concentrations (C0) and area under the concentration–time curve (AUC) during dosage form conversion in stable kidney transplant recipients. However, tablets had a lower whole-blood peak concentration (Cmax) and dose corrected Cmax. The tmax of tablets was longer, but was significant only at 4 weeks post-conversion. The inter-individual variability and percentage fluctuation of sirolimus serum concentration were less in tablets than in liquids (Kelly et al 2000). These results indicate that sirolimus tablets provide more consistent immunosuppressive exposure over the dosing interval in each individual than the liquid formulation.
Sirolimus is metabolized extensively by liver enzymes, with only 2.2% excreted unchanged in urine (PDR 2003). A study by Wu et al (2005) found that the dose-adjusted trough concentration of sirolimus in patients with persistent liver enzyme elevation was significantly higher than in those with normal liver function. Like cyclosporine and tacrolimus, sirolimus is a substrate for both cytochrome P450 (CYP) 3A and P-glycoprotein (P-gp) (Sattler et al 1992; Turgeon et al 1994; Lampen et al 1995; Fricker et al 1996; Yatscoff 1996; Lown et al 1997; Masuda et al 2000), and thus has potential drug interactions similar to those of CNIs. In addition, the potential drug interactions between CNIs and sirolimus have been a concern. Sirolimus did not significantly affect the pharmacokinetics of either an olive oil-based or the microemulsion-formulated cyclosporine in human studies (Zimmerman and Kahan 1997; Kahan et al 1998; MacDonald et al 2000). A limited number of available studies did not reveal an effect of tacrolimus on the pharmacokinetics of sirolimus (MacDonald et al 2000; McAlister et al 2002; Kuypers et al 2003; Undre 2003). However, both an olive oil-based or the microemulsion-formulated cyclosporine may increase the bioavailability of sirolimus (PDR 2003). In addition, the bioavailability and trough concentrations of sirolimus are significantly higher when microemulsion-formulated cyclosporine and sirolimus are administered concomitantly than when they are staggered by 4 hours (Kaplan et al 1998; MacDonald et al 2000; PDR 2003). Thus, the manufacturer of sirolimus, Wyeth Ayerst (Philadelphia, PA), has suggested that the two drugs be given 4 hours apart. However, a randomized, parallel-group study has shown that during multiple-dose administration, the bioavailability and trough concentration of sirolimus were, respectively, 1.46 and 1.42 times higher in the cyclosporine group than in the tacrolimus group, even though sirolimus was administered 6 hours after CNIs (Wu et al 2005). Staggered administration cannot completely prevent a drug interaction between cyclosporine and sirolimus solution. Whether sirolimus tablets exert the same drug interaction with cyclosporine remains to be studied.
Efficacy, comparative studies, and prolonged therapy
In September 1999, sirolimus was approved by the US Food and Drug Administration (FDA) for use in combination with cyclosporine and steroids. In November 2000, the drug was registered by the European Agency as an alternative to CNI for maintenance therapy. In April 2003, the FDA approved a new regimen to reduce the use of cyclosporine by substituting higher doses of sirolimus (Kahan 2004).
The safety and efficacy of sirolimus oral solution and tablets for the prevention of graft rejection following kidney transplantation has been compared in a 477-patient, randomized, multicenter, controlled trial. There was no significant difference between the oral solution and tablet formulation for rejection, graft, and patient survival at 3, 6, and 12 months after transplantation (VanBuren 2000; PDR 2003).
A CNI-free sirolimus-based protocol may avoid CNI nephrotoxicity. The incidences of acute rejection, chronic graft failure, and graft loss associated with this sirolimus-based therapy were 19%, 14%, and 10%, respectively (Kahan 2004). The long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal have been demonstrated by Kreis et al (2004). Lipid profiles were similar between groups, while blood pressures and incidence of abnormal kidney function, edema, hyperuricemia, hyperkalemia, gingival hyperplasia, and Herpes zoster were significantly lower in the sirolimus-based therapy. Recipients with low to moderate rejection risk may consider stopping cyclosporine 2–4 months after transplantation (Halloran 2004; Kreis et al 2004). Compared with other immunosuppressive agents, regimens containing sirolimus have a lower incidence of cytomegalovirus (CMV) and BK polyoma virus infection (Halloran 2004; Kahan 2004).
Different from other immunosuppressive agents, m-TOR inhibitors may have antineoplastic and arterial protective effects. Sirolimus slows the growth of established experimental tumors (Guba et al 2002). According to Kahan’s study group (Kahan 2004), sirolimus therapy oughtweighs other immunosuppressive agents in the low incidence of malignancy. Compared with the general US population, the sirolimus/cyclosporine therapy showed equal incidence of skin cancers, and a 4-fold increase in post-transplant lymphoproliferative disorder (PTLD) and renal cell carcinoma. It was also reported to inhibit the progression of dermal Kaposi’s sarcoma in kidney-transplant recipients while providing effective immunosuppression (Stallone et al 2005). The incidence of Kaposi’s sarcoma among solid organ recipients is about 500 times that in the general population (Hayward 2003). Three months after switching from cyclosporine to sirolimus in 15 kidney-transplant recipients who had biopsy-proven Kaposi’s sarcoma, all cutaneous Kaposi’s sarcoma lesions disappeared in all patients (Guba et al 2002).
Incorporation of sirolimus into coronary stents inhibits restenosis (Morice et al 2002; Dibra et al 2005). Combination of everolimus with CNIs reduced the incidence of graft coronary artery disease associated with heart transplantation (Eisen et al 2003). However, the potential arterial protective effects of m-TOR inhibitors must be weighed against their hyperlipidemia side-effect (Blum 2002).
Tolerability
The major adverse reactions of sirolimus include hyper-lipedemia, thrombocytopenia, and impaired wound healing (PDR 2003). Delayed recovery from acute tubular necrosis in kidney transplants, aggravation of proteinuria, mouth ulcers, pneumonitis, and skin lesions were also reported (Halloran 2004). Theoretically, sirolimus should not cause nephrotoxicity due to lack of CNI activity (Sehgal 1998). Its combination with CNIs was expected to decrease nephrotoxicity of CNIs due to dose reduction of CNIs (McAlister et al 2000; Kahan and Camardo 2001). However, the combinations have been reported to increase nephrotoxicity, the hemolytic-uremic syndrome, and hypertension (Gonwa et al 2003; Halloran 2004). It should be noted that the dose and whole-blood concentrations of CNIs and sirolimus should be taken into consideration in interpreting these data. For example, in the study by Gonwa et al (2003), there was no difference between the two groups in median tacrolimus whole-blood trough concentration (8.5 ng/mL and 8.7 ng/mL in the sirolimus- and mycophenolate-treatment groups, respectively). That is, there was no dose reduction of tacrolimus in the sirolimus-treatment group. The median sirolimus dose was 3 mg/day with a corresponding median whole-blood trough concentration 7.3 ng/mL at 6 months, which was higher than 2 mg/day (the dosage usually recommended). Dose reduction of CNIs as well as minimizing the dose of sirolimus is crucial to minimize toxicity of this combination. Withdrawing one of the drugs in the combination of a m-TOR inhibitor and cyclosporine can also reduce renal dysfunction and hypertension, with a small increase in rejection episodes (Johnson et al 2001).
Pharmacoeconomic data and patient acceptability
With the help of the nanotechnology, tablet formulation of sirolimus was successfully introduced onto the market one year after the launch of the oil-based liquid formulation. Patient satisfaction is greater with sirolimus tablet than oil-based liquid form because of improvement in palatability, less restricted storage conditions, and greater convenience of administration. Tablet sirolimus is now the preferred dosage form except in those who require a lower dose or cannot take tablets.
Sirolimus has been recognized as a potent immunosuppressive agent with less nephrotoxicity than CNIs, such as tacrolimus and cyclosporine. The cost-effectiveness and cost-utility studies of sirolimus versus tacrolimus and cyclosporine in kidney transplant patients have been reported by McEwan et al (2005, 2006). A stochastic simulation model was developed using clinical trial and observational data to forecast the incidence of graft failures, hemodialysis, peritoneal dialysis, retransplants, and acute rejections. Using historical data on 937 renal transplant recipients, the costs of the different events from the perspective of the UK National Health Service, valued at 2003 prices with discount, were then used to evaluate both cost-effectiveness and cost-utility over 10 and 20 years after transplantation (McEwan et al 2005, 2006). Treatment with sirolimus was projected to gain 0.72 and 1.8 discounted years of functioning graft over tacrolimus over 10-year and 20-year time horizons, respectively (McEwan et al 2005). Similarly, when compared with cyclosporine, sirolimus gained 0.6 and 1.59 discounted years of functioning graft and a cost saving of US$486 and US$13033 per patient over 10-year and 20-year time horizons, respectively (McEwan et al 2006).
Conclusions
Sirolimus is one of the most potent immunosuppressive agents in transplantation which is useful in combination with CNI or as an alternative to CNI for maintenance therapy. Although sirolimus is correlated with higher incidence of hyperlipedemia, thrombocytopenia, impaired wound healing, delayed graft function, proteinuria, mouth ulcers, pneumonitis, and skin lesions, it has antineoplastic and arterial protective effects, and is associated with lower incidence of CMV infection in transplant recipients. With the application of nanotechnology, NanoCrystal formulation overcomes the problems of formulation, poor bioavailability, and erratic absorption of sirolimus. The tablet formulation has a better palatability, and is more convenient for long-term use. In addition, cost-effectiveness and cost-utility analysis also demonstrated the benefits of long-term use of sirolimus in kidney transplantation. Using nanocrytalline sirolimus as a successful prototype, nanotechnology is a prominent approach in the formulation of water-insoluble drugs for clinically applicable dosage forms in the future.
Footnotes
Disclosures
Neither of the authors has any conflict of interest.
References
- Aagaard-Tillery KM, Jelinek DF. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 1994;156:493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- Alemdar AY, Sadi D, et al. Liposomal formulations of tacrolimus and rapamycin increase graft survival and fiber outgrowth of dopaminergic grafts. Cell Transplant. 2004;13:263–71. doi: 10.3727/000000004783983936. [DOI] [PubMed] [Google Scholar]
- Ashok B, Arleth L, et al. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–87. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. Am J Transplant. 2002;2:551–9. doi: 10.1034/j.1600-6143.2002.20610.x. [DOI] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–7. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Dibra A, Kastrati A, et al. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–70. doi: 10.1056/NEJMoa044372. [DOI] [PubMed] [Google Scholar]
- Eisen HJ, Tuzcu EM, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- Forrest ML, Won CY, et al. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110:370–7. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Freitas RA. What is namomedicine. Nanomedicine: Nanotechnology, Biology, and Medicine. 2005;1:3–9. doi: 10.1016/j.nano.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fricker G, Drewe J, et al. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br J Pharmacol. 1996;118:1841–7. doi: 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonwa T, Mendez R, et al. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation. 2003;75:1213–20. doi: 10.1097/01.TP.0000062837.99400.60. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- Halloran PF, Miller LW. In vivo immunosuppressive mechanisms. J Heart Lung Transplant. 1996;15:959–71. [PubMed] [Google Scholar]
- Hariharan S, Zimmerman JJ.2000A comparative study of the pharmacokinetic profiles of sirolimus oral solution and tablets in renal allograft patients [abstract] Transplantation 69SupplS154no. 159 [Google Scholar]
- Hayward GS. Initiation of angiogenic Kaposi’s sarcoma lesions. Cancer Cell. 2003;3:1–3. doi: 10.1016/s1535-6108(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Kreis H, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–86. doi: 10.1097/00007890-200109150-00007. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Sirolimus: a ten-year perspective. Transplant Proc. 2004;36:71–5. doi: 10.1016/j.transproceed.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72:1181–93. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Podbielski J, et al. Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation. Transplantation. 1998;66:1040–6. doi: 10.1097/00007890-199810270-00013. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Meier-Kriesche HU, et al. The effects of relative timing of sirolimus and cyclosporine microemulsion formulation coadministration on the pharmacokinetics of each agent. Clin Pharmacol Ther. 1998;63:48–53. doi: 10.1016/S0009-9236(98)90120-5. [DOI] [PubMed] [Google Scholar]
- Kayser O. Nanosuspensions for the formulation of aphidicolin to improve drug targeting effects against leishmania infected macrophages. Int J Pharm. 2000;196:253–6. doi: 10.1016/s0378-5173(99)00434-2. [DOI] [PubMed] [Google Scholar]
- Kelly P, Napoli KL, et al. 2000Comparison of the pharmacokinetics of sirolimus (Rapamune) in renal transplant recipients following administration of the liquid or solid tablet formulations [abstract] Transplantation 69SupplS154no. 158. [Google Scholar]
- Kelly PA, Napoli K, et al. Conversion from liquid to solid rapamycin formulations in stable renal allograft transplant recipients. Biopharm Drug Dispos. 1999;20:249–53. doi: 10.1002/(sici)1099-081x(199907)20:5<249::aid-bdd181>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kreis H, Oberbauer R, et al. Long–term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809–17. doi: 10.1097/01.asn.0000113248.59077.76. [DOI] [PubMed] [Google Scholar]
- Kuypers DR, Claes K, et al. Long-term pharmacokinetic study of the novel combination of tacrolimus and sirolimus in de novo renal allograft recipients. Ther Drug Monit. 2003;25:447–51. doi: 10.1097/00007691-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Lampen A, Christians U, et al. Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variability. Drug Metab Dispos. 1995;23:1315–24. [PubMed] [Google Scholar]
- Lown KS, Mayo RR, et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–60. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Scarola J, et al. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- McAlister VC, Gao Z, et al. Sirolimus-tacrolimus combination immunosuppression. Lancet. 2000;355:376–7. doi: 10.1016/S0140-6736(99)03882-9. [DOI] [PubMed] [Google Scholar]
- McAlister VC, Keshavamurthy M, et al. Oral delivery of liposomal tacrolimus: increased efficacy and reduced toxicity. Transplant Proc. 1999;31:1110. doi: 10.1016/s0041-1345(98)01923-x. [DOI] [PubMed] [Google Scholar]
- McAlister VC, Mahalati K, et al. A clinical pharmacokinetic study of tacrolimus and sirolimus combination immunosuppression comparing simultaneous to separated administration. Ther Drug Monit. 2002;24:346–50. doi: 10.1097/00007691-200206000-00004. [DOI] [PubMed] [Google Scholar]
- McEwan P, Baboolal K, et al. Evaluation of the cost-effectiveness of sirolimus versus cyclosporin for immunosuppression after renal transplantation in the United Kingdom. Clin Ther. 2005;27:1834–46. doi: 10.1016/j.clinthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- McEwan P, Dixon S, et al. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics. 2006;24:67–79. doi: 10.2165/00019053-200624010-00006. [DOI] [PubMed] [Google Scholar]
- Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40:573–85. doi: 10.2165/00003088-200140080-00002. [DOI] [PubMed] [Google Scholar]
- Masuda S, Uemoto S, et al. Effect of intestinal P-glycoprotein on daily tacrolimus trough level in a living-donor small bowel recipient. Clin Pharmacol Ther. 2000;68:98–103. doi: 10.1067/mcp.2000.107912. [DOI] [PubMed] [Google Scholar]
- Merisko-Liversidge E, Liversidge GG, et al. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20. doi: 10.1016/s0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Merrill JP, Murray JE, et al. Successful homortransplantation of the human kidney between identical twins. JAMA. 1956;160:277–82. doi: 10.1001/jama.1956.02960390027008. [DOI] [PubMed] [Google Scholar]
- Morice MC, Serruys PW, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–80. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Morris PJ. Transplantation--a medical miracle of the 20th century. N Engl J Med. 2004;351:2678–80. doi: 10.1056/NEJMp048256. [DOI] [PubMed] [Google Scholar]
- Muller RH, Jacobs C, et al. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:151–70. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–4. [Google Scholar]
- Patravale VB, Date AA, et al. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–40. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- PDR, editor. Rapamune oral solution and tablets Physicians’ Desk Reference. Montvale: Medical Economics Company; 2003. [Google Scholar]
- PDR, editor. Neoral soft gelatin capsules (Novartis) Physicians’ Desk Reference. Montvale: Thomson PDR; 2006. [Google Scholar]
- Rosen H, Abribat T. The rise and rise of drug delivery. Nat Rev Drug Discov. 2005;4:381–5. doi: 10.1038/nrd1721. [DOI] [PubMed] [Google Scholar]
- Sattler M, Guengerich FP, et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–61. [PubMed] [Google Scholar]
- Sayegh MH, Carpenter CB. Transplantation 50 years later--progress, challenges, and promises. N Engl J Med. 2004;351:2761–6. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- Scott LJ, McKeage K, et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–97. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- Simamora P, Alvarez JM, et al. Solubilization of rapamycin. Int J Pharm. 2001;213:25–9. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, et al. Sirolimus for Kaposi’s sarcoma in renal–transplant recipients. N Engl J Med. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Thomas K, Koelwel C, et al. Three generations of cyclosporine a formulations: an in vitro comparison. Drug Dev Ind Pharm. 2005;31:357–66. doi: 10.1081/ddc-54311. [DOI] [PubMed] [Google Scholar]
- Turgeon DK, Leichtman AB, et al. P450 3A activity and cyclosporine dosing in kidney and heart transplant recipients. Clin Pharmacol Ther. 1994;56:253–60. doi: 10.1038/clpt.1994.135. [DOI] [PubMed] [Google Scholar]
- Undre NA. Pharmacokinetics of tacrolimus-based combination therapies. Nephrol Dial Transplant. 2003;18(Suppl 1):i12–5. doi: 10.1093/ndt/gfg1029. [DOI] [PubMed] [Google Scholar]
- Valente JF, Alexander JW. Immunobiology of renal transplantation. Surg Clin North Am. 1998;78:1–26. doi: 10.1016/s0039-6109(05)70631-9. [DOI] [PubMed] [Google Scholar]
- VanBuren CT.2000Sirolimus oral solution and tablets demonstrate equivalent safety and efficacy in real allografts [abstract] Transplantation 69SupplS153no 157. [Google Scholar]
- Vasquez EM.2000Sirolimus: a new agent for prevention of renal allograft rejection Am J Health Syst Pharm 57437–48.quiz 449–51. [DOI] [PubMed] [Google Scholar]
- Vathsala A, Chou TC, et al. Analysis of the interactions of immunosuppressive drugs with cyclosporine in inhibiting DNA proliferation. Transplantation. 1990;49:463–72. doi: 10.1097/00007890-199002000-00044. [DOI] [PubMed] [Google Scholar]
- Vezina C, Kudelski A, et al. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–6. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Weber DO. Nanomedicine. Health Forum J. 1999;42:32, 36–7. [PubMed] [Google Scholar]
- Wu FL, Tsai MK, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy. 2005;25:646–53. doi: 10.1592/phco.25.5.646.63593. [DOI] [PubMed] [Google Scholar]
- Wu FL, Tsai MK, et al. Effects of conversion from sirolimus oral solution to tablets in stable Taiwanese renal transplant recipients. J Formos Med Assoc. 2005;104:22–8. [PubMed] [Google Scholar]
- Yatscoff RW. Pharmacokinetics of rapamycin. Transplant Proc. 1996;28:970–3. [PubMed] [Google Scholar]
- Yokoyama M. Drug targeting with nano-sized carrier systems. J Artif Organs. 2005;8:77–84. doi: 10.1007/s10047-005-0285-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997;37:405–15. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]