Abstract
Increasing attention is being paid on synthetic DNA delivery systems considering some potential life-threatening effects of viral particles, for development of gene-based nano-medicine in the 21st century. In the current nonviral approaches, most of the efforts have been engaged with organic macromolecules like lipids, polymers, and peptides, but comparatively fewer attempts were made to evaluate the potential of inorganic materials for gene delivery. We recently reported that biodegradable nanoparticles of carbonate apatite are highly efficient in transfecting a wide variety of mammalian cells. Here we show that a number of parameters actively regulate synthesis of the nanoparticles and their subsequent transfection efficacy. Development of “supersaturation”, which is the prerequisite for generation of such particles, could be easily modulated by reactant concentrations, pH of the buffered solution, and incubation temperatures, enabling us to establish a flexible particle generation process for highly productive trans-gene delivery. Carbonate incorporation into the particles have been proposed for generating nano-size particles resulting in cellular uptake of huge amount of plasmid DNA as well as endosome destabilization facilitating significant release of DNA from the endosomes.
Keywords: carbonate apatite, gene delivery, transfection, nanoparticles, supersaturation, DNA uptake, endosome, DNA release
Introduction
Development of nonviral gene-delivery devices as alternatives to the viral vectors is essentially needed for the safe implementation of gene delivery concepts in clinical medicine (Luo and Saltzman 2000). Despite existence of a wide variety of nonviral techniques, there has been limited focuses on synthesis of inorganic nano-size DNA carriers which could have better performances for trans-gene delivery compared with the organic materials being extensively used so far (Chowdhury and Akaike 2005a). As inorganic materials, gold, silica, iron-oxide, carbon nanotube, clay minerals, and apatite have been used so far to carry genetic materials to mammalian cells (Luo and Saltzman 2000; Thomas and Klibanov 2003; Chowdhury and Akaike 2005). However, among the synthetic inorganic carriers, apatite particles have received considerable attention by virtue of their biodegradability and resemblance to body hard tissue components (Chowdhury and Akaike 2005b; Chowdhury et al 2006). In fact, calcium phosphate precipitation, one of the pioneering gene delivery methods, is based on generation of hydroxyapatite particles (Chowdhury et al 2006). Low-level of transgene expression due to the inefficiency in the cellular uptake and the endosomal escape of DNA (Loyter et al 1982; Orrantia and Chang 1990; Jordan et al 1996; Lee and Welsh 1999) has limited the potential applications of this traditional technique. We have recently focused on the generation of carbonate apatite particles which, like hydroxyapatite particles, adsorbed DNA, but unlike the latter, could enhance transgene expression to a significant extent in both cancer and primary cells (Chowdhury and Akaike 2005b; Chowdhury et al 2006). Here we describe that a number of factors influence the generation of carbonate apatite particles by either inducing or inhibiting the development of “supersaturation” in the bicarbonate-buffered solution of calcium, phosphate, and DNA. Thus, a number of routes have been demonstrated for the synthesis of high transfec-tion-promoting nanoapatite particles for a particular cell culture environment. Moreover, we proposed that efficient endocytosis of nano-size DNA/apatite particles, followed by endosomal release of DNA contributed to the remarkable transfection efficiency of carbonate apatite.
Materials and methods
Reagents
Plasmid pGL3 (Promega, Tokyo, Japan) containing a luciferase gene under SV40 promoter was propagated in the bacterial strain XL-1 Blue and purified by QIAGEN plasmid kits (QIAGEN, Tokyo, Japan) for being used in gene delivery and subsequently quantitative measurement of luciferase protein expression. Propidium iodide (PI), a DNA-labelling dye and LysoSensor™Green DND-189, an endosome-specific marker were purchased from Sigma-Aldrich (Tokyo, Japan) and Molecular Probes (Invitrogen, Tokyo, Japan), respectively. Lipofectamine 2000 and DMEM were purchased from Invitrogen and Gibco BRL (Tokyo, Japan), respectively.
Cell culture
HeLa, a human cervical cancer cell widely used in gene delivery study, was cultured in 75-cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) supplemented with 10% fetal bovine serum (FBS), 50 μg penicillin ml−1, 50 μg streptomycin ml−1, and 100 μg neomycin ml−1 at 37 °C in a humidified 5% CO2-containing atmosphere.
Transfection of cells
Cells from the exponential growth phase were seeded at 50,000 cells per well into 24-well plates the day before transfection. Three to 6 μl of 1 M CaCl2 was mixed with 2 μg of plasmid DNA in 1 ml of fresh serum-free HCO3− –buffered (pH 7.5) medium (DMEM) followed by incubation for 30 min at 37 °C for complete generation of DNA/carbonate apatite particles. Medium with generated DNA-containing particles was added with 10% FBS to the rinsed cells. After 4-h incubation, the medium was replaced with serum supplemented medium and the cells were cultured for 1 day. Luciferase gene expression was monitored by using a commercial kit (Promega) and photon counting (TD-20/20 Luminometer, USA). Each transfection experiment was done in triplicate and transfection efficiency was expressed as mean light units per mg of cell protein. Transfection by calcium phosphate-DNA co-precipitation was performed according to Jordan and colleagues (1996). Briefly, 12 μg of plasmid DNA was added to 300 μl of a solution containing 250 mM CaCl2. This solution was added to 300 μl of a 2 × HBS (50 mM Hepes, 140 mM NaCl, 1.5 mM Na2HPO4.2H2O, pH 7.05) and mixed rapidly by gentle pipetting twice. The DNA/CaPi mixture was incubated at room temperature for the period of time indicated. After addition of 100 μl of the incubated mixture drop-wise to 1 ml serum supplemented media of each well, cells were grown for 4 h in the incubator. After removal of the particle-containing medium, fresh medium was added to the cells which were subsequently grown for 1 day. For lipofectamine-mediated transfection, protocol provided by Invitrogen was followed. A 1:6 ratio of DNA (2 μg) to lipofectamine provided the best transfection efficiency in our study. Cells were incubated with DNA/lipofactamine complexes in serum media for 4 h and like above, grown for 1 day after replacement with fresh serum media.
Particle size measurements
For visualization by a scanning electron microscope (SEM), a drop of DNA-carbonate apatite suspension prepared according to the instructions in transfection protocol, was added to a carbon-coated SEM stage and dried by keeping the stage at 50°C for 10 min, followed by observation by a high resolution SEM (S-800, Hitachi, Japan). Dynamic light scattering (DLS) measurement for particle suspension was carried out with a Super-dynamic Light Scattering Spectrophotometer, ‘Photal’ (Otsuka Electronics, Osaka, Japan) at 75 mW Ar laser.
Confocal laser scanning microscopy
pGL3 vector was labeled with PI at a PI/DNA ratio of 1:1 and particles generated with this labeled plasmid were incubated with HeLa cells. Membrane-bound precipitates were removed by 5 mM EDTA in PBS before observation by LEICA TCS-NT.
Results and discussion
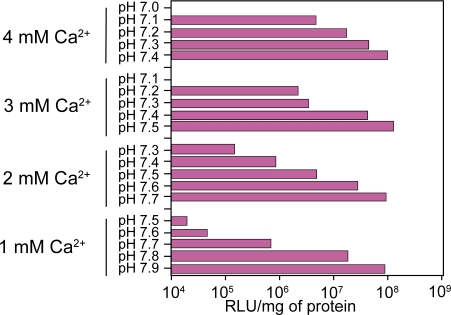
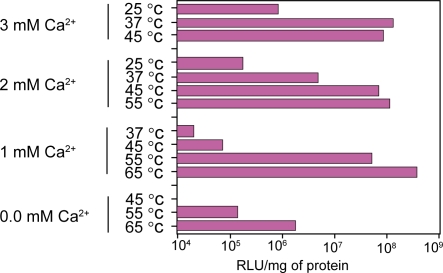
Generation of carbonate apatite particles was governed by exogenously added Ca2+ concentrations, pH of the HCO3− –buffered media and incubation temperatures. We have investigated a long range of pH (7.0 to 7.9) of the HCO3− –buffered medium as well as incubation temperatures (25 °C to 65 °C) in order to make particles by exogenously added Ca2+ and subsequently transfect HeLa cells using the generated particles.
Interestingly, the optimal Ca2+ concentrations required for generation of effective number of DNA/carbonate apatite particles leading to the high transfection efficiency, were inversely related to the pHs of the media (Figure 1) and the incubation temperatures (Figure 2). Thus, while 4 mM Ca2+ was sufficient to induce particle formation at pH 7.4 by incubating the Ca2+-supplemented buffered medium for 30 min at 37 °C, only 1 mM2+ was enough to stimulate particle generation to the similar level at pH 7.9. Like pH, incubation temperatures have also profound and sensitive effects on particle formulation and subsequent trans-gene delivery. Thus, at the incubation temperature of 37 °C, 3 mM Ca2+ was able to induce the proper “supersaturation” whereas at 65 °C, only 1 mM Ca2+could stimulate “supersaturation” development to a similar extent – a prior need for generation of the particles (Jordan et al 1996; Chowdhury, Kunou, et al 2004). The decline below the high efficiency level of transfection (Figures 1 and 2) was due to the formation of too few particles (microscopically observed) since increase in pH or temperature contributed to the development of “supersaturation” by increasing the ionization of phosphate and carbonate in the solution. The new system of particle synthesis is, therefore, very flexible since it allows us to make particles at a wide range of pH and temperatures. The analysis also indicates that induction of “supersaturation” as required for particle formation, can be delicately controlled by manipulating the parameters. Thus, one of the big challenges of using inorganic particles to regulate “supersaturation” development for reproducing particle synthesis and trans-gene delivering efficacy, could be resolved.
Figure 1.
Regulation of trans-gene expression by the nanoparticles of carbonate apatite generated at a wide range of pH. DNA/carbonate apatite particles were generated by addition of 1 to 4 mM Ca2+ and 2 μg plasmid DNA to 1 ml HCO3− (40 mM)-buffered DMEM medium with a pH range from 7.0 to 7.9, followed by incubation for 30 min at 37 °C. Transfection of HeLa cells was performed in the same manner as mentioned in ‘Materials and methods’ section.
Figure 2.
Regulation of trans-gene expression by the nano-particles of carbonate apatite generated at different temperatures. DNA/carbonate apatite particles were generated by addition of 0 to 3 mM Ca2+ and 2 μg plasmid DNA to 1 ml HCO3− (40 mM)-buffered DMEM medium with a pH of 7.5, followed by incubation for 30 min at 25 °C to 65 °C. Transfection of HeLa cells was performed in the same manner as mentioned in ‘Materials and methods’ section.
In addition to pH and temperature, concentrations of initially added Ca2+, inorganic phosphate and HCO3−. should have profound influences on particle synthesis as the major constituents of carbonate apatite particles. Generation of DNA/apatite particles was usually performed in a ready-to-use cell culture medium (DMEM) already containing sufficient amount of phosphate (0.9 mM) and insufficient amount of Ca2+.
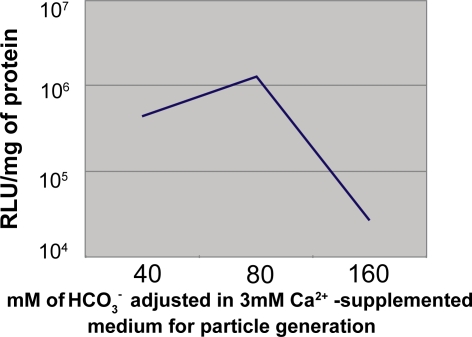
We investigated the effects of externally added Ca2+as well as HCO3−. on the synthesis of particles as evaluated by the subsequent particle-mediated trans-gene expression efficiency. A significantly high number of particles resulted from the excessive induction of “supersaturation” by too high pH, temperature and Ca2+ concentrations, could adversely affect transfection of the cells being cultured in a dish of particular size (24-well plate) and so a 5X dilution of prepared particle suspension was used to transfect HeLa cells for interpreting the effects of Ca2+ and HCsO3−. As shown in Figure 3, above 6 mM Ca2+, a gradual decline in transgene expression was observed with a drastic reduction at 15 mM Ca2+. This could be explained by the fact that higher concentrations of Ca2+ could accelerate development of “supersaturation” leading to formation of too many particles and growth of big size particles with the consequence of gradually reduced DNA uptake by the cells. The relatively lower gene expression by the particles formed at 3 mM Ca2+ could simply be explained by the presence of very low number of the particles with insufficient amount of bound DNA as expected for the dilution of particle suspension prior to transfection in a 24-well plate. Thus, the transfection potential of carbonate apatite sustained even at higher doses of Ca2+ (up to 12 mM). On the other hand, keeping Ca2+ concentration constant (3 mM), increasing HCO3− concentrations from 40 to 80 mM caused a shift to the synthesis of higher number of particles (microscopically observed) probably due to reaction accelerating effect of HCO3− as one of the reactants and at a sufficiently higher dose (160 mM), fewer particles appeared due to the continuous inhibition of particle growth at higher HCO3− concentrations (80 to 160 mM) as also evident from the particle size profiling (not shown). Thus, higher gene expression at 80 mM HCO3− was indicative of higher number of very small particles and reduced expression at 160 mM was reminiscent of very few small particles (Figure 4).
Figure 3.
Luciferase expression using the particles generated with different concentrations of Ca2+ (3 to 24 mM) and 2 μg plasmid DNA added to HCO3− (40 mM)-buffered DMEM medium (pH 7.5). One-fifth of the total particle suspension (1 ml) representing 400 ng of plasmid DNA was used to transfect HeLa cells according to protocol described in ‘Materials and methods’ section.
Figure 4.
Luciferase expression using the particles generated in 3 mM Ca2+ and 2 μg plasmid DNA-supplemented DMEM medium buffered with different concentrations of HCO3− (40 to 160 mM) (pH 7.5). One-fifth of the total particle suspension (1 ml) representing 400 ng of plasmid DNA was used to transfect HeLa cells according to protocol described in ‘Materials and methods’ section.
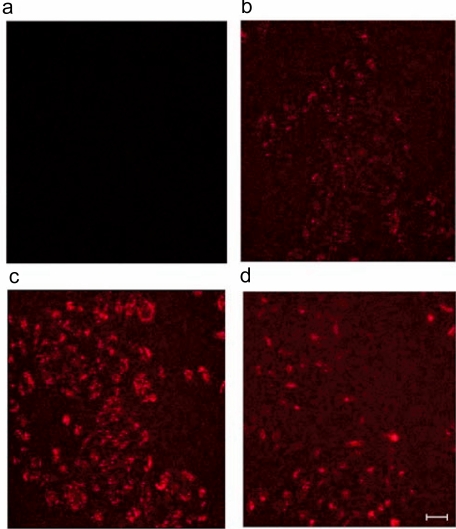
Next, we explored the contribution of sizes of carbonate apatite particles generated under standard conditions, to the delivery of a trans-gene. Carbonate when present in the apatite structure is known to limit the size of the growing apatite crystals (Legeros and Trautz 1967). We carried out scanning electron microscopy of generated carbonate apatite (Figure 5A) which revealed reduced growth of the crystals, most of which had diameters of 50 to 300 nm. Similar results were found by dynamic light scattering measurement (Figure 5B). We verified this size limiting effect of carbonate by observing cellular uptake of the PI-labeled plasmid DNA adsorbed to the apatites, since large particles are phagocytosed less efficiently than small ones (Jordan et al 1996). DNA was carried into the cells by carbonate apatite (Figure 6C) at least 10 times more efficiently than hydroxyapatite, generated by 1 min incubation (Jordan et al 1996) (Figure 6D). Longer period (30 min) incubation resulted in large hydroxyapatite particles (Jordan et al 1996), showing extremely low cellular uptake of DNA (not shown). Our findings, therefore, clearly suggest that carbonate apatite is superior over hydroxyapatite for its intrinsic property of preventing crystal growth (Legeros and Trautz 1967), leading to high efficiency cellular uptake of DNA.
Figure 5.
Scanning electron microscopy and dynamic light scattering for size measurement of the particles generated by addition of 3 mM Ca2+ and 2 μg plasmid DNA to 1 ml HCO3− buffered medium (pH 7.5), followed by incubation for 30 min at 37 °C. Scale bar, 600 nm.
Figure 6.
Cellular uptake of PI-labeled plasmid DNA associated with carbonate apatite and hydroxyapatite. (a) no uptake of DNA (control), since endocytosis was blocked by energy depletion (50 mM 2-deoxy glucose and 1 mM Na-azide). DNA/carbonate apatite particles were prepared in 1 ml serum-free medium using 6 mM Ca2+ and 2 μg DNA by incubating the medium at 37 °C for 30 min. 40 ng (b) and 200 ng (c) of DNA in 20 μl and 100 μl of 1ml suspension respectively, were allowed for cellular uptake for 4 h. (d) 2 μg of DNA adsorbed to hydroxyapatite (described in ‘Methods and materials’ section) was allowed for uptake for the same period of time. Scale bar, 50 μm.
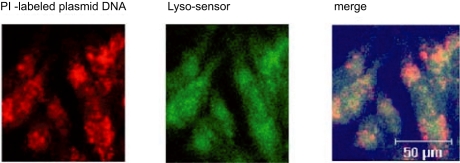
To elucidate the role of endosomal escape of DNA in transgene expression, following endocytosis of PI-labeled plasmid DNA for different period of time, we labelled endosomes with LysoSensor (a fluorescence probe for endosomes). A significant portion of plasmid DNA (red color) appeared to be released from the endosomes (green color) after 6 h of DNA uptake by cells, as shown by colocalization of DNA with endosomes (Figure 7). The high dissolution rate of carbonate apatite (Chowdhury et al 2006) might contribute to the destabilization of endosomes releasing DNA to the cytoplasm, since v-ATPase-driven massive proton accumulation for crystal dissolution could lead to passive chloride influx to endosomes and subsequent endosome swelling and rupture (Boussif et al 1995).
Figure 7.
Endosomal escape of endocytosed PI–labeled plasmid DNA, as evident after colocalization with a fluorescence probe (Lyso-Sensor) specific for endosomes. HeLa cells were transfected for 6 hrs with PI-labeled DNA/carbonate apatite particles generated using 3 mM Ca2+ in the same manner described above and labeled for endosomes by Lyso-Sensor before observation by confocal laser scanning microscopy.
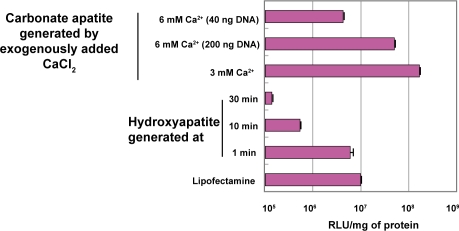
To evaluate the role of carbonate apatite as a powerful carrier of genetic material, we compared transfection efficiency of different techniques including two frequently used ones: CaP co-precipitation method and lipofection. In HeLa cell, luciferase expression level for carbonate apatite-mediated transfection was over 25-fold higher than for lipofection and CaP co-precipitation method (Figure 8) in 10% serum-containing medium. Nano gram level of DNA was even sufficient for efficient transgene expression (Figure 8). However, in the case of conventionally used CaP precipitation technique, as mentioned before, with passage of time, particles continue to grow, creating large size particles which are extremely inefficient for cellular endocytosis (Jordan et al 1996; Chowdhury, Zohra, et al 2004). Thus, with increasing the incubation time from 1 min to 30 min, particles become larger resulting in lower cellular uptake of DNA and gradually reduced transfection efficiency (Figure 8).
Figure 8.
Comparison of transfection efficiency of carbonate apatite nano-carriers with other conventionally used chemicals. DNA/carbonate apatite particles were generated by addition of 3 mM Ca2+ and 2 μg plasmid DNA to 1 ml HCO3− buffered medium (pH 7.5), followed by incubation for 30 min at 37 °C. In few cases, 6 mM Ca2+ was added along with 2 μg DNA to generate particles in 1 ml serum-free media (described above) and 100 μl (200 ng DNA) and 20 μl (40 ng DNA) of 1 ml suspension were applied for transfection. Preparation of DNA/hydroxyapa-tite particles and DNA/lipofectamine complexes and subsequent all transfection experiments were performed according to the protocol described in ‘Methods and materials’ section.
Since endocytosed apatite particles are dissolved in endosomes finally releasing Ca2+ in cytoplasm, it is necessary to address the possible cellular mechanisms for processing these additional Ca2+ ions. Indeed, cells possess machinery to precisely regulate cytoplasmic Ca2+ level (Blaustein and Lederer 1999). In order to remove excessive Ca2+ from the cytosol, there are ATP-driven Ca2+ pumps in the endoplasmic reticulum for sequestering Ca2+ and in the plasma membrane for extruding Ca2+. In addition, the plasma membrane of most animal cells also contain another Ca2+ transport system, the Na+/Ca2+ exchanger, that operates in parallel with the Ca2+-selective channels and the ATP-driven Ca2+ pump. Thus both ATP-driven Ca2+ pumps and Na+/Ca2+ exchanger might actively be engaged to maintain Ca2+ homeostasis and functionality of a cell after transfection with apatite nanocrystals.
Thus, we have unveiled the basic parameters which regulate delicately the generation of carbonate apatite nanoparticles in a wide range of experimental conditions, leading to the development of a highly simplified, flexible, and efficient gene delivery technology having wide applications from laboratories to clinical medicine (Fasbender et al 1998; Toyoda et al 2004).
Acknowledgments
This work was financially supported by the Japanese Society for Promotion of Sciences (JSPS).
References
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: Its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyeth-ylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury EH, Akaike T. Bio-functional inorganic materials: An attractive branch of gene-based nano-medicine delivery for 21st century. Curr Gene Ther. 2005a;5:669–76. doi: 10.2174/156652305774964613. [DOI] [PubMed] [Google Scholar]
- Chowdhury EH, Maruyama A, Kano M, et al. pH-sensing nano-crystals of carbonate apatite: Immense effects on intracellular delivery and release of DNA for efficient expression into mammalian cells. Gene. 2006;376:87–94. doi: 10.1016/j.gene.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Chowdhury EH, Akaike T. A bio-recognition device developed onto nano-crystals of carbonate apatite for cell-targeted gene delivery. Biotechnol Bioeng. 2005b;90:414–21. doi: 10.1002/bit.20398. [DOI] [PubMed] [Google Scholar]
- Chowdhury EH, Kunou M, Nagaoka M, et al. High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca-Mg phosphate. Gene. 2004;341:77–82. doi: 10.1016/j.gene.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Chowdhury EH, Zohra FT, Tada S. Fibronectin in collaboration with Mg2+ enhances transgene expression by calcium phosphate coprecipitates. Anal Biochem. 2004;335:162–4. doi: 10.1016/j.ab.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Fasbender A, Lee JH, Walters RW, et al. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway Epithelia in vitro and in vivo. J Clin Invest. 1998;102:184–93. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Welsh MJ. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther. 1999;6:676–82. doi: 10.1038/sj.gt.3300857. [DOI] [PubMed] [Google Scholar]
- Legeros RZ, Trautz OR. Apatite crystallites: effects of carbonate on morphology. Science. 1967;155:1409–11. doi: 10.1126/science.155.3768.1409. [DOI] [PubMed] [Google Scholar]
- Loyter A, Scangos G, Juricek D, et al. Mechanisms of DNA entry into mammalian cells. Exp Cell Res. 1982;139:223–34. doi: 10.1016/0014-4827(82)90336-6. [DOI] [PubMed] [Google Scholar]
- Luo D, Saltzman WM. Synthetic DNA delivery systems. Nature Biotech. 2000;18:33–7. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- Orrantia E, Chang PL. Intracellular distribution of DNA internalized through calcium phosphate precipitation. Exp Cell Res. 1990;190:170–4. doi: 10.1016/0014-4827(90)90181-9. [DOI] [PubMed] [Google Scholar]
- Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimines transfer of plasmid DNA into mammalian cells. PNAS. 2003;100:9138–43. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Andresen J, Zabner J, et al. Calcium phosphate precipitates augment adenovirus-mediated gene transfer to blood vessels in vitro and in vivo. Gene Ther. 2004;7:1284–91. doi: 10.1038/sj.gt.3301214. [DOI] [PubMed] [Google Scholar]