Abstract
A low-protein (LP) diet induces injury from energy depletion in renal epithelial cells. Overexpression of heat-shock proteins has been implicated in the restoration of the cytoskeletal anchorage of Na+/K+-ATPase. We tested if Hsp70 stabilizes renal Na+/K+-ATPase attachment to the cytoskeleton from the cortex and the outer stripe of the outer medulla (OSOM) in rats during recovery from a LP diet. Rats were fed with a LP diet (8% protein) for 14 days, and then the rats were recovered with a 24% protein (RP) diet. The control group received a 24% protein (NP) diet. Increased Na+/K+-ATPase dissociation was demonstrated in soluble fraction from OSOM with lower ATP content as a result of LP diet vs NP. Meanwhile, decreased Hsp70 levels in the same fraction were shown. Translocation of Hsp70 to the cytoskeletal injured fraction associated with stabilization of Na+/K+-ATPase was shown in OSOM from LP after in vitro co-incubation of the cytoskeletal fraction of LP and non-cytoskeletal fraction of RP. These effects were abolished by the addition of the anti-Hsp70 antibody. Absence of Na+/K+-ATPase detachment from its cytoskeletal anchorage was demonstrated in proximal duct segments from cortex in LP. Co-immunoprecipitation showed that the amount of Na+/K+-ATPase co-precipitating with Hsp70 increased in the OSOM as a result of the LP diet. In the cortex tissues from rats fed the LP and the RP diet, the interaction of both proteins were similar to the control groups. Our results indicate that Hsp70 has a critical role in protecting the integrity of the cytoskeletal anchorage of Na+/K+-ATPase during recovery from ATP-depleted injury resulting from LP in OSOM.
Introduction
Cellular perturbations in renal epithelia are produced by energy deprivation from hypoxia, ischemia, or metabolic inhibition. Early in the injury process, renal ischemia induces the rapid duration-dependent relocation of apical and basolateral membrane proteins into the alternate domain (Spiegel et al. 1989; Fish and Molitoris 1994). For Na+/K+-ATPase to be translocated to the apical domain, it must first be detached from its cytoskeletal anchorage, which has been defined functionally by detergent extractability (Spiegel et al. 1989; Molitoris et al. 1991).
Early events in renal epithelia injured by ATP depletion result in a rapid and duration-dependent alteration in cytoskeletal proteins that disrupts membrane–cytoskeletal protein interactions, and it is manifested by membrane blebbing and loss of cell polarity (Molitoris et al. 1998). Na+/K+-ATPase dissociation from the cytoskeleton progressively increases when ATP is intensively reduced (Van Why et al. 1999). In addition to the duration of the energy depletion, the severity of the ATP depletion affects the degree of cellular disruption (Siegel et al. 1994). Reestablishment of the membrane–cytoskeletal complex, and thus cell polarity, appears to occur by recycling of misplaced Na+/K+-ATPase subunits (Van Why et al. 1994a).
Heat-shock protein (Hsp70) has been implicated in the restoration of the cytoskeletal anchorage of Na+/K+-ATPase (Aufricht et al. 1998; Riordan et al. 2005). Hsp70 binds to nascent and immature proteins to prevent premature and improper binding and folding (Glover and Lindquist 1998). Therefore, a role in the reassembly of disrupted or denatured proteins during post-ischemic cellular reorganization by induced Hsp72 has been suggested (Pelham 1986).
Induction of heat-shock protein synthesis has been well characterized in cell injury from a variety of insults (Morimoto et al. 1994a). The relationship of stress response initiation, to specific decrements in ATP in renal cortex in vivo, has been previously studied. As indicated by activation of heat-shock transcription factor (HSF) and expression of inducible Hsp70, the stress response was initiated when renal cortical ATP was reduced below a threshold of 50% of control. Further reductions in renal ATP resulted in a more vigorous stress response (Van Why et al. 1994b). In LLC-PK1 cells, graded ATP depletion resulted in a stepwise dissociation of Na+/K+-ATPase from the cytoskeleton and the activation of the HSF (Van Why et al. 1999). Either cellular ATP or the metabolic consequences associated with its depletion may be threshold factors for the initiation of the stress response in the kidney.
Hypoxia and ATP depletion are involved in renal ischemia damage. Injury events in LP feeding, an in vivo model of energy deprivation, include ATP depletion on epithelial cells from duct segments (Seney and Marver 1989; Vallés et al. 2005) besides renal hemodynamic changes (Martinez-Maldonado et al. 1993). Previously, we provided evidence for the apoptosis induction in epithelial cells from medullary collecting duct segments in LP and for the anti-apoptotic, cytoprotective mechanism of Hsp70 during protein recovery (Carrizo et al. 2006).
In the present study, we tested whether Hsp70 interacts with Na+/K+-ATPase by stabilizing its attachment to the cytoskeleton in the outer stripe of the outer medulla during recovery from low-protein feeding.
Methods
Experimental animals and protocol
Female Wistar rats weighing 60–70 g were used. Rats had free non-restricted access to water and food consumption. The body weight of each animal was measured daily.
The animals were divided into three dietary groups. The normal protein (NP) group (n = 12) received an isocaloric 24% protein diet during 14 days (NP14; age-matched control group of the LP) or during 30 days (NP30; age-matched control group of the RP). The control group’s diet was composed of casein (24%), cornstarch (36%), sucrose (21.3%). The low protein (LP) group (n = 12) received an isocaloric 8% protein diet for 14 days. This group’s diet was composed of casein (8%), cornstarch (48.45%), and sucrose (24.3%). Both diets contained cellulose fiber (10%), choline (0.2%), mineral mix (2%), vitamin mix (0.5%), corn oil (6%), 0.069 mEq of Na+ per gram, and 0.16 mEq of K+ per gram. The recovery protein (RP) group (n = 12) received a re-administration of 24% protein for 14 days after being fed with 8% LP diet for 14 days.
Blood pressure was measured by tail-cuff plethysmography (Grass model 7B Poligraph, Grass Instruments, MA, USA) in the rats on days 14 and 30 after the initiation of the experimental protocol.
Tissue preparation
Rats were anaesthetized with sodium pentobarbital (60 mg/kg IP). Then, kidneys were perfused through the abdominal aorta with ice-cold phosphate buffered saline (PBS) solution to rinse away all the blood. Left kidney cortex and outer stripe of the outer medulla from all groups were isolated and homogenized in chilled extraction buffer containing 0.1% Triton X-100, 30 mM imidazole, 10 mM ethylenediaminetetraacetic acid, 2 mM MgCl2, 0.1 mM dithiothreitol, 0.5 mM phenylmethanesulphonylfluoride, 10 μg/ml leupeptin, pH 7.4, with a Duonce style tissue homogenizer. The homogenate was centrifuged at 35,000×g for 10 min at 4°C to separate the Triton-soluble supernatant (non-cytoskeletal) protein fraction from the Triton-insoluble pelleted fraction (cytoskeletal). The pellets were resuspended in an extraction buffer of half the volume of the original homogenate, resulting in similar protein concentration as in supernatants. Aliquots of each were saved at −70°C.
Incubation procedures
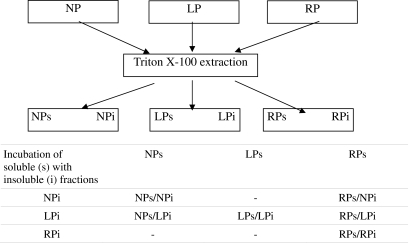
One hundred microliters of aliquots of isolated pellet (i) were thawed on ice in 200 μl of isolated supernatant (s) aliquots. After thawing, the mixture was resuspended and kept for 20 min at room temperature. The samples were centrifuged at 35,000×g for 15 min at 4°C. The repelleted cytoskeletal fraction and non cytoskeletal supernatant were stored at −70°C (Table 1).
Table 1.
Schematic incubation procedures
Cellular proteins are fractionated into cytoskeletal pellets (i) and non-cytoskeletal supernatants (s) by Triton X-100 extraction of renal tissue from normal protein (NP), low protein (LP) and recovery protein (RP) groups. Aliquots of isolated pellets and isolated supernatant were incubated in different combinations.
For the assessment of cytoskeletal injury, aliquots from NP and LP Triton X-100 insoluble were incubated in their own NP (NPs/NPi) or LP (LPs/LPi) Triton X-100 soluble, respectively. At the same time, aliquots from LP pellets were incubated in NP supernatants (NPs/LPi; Table 1). The second set of experiments consisted in parallel incubation of aliquots from the same LP pellet in both LP (LPs/LPi) and RP (RPs/LPi) supernatants, and aliquots from RP pellet were incubated in RP supernatant (RPs/RPi; Table 1). To assess the translocation of Hsp70 into the injured cytoskeletal fraction, incubation of LP pellet in RP supernatant (RPs/LPi) and of NP pellet in RP supernatant (RPs/NPi) were conducted (Table 1). The third set of experiments consisted of parallel incubation of LPi in both RPs (RPs/LPi) and RPsAnti-Hsp70 Ab plus 25 μg of anti-Hsp70 antibody (Sigma; RPsAnti-Hsp70 Ab/LPi). This mixture was resuspended and incubated for 20 min at room temperature. Differential centrifugation was then repeated at 35,000×g for 15 min at 4°C. Repelleted cytoskeletal fractions and dissociated supernatant fractions were saved for further analysis.
Na+/K+-ATpase immunoprecipitation—Hsp70 co-precipitation
Co-immunoprecipitation was carried out using Dynabeads M-280 Tosylactivated (Dynal, Biotech). The antibody (Na+/K+-ATPase) was dissolved in a 0.1 M borate buffer pH 9.5, added to the Dynabeads, and then vortexed for 1 min. After 48-h incubation, rotating at 4°C, samples were placed on the magnet, and the supernatants were removed and discarded. The coated beads were washed with a buffer containing PBS pH 7.4 with 0.1% bovine serum albumin (BSA) and then with 0.2 M Tris pH 8.5 with 0.1% BSA. Subsequently, equal volumes of membrane samples adjusted to contain equal quantities of protein were added to the coated beads. After 1-h rotating incubation at 2–8°C, membrane samples were placed on the magnet, and the supernatants were removed and discarded. The beads were washed with a 0.1 M Na phosphate pH 7.4, resuspended in an equal volume of 2X sample buffer, and boiled for 3 min. The supernatant was removed, and membrane samples were stored at −70°C. The Hsp70 level was normalized against Na+/K+-ATPase level for each experimental condition. The results were expressed as a ratio between Hsp70/Na+/K+-ATPase levels.
Protein determination and Western blot analysis
We quantified the protein concentrations from the cortex and the outer stripe of the outer medulla by Lowry assay. We used BSA as a standard. We electrophoresed 20 μg of proteins in 0.1% sodium dodecyl sulfate (SDS) and 8% polyacrylamide gel with 4% stacking gel. For each gel, an identical gel was run in parallel. The first gel was subjected to Coomassie blue staining to assure identical loading. The second one was subjected to immunoblotting. Proteins were electrophoretically transferred from gels to nitrocellulose membranes. Non-specific reactivity was blocked by incubation for 1 h at room temperature with 5% nonfat dry milk dissolved in PBS (pH 7.6, 0.1% Tween 20). Blots were incubated overnight at 4°C with primary antibodies against Hsp70 (dilution 1:2,000, Sigma) or the alpha-subunit Na+/K+-ATPase (dilution 1:1,000, Chemicon). The labeling was visualized with secondary biotinylated antibodies and then with horseradish peroxidase-conjugate streptavidin (DAKO). The signal was detected with an enhanced chemiluminescence system and exposure to X-ray film (Amersham). Densitometric analysis was carried out by image analysis software. The photographs were digitalized using a scanner. Densitometric analysis was performed using NIH Image software.
Assay for ATP content
Frozen cortex and OSOM samples (~15 mg) were homogenized with 200 μl ice-cold trichloracetic acid (2.5% vol./vol.). The homogenate was centrifuged at 1,000×g 10 min at 4°C. The supernatant was neutralized with 1 M Tris base (120 μl/ml supernatant) and then used for assay of ATP content (FL-AA Kit, Sigma). The pellet was neutralized with 75 μl of 0.5 M NaOH, and the protein content was determined by the Lowry method. The tissue content of ATP was expressed as micromoles of ATP per gram of protein.
Preparation of tissue for immunofluorescence
To rinse all the blood, we perfused the kidneys through the abdominal aorta with ice-cold PBS solution. Then, the kidneys were fixed by retrograde perfusion with 40 ml of 4% paraformaldehyde in 9.4 mM Na2B4O7, 0.34 mM Na2SO3, 0.16 M H3BO3, pH 7.4. The kidneys were removed and placed in paraformaldehyde for 4 h at room temperature and overnight at 4°C. Fixed tissues were cryoprotected in 0.9 M sucrose, washed in PBS several times, frozen in isopentane, and stored at −70°C.
Indirect immunofluorescence
At the time of staining, kidneys were cut into 5-μm sections using a Reichert Frigocut microtome. Sections were permeabilized with 1% SDS for 5 min, rinsed with PBS, and then incubated with PBS plus 1% bovine serum albumin to block non-specific background staining. Sections were then incubated with antibody against the α-subunit of the Na+/K+-ATPase (diluted 1:100) overnight at 4°C. Then, the sections were washed twice in PBS containing an additional 2.7% NaCl and then once with plain PBS, 5 min each. Sections were then incubated with fluorescein isothiocyanate-conjugated secondary antibody (goat anti-rabbit diluted 1:100) for 1 h. Excess antibody was washed away, and the sections were mounted with glycerol/PBS (1:1) and then observed in a microscope, Nikon Eclipse TE-2000-U, with a camera Hamamatsu-ORCA C4742-95-12MR. Software: Metamorph v6, Molecular Devices.
Statistical analysis
The average values for all experimental conditions were calculated and normalized in terms of the expression of proteins in the control tissues. Data were assessed by the analysis of variance test. Statistical significance was assessed by Bonferroni post-test. A P < 0.05 was considered significant. Values are expressed as means ± SEM.
Results
For the 14-day period of pair feeding, average daily food intakes were 13.25 ± 0.54 and 13.43 ± 0.45 g/100 g body weight for rats fed with the 24% and 8% protein diets, respectively. The body weight in the LP group after 14 days showed a significant decrease compared to NP (65.9 ± 2.2 vs. 121.3 ± 3.6, p < 0.05). No differences were observed in blood pressure among groups during experimental conditions (147.8 ± 4.98 vs. 152.1 ± 5.57, p > 0.05).
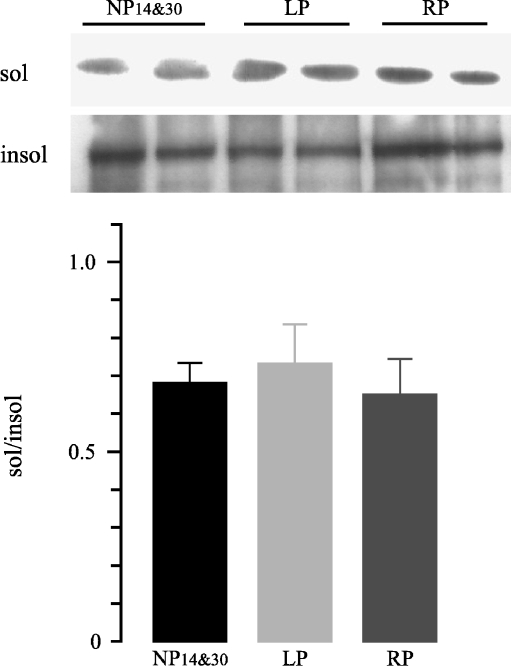
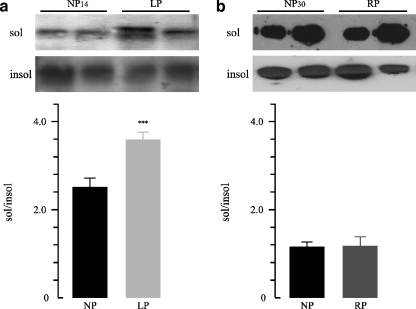
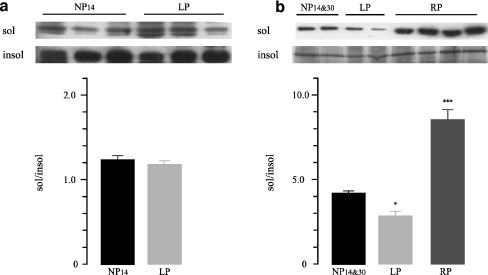
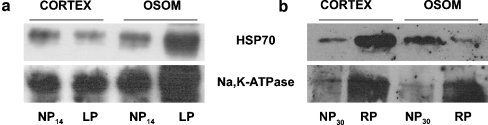
To analyze whether Na+/K+-ATPase was detached in cortex and OSOM from LP group, Western blot analysis of Triton X-100 extracts was performed. No significant differences in Triton X-100 soluble to insoluble Na+/K+-ATPase ratio (sol/insol) in the cortex were observed among groups (Fig. 1). On the contrary, the densitometric analysis revealed higher protein levels of Triton X-100-extractable Na+/K+-ATPase in the OSOM from the LP group compared to the NP group (1.4-fold increase, 3.56 ± 0.18 vs. 2.48 ± 0.02, n = 12, p < 0.001, Fig. 2a). We also analyzed whether recovered protein diet for 14 days stabilized the cytoskeletal association of Na+/K+-ATPase in the OSOM. When rats were fed with the recovery diet, OSOM Triton X-100-extractable Na+/K+-ATPase had returned to control 1.17 ± 0.04 vs. 1.15 ± 0.01, n = 12, p > 0.05 (Fig. 2b).
Fig. 1.
Representative Western blot and densitometry of TritonX100-soluble and -insoluble Na+/K+-ATPase in rat renal cortex obtained from control 14 and 30 days (NP14&30), low-protein diet group (LP), and recovery group (RP; n = 12). No significant differences were observed among groups. Data are shown as mean ± SEM
Fig. 2.
Representative Western blot and densitometry of TritonX100-soluble and -insoluble Na+/K+-ATPase in outer stripe of the outer medulla (OSOM) a OSOM renal tissue obtained from control 14 days (NP14) group compared to low protein diet group (LP). b OSOM renal tissue obtained from control 30 days (NP30) group compared to recovery group (RP; n = 12). Increased sol/insol Na+/K+-ATPase ratio was demonstrated in OSOM from LP compared to NP14, ***p < 0.01. Data are shown as mean ± SEM
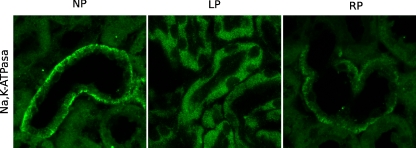
To further demonstrate Na+/K+-ATPase displacement during low-protein feeding, inmunocytochemical localization was used. Antibody against Na+/K+-ATPase protein brightly stained the basolateral membranes of tubular epithelial cells of the OSOM. ATP depletion in LP resulted in Na+/K+-ATPase detachment from the basolateral membrane and relocation to the apical membrane of tubular cells of OSOM. The basolateral Na+/K+-ATPase staining pattern of tubular cells from OSOM during recovery of 24% in diet suggests that the Na+/K+-ATPase localization is stabilized during RP (Fig. 3).
Fig. 3.
Immunofluorescence staining using antibody against the Na+/K+-ATPase in sections of rat kidney OSOM. Magnification 600×. Tubular epithelial cells of outer stripe of the outer medulla (OSOM) were labeled by indirect immunofluorescence under control, LP, and RP conditions. In control OSOM, basolateral Na+/K+-ATPase expression was present. After LP, dislocation of Na+/K+-ATPase from basolateral domain into apical domain was shown. A pattern of basolateral Na+/K+-ATPase distribution during RP similar to control was shown
No-significant differences were observed in Triton X-100 sol/insol Hsp70 ratio in the cortex among groups. Meanwhile, we found lower levels of sol/insol Hsp70 ratio from OSOM in the LP group than in the NP group (2.84 ± 0.24 vs. 4.18 ± 0.11, n = 12, p < 0.05; Fig. 4b). After protein recovery for 14 days, higher abundance of sol/insol Hsp70 ratio in the RP group than in the LP group (8.44 ± 0.55 vs. 2.84 ± 0.24, n = 12, p < 0.001; Fig. 4b) was demonstrated. The majority of the Hsp70 protein levels were detected in the Triton X-100 soluble fraction. These results indicate lower levels of Hsp70 during the LP diet and higher abundance of the protein during the recovery period in OSOM.
Fig. 4.
Representative Western blot and densitometry of TritonX100-soluble and -insoluble Hsp70 in cortex (a) and outer stripe of the outer medulla (OSOM) (b) obtained from control 14 (NP14) and 30 days (NP30), low-protein diet group (LP), and recovery group (RP; n = 12). Decreased Hsp70 levels were demonstrated in non-cytoskeletal supernatants from OSOM in LP compared to NP14&30, *p < 0.05. Increased levels in RP compared to NP14&30 and LP were shown, ***p < 0.001. Data are shown as mean ± SEM
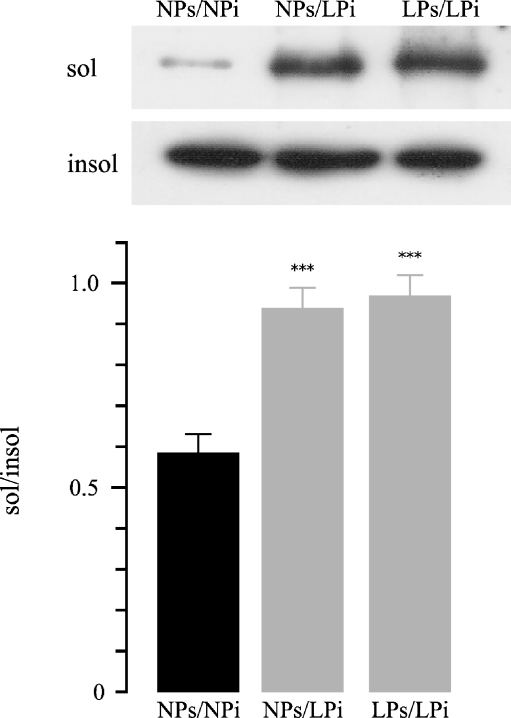
In the second part of the study, aliquots from NPi and LPi Triton X-100 insoluble were incubated in their own NPs (NPs/NPi) or LPs (LPs/LPi) Triton X-100 soluble, respectively. At the same time, aliquots from LPi pellets were incubated in NPs supernatants (NPs/LPi; Table 1). Repeat Triton X-100 extraction resulted in a significant increase in Triton extractability of Na+/K+ ATPase from the low-protein cytoskeletal fraction after the incubation with LPs compared to NPs, sol/insol ratio: NPs/LPi 0.93 ± 0.052 vs. NPs/NPi 0.58 ± 0.043, n = 12;, p < 0.001; LPs/LPi 0.96 ± 0.049 vs. NPs/NPi 0.58 ± 0.043, n = 12, p < 0.001 (Fig. 5). These studies indicate that the incubation of LP non-cytoskeletal fraction (LPi), either with NPs or LPs, resulted in higher Na+/K+-ATPase dissociation from the cytoskeletal anchorage.
Fig. 5.
Representative Western blot and densitometry of Na+/K+-ATPase in low-protein injured insoluble fractions. Cytoskeletal pellets isolated from controls or from low-protein rat renal outer stripe of the outer medulla were resuspended in their own supernatant (NPs/NPi and LPs/LPi, respectively); another aliquot of low protein cytoskeletal pellet was resuspended in control supernatant (NPs/LPi). These mixtures were incubated, and repeated Triton extraction was performed. Statistical analysis from three experiments confirmed the increased Triton extractability of Na+/K+-ATPase (sol/insol Na+/K+-ATPase ratio) in LPs/LPi vs. NPs/NPi (***p < 0.001) and NPs/LPi vs. NPs/NPi (***p < 0.001). Data are shown as mean ± SEM
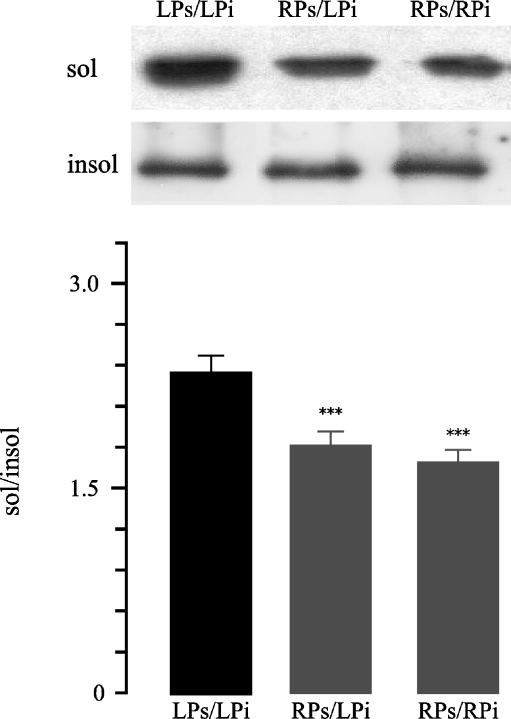
To assess the in vitro repair, aliquots from the same LPi pellet were incubated in both LPs (LPs/LPi) and RPs (RPs/LPi) supernatants, and aliquots from RPi pellet were incubated in RPs supernatant (RPs/RPi; Table 1). Incubation of LPi and RPi in RPs resulted in less Na+/K+-ATPase levels in soluble fraction during the repeated Triton X-100 extraction; RPs/LPi 1.78 ± 0.09 vs. LPs/LPi 2.31 ± 0.11, n = 12, p < 0.001; RPs/RPi 1.66 ± 0.086 vs. LPs/LPi 2.31 ± 0.11, n = 12, p < 0.001 (Fig. 6). The incubation of LPi with RPs resulted in Na+/K+-ATPase reestablishment to the cytoskeletal anchorage.
Fig. 6.
Representative Western blot and densitometry of Triton extractability of Na+/K+-ATPase in low-protein renal outer stripe of the outer medulla injured insoluble protein fractions after co-incubation with Hsp-rich protein extracts. Cytoskeletal pellets isolated from low-protein or recovery rat renal OSOM were resuspended in their own supernatant (LPs/LPi and RPs/RPi, respectively); another aliquot of low protein cytoskeletal pellet was resuspended in recovery HSP-rich supernatant (RPs/LPi). These mixtures were incubated, and repeat Triton extraction was performed. Statistical analysis from three experiments confirmed the decreased Triton extractability of Na+/K+-ATPase (sol/insol Na+/K+-ATPase ratio) in RPs/LPi vs. LPs/LPi, ***p < 0.001 and RPs/RPi vs. LPs/LPi, ***p < 0.001. Data are shown as mean ± SEM
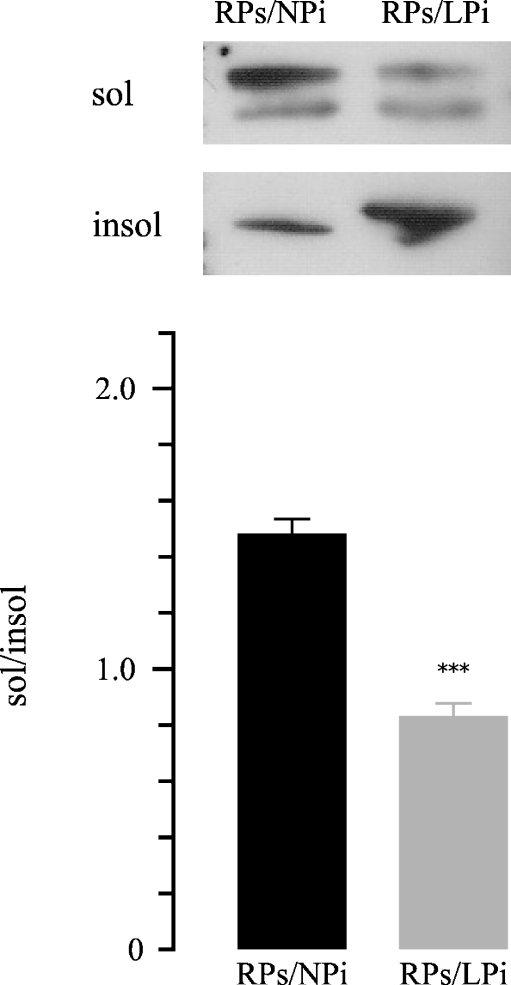
The Hsp70 translocation into the injured cytoskeletal fraction was also evaluated through the in vitro incubation of LPi in RPs (RPs/LPi), resulting in the appearance of Hsp70 Triton-insoluble higher signal in the blot than the one shown in the incubation of NPi in RPs (RPs/NPi). At this time, the significant translocation of Hsp70 into the cytoskeletal fraction shifted the Triton X-100 soluble to insoluble ratio in the densitometric analysis from 1.46 ± 0.048 to 0.82 ± 0.041;, n = 12, p < 0.001 (Fig. 7).
Fig. 7.
Representative Western blot and densitometry of differential Triton extractability of Hsp70 from insoluble and soluble fractions from outer stripe of the outer medulla. Aliquots of Hsp-rich supernatant were incubated with cytoskeletal pellets isolated after low protein (RPs/LPi) or isolated from controls (RPs/NPi). Repeat Triton extraction was performed. Statistical analysis from three experiments confirmed the Hsp70 translocation into the injured cytoskeletal fraction (insoluble) after incubation of LPi in RPs (RPs/LPi), resulting in the appearance of Hsp70 Triton-insoluble signal higher than in the incubation of NPi in RPs (RPs/NPi) in the blot. Meanwhile, densitometric analysis showed a lower sol/insol Hsp70 ratio in RPs/LPi vs. RPs/NPi (***p < 0.001). Data are shown as mean ± SEM
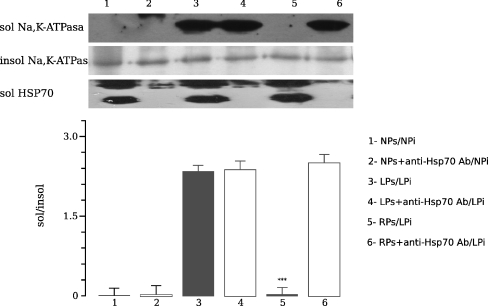
We next compared the amount of non-cytoskeletal Na+/K+-ATPase after the incubation in vitro of OSOM cytoskeletal fraction from LP fed rats (LPi) with RPs in absence or presence of anti-Hsp70 antibody. Aliquots from the same LPi were incubated in both RPs (RPs/LPi) and RPs plus anti-Hsp70 antibody (RPsAnti-Hsp70 Ab/LPi) supernatants. Translocation of Hsp70 to the cytoskeletal injured fraction associated with stabilization of Na+/K+-ATPase was shown in OSOM from LP after in vitro co-incubation of the cytoskeletal fraction of LP (LPi) and non-cytoskeletal fraction of RP (RPs; Fig. 8). These effects were abolished by the addition of anti-Hsp70 antibody (RPs/LPi vs. RPsAnti-Hsp70 Ab/LPi 0.04 ± 0.13 vs. 2.5 ± 0.14, n = 12, p < 0.001). Non-cytoskeletal Na+/K+-ATPase levels remained unchanged.
Fig. 8.
Representative Western blot and densitometry demonstrating the effects of anti-Hsp70 antibody on Triton extractability of Na+/K+-ATPase in low-protein cytoskeletal fractions from outer stripe of the outer medulla. Aliquots of low protein pellets were either incubated in their own supernatant (LPs/LPi) or in recovery supernatants (RPs/LPi) with or without anti-Hsp70 antibody. These mixtures were incubated, and a repeat Triton extraction was performed. Translocation of Hsp70 showed lower Na+/K+-ATPase dissociation compared to same fraction in the presence of the antibody against Hsp70 (RPs/LPi vs. RPsAnti-Hsp70 Ab/LPi, ***p < 0.001). Data are shown as mean ± SEM
To further evaluate the interaction between Na+/K+-ATPase and Hsp70, membrane extracts from the cortex and the OSOM were immunoprecipitated with anti-Na+/K+-ATPase antibody, and then they were analyzed for the presence of co-precipitating protein Hsp70. Interaction of Na+/K+-ATPase and Hsp70 was observed under control and experimental conditions. In the cortex membranes, no significant differences were observed. In contrast, in the OSOM membranes from the LP group, the amount of Hsp70 co-precipitated with Na+/K+-ATPase, expressed as a ratio, rose to 25.4% of control (NP14 0.8 vs. LP 1.06, p < 0.05; Fig. 9a). Interaction of Na+/K+-ATPase and Hsp70 was reversible after recovery period (RP); the level of Hsp70 that co-precipitated with Na+/K+-ATPase was similar to that seen in the uninjured controls (Fig. 9b). Co-precipitation was not observed in membrane samples from the cortex and the OSOM incubated without Na+/K+-ATPase antibody.
Fig. 9.
Representative immunoprecipitation of Na+/K+-ATPase. Membrane extracts from rat cortex and outer stripe of the outer medulla were immunoprecipitated with Na+/K+-ATPase antibody and were co-precipitated and analyzed for Hsp70. The amount of Hsp70 co-precipitating with Na+/K+-ATPase was expressed as a ratio. (a) Higher ratio between both proteins was shown in membrane OSOM from low protein diet (LP). (b) In RP, the Hsp70 that co-precipitated with Na+/K+-ATPase was similar to control. In LP and RP cortex membrane tissues, interaction of both, Na+/K+-ATPase and Hsp70 proteins by co-immunoprecipitation was similar to control
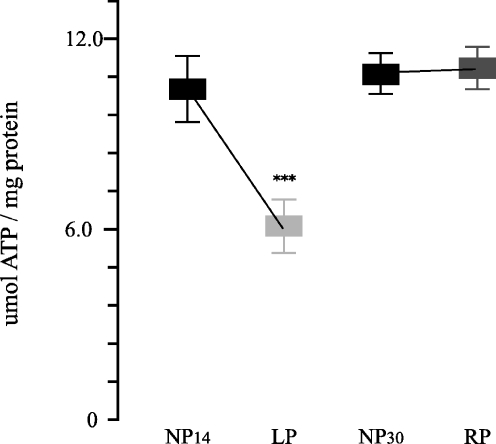
Measurement of tissue ATP levels was performed to confirm ATP depletion during the low-protein period. We found that tissue ATP content in the OSOM from LP group fell to 58.9% of NP group (6.34 ± 0.55 vs. 10.7 ± 0.67 μmol ATP per milligram protein, n = 8,; p < 0.001) and then increased to values near control during the recovery period (11.33 ± 0.32 vs. 11.2 ± 0.29 μmol ATP per milligram protein, n = 8; Fig. 10). No significant differences were observed on ATP levels in the cortex tissues among NP, LP, and RP groups.
Fig. 10.
ATP content in outer stripe of the outer medulla renal tissue from control 14 (NP14) and 30 days (NP30), low protein (LP), and recovery protein (RP) groups. Decreased ATP levels on OSOM from LP compared to NP14 and RP (***p < 0.001, both). Data are shown as mean ± SEM
Discussion
Cellular localization and distribution of the Na+/K+-ATPase after in vivo renal ischemia represents a marker for tubule cell injury (Atkinson and Molitoris 2001). Under normal circumstances, the sodium pump is a basolaterally located, integral membrane protein attached to the cytoskeleton but dissociates and redistributes to apical domains with in vivo ischemia and ATP depletion in cultured renal epithelia (Molitoris et al. 1991; Riordan et al. 2004). For Na+/K+-ATPase to be translocated from its basolateral membrane domain, it must be first detached from its cytoskeletal anchorage, which has been defined by Triton X-100 extractability (Molitoris 1991). The transient disruption of the Na+/K+-ATPase from its cellular localization in the cytoskeleton anchorage and migration to the apical membrane is a cardinal feature of early ischemic renal cell injury (Siegel et al. 1994). During recovery from reversible renal injury, restitution of cellular polarity appears to be through recycling of displaced Na+/K+-ATPase into the basolateral membrane (Spiegel et al. 1989). HSPs have been implicated in the modulation of cellular injury acting as molecular chaperones for damaged or displaced proteins. Overproduction of 70-kDa Hsp has been associated with cytoprotection in a variety of renal epithelial cell lines (Turman and Rosenfeld 1999).
Our present results demonstrate that low-protein feeding, in renal outer stripe of the outer medulla (OSOM) with ATP content reduction, caused in vivo and in vitro transient dissociation of Na+/K+-ATPase from its cytoskeletal anchorage. During recovery from LP with 24% protein in the diet, higher Hsp70 levels were demonstrated, while detergent-soluble Na+/K+-ATPase decreased, suggesting that reestablishment of Na+/K+-ATPase anchorage to the cytoskeleton may be facilitated by the action of Hsp70 in renal OSOM. Moreover, the in vitro increased Na+/K+-ATPase dissociation in the presence of anti-Hsp70 antibody suggested a specific effect of Hsp70 on the preservation of Na+/K+-ATPase attachment to the cytoskeleton.
Renal ischemic injury events include hypoxia and ATP depletion on epithelial cells from duct segments (Riordan et al. 2005). In low-protein-fed rats, enhanced expression of the genes that encode for components of the renin–angiotensin system has been demonstrated (Martinez-Maldonado et al. 1993; Benabe et al. 1993). Involvement of local angiotensin II on renal hemodynamics owing to increased vascular resistance and reduced prostaglandin synthesis contributing to renal ischemia have been previously reported in LP feeding (Ichikawa et al. 1980; Kapoor and Krishna 1991).
Previously, we have shown energy depletion through the continued H+-ATPase activity inhibition in outer and inner medullary collecting duct segments from kidneys of low-protein-fed rats (Vallés et al. 2005). Recently, we provided evidence for the apoptosis induction in epithelial cells from medullary collecting duct segments in LP and for the anti-apoptotic cytoprotective mechanism of Hsp70 during protein recovery (Carrizo et al. 2006).
Our in vivo results showed that Triton X-100 extractable Na+/K+-ATPase was higher in renal OSOM after 14 days with a low-protein diet avoiding the Hsp70 cytoprotection role due to the decreased Hsp70 protein levels. After recovery for the same period of time with 24% protein in the diet, returning of non-cytoskeletal Na+/K+-ATPase to basal levels and increased Hsp70 expression in the same fraction were shown.
By in vitro assay, co-incubation of cytoskeletal proteins from OSOM obtained during LP exhibiting severe injury of the cytoskeletal anchorage of Na+/K+-ATPase, with non-cytoskeletal proteins obtained during the recovery period, resulted in translocation of Hsp70 from the non-cytoskeletal fraction into the cytoskeletal fraction and Na+/K+-ATPase stabilization.
Furthermore, the immunohistochemical study showed that LP feeding resulted in re-localization of Na+/K+-ATPase into the apical membrane from the basolateral membrane domain in OSOM tubular epithelial cells. Stabilization of Na+/K+-ATPase in the basolateral membrane, with the reestablishment of the protein polarity was shown during recovery of 24% in diet.
Our findings of released Na+/K+-ATPase from its cytoskeletal attachment allow us to suggest that during LP, as it has been described during mild ischemia (Molitoris et al. 1992), Na+/K+-ATPase would be free to diffuse within the bilayer through an open tight junction into the apical membrane domain.
Conversely, non-significant detachment of Na+/K+-ATPase was demonstrated in proximal duct segments from the cortex in LP. A possible explanation for these results may include differences in oxygen tension between the cortex and the medulla. Cells in the outer medulla suffer more extreme oxygen deprivation than cells in the cortex with a falling gradient of oxygen tension in the cells of the deepest zone of the outer medulla, the latter being more susceptible to ischemic injury (Brezis and Rosen 1995).
In our LP experimental model, the reduced medullary interstitial urea might be involved in the Hsp70 downregulation during the period of low-protein diet. This suggestion may be inferred from the previous demonstration of accumulation of compatible organic osmolytes and enhanced synthesis of Hsp70 being related to the protection process against high interstitial urea concentration in the medulla (Neuhofer et al. 2005).
Hsp70 binds to nascent and immature proteins to prevent premature and improper binding and folding. The Hsp70 are ideal candidates for post-translational repair mechanisms (Morimoto et al. 1994b). If increased expression of HSP is protective, then downregulation should augment cellular injury or impair restitution of cellular integrity. Attempts to inhibit Hsp70 have focused in the use of an anti-Hsp70 antibody. In our study, a specific effect of Hsp70 on the preservation of Na+/K+-ATPase attachment to the cytoskeleton was suggested because addition of anti-Hsp70 antibody in vitro reduced Na+/K+-ATPase stabilization. Increased expression of Hsp70, by in vitro co-incubation of recovery fraction Hsp70-rich non-cytoskeletal supernatant with LP fraction injured cytoskeletal pellet, did not completely prevent but significantly reduced the dissociation of Na+/K+-ATPase in response to a LP diet compared to control. These results suggest that the stress protein Hsp70 may preserve and help to restore cell architecture in this region during recovery with 24% protein after low-protein injury.
To further demonstrate the interaction between both proteins, an antibody directed against the α-subunit of the Na+/K+-ATPase was used to precipitate native Na+/K+-ATPase. In injured OSOM tissue from LP, Hsp70 was induced, and its protein levels were higher than in controls or during the recovery period. Co-precipitation of both proteins rose to 25% of control; these observations are consistent with increased Hsp70 interacting with Na+/K+-ATPase during low protein injury. On the contrary, in agreement with the in vivo and in vitro results, no significant difference was shown throughout co-immunoprecipitation in the cortex in the same experimental conditions.
In relation to the cytoprotective role of Hsp70, support for functional interaction of Hsp70 with specific proteins can be provided by taking advantage of a cardinal feature of stress protein activity. Molecular chaperones such as Hsp70 readily bind to other proteins in the absence of ATP hydrolysis, but do not act and release the attached protein without hydrolysis of ATP (Skowyra et al. 1990; Brown et al. 1993; Di et al. 1995). These HSPs use the energy of ATP hydrolysis to undergo a conformational change, which may result in refolding or partial stabilization of denatured proteins and release of reconformed proteins (Pelham 1986). ATP binds to the NH2 terminus of Hsp70, causing a conformational change in Hsp70 (Brehmer et al. 2001). In fact, co-precipitation of Hsp70 with Na+/K+-ATPase after ATP depletion occurs as a consequence of low-protein injury in our study; higher abundance of Hsp70 to Na+/K+-ATPase was found.
Conclusion
Our results showed in vivo and in vitro overexpression of Hsp70 associated with stabilization of Na+/K+-ATPase in the cytoskeletal fraction from OSOM after recovery from LP with 24% protein in the diet.
These results allow us to suggest that Hsp70 has a critical protective role in the integrity of the cytoskeletal anchorage of Na+/K+-ATPase during recovery from ATP depletion injury resulting from LP diet in the outer stripe of the outer medulla.
Acknowledgments
This work was performed with financial support from CONICET: National Council of Scientific Research and Technology and from Agencia Nacional de Promoción Científica y Tecnológica /PICT 2002 N: 05-12620 to P. Vallés.
Footnotes
Portions of this study were presented in abstract form at the World Congress of Nephrology in Rio de Janeiro, Brasil. April 21–25, 2007.
References
- Atkinson SJ, Molitoris BA (2001) Cytoskeletal alterations as a basis of cellular injury in acute renal failure. In: Molitoris BA, Finn WF (eds) A companion to Brenner & Rector’s The Kidney. Saunders, Philadelphia, pp 119–131
- Aufricht C, Lu E, Thulin G, Kashgarian M, Siegel NJ, Van Why SK (1998) ATP releases HSP72 from protein aggregates after renal ischemia. Am J Physiol Renal Physiol 274:F268–F274 [DOI] [PubMed]
- Benabe JE, Wang S, Wilcox JN, Martinez-Maldonado M (1993) Modulation of ANG II receptor and mRNA in normal rat low-protein feeding. Am J Physiol 265:F660–F669 [DOI] [PubMed]
- Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies D, Reinstein J, Mayer MP, Bukau B (2001) Tuning of chaperone activity of HSP70 proteins by modulation of nucleotide exchange. Nat Struct Biol 8:427–432 [DOI] [PubMed]
- Brezis M, Rosen S (1995) Hypoxia of the renal medulla: its implications for disease. N Engl J Med 332:647–655 [DOI] [PubMed]
- Brown CR, Martin RL, Hansen WJ, Hansen WJ, Beckmann RP, Welch WJ (1993) The constitutive and stress inducible forms of hsp-70 exhibit functional similarities and interact with one another in an ATP-dependent fashion. J Cell Biol 120:1101–1112 [DOI] [PMC free article] [PubMed]
- Carrizo L, Ruete C, Manucha W, Ciocca DR, Vallés P (2006) Heat shock protein 70 (HSP70) expression is associated with inhibition of renal tubule epithelial cell apoptosis during recovery from low protein feeding. Cell Stress Chaperones 11(4):309–324 [DOI] [PMC free article] [PubMed]
- Di YP, Repasky E, Laszlo A, Calderwood S, Subjeck J (1995) HSP70 translocates into a cytoplasmic aggregate during lymphocyte activation. J Cell Physiol 16:5:228–238 [DOI] [PubMed]
- Fish EM, Molitoris BA (1994) Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med 330:1580–1588 [DOI] [PubMed]
- Glover JR, Lindquist S (1998) HSP104, Hsp70, Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73–82 [DOI] [PubMed]
- Ichikawa I, Pukerson ML, Klahr S, Klahr S, Troy JL, Martinez-Maldonado M, Brenner BM (1980) Mechanism of reduced glomerular filtration rate in chronic malnutrition. J Clin Invest 65:982–988 [DOI] [PMC free article] [PubMed]
- Kapoor SC, Krishna GG (1991) Protein-induced modulation of renin secretion is mediated by prostaglandins. Am J Physiol 2:60:F688–F694 [DOI] [PubMed]
- Martinez-Maldonado M, Benabe JE, Wilcox JN, Wang S, Luo C (1993) Renal renin, angiotensinogen, and ANG I-converting-enzyme gene expression: influence of dietary protein. Am J Physiol 264:F981–F988 [DOI] [PubMed]
- Molitoris BA (1991) New insights into the cell biology of ischemic acute renal failure. J Am Soc Nephrol 1:1263–1270 [DOI] [PubMed]
- Molitoris BA, Geerdes A, McIntosh JR (1991) Dissociation and redistribution of Na, K-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest 88:462–469 [DOI] [PMC free article] [PubMed]
- Molitoris BA, Dahl R, Geerdes A (1992) Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am J Physiol Renal Physiol 263:488–495 [DOI] [PubMed]
- Molitoris BA, Dahl R, Hosford M (1998) Cellular ATP depletion induces disruption of the spectrin cytoskeletal network. Am J Physiol Renal Physiol 274:F790–F798 [DOI] [PubMed]
- Morimoto RI, Tissieres A, Georgopoulos C (eds) (1994a) The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, NY, pp 1–30
- Morimoto RI, Tissieres A, Georgopoulos C (eds) (1994b) The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, NY, pp 1–594
- Neuhofer W, Fraek ML, Ouyang N, Beck FX (2005) Differential expression of heat shock protein 27 and 70 in renal papillary collecting duct and interstitial cells implications for urea resistance. J Physiol 564:715–722 [DOI] [PMC free article] [PubMed]
- Pelham HR (1986) Speculation on the functions of the major heat shock and glucose-related proteins. Cell 46:959–961 [DOI] [PubMed]
- Riordan M, Garg V, Thulin G, Kashgarian M, Siegel NJ (2004) Differential inhibition of HSP72 and HSP25 produces profound impairment of cellular integrity. J Am Soc Nephrol 15:1557–1566 [DOI] [PubMed]
- Riordan M, Sreedharan R, Wang S, Thulin G, Mann A, Stankewich M, Van Why S, Kashgarian M, Siegel NJ (2005) HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol 288:F1236–F1242 [DOI] [PubMed]
- Seney FD, Marver D (1989) Protein intake and cation transport in the loop of Henle. J Lab Clin Med 114:587–599 [PubMed]
- Siegel NJ, Devarajan P, Van Why SK (1994) Renal cell injury: metabolic and structural alterations. Pediatr Res 36:129–136 (invited review) [DOI] [PubMed]
- Skowyra D, Georgopoulos C, Zylicz M (1990) The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell 62:939–944 [DOI] [PubMed]
- Spiegel DM, Wilson PD, Molitoris BA (1989) Epithelial polarity following ischemia: a requirement for normal cell function. Am J Physiol Renal Fluid Electrolyte Physiol 256:F430–F436 [DOI] [PubMed]
- Turman MA, Rosenfeld SI (1999) Heat shock protein 70 overexpression protects LLC-PK1 tubular cells from heat shock but not hypoxia. Kidney Int 55:189–197 [DOI] [PubMed]
- Vallés P, Carrizo L, Seltzer A, Manucha W (2005) H+-ATPase activity in collecting duct segments in protein-deprived rats. Role of angiotensin II on its regulation. Nephron Physiol 99:90–100 [DOI] [PubMed]
- Van Why SK, Mann AS, Ardito T, Siegel NJ, Kashgarian M (1994a) Expression and molecular regulation of Na-K-ATPase after renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 26:7:F75–F85 [DOI] [PubMed]
- Van Why SK, Mann AS, Thulin G, Zhu XH, Kashgarian M, Siegel NJ (1994b) Activation of heat-shock transcription factor by graded reductions in renal ATP, in vivo, in the rat. J Clin Invest 94:1518–1523 [DOI] [PMC free article] [PubMed]
- Van Why SK, Sunmi Kim S, Geibel J, Seebach FA, Kashgarian M, Siegel NJ (1999) Thresholds for cellular disruption and activation of the stfigure gel.epsress response in renal epithelia. Am J Physiol 277:F227–F234 [DOI] [PubMed]