Abstract
Gain-of-function mutations in FGF receptor 3 (FGFR3) have been implicated in severe skeletal dysplasias and in a variety of cancers. In their study in this issue of the JCI, Qing et al. used specific shRNA probes to demonstrate that FGFR3 functions as an important driver of bladder carcinoma cell proliferation (see the related article beginning on page 1216). A unique anti-FGFR3 mAb was shown to exhibit antitumor activity in human bladder carcinoma cells in vitro and in mouse bladder cancer or multiple myeloma xenograft tumor models bearing either wild-type or mutant FGFR3. These results suggest that clinical development of anti-FGFR3 mAbs should be considered for targeted therapy of cancer and other diseases.
FGFs are members of a large family of growth factors that play an important role in the control of diverse cellular processes (e.g., cell proliferation, differentiation, survival, and migration) during embryonic development and in the maintenance of cellular homeostasis in virtually all organs and tissues (1, 2). FGFs act in concert with heparan sulfate proteoglycans (HSPGs) to activate a family of four receptor tyrosine kinases (RTKs) designated FGF receptors 1–4 (FGFR1–4). Each FGFR consists of an extracellular region composed of three Ig-like domains designated D1, D2, and D3; a short stretch of amino acids in the D1–D2 linker region, designated the acid box; and a single transmembrane domain followed by an intracellular tyrosine kinase domain with additional regulatory sequences (1). Diversity in ligand-binding specificity and tissue expression patterns of FGFR1–3 are further enhanced by alternative RNA splicing in the C-terminal half of D3 to generate either the IIIb or the IIIc splice isoform. It was previously shown that the IIIb isoform is expressed in epithelial cells and the IIIc isoform is expressed in mesenchymal cells (1). Interestingly, FGFs that activate the epithelial IIIb FGFR isoforms are expressed in mesenchymal cells, whereas FGFs that activate the mesenchymal IIIc FGFR isoforms are produced in epithelial cells (1).
A variety of human skeletal dysplasias associated with severe impairment in cranial, digital, and skeletal development have been shown to be driven by gain-of-function mutations in FGFR1–3 (1). Gain-of-function mutations in FGFRs have also been identified in a variety of human cancers, including myeloproliferative syndromes, lymphomas, glioblastoma, as well as prostate, bladder, and mammary carcinomas (1). Importantly, the t(4;14)(p16.3;q32) chromosomal translocation that results in FGFR3 overexpression in 20% of multiple myeloma patients is correlated with poor clinical response and patient survival (3). FGFR3 overexpression has also been identified in bladder carcinoma (4). Moreover, somatic gain-of-function mutations in FGFR3 have been identified in 65% of papillary and in 20% of muscle-invasive bladder carcinomas (4). These studies suggest that FGFR3 could be considered a candidate for targeted therapies for the treatment of cancers and skeletal dysplasias driven by gain-of-function FGFR3 mutations. Several small molecule inhibitors of the tyrosine kinase activity of FGFR3 and other members of the FGFRs have been described, some of which are currently in clinical development (5, 6). However, potent small molecule inhibitors that specifically interfere with the tyrosine kinase activity of FGFR3 are difficult to develop because of the close structural similarity of the FGFR3 tyrosine kinase domain to that of other members of the FGFR family and other RTKs. An alternative approach for developing a selective inhibitor of FGFR3 that does not interfere with the activity of other FGFRs and RTKs is to raise inhibitory mAbs that selectively bind to the extracellular domain of FGFR3.
Development of a specific anti-FGFR3 mAb
Since the therapeutic potential of targeting FGFR3 for the treatment of bladder cancer has not been well defined, in their study in this issue of the JCI Qing et al. first examined the role played by activating FGFR3 mutations in bladder cancer by employing specific shRNA probes that silence FGFR3 expression in the tumor cells (7). Using several human bladder cancer cell lines expressing either wild-type or activated FGFR3 mutants, Qing et al. demonstrated that FGFR3 plays a major role in bladder cancer cell proliferation and that silencing of FGFR3 expression leads to attenuation of cell-cycle progression rather than stimulation of cell apoptosis.
Qing et al. went on to generate an anti-FGFR3 mAb, via the immunization of mice and by screening phage display libraries for mAbs that bind to the extracellular region of FGFR3 and thereby interfere with FGF binding (7). The most effective antagonist of FGFR3 activity identified in these experiments was an anti-FGFR3 mAb the authors termed R3Mab. The authors demonstrate that R3Mab binds selectively to FGFR3 and interferes with ligand binding to either the IIIb or IIIc isoforms of FGFR3. Moreover, R3Mab blocked FGF1-mediated stimulation of FGFR3 activation, tyrosine phosphorylation of the docking protein FGFR substrate 2 α, the MAPK response, and FGFR3-dependent proliferation of normal and transformed cells (Figure 1).
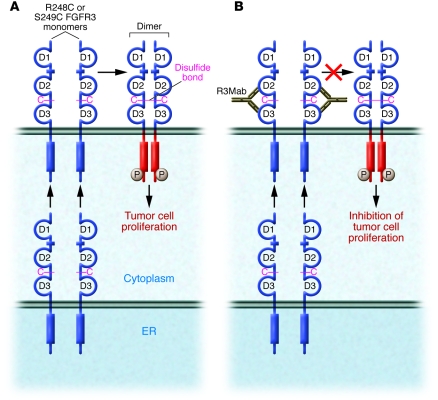
Figure 1. Potential mechanism of inhibition of the activity of disulfide-linked oncogenic FGFR3 mutants by treatment with an anti-FGFR3 mAb (R3Mab).
Oncogenic FGFR3 mutants R248C and S249C remain monomeric as long as they remain inside the cell in the endoplasmic reticulum and other intracellular compartments. Upon migration to the cell surface and exposure to the oxidative extracellular environment, disulfide-linked activated dimers of FGFR3 are formed, resulting in tyrosine phosphorylation and triggering of intracellular signaling pathways that result in bladder cell carcinoma proliferation (A). In their study in this issue of the JCI, Qing et al. (7) report that binding of their anti-FGFR3 mAb (R3Mab) to D2 and D3 of the extracellular ligand-binding region of FGFR3 before intermolecular disulfide linkages are formed prevents formation of disulfide-linked FGFR3 dimers with constitutively stimulated tyrosine kinase activity, resulting in the inhibition of bladder cell carcinoma proliferation and of tumor formation (B). Cysteines of R248C or S249C FGFR3 are shown in magenta. The stimulated tyrosine kinase domain is shown in red.
Detailed studies of R3Mab binding to a variety of synthetic peptides derived from the extracellular region of FGFR3, as well as the determination of the X-ray crystal structure of R3Mab in complex with the D2 and D3 domains of FGFR3, revealed that R3Mab binds to epitopes corresponding to FGF- and HSPG-binding sites in D2 and D3 and to epitopes in a region responsible for mediating receptor-receptor contacts between neighboring dimeric FGFR3 molecules (7, 8). The X-ray crystal structure also showed that R3Mab binds preferentially to an alternate conformation of D3 relative to D2, one that is different from the orientation of D3 relative to D2 that was seen in the crystal structure of FGF1 in complex with D2 and D3 of FGFR3 (7–9).
Most FGFR3-activating mutations identified in bladder cancer are located in the extracellular domain of the receptor (4). These mutations (R248C or S249C) give rise to a new, unpaired cysteine residue, leading to formation of disulfide-linked FGFR3 dimers in a ligand-independent manner. The disulfide-linked FGFR3 dimers exhibit constitutive tyrosine kinase activity and ligand-independent stimulation of cellular signaling pathways (4) (Figure 1). Qing et al. show that, remarkably, R3Mab exhibits a robust inhibitory activity not only against wild-type FGFR3 but also against a variety of oncogenic FGFR3 mutants, including the oncogenic, disulfide-linked R248C or S249C FGFR3 mutants (7) (Figure 1). Interestingly, it is demonstrated that formation of disulfide-linked FGFR3 dimers is prevented by R3Mab treatment or by treating the cells with a non–cell-permeating agent that blocks free sulfhydryl groups from forming disulfide linkages. Qing et al. propose that a reversible equilibrium between the monomeric and disulfide-linked oncogenic forms of FGFR3 exists and that R3Mab binding shifts the equilibrium toward the monomeric inactive state, resulting in inhibition of FGFR3 activity. Since disulfide bond formation is not a reversible chemical reaction, an alternative and more plausible mechanism is that the R248C or S249C FGFR3 mutants remain monomeric as long as they are located inside the cell due to the reducing intracellular environment. After the R248C or S249C FGFR3 mutants are transported to the cell surface, the unpaired cysteine of the (R248C or S249C) FGFR3 mutant pairs with the unpaired cysteine of another FGFR3 mutant molecule to generate a disulfide-linked FGFR3 dimer — a process likely facilitated by the oxidative nature of the extracellular environment. As mutant FGFR3 is transported to the cell surface in a monomeric configuration, the rate of disulfide bond formation between neighboring mutant monomers must be sufficiently slow to permit R3Mab binding and prevention of disulfide bond formation between the R248C or S249C FGFR3 mutants, even in the oxidative extracellular environment (Figure 1).
Efficacy of R3Mab therapy in murine tumor models
Qing et al. show that R3Mab treatment of bladder cancer or multiple myeloma xenografts in mice has an antitumor effect on tumors bearing either wild-type or mutant FGFR3 (7). These experiments demonstrate that R3Mab is a potent inhibitor of FGFR3-driven tumors in vivo and suggest that R3Mab is a good candidate for clinical development. However, whereas accumulation of mutant FGFR3 monomers was detected in lysates from cultured human bladder cancer cells treated with R3Mab, Qing et al. were not able to detect a similar accumulation of mutant FGFR3 monomers in lysates from tumors isolated from mice treated with R3Mab. This difference may be due to technical reasons; alternatively, the antitumor activities of R3Mab in vitro and in vivo may be mediated by different mechanisms. An intriguing possibility is that treatment of the tumor-bearing mice with antioxidants may potentiate the in vivo antitumor activity of R3Mab.
As noted above, robust antitumor activity of R3Mab was also detected in mice bearing multiple myeloma xenografts (7). The multiple myeloma tumors that were treated with R3Mab overexpress wild-type FGFR3. It was convincingly demonstrated that the antitumor effect of R3Mab treatment of tumor cells bearing high amounts of wild-type FGFR3 on their cell surface was mediated in part by Ab-dependent cell-mediated cytotoxicity (ADCC). By contrast, ADCC did not seem to play a role in the antitumor effect of R3Mab on human bladder cancer cells, which express at least a 5-fold lower level of wild-type or mutant FGFR3 compared with the multiple myeloma cells studied.
Finally, since similar gain-of-function mutations in FGFR3 cause skeletal dysplasias and other developmental disorders, it will be important in future studies to examine whether R3Mab treatment may prevent or attenuate developmental disorders caused by FGFR3-activating mutations in neonatal murine models of these diseases (10–12).
Acknowledgments
This work was supported by NIH grants R01-AR051448, R01-AR051886, and P50-AR054086 to Joseph Schlessinger.
Footnotes
Conflict of interest: Y. Hadari is an employee of and owns equity in Kolltan Pharmaceuticals Inc. J. Schlessinger owns equity in and has received consultancy fees from Plexxikon Inc. and Kolltan Pharmaceuticals Inc., which develop drugs for the treatment of cancer and other diseases.
Nonstandard abbreviations used: FGFR, FGF receptor; RTK, receptor tyrosine kinase.
Citation for this article: J. Clin. Invest. 119:1077–1079 (2009). doi:10.1172/JCI38948
See the related article beginning on page 1216.
References
- 1.Eswarakumar V.P., Lax I., Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Dailey L., Ambrosetti D., Mansukhani A., Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Chesi M., et al. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat. Genet. 1997;16:260–264. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles M.A. Novel therapeutic targets in bladder cancer: mutation and expression of FGF receptors. Future Oncol. 2008;4:71–83. doi: 10.2217/14796694.4.1.71. [DOI] [PubMed] [Google Scholar]
- 5.Trudel S., et al. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2004;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- 6.Grand E.K., Chase A.J., Heath C., Rahemtulla A., Cross N.C. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–966. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- 7.Qing J., et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J. Clin. Invest. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotnikov A.N., Schlessinger J., Hubbard S.R., Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–650. doi: 10.1016/S0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 9.Schlessinger J., et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. . Mol. Cell. 2000;6:743–750. doi: 10.1016/S1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., et al. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J. Clin. Invest. 1999;104:1517–1525. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twigg S.R., et al. Skeletal analysis of the Fgfr3(P244R) mouse, a genetic model for the Muenke craniosynostosis syndrome. Dev. Dyn. 2009;238:331–342. doi: 10.1002/dvdy.21790. [DOI] [PubMed] [Google Scholar]
- 12.Pannier S., et al. Activating Fgfr3 Y367C mutation causes hearing loss and inner ear defect in a mouse model of chondrodysplasia. Biochim. Biophys. Acta. . 2009;2:140–147. doi: 10.1016/j.bbadis.2008.11.010. [DOI] [PubMed] [Google Scholar]