Abstract
Despite recent advances in technology, targeting, and chemotherapy, brain metastasis from non-small cell lung cancer (NSCLC) remains a significant problem. The vast majority of patients with this diagnosis undergo whole brain radiation therapy (WBRT). However, outcomes are still quite poor with median survivals measured in only months. In an effort to enhance outcomes from external beam radiation treatments, radiosensitizers have been investigated. Motexafin gadolinium (MGd) (Xcytrin®, Sunnyvale, CA, USA) is a novel radiation sensitizer with a unique mechanism of action that may increase the therapeutic index of WBRT for patients with brain metastases, particularly in those with NSCLC histologies. Here we review the rationale for the use of this drug as well as its current and future role as a radiation enhancer in the management of NSCLC brain metastasis.
Keywords: radiation sensitizers, motexafin gadolinium, brain metastasis, redox modulators
Introduction
Scope of the problem
Brain metastasis is the most common type of malignancy found in the brain, as well as the most frequent neurologic complication a cancer patient will encounter (Lassman and DeAngelis 2003). Annually, an estimated 170 000 patients develop brain metastases in the United States alone, most commonly presenting with headache (24%–53%), focal weakness (16%–40%), altered mental status (24%–31%), seizures (15%), and ataxia (9%–20%) (Nussbaum et al 1996; Schellinger et al 1999). The lung is the most common primary tumor site, with over 25% of these patients encountering brain metastases during the course of their illness (Sheehan et al 2002). The other most frequent primary tumor sites in decreasing order of incidence include breast, unknown primary, colorectal, melanoma, thyroid, and renal cell carcinoma (Mehta and Khuntia 2005). Approximately 80% of these lesions occur in the cerebral hemispheres, most often in watershed areas between middle and posterior cerebral arteries, 10%–15% in the cerebellum, and 3% in the brainstem (Kufe et al 2003).
Unfortunately, survival even in patients with the best prognostic factors is dismal, with untreated patients showing a median survival of only one month. To better characterize outcomes, the Radiation Therapy Oncology Group (RTOG) performed a Recursive Partitioning Analysis (RPA) on 1200 patients treated in three consecutive RTOG trials conducted between 1979 and 1993, analyzing a number of pretreatment characteristics and treatment-related variables. As a result of this analysis, patients can be divided into three prognostic groups using the four pretreatment factors: Karnofsky Performance Score (KPS), age, control of primary tumor, and presence of extracranial metastases. Patients with KPS ≥ 70, age <65 years, primary tumor control, and no extra-cranial metastases comprise the most favorable prognostic group, RPA class I, with median survival of 7.1 months (Gaspar et al 1997). Patients with KPS <70 comprise RPA class III, and have the worst prognosis, with median survival of 2.3 months. All other patients fall into RPA class II, with median survival of 4.2 months (see Table 1). Although tumor histology does not have a substantial impact on survival, certain primaries such as SCLC are much more responsive to radiation than other histologies such as renal cell carcinoma or malignant melanoma (Nieder et al 1997).
Table 1.
Recursive partitioning analysis (Gaspar et al 1997)
| RPA I | KPS ≥70, 1° controlled, age <65, no extracranial mets | 7.1 months |
| RPA II | Uncontrolled 1°, age ≥65, extracranial mets | 4.2 months |
| RPA III | KPS <70 | 2.3 months |
Treatment strategies
Though several trials have demonstrated that whole brain radiation therapy (WBRT) effectively increases survival to 4–6 months, little further improvement with altered fractionation schedules or the addition of other sensitizers such as misonidazole and bromodeoxyurine has been realized (Sause et al 1990; Komarnicky et al 1991; Phillips et al 1995). The current approach to patients with brain metastases is based on many factors, including performance status, extent of disease, and the presence of single vs multiple intracranial lesions. Management of a single brain metastasis is largely based on the compelling results of two randomized trials conducted by Patchell and colleagues. They first randomized 48 patients to needle biopsy plus WBRT vs surgical resection followed by WBRT (Patchell et al 1990). This trial demonstrated a dramatic benefit in overall survival with resection and WBRT, 40 weeks vs 15 weeks (p < 0.01), as well as a statistically significant improvement in local recurrence, 20% vs 52% (p < 0.02). In a subsequent study, 98 patients were randomized to surgical resection with or without post-operative WBRT (Patchell et al 1998). Although the addition of radiotherapy did not impact overall survival, WBRT significantly lowered local recurrence (10% vs 46%, p < 0.001), the primary endpoint, and decreased the incidence of neurologic death (14% vs 44%, p = 0.003).
As surgical resection for single brain metastases became standard treatment, investigators began exploring the role of stereotactic radiosurgery for these lesions. In the RTOG 90-05 dose escalation trial, Shaw et al sought to determine the safety, efficacy, and maximum-tolerated-dose of stereotactic radiosurgery (SRS) in patients with recurrent primary or metastatic brain tumors previously treated with EBRT (Shaw et al 2000). No unacceptable acute toxicity levels were achieved with 12–24 Gy, prescribed based on size, demonstrating the safety of SRS within this dose range.
Following this, the RTOG 95-08 trial was designed to determine the benefit of WBRT followed by SRS boost over WBRT alone in patients with 1–3 brain metastases ≤ 4 cm in diameter (Andrews et al 2004). Patients were stratified by 1 vs 2–3 metastases and also by the presence of extracranial metastasis. This multi-institutional trial demonstrated a survival advantage with SRS boost for patients with a solitary brain metastasis (6.5 vs 4.9 months, p = 0.0393), as well as better local control at one year (82% vs 71%, p = 0.01). SRS boost did not provide a survival advantage overall in patients with multiple metastases. However, radiosurgery did improve survival in RPA class I patients (p = 0.0453) and those with NSCLC primaries (p = 0.0508) in subset analysis. RPA and histology were not stratifications of the trial, however. Improvement in performance status and reduction in steroid use was realized in patients receiving SRS. As a result of these trials, RPA class I patients with a single brain metastasis are currently treated with surgical resection or SRS followed by whole-brain radiation. Most patients with multiple intracranial lesions are treated with WBRT alone, with selected patients undergoing SRS for possible improvement in performance status.
Radiation sensitizers
Biochemical agents that may be combined with radiotherapy to improve patient outcomes have been of interest to oncologists for decades. These agents provide an advantage via four basic mechanisms: spatial co-operation (each modality treats a different anatomical site), toxicity independence, protection of normal tissue, and enhancement of tumor response to radiation (Coleman and Mitchell 1999). The last of these, known as radiation sensitizers, involves the administration of an agent which discriminates between tumors cells and normal tissue, and improves the effectiveness of the targeted radiation. Brain metastases can be effectively targeted by systemic administration of these agents, as these lesions are characterized by upregulation of angiogenesis and neovascularization, interrupting the blood–brain barrier.
Radiosensitizers fall into three major categories: non-hypoxic cell sensitizers, hypoxic cell sensitizers, and hypoxic cytotoxins. Non-hypoxic cell sensitizers, principally halogenated pyrimidines, are readily incorporated into the DNA of rapidly dividing tumors cells and weaken DNA bonds, increasing the sensitivity to ionizing radiation (Mehta and Khuntia 2005). Hypoxic cell sensitizers enhance tumor response to radiation primarily by inducing the formation and stabilization of toxic DNA radicals, mimicking the effects of oxygen (Rowinsky 1999). Tumor cells are hypoxic relative to surrounding normal tissue due to obstruction of blood flow, defective or inadequate angiogenesis, and outstripping of capillary blood supply from lack of cellular growth control. Hypoxic cell sensitizers include the nitroimidazoles, misonidazole, etanidazole, nimorazole, and efaproxaril (also known as RSR-13 [Efaproxyn™, Allos Therapeutics, Inc., Westminster, CO, USA]). Unfortunately, the nitroimidazoles are largely limited by their neurotoxicity. However, RSR-13 is of particular interest, as recent studies have demonstrated its clinical utility.
RSR-13 is a synthetic allosteric modifier of hemoglobin (Hb), which decreases Hb-oxygen binding affinity, resulting in increased tumor oxygen concentration in tumor cells (Stea et al 2006). Suh et al conducted a Phase III trial, randomizing 538 patients with brain metastases to 30 Gy WBRT with supplemental oxygen with or without RSR-13 (Suh et al 2004). In this trial, addition of RSR-13 did not result in a survival benefit overall, but did improve survival in the subset of patients with primary breast cancer (8.67 vs 4.57 months, p = 0.006). As a result of this finding, an ongoing international multi-institutional phase III clinical trial looking at WBRT +/−RSR-13 is currently underway.
Finally, hypoxic cytotoxins including quinone antibiotics, nitroaromatic compounds, and benzotriazine di-N-oxides have failed to demonstrated substantial clinical efficacy. The ineffectiveness of many of these radiosensitizers led to the development of a novel hypoxic cell sensitizer and redox modulator, motexafin gadolinium (MGd). Two major features of MGd make this agent an ideal candidate for radiosensitization: high electron affinity and tumor selectivity. MGd may prove to have substantial clinical efficacy as the utility of this agent in conjunction with radiotherapy has been already been demonstrated in recent clinical trials.
Structure, mechanism, and pharmacokinetics
Structure
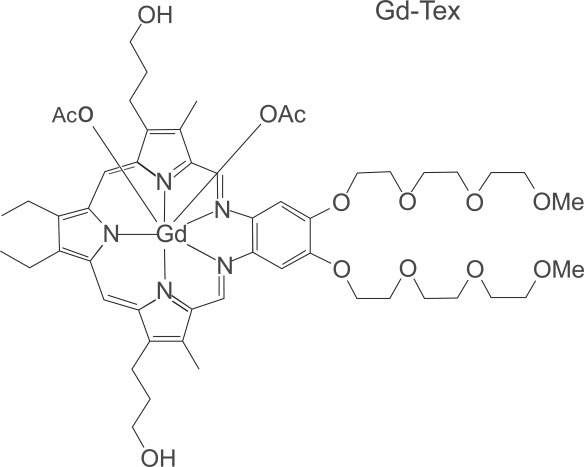
Formally known as gadolinium texaphyrin, MGd is in a class of drugs known as texaphyrins. The chemical structure is shown in Figure 1. The chemistry of texaphyrins was first described by Sessler and Miller as a fully aromatic porphyrin analog (Sessler and Miller 2000). Texaphyrins, which are large porphyrin-like macromolecules, form stable complexes with large metal cations such as gadolinium (and other lanthanides). These large metal cations have a very high avidity for accepting electrons, which allows them to disrupt the cancer cell at the cellular level.
Figure 1.
Chemical structure of motexafin gadolinium.
Mechansim
Magda and colleagues have suggested, through in vitro studies, that oxidative stress caused by redox cycling is the mechanism by which MGd enhances the radiation response (Magda et al 2001). MGd is a redox active drug that has an exceptionally high electron affinity. This drug preferentially deposits within tumor cells and catalyzes important intracellular metabolites (Young et al 1994). These compounds, such as ascorbate, glutathione, dihydrolipoate, nicotinamide adenine dinucleotide phosphate, and protein thiols, are necessary to maintain the appropriate energy balance necessary for repairing cellular radiation injury (Rowinsky 1999). In addition, MGd inhibits thioredoxin reductase, which is a crucial enzyme required to restore reducing agents back into the cell. By depleting the cells of energy as well as these protective metabolites, the cell dies.
More recently, additional research supporting the cytotoxicity of MGd on tumor cells has been reported. MGd has been found to increase intracellular oxygen levels in EMT6 mouse mammary tumors (Donnelly et al 2004). Overcoming hypoxia will allowed the radiation damage to become “fixed” so that they cannot be repaired. In another study MGd was found to inhibit heme oxygenase-1 (HO1). HO1 is protein that that typically protects a cell from oxidative stress and is also an antiapoptic (Evans et al 2006). Evens and colleagues have also showed that MGd can induce apoptosis in fresh malignant cells from patients with multiple myeloma (Evens et al 2005). Recently, MGd had been reported to disrupt the zinc metabolism, which is responsible for regulating cancer cell growth. This has shown enhanced cell killing in both lung cancer cell lines and B-cell hematologic malignancies (Lecane et al 2005; Magda et al 2005).
Pharmacokinetics and metabolism
The pharmacokinetics and metabolism of MGd is similar to those of other porphyrin molecules. The drug is excreted through the bowel, but levels in the biliary tree tend to be elevated (Miller et al 1999; Sessler and Miller 2000). MGd has linear pharmacokinetics and is cleared in plasma over time without buildup (Khuntia and Mehta 2004).
As gadolinium is paramagnetic, magnetic resonance imaging (MRI) is exquisitely sensitive in depicting uptake of the drug. MGd selectively introduces itself into tumors and may be seen on MRI up to 24 hours after injection and may persist for months if multiple doses are delivered (Young et al 1994). At least 30% of enhancement has been seen even 5 hours after injection of MGd.
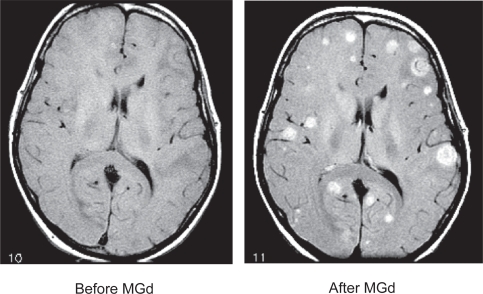
In the original phase Ib/II trial of MGd for brain metastasis, a paradoxical increased number of metastasis was found after the introduction of the drug into the patient (Viala et al 1999) (Figure 2). This was not due to increased number of metastases, but rather a higher concentration of MGd in the brain which allowed for increased sensitivity on MRI. Nearly 70% of the patients in the trial had at least a 50% reduction in the size of the brain metastasis.
Figure 2.
Before and after 10 doses of motexafin gadolinium (MGd). MRI scans; both are non-contrast MR images. Selective uptake in the brain metastases is clearly evident (courtesy of Minesh P Mehta, University of Wisconsin).
Clinical efficacy in brain metastasis
The safety and utility of MGd in patients with brain metastases was first reported by Carde et al in the phase Ib/II trial above, combining 30 Gy WBRT with daily pre-radiation MGd administration (Carde et al 2001). The results showed a maximum-tolerated-dose (MTD) of 6.3 mg/kg, due to dose-limiting reversible hepatotoxicity. In addition, combined treatment resulted in a 72% radiological response rate and only 12% neurologic death vs a 47% radiologic response rate and 33%–50% neurological death in patients treated with WBRT alone (30–70.4 Gy) in RTOG trials 7916, 8528, and 8905 (Gaspar et al 1997).
Following this trial, Mehta and colleagues randomized 401 patients with brain metastases to 30 Gy WBRT with or without 5 mg/kg/day MGd and analyzed impact on survival, as well as neurological and neurocognitive function (NCF) (Mehta et al 2003). Using survival alone as the primary endpoint in trials for patients with brain metastases can be limiting, as very few novel fractionation schedules or radiosensitizing agents have resulted in a survival benefit and many patients die from progression of systemic disease. The impressive battery of neurological testing conducted in this trial, including FACT-BR, Barthel Index of Activities of Daily Living, specialized neurocognitive tests, and systematic recording of neurologic signs and symptoms, revealed MGd’s positive effect on neurological function and NCF, which have been correlated with functional independence and quality of life.
The results of this trial revealed no overall difference in median survival (5.2 vs 4.9 months, p = 0.48) or time to neurologic progression (median 9.5 vs 8.3 months, p = 0.95) for WBRT alone vs WBRT with MGd respectively. However, patients with NSCLC did demonstrate improved time to neurological progression (median not reached for MGd vs 7.4 months, p = 0.048), time to neurocognitive progression for memory and executive functioning (p = 0.047), and incidence of neurological death (36.4% vs 51.5%, p = 0.037) (Meyers et al 2004). In this study, NCF at baseline was predictive of overall survival and in concordance with previous trials, the combination of tumor prognostic variables and brain function assessments was more predictive of survival than the tumor variables alone.
The correlation between neurocognitive functioning (NCF) and survival was further characterized in a recent study by Li et al (2006). The authors analyzed 208 patients treated with 30 Gy WBRT as part of a phase III trial (PCYC-9801). The authors correlated the extensive neurocognitive assessments performed in this study with brain metastases volume change as documented by MRI. The results of this analysis showed that a “good response” on imaging correlated with longer median time to NCF deterioration, and “good responders” demonstrated significantly longer median survival (p = 0.03). In addition, 15-month survivors in relation to 4-month survivors showed a greater reduction in cumulative brain metastases volume and had stable or improving NCF scores. This analysis demonstrated that the ability of WBRT to cause tumor shrinkage correlates with NCF preservation and improved survival. The extensive neurologic and neurocognitive testing performed in these two trials highlights a new endpoint which appears to have significant clinical utility. Neurological function and NCF can be objectively measured and correlates with important established endpoints including functional independence, quality of life, and survival.
There are several explanations for the greater benefit achieved in lung cancer patients treated with WBRT + MGd patients vs patients with other primary lesions in the recent study by Mehta and colleagues (Mehta et al 2006). Lung cancer patients are more likely to present with brain metastases, to have smaller and isolated intracranial metastases, and to have had less exposure to chemotherapy (Meyers et al 2004). To confirm the benefit demonstrated in this trial, Mehta and colleagues recently conducted a phase III trial (known as the SMART study) involving non-small cell lung cancer (NSCLC) patients from North America (NA), Europe, and Australia (Mehta et al 2006). They randomized 554 NSCLC patients to 30 Gy WBRT ± 5 mg/kg/day MGd with time to neurological progression (TNP) as the primary endpoint. Overall, combination therapy did not result in a statistically significant improvement in TNP. However, when analysis was limited to NA patients, WBRT with MGd significantly improved TNP (24.2 vs 8.8 months, p = 0.004). This unexpected finding can largely be explained by one factor: time between diagnosis and treatment was shorter in NA (2.2 weeks) than in Europe (6.5 weeks). This is largely due to a tendency to first treat with chemotherapy before WBRT in Europe (see Tables 2 and 3). The benefit of the addition of MGd in patients treated within 3 weeks of diagnosis was largely not observed when treatment was delayed beyond this point. Thus, this study suggests the benefit of WBRT +MGd in lung cancer patients treated in the US and has highlighted the impact of other parameters (time to treatment and prior chemotherapy exposure) on treatment efficacy. As a result of this finding, MGd is currently under review of the FDA for approval in the US for patients with brain metastases from NSCLC.
Table 2.
Distribution of patients from brain metastasis diagnosis to randomization (courtesy of Minesh P Mehta, University of Wisconsin)
| 0–2 weeks | 2–4 weeks | >4 weeks | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Overall | 274 | 49.5% | 161 | 29.1% | 119 | 21.5% |
| North America | 208 | 59.8% | 106 | 30.5% | 34 | 9.8% |
| Rest of world | 66 | 32.0% | 55 | 26.7% | 85 | 41.3% |
| France | 26 | 22.2% | 25 | 21.4% | 66 | 56.4% |
Table 3.
Patients receiving chemotherapy as initial treatment for brain metastasis (courtesy of Minesh P Mehta, University of Wisconsin)
| Country/Region/Site | Patients enrolled
|
Treated with chemotherapy
|
|
|---|---|---|---|
| N | N | % | |
| North America | 348 | 6 | 1.7 |
| USA | 185 | 6 | 3.2 |
| Canada | 163 | 0 | 0.0 |
| Rest of world | 206 | 36 | 17.5 |
| France | 117 | 25 | 21.4 |
| Site 186 | 34 | 13 | 38.2 |
| Site 211 | 10 | 2 | 20.0 |
In response to the encouraging results of several studies combining WBRT and MGd, Mehta and colleagues have begun to investigate the utility of MGd in combination with additional treatment modalities, specifically, stereotactic radiosurgery (SRS). In this trial, patients with 1–4 brain metastases are being treated with 37.5 Gy WBRT +10 doses MGd 5 mg/kg/day during weeks 2 and 3, followed by SRS boost +MGd 5 mg/kg within 14 days of completing WBRT. This study is designed primarily to determine the safety and tolerability of this combination, and secondarily to evaluate the impact of this treatment on lesion size and number, neurological and neurocognitive progression, and survival. An additional exploratory objective is evaluation of lesion size and number after 11 doses of MGd in comparison with standard contrast-enhanced MRI. The results of this trial will hopefully reveal yet another use for this promising agent.
Future directions
As efficacy has been established for patients with NSCLC brain metastases, future indication in other cancers where redox modulation plays an important role is being investigated. Table 4 shows the current ongoing trials looking at other indications for the drug. Promising data currently exists for MGd in patients with glioblastoma multiforme (GBM). Benefit in GBM’s from MGd may be a result of imaging effects, radiation sensitization effects, and also synergy with other drugs.
Table 4.
Selected ongoing clinical trials using motexafin gadolinium (MGd)
| Trial | Principal investigator |
|---|---|
| Randomized phase III trial of Xcytrin® MGd. Injection for the treatment of brain metastases in patients with NSCLC undergoing whole brain radiation therapy completed. | Minesh Mehta, University of Wisconsin; Paul P. Carbone Comprehensive Cancer Center, Madison, WI; Corey Langer, Fox Chase Cancer Center, Philadelphia, PA, USA |
| Phase II trial of Xcytrin MGd with WBRT and SRS for patients with NSCLC and brain metastases | Minesh Mehta, University of Wisconsin; Paul P Carbone, Comprehensive Cancer Center, Madison, WI, USA |
| Phase II Trial of Xcytrin MGd. injection for the treatment of metastatic renal cell carcinoma | Robert Amato, Scott Department of Urology at Baylor College of Medicine in Houston, TX, USA |
| A phase I/II trial of redox regulation in patients with relapsed or refractory CD20 positive non-Hodgkin’s lymphoma NHL: combining 90yttrium-zevalin and the redox-modulating agent MGd | Andrew Evens, Leo I Gordon, Robert H Lurie, Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA |
| An open-label phase II trial of MGd in patients with relapsed or refractory multiple myeloma | Andrew Evens, Seema Singhal, Robert H Lurie, Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA |
| Phase II trial of MGd in patients with relapsed or refractory indolent non-Hodgkin’s lymphoma | Brad Kahl, University of Wisconsin Comprehensive Cancer Center, Madison, WI, USA |
| Phase II trial MGd in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma with refractory or relapsed disease | Andrew Evens, Robert H Lurie, Comprehensive Cancer Center, Northwestern University, Chicago, IL; Neil Kay, MD, Mayo Clinic, Rochester, MN, USA |
| Phase I trial of MGd and chemoradiation in locally advanced, squamous cell carcinoma of the head and neck | Principal Investigator: David Brizel, Duke University, Durham, NC, USA |
| Phase I study of gadolinium texaphyrin PCI-0120. and radiotherapy in children with intrinsic pontine glioma | Children’s Cancer Group |
| A phase I trial of MGd and docetaxel chemotherapy in the treatment of advanced solid tumors | Gurkamal Chatta, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA |
| Phase I Trial of MGd and docetaxel administered at 3-week intervals for advanced solid tumors | Kishan Pandya, University of Rochester Cancer Center, Rochester, NY, USA |
| Phase I trial of MGd in combination with temozolomide for treatment of malignant gliomas | William R Shapiro, Barrow Neurological Institute, Phoenix, AZ, USA |
| Phase I trial of MGd in combination with docetaxel and cisplatin for treatment of NSCLC. | David Stewart, Anderson Cancer Center, Houston, TX, USA |
Manon and colleagues have shown that mid treatment MRI imaging in patients with GBM may allow for improved target delineation for the boost volumes (Manon et al 2004). This may allow for a reduction in geographic misses with external beam radiation. Recent phase I and II data also show potential improvements in GBM patients treated with EBRT and MGd concurrently with approximately a 6 month gain in survival compared to standard controls (Woodburn 2001; Suh et al 2002). More recently, preclinical data suggests that there may be a synergistic relationship between MGd and the alkylating agent temozolomide (Khuntia and Mehta 2004). Temozolamide has recently been validated for concurrent and adjuvant use with radiation in patients with newly diagnosed GBM, portending a survival advantage of 14.6 months vs 12.1 months (p < 0.001) (Stupp et al 2005). Since both drugs cross the blood brain barrier, a synergistic response in GBM may be realized and is currently under active investigation.
Another avenue of exploration includes the combination of other drugs with MGd to further enhance MGd’s cytotoxic potential. An important mechanism in cell kill is via apoptosis. Apoptosis is mediated, at least in part, by the Akt/protein kinase B pathway (Franke et al 2003). Akt is an antiapoptotic factor that is activated by phosphorylation after the cell is stressed. Since the activation of Akt to pAkt involves a redox pathway, MGd has a unique ability to potentially interrupt the phosphorylation and decrease the amount pAKT, which may be cytoprotective in the tumor cell. Ramos and colleagues have shown in the presence of MGd, levels of pAKT are initially increased and then decreased right before cell death (Ramos et al 2006). This initial increase may be the tumor cells protective response to a stress and it is postulated that attenuating pAKT levels may lead to a synergistic response if inhibitors of the Akt kinase phosphorylation process is introduced in conjunction with MGd. They have found that when combining SH-5, an inhibitor of Akt kinase phosphorylation with MGd, synergistic toxicity to the cell was realized. This groundbreaking research may allow for the development of many more drugs that modulate pAkt, such as celecoxib or docetaxol (Ramos et al 2006).
Finally, MGd is also being evaluated for use as an agent in neutron capture therapy (GdNCT). Traditionally, boron neutron capture therapy has only modest efficacy in CNS tumors. This was largely because of a lack of tumor specificity to the boronated compounds. By incorporating a selective drug like MGd, it is postulated the gadolinium neutron capture may be able penetrate tumor cells more readily. Efforts are currently under way to find a gadolinium compound with high nuclear affinity that may translate to improved tumor penetration (De Stasio et al 2001, 2005).
Conclusion
MGd is a novel radiation sensitizer with a unique mechanism of action that has recently shown efficacy in patients with NSCLC in North America. Because of its unique mechanism of action, its use in other diseases is actively under investigation. Synergy with other drugs remains investigational, but mechanistically, combination with other drugs may improve MGd’s cytotoxicity.
Acknowledgments
We would like to acknowledge the assistance of Minesh P Mehta for providing recent data on MGd for this paper.
References
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- Carde P, Timmerman R, Mehta MP, et al. Multicenter phase Ib/II trial of the radiation enhancer motexafin gadolinium in patients with brain metastases. J Clin Oncol. 2001;19:2074–83. doi: 10.1200/JCO.2001.19.7.2074. [DOI] [PubMed] [Google Scholar]
- Coleman CN, Mitchell JB. Clinical radiosensitization: why it does and does not work. J Clin Oncol. 1999;17:1–3. doi: 10.1200/JCO.1999.17.1.1. [DOI] [PubMed] [Google Scholar]
- De Stasio G, Casalbore P, Pallini R, et al. Gadolinium in human glioblastoma cells for gadolinium neutron capture therapy. Cancer Res. 2001;61:4272–7. [PubMed] [Google Scholar]
- De Stasio G, Rajesh D, Casalbore P, et al. Are gadolinium contrast agents suitable for gadolinium neutron capture therapy? Neurol Res. 2005;27:387–98. doi: 10.1179/016164105X17206. [DOI] [PubMed] [Google Scholar]
- Donnelly ET, Liu Y, Fatunmbi YO, et al. Effects of texaphyrins on the oxygenation of EMT6 mouse mammary tumors. Int J Radiat Oncol Biol Phys. 2004;58:1570–6. doi: 10.1016/j.ijrobp.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Evans JP, Xu F, Sirisawad M, et al. Motexafin gadolinium-induced cell death correlates with heme oxygenase-1 expression and inhibition of P450 reductase-dependent activities. Mol Pharmacol. 2006;71:193–200. doi: 10.1124/mol.106.028407. [DOI] [PubMed] [Google Scholar]
- Evens AM, Lecane P, Magda D, et al. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–73. doi: 10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis RPA. of prognostic factors in three Radiation Therapy Oncology Group RTOG. brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- Khuntia D, Mehta M. Motexafin gadolinium: a clinical review of a novel radioenhancer for brain tumors. Expert Rev Anticancer Ther. 2004;4:981–9. doi: 10.1586/14737140.4.6.981. [DOI] [PubMed] [Google Scholar]
- Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases RTOG-7916. Int J Radiat Oncol Biol Phys. 1991;20:53–8. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- Kufe D, Pollock R, Weichselbaum RR, et al. Section 23: Central Nervous System. Hamilton, ON, Canada: BC Decker; 2003. [Google Scholar]
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21:1–23. vii. doi: 10.1016/s0733-8619(02)00035-x. [DOI] [PubMed] [Google Scholar]
- Lecane PS, Karaman MW, Sirisawad M, et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 2005;65:11676–88. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- Li J, Bentzen SM, Renschler M, Mehta MP. Improvement in neurocognitive function (NCF) correlates with tumor regression after whole brain radiation therapy (WBRT) for brain metastasees (BM) J Clin Oncol, Annual Meeting Proceedings Pt I. 2006 Jun 20;24(Supplement):1504. [Google Scholar]
- Magda D, Lecane P, Miller RA, et al. Motexafin gadolinium disrupts zinc metabolism in human cancer cell lines. Cancer Res. 2005;65:3837–45. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- Magda D, Lepp C, Gerasimchuk N, et al. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys. 2001;51:1025–36. doi: 10.1016/s0360-3016(01)01810-7. [DOI] [PubMed] [Google Scholar]
- Manon R, Hui S, Chinnaiyan P, et al. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol Cancer Res Treat. 2004;3:303–7. doi: 10.1177/153303460400300308. [DOI] [PubMed] [Google Scholar]
- Mehta M, Gervais R, Chabot P, et al. Motexafin gadolinium MGd. combined with prompt whole brain radiation therapy RT. prolongs time to neurologic progression in non-small cell lung cancer NSCLC. patients with brain metastases: Results of a phase III trial [abstract] Proc Am Soc Clin Oncol. 2006;24:7014. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Mehta MP, Khuntia D.2005Current strategies in whole-brain radiation therapy for brain metastases Neurosurgery 57S33–44.discusssion S1–4. [DOI] [PubMed] [Google Scholar]
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. Journal of Clinical Oncology. 2003;21:2529–36. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium:results of a randomized phase III trial. J Clin Oncol. 2004;22:157–65. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- Miller RA, Woodburn K, Fan Q, et al. In vivo animal studies with gadolinium III. texaphyrin as a radiation enhancer. Int J Radiat Oncol Biol Phys. 1999;45:981–9. doi: 10.1016/s0360-3016(99)00274-6. [DOI] [PubMed] [Google Scholar]
- Nieder C, Berberich W, Schnabel K. Tumor-related prognostic factors for remission of brain metastases after radiotherapy. Int J Radiat Oncol Biol Phys. 1997;39:25–30. doi: 10.1016/s0360-3016(97)00154-5. [DOI] [PubMed] [Google Scholar]
- Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–8. [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89–05. Int J Radiat Oncol Biol Phys. 1995;33:339–48. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- Ramos J, Sirisawad M, Miller R, et al. Motexafin gadolinium modulates levels of phosphorylated Akt and synergizes with inhibitors of Akt phosphorylation. Mol Cancer Ther. 2006;5:1176–82. doi: 10.1158/1535-7163.MCT-05-0280. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK. Novel radiation sensitizers targeting tissue hypoxia. Oncology (Williston Park) 1999;13:61–70. [PubMed] [Google Scholar]
- Sause WT, Crowley JJ, Morantz R, et al. Solitary brain metastasis: results of an RTOG/SWOG protocol evaluation surgery + RT versus RT alone. Am J Clin Oncol. 1990;13:427–32. [PubMed] [Google Scholar]
- Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44:275–81. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- Sessler JL, Miller RA. Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol. 2000;59:733–9. doi: 10.1016/s0006-2952(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–8. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Sun MH, Kondziolka D, et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97:1276–81. doi: 10.3171/jns.2002.97.6.1276. [DOI] [PubMed] [Google Scholar]
- Stea B, Shaw E, Pinter T, et al. Efaproxiral red blood cell concentration predicts efficacy in patients with brain metastases. Br J Cancer. 2006;94:1777–84. doi: 10.1038/sj.bjc.6603169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Suh J, Chang E, Timmerman R, et al. Phase II trial of motexafin gadolinium MGd, Xcytrin. and cranial radiation in newly diagnosed glioblastoma multiforme GBM [abstract] Proc Am Soc Clin Oncol. 2002;21:2108. [Google Scholar]
- Suh J, Stea B, Nabid A. Standard whole brain radiation therapy WBRT. with supplemental oxygen O2., with or without RSR-13 efaproxiral. in patients with brain metastasis: Results of the randomzied REACH RT-009 study [abstract] Proc Am Soc Clin Oncol. 2004;22(Suppl 14):115s. [Google Scholar]
- Viala J, Vanel D, Meingan P, et al. Phases IB and II multidose trial of gadolinium texaphyrin, a radiation sensitizer detectable at MR imaging: preliminary results in brain metastases. Radiology. 1999;212:755–9. doi: 10.1148/radiology.212.3.r99se10755. [DOI] [PubMed] [Google Scholar]
- Woodburn KW. Intracellular localization of the radiation enhancer motexafin gadolinium using interferometric Fourier fluorescence microscopy. J Pharmacol Exp Ther. 2001;297:888–94. [PubMed] [Google Scholar]
- Young SW, Sidhu MK, Qing F, et al. Preclinical evaluation of gadolinium III. texaphyrin complex. A new paramagnetic contrast agent for magnetic resonance imaging. Invest Radiol. 1994;29:330–8. doi: 10.1097/00004424-199403000-00013. [DOI] [PubMed] [Google Scholar]