Abstract
Uveitis is a potentially sight-threatening inflammatory eye disease caused by multiple infectious and non-infectious etiologies for which the standard of care involves corticosteroids or various immunomodulary therapy (IMT) drugs. These available treatments, although effective, may cause significant morbidity and sometimes mortality in uveitis patients due to their toxic side-effects and the necessity of long-term therapy to prevent recurrences. In order to avoid the systemic toxicity of corticosteroids and IMT or the repeated injections of local steroids necessary to control ocular inflammation, and to prevent development of cumulative damage resulting from recurrent episodes of inflammation, researchers have developed a number of local corticosteroid sustained-release devices that can be implanted directly into the vitreous of the eye, at the site of the inflammatory disease. Preliminary studies of such a device, the fluocinolone acetonide (Retisert™) implant, have shown significant reductions in the number of inflammatory episodes and decreased reliance on systemic corticosteroids or other IMT. This review explores the current research evaluating the fluocinolone sustained-release intravitreal implant in the treatment of posterior uveitis and the implications for its future use on a wider scale.
Keywords: uveitis, immunomodulary therapy drugs, fluocinolone acetonide implant, sustained-release implant
Introduction
Uveitis is an inflammatory eye disease affecting the iris, ciliary body, and choroid that can lead to symptoms ranging from redness, pain, and blurred vision to markedly diminished acuity in the setting of severe or chronic disease (Opremcak et al 2004). Corticosteroids comprise the mainstay of uveitis therapy, but current methods of systemic, topical, and periocular administration can pose challenges to physicians and patients. Such difficulties have led investigators to explore new techniques for intraocular corticosteroid delivery, including dexamethasone devices and the sustained-delivery fluocinolone acetonide intravitreal implant (Retisert™, Bausch and Lomb, Rochester, NY, USA). The latter device has shown promise in several clinical trials, including pivotal trials that led to FDA approval of Retisert, and may become a long-term alternative for patients suffering from posterior non-infectious uveitis.
Intraocular drug delivery systems: an ever-developing technology
The concept of intraocular drug-delivery systems is not novel to uveitis; such devices have already improved the treatment of cytomegalovirus retinitis in immunocompromised individuals. In a randomized trial, the intravitreal gancyclovir sustained-release implant was shown to be superior to intravenous administration in preventing the progression of cytomegalvirus (CMV) retinitis in AIDS patients (Musch et al 1997). The fellow eyes of implant patients showed higher rates of progression compared with patients given intravenous gancyclovir, however, and implant patients were also more likely to develop extraocular manifestations of CMV infection. A subsequent randomized trial demonstrated that the addition of oral gancyclovir to patients with the implant decreased the development of new cases of CMV retinitis, delayed progression of existing cases, and decreased the risk of Kaposi’s sarcoma (Martin et al 1999). The most common complication of gancyclovir implantation is vitreous hemorrhage, with an incidence of 10% (Dunn et al 2004). Less common complications include hypotony, uveitis, retinal detachment, choroidal effusion, and cataract.
The success of sustained-release delivery systems in the treatment of CMV retinitis led scientists to develop similar devices for use in patients with steroid-dependent chronic uveitis. In an experimental rabbit model of uveitis, Cheng et al (1995) demonstrated that an intravitreal sustained-release dexamethasone implant was significantly more effective at controlling inflammation by physical exam and objective measures than sham treatment of fellow eyes. In addition, implanted eyes had fewer instances of cataract, hypotony, and corneal neovascularization than fellow eyes. A case report describing implantation of a dexamethasone sustained-release device into a patient noted complete resolution of intraocular inflammation for a 10-month period following surgery (Jaffe et al 2000b). After this time, however, the patient experienced recurrent disease suggesting that the dexamethasone was expended.
Uveitis: a challenging condition to diagnose and manage
Classification and epidemiology
Previous studies of the incidence and prevalence of uveitis in the United States estimated that 38 000 Americans were newly diagnosed each year, though a recent study by Gritz and Wong reports figures in Northern California that may require the national incidence to be re-examined (Foster and Vitale 2002; Gritz and Wong 2004). In a 2004 study examining a large Health Maintenance Organization population, the incidence of uveitis was 52.4 per 100 000 person-years, with a prevalence of 115.3 per 100 000 (Gritz and Wong 2004). The age range of patients suffering from uveitis encompasses the entire lifespan, but mean ages from several studies were concentrated at 40 years with an approximately equal proportion of males and females affected and no particular racial tendencies (Foster and Vitale 2002). In the study of Northern California residents, however, women had a higher incidence and prevalence of uveitis, though only prevalence was statistically significant (Gritz and Wong 2004). The broad disease entity of uveitis can be further classified into the anatomical divisions of anterior, intermediate, posterior, and panuveitis (Foster and Vitale 2002).
Anterior uveitis primarily affects the iris, ciliary body, cornea, or sclera and usually has a non-infectious, and often idiopathic, etiology (Opremcak et al 2004). Overall, anterior uveitis is the most common form of uveitis, representing 28%–61% of all cases (Foster and Vitale 2002).
Intermediate uveitis affects the anterior vitreous and pars plana, causing floaters and vision loss from cystoid macular edema (Opremcak et al 2004). Intermediate uveitis is responsible for the smallest proportion of overall cases, ranging from 3%–17% in different studies (Foster and Vitale 2002). Most are idiopathic (69.1%), but common definable etiologies are sarcoidosis, 22.2%, multiple sclerosis, 8%, and Lyme disease, 0.6% (Foster and Vitale 2002).
Posterior uveitis involves the retina and choroid, making up 9.3%–38% of all uveitis cases (Foster and Vitale 2002). Inflammation of the retina and choroid results in decreased visual acuity and distorted vision with associated scotomata and floaters (Opremcak et al 2004). This form of uveitis accounts for more visual loss than other forms, often due to cystoid macular edema, retinal detachment, cataract, glaucoma, subretinal fibrosis, and optic disc atrophy (Sabrosa and Pavésio 2000). Posterior uveitis is often infectious, with toxoplasmosis representing 24.6% of cases and cytomegalovirus, 11.6%. Other etiologies are idiopathic, systemic lupus erythematosus (SLE), birdshot retinochoroidopathy, sarcoidosis, Adamantiades-Behcet’s disease (ABD), and syphilis (Foster and Vitale 2002).
Panuveitis affects all three sections of the uveal tract and is responsible for 7%–38% of all cases (Foster and Vitale 2002). The most common cause is idiopathic (22.2%), followed by sarcoidosis, 14.1%, and multifocal choroiditis and panuveitis, 12.1%. Other causes include ABD, SLE, syphilis, Vogt-Koyanagi-Harada (VKH) syndrome, and sympathetic ophthalmia (Foster and Vitale 2002).
Current treatment
The mainstay of treatment of posterior uveitis is corticosteroids, which are administered in 3 forms: topically (most often used in conjunction with other forms), locally via sub-Tenon’s or intravitreal injection, and systemically (Jabs et al 2005). Each of these treatment modalities has strengths and weaknesses that must be weighed to individualize treatment for a particular patient. Topical steroid drops and ointment are primarily useful for anterior uveitis or panuveitis, as penetration into the posterior segment is minimal (Jabs et al 2005).
Local injection of long-acting steroids such as methylprednisolone or triamcinolone, however, results in therapeutic concentrations in the posterior segment and therefore can be useful for treatment of posterior uveitis in some individuals (Sabrosa and Pavésio 2000). The relatively low systemic concentration of steroids achieved by this method spares patients from the usual complications of steroid therapy, such as Cushingoid features, bone marrow suppression and infection, avascular necrosis, hyperglycemia, and osteopenia (Sabrosa and Pavésio 2000). Disadvantages to this form of therapy, however, include the necessity of repeated local injections as the steroid concentration in the posterior segment declines and the recurrence of inflammation every 2–3 months (Jabs et al 2005). These patients may suffer poorer outcomes than patients treated with systemic steroids due to visual loss in the interval between steroid injections (Jabs et al 2005), as recurrences of inflammation and repeated attacks may lead to cumulative damages and permanent visual compromise. In addition, intraocular steroid injections can lead to increased intraocular pressure, ptosis, strabismus, and carry a risk of globe penetration (Sabrosa and Pavésio 2000).
Many patients with posterior uveitis depend on systemic steroids to achieve long-term control of intraocular inflammation, and this form of therapy is considered first-line for posterior non-infectious uveitis (Jabs et al 2005). Oral prednisone is most often used, starting at a dosage of 1–2 mg/kg/day until inflammation abates, and then tapered gradually to a maintenance level of 15 mg/day or less (Sabrosa and Pavésio 2000). However, patients often tolerate oral prednisone best with the lowest risk for experiencing side-effects if the maintenance dose is 10 mg/day or less. In cases of vision-threatening inflammation, pulsed intravenous methylprednisolone of 1 g/day for 3 days can be used for rapid resolution, and patients may be switched to lower doses of oral prednisone thereafter (Sabrosa and Pavésio 2000). Due to the recurrent nature of the majority of uveitic entities, many patients must be maintained on high doses of long-term oral prednisone (>10 mg/day) in order to suppress active inflammation. Unfortunately, this course of treatment can lead to numerous adverse effects, including Cushingoid features, hyperglycemia, osteopenia and osteoporosis, bone marrow suppression and infection, avascular necrosis, hypertension, insomnia, and psychosis, among others. While weight gain and Cushingoid features may not meet the standard of adverse events, they are quite difficult for most patients to endure. On the other end of the continuum, however, avascular necrosis and osteoporosis can be debilitating and bone marrow suppression and infection may be life threatening. These conditions necessitate the cessation of corticosteroid therapy.
Additional immunomodulating drugs may be added to the corticosteroid regimen or used alone when steroids are not tolerated in order to control the inflammation. There are three classes of immunomodulatory therapy (IMT) that are commonly used in the management of ocular inflammatory diseases: antimetabolites, T-cell inhibitors, and alkylating agents. The common antimetabolites include methotrexate, azathioprine, and mycophenolate mofetil. Methotrexate is a folic acid analogue that inhibits dihydrofolate reductase, which is necessary for DNA and RNA synthesis. Its role in posterior uveitis is primarily for steroid-resistant pars plinitis and sympathetic ophthalmia (Sabrosa and Pavésio 2000). Methotrexate is given weekly; adverse effects include gastrointestinal upset, reversible hepatotoxicity, pulmonary fibrosis, and teratogenicity (Sabrosa and Pavésio 2000; Jabs et al 2005). Azathioprine is a purine analogue that has also been used for the treatment of ABD, in addition to Vogt-Koyanagi-Harada and sympathetic ophthalmia (Sabrosa and Pavésio 2000). Mycophenolate mofetil is also a purine synthesis inhibitor, originally developed for the prevention of kidney transplant rejection. It can be used for the treatment of posterior uveitis in patients intolerant to steroids, though it has its own treatment-limiting side-effects, including diarrhea (Jabs et al 2005).
Among the T-cell inhibitors, cyclosporine, an inhibitor of the inflammatory cytokine interleukin-2, has been shown to be effective in the treatment of ABD, though it is often combined with corticosteroids for optimal results (Sabrosa and Pavésio 2000). Its usefulness is limited by the common side-effects of nephrotoxicity and hypertension.
Cyclophosphamide, an alkylating agent used in the treatment of neoplastic disease, can be used for Wegener’s granulomatosis and necrotizing scleritis, though it is known to cause sterility and hemorrhagic cystitis, which may be associated with bladder cancer (Sabrosa and Pavésio 2000). Chlorambucil, another alkylating anti-neoplastic agent, has also been used in the treatment of posterior uveitis, including ABD (Sabrosa and Pavésio 2000). Although it shares some of the adverse effects of cyclophosphamide, including teratogenicity and sterility, it has not been related to cystitis or bladder cancer (Jabs et al 2005).
Unfortunately, these systemic immunomodulating therapies share with prednisone many potential systemic side-effects including the risks of bone marrow suppression and the possibility of infection by opportunistic organisms. If only local therapy with periocular or intravitreal delivery of steroids is used in the management of uveitis without the use of systemic steroids and/or IMT, disease often occurs. Repeated bouts of inflammatory episodes can lead to cumulative damage of ocular structures and functions, potentially leading to permanent visual loss. Thus, the risks of adverse events associated with prednisone and IMT have led to research efforts focused on developing effective local techniques of drug delivery that avoid the necessity of repeated intraocular injections and reduce the side-effects from systemic therapy, thus decreasing the risk for cumulative damage and visual loss.
Fluocinolone acetonide intravitreal implant: the first FDA-approved drug for the treatment of uveitis
The beneficial effects of the gancyclovir intravitreal implant device for CMV retinitis led investigators to explore the use of corticosteroid implants in the treatment of uveitis. The dexamethasone implant, detailed above, showed promise in a rabbit model of uveitis and proved to be effective at resolving a severe case of granulomatous iridocyclitis in a case report (Jaffe et al 2000b). Ten months after implantation of the device, however, the patient’s uveitis recurred, indicating the need for longer-acting implants (Jaffe et al 2000b). Such results led Jaffe and colleagues to examine fluocinolone acetonide, a corticosteroid with 1/24 the solubility of dexamethasone in aqueous solution, which presumably would allow steroid release over a much longer time period (Jaffe et al 2000c).
Pre-clinical studies
To demonstrate the safety and feasibility of a fluocinolone acetonide implant, Jaffe and colleagues constructed devices containing 15 mg of steroid and implanted them into the right eyes of 14 rabbits, using the fellow eyes as controls (Jaffe et al 2000c). Electroretinograms over a 54-week period showed no evidence of retinal toxicity, and histopathologic analysis of the implanted eyes was normal. The mean release rate from the devices was 3.26 μg/day, which remained relatively constant over the 54-week analysis period. Finally, throughout the study, there was no clinical evidence of toxicity by ophthalmoscopy. The authors estimated from the linear release kinetics that the 15-mg implant could last 18.6 years in the rabbit eye and 2.7 years in the human eye, making it a potential candidate for the treatment of chronic uveitis.
Pharmacokinetics
A follow-up study of rabbits implanted with 0.5- and 2-mg implants found constant levels of fluocinolone acetonide in the vitreous at all time points tested from 2 hours to 12 months post-implantation, indicating zero-order kinetics (Driot et al 2004). A dose-concentration relationship was also demonstrated, with vitreous concentrations 7–8 times higher in rabbits fitted with 2 mg implants compared with those with 0.5 mg implants. Steroid concentrations in the retina and vitreous were considerably higher than those measured in the aqueous humor, indicating posterior localization. Urine and plasma levels of fluocinolone were below the threshold of detection of 200 pg/mL, indicating the lack of systemic absorption.
The findings have been confirmed in human trials, where fluocinolone was also undetectable in blood samples (Jaffe 2006). In the rabbit trial, estimations of the implant lifespan were similar to that predicted by Jaffe et al (2000c), with approximately 38% of the steroid delivered after 1 year for the 0.5 mg implant and approximately 65% delivered in the 2 mg implant (Driot et al 2004). These results reinforce the local activity of the fluocinolone implant and the accompanying low risk of systemic adverse effects compared with current therapies.
Early clinical trials
To evaluate the safety and efficacy of the fluocinolone implant in humans, Jaffe and colleagues (2000a) recruited 5 patients with severe uveitis involving the posterior segment who had previously responded well to corticosteroids. The study group included 3 patients with panuveitis, 1 with iridocyclitis and intermediate uveitis, and 1 with Behcet’s syndrome. Seven eyes of the 5 study patients were implanted with fluocinolone acetonide intravitreal sustained-delivery devices and followed for an average of 10 months (Figure 1). All implanted eyes demonstrated stable or increased visual acuity, improving from a median of 20/200 to 20/30, and significant reduction in corticosteroid requirements. Before implantation, all 7 eyes needed local steroid injections, 6 required topical steroid drops, and 1 patient was treated with high-dose systemic steroids. After implantation of the devices, no patients needed periocular injections or topical steroids, and 1 patient used 10 mg prednisone systemically on alternating days to facilitate recovery of endogenous glucocorticoid production. No implanted eyes showed any signs of inflammation during the study. Three of the 4 non-implanted, intact fellow eyes (1 patient had undergone a previous enucleation), however, developed worsening inflammation during the study period, including episodes of necrotizing retinitis and reactivated chorioretinitis. These results indicated that the fluocinolone acetonide implant was effective at controlling posterior uveitis in steroid-responders during an average follow-up period of 10 months. One limitation of the study, the authors noted, was the unanswered question of whether non-steroid responders would experience uveitis regression with the fluocinolone acetonide implant.
Figure 1.
The fluocinolone acetonide device is inserted into the vitreous cavity through a scleral incision and anchored with a suture.
Increased intraocular pressure is a known complication of local corticosteroid use that limits the efficacy of topical steroid drops and local injections. In eyes implanted with the steroid-releasing device, mean intraocular pressure rose from a baseline value of 13.1 mm Hg to 15.7 mm Hg at the end of the observation period. Although this difference was not statistically significant, new pressure-lowering topical drops were needed in 4 of the 5 implanted eyes during the study, implying a relationship between device implantation and increased intraocular pressure.
In 2005, Jaffe and colleagues reported the results of a larger follow-up study to their pilot trial of the fluocinolone acetonide implant. In this trial, the study population included 5 eyes of 4 patients from the pilot study and added 31 eyes of 28 new patients. The patients from the pilot study all received the 2.1 mg device, and the new patients’ eyes were randomized to receive a 0.59 mg or 2.1 mg implant and observed for an average of 683 days (range 204–1817). Investigators remained blinded as to the dosage assignments at the time of manuscript preparation, so the combined results of the 2 groups were presented. Inclusion criteria for the follow-up study included incomplete response or inability to tolerate adverse effects of corticosteroids or immunosuppressants, visual acuity of light perception or better, intraocular pressure less than or equal to 21 mm Hg, and a history of noninfectious posterior or intermediate uveitis.
Of the study participants, 38.9% were diagnosed with idiopathic panuveitis and 19.4% with sarcoidosis. Women represented a large majority of the study population at 80.6%. The principal outcome measures were inflammation, visual acuity, anti-inflammatory medication use, and safety.
A comparison of inflammation before and after surgery revealed a markedly decreased number of episodes with the fluocinolone acetonide implant. In the 12-month period while study eyes were treated with standard therapy, each eye averaged 2.5 inflammatory episodes. In contrast, only 5 inflammatory episodes were observed during the entire post-implant follow-up period–all of which occurred in the same 2 eyes. These 5 episodes all occurred at 29 months after device implantation or later, and no inflammation was observed in the other 34 implanted eyes. Notably, non-implanted fellow eyes exhibited high rates of inflammatory episodes. At 1 year after device implantation, inflammation recurred in 9 of 23 eyes, and at 24 months, 5 out of 15 eyes had inflammatory episodes.
In addition to experiencing fewer inflammatory episodes, implanted eyes demonstrated substantially improved visual acuity, starting at a baseline of +1.11 logMAR units and improving to +0.72 at 24 months and +0.81 at 30 months. Mean visual acuity improvement in implanted eyes was 0.44 logMAR units between months 6 and 9, 0.47 from months 9 to 18, and 0.68 from months 18 to 30. In contrast, the fellow eyes did not improve significantly during the course of the trial, and by 6 months the implanted and fellow eyes did not statistically differ in visual acuity despite the fellow eye having significantly better vision at baseline. Greater than 90% of implanted eyes had stable or improved visual acuity during 2 years of follow up.
The proportion of patients requiring systemic corticosteroids or other immunosuppressive drugs declined significantly from 53.1% at baseline to 44.4% at 12 months and 38.8% at 24 months. Of these patients, none required systemic medication for inflammation in the implanted eye, but instead needed treatment for systemic disease or inflammation in the fellow eye. In addition, the dose requirement decreased in 70% of patients receiving systemic medication at 12 months, and in 85% at 24 months.
Implanted eyes also saw considerable reduction in local corticosteroid injections following implantation. The average injection rate at baseline was 2.2 per eye per year, but after device insertion, no injections were required for the first 24 months of follow up. At 36 months the injection rate was 0.03, increasing to 0.07 at 36 months. Topical corticosteroid drop use demonstrated a similar decline in implanted eyes, moving from 6.1 drops per patient per day at baseline to 1.1 at 24 months and 0.3 at 30 months. The proportion of implanted eyes requiring drops decreased from 78.1% before implantation to 21% at 2 years.
The pilot study of the fluocinolone acetonide implant demonstrated no statistically significant increase in intraocular pressure, but showed that an increased proportion of implanted eyes required pressure-lowering topical medication (Jaffe et al 2000a). Safety evaluation in the follow-up study mirrored these findings, with a statistically insignificant increase in intraocular pressure in implanted eyes from 14 mm Hg at baseline to 18.8 mm Hg at 24 months. Similarly to the pilot study, however, the proportion of implanted eyes requiring topical pressure-lowering drops increased from 11% at baseline to 56.1% between months 18 and 27, when they needed a mean of 3.3 types of drops each. In addition to topical therapy, glaucoma filtering procedures were required to lower intraocular pressure in 19.4% of implanted eyes during the study, compared with 0% in fellow eyes.
Cataract development is a known complication of corticosteroids, but a high rate of prior cataract extraction in study patients made it difficult to assess this relationship with the implant. Fifty percent of implanted eyes had undergone cataract surgery before entering the study and an additional 27.7% had cataract surgery during the implantation procedure. Only 8 phakic eyes remained following implantation, and 4 of these underwent cataract extraction during the study. The only other complications observed during the study follow-up period were 7 cases of hypotony (19.4% of implanted eyes), of which 5 resolved without treatment. No endophthalmitis, severe vitreous hemorrhage, or other complications were seen.
Fluocinolone acetonide uveitis study group
In response to the positive results reported in the pilot and follow-up trials, a multi-centered, randomized controlled trial was designed to study the efficacy and safety of the fluocinolone acetonide implant in a wider population. Patients were randomly assigned at study entry to a 2.1 mg implant, releasing 1.8–2.0 μg/day, or a 0.59 mg implant, releasing 0.4–0.5 μg/day. Patients would subsequently be followed for the lifespan of the implant, estimated to be 1000 days for both dosages. Any inflammatory recurrences were to be managed with local therapy (Jaffe et al 2006).
Eligible patients included those with 1 or more years of recurrent, non-infectious posterior uveitis who had either been treated with systemic medication for at least 3 months or who had 2 or more recurrences in a 6 month period. Study participants were also required to have visual acuity of at least 15 letters on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart and a quiet eye at the time of implantation surgery. Patient were excluded from the study if their intraocular pressure was greater than 25 on 2 or more pressure-lowering medications, if they had a history of uncontrolled intraocular pressure while using corticosteroids, or if they required systemic corticosteroids or other immunosuppressants for non-ocular disease. The primary outcome measure for the trial was a comparison of uveitis recurrence rates before and after device implantation. Secondary measures included improvement of visual acuity, adjunctive therapy requirement, and a comparison of uveitis recurrence rates in the implanted and fellow eyes.
A total of 278 patients at 27 clinical centers met all screening requirements and were randomized into treatment groups, including 110 receiving the 0.59 mg implant and 168 fitted with the 2.1 mg device. The average age of study participants was 43.5 years, ranging from 7 to 84. Bilateral disease was present in 77.3%, and 72.3% of study participants were female.
As the investigators remain masked to the treatment group assignment, the composite results are discussed. With 2 years of follow-up after implantation, the proportion of study eyes experiencing recurrence decreased considerably, from 59.7% in the 12 month period prior to implantation to 5.4% during the 12 months following surgery and 11.2% after 24 months of follow-up (Jaffe et al 2006). Conversely, the proportion of fellow eyes suffering inflammatory episodes increased from 25.4% 1 year prior to study entry to 42.4% at 1 year and 50% at 2 years. Thus the fluocinolone acetonide implant was significantly more successful at treating posterior uveitis compared with standard therapy, according to the main outcome measure.
The secondary measures of the study, visual acuity and necessity of adjunctive therapy, further demonstrated the efficacy of the fluocinolone implant. Average ETDRS visual acuity improved 5.1 letters in implanted eyes, compared with a drop of 3.9 letters in non-implanted fellow eyes. The proportion of patients needing systemic corticosteroids or other immunosuppressive drugs dropped markedly from 52.5% at study entry to 12.5% after 2 years of observation. The proportion of study eyes receiving local injections of corticosteroids to control intraocular inflammation decreased from 68% in the 12 months before study entry to 9.7% during 24 months following implantation. This is in contrast to the increase in injection rate in fellow eyes from 30.4% to 45.3%. Finally, a smaller proportion of implanted eyes required topical steroid treatment after implantation, 27.8%, compared with 35.7% at baseline. More fellow eyes required topical steroids, as the rate increased from 25.3% to 35.6%.
The early studies of the fluocinolone acetonide implant found that eyes with the device required more topical pressure-lowering drops than before surgery, though the mean increase in intraocular pressure was not significant. These findings were paralleled in the multi-centered study, where the proportion of implanted eyes requiring pressure-lowering drops increased from 14% at baseline to 53.7% at 2 years after surgery. Additionally, 30.6% of implanted eyes underwent pressure-lowering filtration surgery during the course of the study. Interestingly, the proportion of non-implanted fellow eyes needing pressure-lowering drops also increased after 2 years of follow-up, though noticeably less than study eyes, illustrating that eyes with ocular inflammatory diseases are at risk for developing ocular hypertension and glaucoma. At the time of enrollment, 10.9% of fellow eyes needed pressure-lowering drops, and this proportion increased to 20.2% at 2 years, which was statistically significant.
Unlike previous clinical trials of the fluocinolone acetonide implant, the multi-centered study enrolled sufficient patients to compare rates of cataract development in implanted and fellow eyes. Of implanted phakic eyes in a 1 year period following implantation, 27.3% developed cataract and all but 2 (25.9%) underwent cataract extraction. This proportion was relatively high compared with fellow eyes, in which only 5.8% developed cataract and 3.2% underwent extraction surgery. After 2 years of follow-up, the rate of cataract development increased markedly to 89.4% in implanted eyes, compared to 13.3% in fellow eyes. These results confirm earlier hypotheses that the intravitreal implantation of a corticosteroid-releasing device would lead to a higher rate of cataract formation. In addition to the more common adverse effects of rising intraocular pressure and cataract development, a number of less common complications were observed during the post-implant observation period, including hypotony in 6.1%, retinal detachment in 2.9%, endophthalmitis in 0.4%, and explantation at 2 years in 3.6% (Jaffe et al 2006).
Summary and discussion
As the result of several clinical trials demonstrating safety and efficacy, the United States FDA approved the fluocinolone acetonide intravitreal implant, 0.59 mg (Figure 2), as a single-indication orphan drug for the treatment of non-infectious posterior uveitis in April 2005. It received fast-track status due to the lack of drugs currently on the market indicated specifically for posterior uveitis.
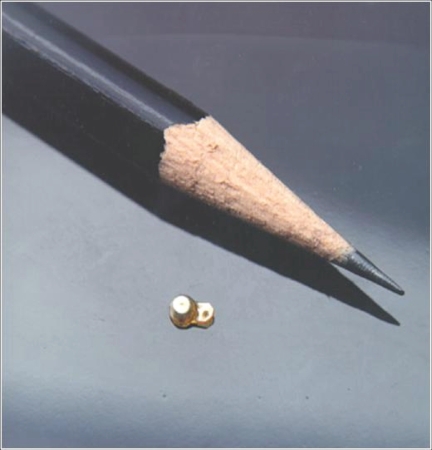
Figure 2.
The fluocinolone acetonide (Retisert™) sustained-release intravitreal implant, shown relative to the tip of a pencil.
Comparison with current therapy
The mass of evidence produced by the clinical trials detailed above suggests that the fluocinolone acetonide intravitreal implant can play a substantial role in the management of non-infectious posterior uveitis. It holds several advantages over current methods of corticosteroid delivery that may produce intolerable systemic side-effects or require frequent administration to maintain quiescence of inflammation. Systemic corticosteroids – often the most effective, though suboptimal, treatment for posterior uveitis – produce adverse effects ranging from mild to extremely serious and life threatening.
The search for alternatives to long-term systemic corticosteroid use has produced several candidates with concurrent advantages and disadvantages. Topical corticosteroid therapy is a poor choice for posterior uveitis due to the low concentrations achieved in the posterior segment. Local sub-Tenon’s injections solve the concentration problem but only last a few months, necessitating several injections per year in order to keep inflammation at bay. Additionally, with repeated injections, the risk of serious adverse effects, such as globe penetration or endophthalmitis, increases. Recurrent bouts of inflammation between injections can lead to cumulative damage causing irreversible visual loss. The use of IMT as a steroid-sparing approach has been successful in controlling uveitis, but the patients are subjected to the potentially significant side-effects of IMT.
The fluocinolone acetonide implant effectively addresses many of the concerns presented by current therapies by providing long-term control of posterior segment inflammation without the systemic complications of corticosteroids. In the studies detailed above, the rates of recurrence plummeted after insertion of the implant, and study eyes remained quiet for an extended period of time, demonstrating the efficacy of the fluocinolone device. In the long-term follow-up to their pilot study, Jaffe and colleagues did not observe a recurrence of posterior uveitis over a 24 month period in 36 implanted eyes (Jaffe et al 2005). During the same time period in the ongoing multi-centered trial, only 11.2% of implanted eyes had experienced a recurrence. These results demonstrate that greater than 88% of eyes receiving the implant could be inflammation-free for 2 years without additional intervention–a significant improvement over local injections, which may last only 2–3 months. In addition to a decrease in the recurrence of uveitis, both studies showed improved visual acuity in implanted eyes and decreased reliance on adjunctive therapy, including systemic steroids and local injections.
Adverse effects
The major adverse effect related to the implant, as demonstrated in clinical trials, is increased intraocular pressure, requiring patients to use pressure-lowering topical medications. In both the pilot studies and the randomized-control trial, intraocular pressure in implanted eyes increased throughout the study, though these increases were not statistically significant. A larger percentage of study patients did, however, require the addition of pressure-lowering drops after implantation. The proportion of eyes requiring drops quadrupled in Jaffe and colleagues’ follow-up trial, from 11% at baseline to 56.1% between months 18 and 27. Similar proportions were seen in the multi-centered trial, with 14% using drops at baseline, increasing to 53.7% at 24 months. Filtering procedures were required by 30.6% of implant patients in this study. These findings are not unexpected, given the long-known association between corticosteroids and increased intraocular pressure, but they underscore the importance of using alternative therapy in patients with significant glaucoma, refractory ocular hypertension, or a history of increased intraocular pressure response to steroids. However, the results of the randomized trial showed that the percentage of patients who require glaucoma surgery at 36 months is approximately 39.9% (authors’ unpublished data). Certainly, there has been an increase in the rates of glaucoma surgery needed from 24 to 36 months, but the rates are less than those seen from baseline to 12 months and 24 months, which may reflect the termination of drug release at approximately 30 months.
Uveitic eyes may be predisposed to increased intraocular pressure due to shifting fluid dynamics caused by inflammation. Investigators have reported a 10% incidence of secondary glaucoma in uveitis, which is postulated to be caused by aqueous outflow obstruction through the trabecular meshwork (Kuchtey et al 2005). Inflammation of the ciliary body in uveitic eyes leads to underproduction of aqueous humor and decreased perfusion of the trabecular meshwork, which blocks the outflow tract and raises intraocular pressure (Kuchtey et al 2005). Adding corticosteroids, which increase intraocular pressure by unclear mechanisms, exacerbates the situation in uveitic eyes that already have underperforming aqueous outflow tracts (Kuchtey et al 2005). It has also been hypothesized that the implant may effectively control inflammation and thus restore the normal aqueous production by the ciliary body (Jaffe et al 2005). In the setting of a chronically blocked outflow tract, intraocular pressure can rise, requiring the use of pressure-lowering medications.
Cataracts are the other common adverse effect of the fluocinolone acetonide implant. The rate of cataract formation was difficult to determine in earlier studies due to a high proportion of pseudophakic eyes at study enrollment. Most patients had previously been treated with systemic or local corticosteroids that likely contributed to prior cataract formation. The multi-centered trial conducted by the Fluocinolone Acetonide Study Group found a notable disparity between implanted and fellow eyes, however, with 12 month post-implant cataract development rates of 27.3% and 5.8% respectively. This rate is comparable to patients receiving oral prednisone for ocular inflammation, in which cataracts developed in 6.4–38.7% (Carnahan and Goldstein 2000). At 24 months of follow-up, however, cataract extraction rates climbed to 89.4% in implanted eyes, compared with 13.3% in fellow eyes. These findings indicate that worsening of cataracts is most likely in patients who were implanted with the fluocinolone implant and that cataract extraction is necessary in a large proportion of phakic patients treated with the implant.
Studies involving patients receiving local corticosteroid injections have reported lower rates of cataract development, but these findings are limited by shorter follow-up time and a low number of injections. A study of 53 patients with posterior ocular inflammation who received an average of 2.5 sub-Tenon’s steroid injections found a cataract development rate of 17.5% among previously clear lenses, however the follow-up period was only 12 months (Lafranco Dafflon et al 1999). Another study involving a single intravitreal injection of triamcinolone acetate reported a cataract development rate of 14.3% in eyes with previously clear lenses and a 5.9% progression rate among eyes that already showed lens changes (Kok et al 2005). Interestingly, eyes in which cataract developed had a mean follow-up time of 17.1 months, compared with a follow-up period of only 7 months for eyes without lens changes. This suggests that longer follow-up periods may reveal greater rates of cataract formation, making it difficult to compare rates across studies with differing methodologies.
Role in future treatment of uveitis
The positive results reported in the pilot, follow-up, and multi-centered trials suggest that the fluocinolone acetonide intravitreal implant may play a significant role in the treatment of non-infectious posterior uveitis. The population that would most benefit from the implant is likely to be patients requiring long-term systemic corticosteroids for posterior uveitis who become intolerant to the adverse effects of these drugs. As shown in the clinical trials, patients receiving the implant are usually able to discontinue systemic corticosteroids unless they are needed for systemic disease or inflammation in the fellow eye. In addition to alleviating the negative effects of systemic steroids, the implant appears to achieve superior inflammatory control compared with other methods of steroid delivery. The implant may also be an alternative for patients with unilateral or asymmetric posterior uveitis, in whom the dosage of systemic corticosteroids required for control of inflammation in the fellow eye could be significantly reduced or discontinued. Contraindications for the implant include uveitis with infectious etiology, significant glaucoma, refractory ocular hypertension, or a history of increased intraocular pressure in response to steroids.
Ongoing studies
The Fluocinolone Acetonide Uveitis Study Group will continue following the study patients in their multi-centered trial for an additional year, with final results of the study to be published at that time. The National Eye Institute is recruiting patients for an ongoing phase IV trial studying the efficacy of the fluocinolone acetonide implant versus oral corticosteroids and immunosuppressive agents in the treatment of non-infectious posterior, intermediate, or panuveitis: the Multicenter Uveitis Steroid Treatment (MUST) trial seeks to enroll 400 patients and randomize them to the implant or standard systemic corticosteroids, with supplemental immunosuppressants as needed. Twenty centers in the United States and 1 in Canada are enrolling patients for the study, which is designed to follow patients over an extended period of time. As this is the first large-scale trial to directly compare the implant to standard therapy, its findings have the potential to be influential in future therapeutic guidelines for posterior uveitis.
The potential of sustained-release devices in targeted delivery of pharmacologic agents is vast. In addition to corticosteroids for ocular inflammatory diseases, other classes of therapeutic agents such as antibodies for choroidal neovascularization can potentially be packaged, stored, and delivered using such technology. Moreover, current research also focuses on technology that will deliver therapeutic agents in a sustained fashion, but unlike the fluocinolone implant, will not require a scleral incision to place the implant into the vitreous cavity. Rather, the implant, which may be self-dissolvable or replaceable, can be inserted as an in-office procedure, allowing the chronic delivery of medications through only a mildly invasive procedure.
References
- Carnahan MC, Goldstein DA. Ocular complications of topical, periocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478–83. doi: 10.1097/00055735-200012000-00016. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Berger AS, Pearson PA, et al. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci. 1995;36:442–53. [PubMed] [Google Scholar]
- Driot JY, Novack GD, Rittenshouse KD, et al. Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implanation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004;20:269–75. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- Dunn JP, Van Natta M, Foster G, et al. Complications of ganciclovir implant surgery in patients with cytomegalovirus retinitis: the Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Retina. 2004;24:41–50. doi: 10.1097/00006982-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Foster CF, Vitale AT. Diagnosis and treatment of uveitis. Philadelphia: W.B. Saunders; 2002. [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study, 2004. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Jabs DA, Akpek EK. Immunosuppression for posterior uveitis. Retina. 2005;25:1–18. doi: 10.1097/00006982-200501000-00001. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Ben-nun J, Guo H, et al. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000a;107:2024–33. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–7. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, McCallum RM, Branchaud B, et al. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–8. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Pearson PA, Ashton P. Dexamethasone sustained drug delivery implant for the treatment of severe uveitis. Retina. 2000b;20:402–3. doi: 10.1097/00006982-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Yang CH, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000c;41:3569–75. [PubMed] [Google Scholar]
- Kok H, Lau C, Maycock N, et al. 2005Outcome of intravitreal triamcinolone in uveitis Ophthalmology 1121916.e1–7. [DOI] [PubMed] [Google Scholar]
- Kuchtey RW, Lowder CY, Smith SD. Glaucoma in patients with ocular inflammatory disease. Ophthalmol Clin N Amer. 2005;18:421–30. doi: 10.1016/j.ohc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lafranco Dafflon M, Tran VT, Guex-Crosier Y, et al. Posterior sub-Tenon’s steroid injections for the treatment of posterior ocular inflammation: indications, efficacy and side effects. Graefes Arch Clin Exp Ophthalmol. 1999;237:289–95. doi: 10.1007/s004170050235. [DOI] [PubMed] [Google Scholar]
- Martin DF, Kuppermann BD, Wolitz RA, et al. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340:1063–70. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- Musch DC, Martin DF, Gordon JF, et al. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337:83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- Opremcak EM, Cunningham ET, Foster CS, et al. San Francisco: American Academy of Ophthalmology; 2004. Basic and clinical science course, section 9: intraocular inflammation and uveitis. [Google Scholar]
- Sabrosa NA, Pavésio C. Treatment strategies in patients with posterior uveitis. Int Ophthalmol Clin. 2000;40:153–61. doi: 10.1097/00004397-200004000-00012. [DOI] [PubMed] [Google Scholar]