Abstract
The small heat shock proteins (sHsps), which are ubiquitous stress proteins proposed to act as chaperones, are encoded by an unusually complex gene family in plants. Plant sHsps are classified into different subfamilies according to amino acid sequence similarity and localization to distinct subcellular compartments. In the whole Arabidopsis thaliana genome, 19 genes were annotated to encode sHsps, of which 14 belong to previously defined plant sHsp families. In this paper, we report studies of the five additional sHsp genes in A. thaliana, which can now be shown to represent evolutionarily distinct sHsp subfamilies also found in other plant species. While two of these five sHsps show expression patterns typical of the other 14 genes, three have unusual tissue specific and developmental profiles and do not respond to heat induction. Analysis of intracellular targeting indicates that one sHsp represents a new class of mitochondrion-targeted sHsps, while the others are cytosolic/nuclear, some of which may cooperate with other sHsps in formation of heat stress granules. Three of the five new proteins were purified and tested for chaperone activity in vitro. Altogether, these studies complete our basic understanding of the sHsp chaperone family in plants.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-008-0032-6) contains supplementary material, which is available to authorized users.
Keywords: Heat stress, Small heat shock proteins, Chaperones, Stress granules, Plants
Introduction
Members of the small heat shock protein (sHsp) family are ubiquitous in nature and have been ascribed an unusual diversity of functions in the cellular response to environmental stress and disease, as well as during normal growth and development. Common to all sHsps is a conserved domain of ~90 amino acid residues found toward the C terminus of the protein and referred to as the α-crystallin domain (ACD; de Jong et al. 1998). This domain is characterized by a β-strand structure and is folded into an IgG-like β-sandwich conformation with a topology identical to that of the Hsp90 cochaperone p23 (Kim et al. 1998b; van Montfort et al. 2001b; Stamler et al. 2005). The ACD is flanked by mostly unstructured variable N- and C-terminal extensions leading to sHsp monomer molecular sizes ranging from ~16 to 42 kDa. Another characteristic feature of sHsps is that in their native state, they typically exist as multimeric complexes of 8 to 24 or more subunits, although dimeric forms have also been described (Kim et al. 1998a; van Montfort et al. 2001a; Stamler et al. 2005). X-ray crystal structures are available for two oligomeric sHsps, the 24-subunit Hsp16.5 from Methanococcus jannaschii and the dodecamer Hsp16.9 from wheat (Triticum aestivum), and they show that despite different quaternary structures and oligomeric sizes, the monomers have a very similar fold, and both oligomers are built from the same basic dimer involving strand exchange of β6 into the ACD β-sandwich of the reciprocal monomers (Kim et al. 1998b; van Montfort et al. 2001b).
A current model for sHsp function proposes that they protect thermo-sensitive substrates from irreversible heat stress (hs)-induced denaturation and aggregation. By binding hydrophobic amino acid residues exposed on partially denatured proteins, sHsps keep bound substrates in a soluble, folding-competent state during stress. During recovery from stress, when conditions are permissive for folding, the sHsp-associated substrates are acted on by the adenosine triphosphate (ATP)-dependent chaperones Hsp70/40 (DnaK/DnaJ), which assist in substrate refolding. In addition to Hsp70/40, substrate refolding can be assisted by the Hsp100/ClpB and Hsp60/GroE chaperones in cells and compartments where these proteins are found. Several in vitro and in vivo studies have demonstrated that renaturation of denatured model substrates by high-molecular-weight chaperones was more efficient in the presence of sHsps (Kampinga et al. 1994, 1995; Stege et al. 1995; Lee et al. 1997; Lee and Vierling 2000; Mogk et al. 2003; Haslbeck et al. 2005).
The sHsp chaperone family is highly divergent compared to chaperones of the Hsp70/DnaK, Hsp90/HptG, and Hsp60/GroE classes. Evolutionary analysis indicates that plant, animal, and bacterial sHsps have evolved separately while retaining the characteristic ACD domain. Gene numbers of sHsps also vary significantly, with only two sHsps in E. coli and Saccharomyces cerevisiae (IbpA and B and Hsp26 and Hsp42, respectively) to, for example, 11 Hsps in humans and 19 in Drosophila melanogaster (Narberhaus 2002; Fontaine et al. 2003; Kappè et al. 2003). Seed plants have long been recognized as having the most complex sHsp gene family (Nover 1991; Vierling 1991; Waters et al. 1996). Analysis of the whole genome sequence of Arabidopsis thaliana, one of the smallest plant genomes known, identified 19 genes coding for putative sHsps (Scharf et al. 2001). Recent genome analysis of rice (Oryza sativa) and poplar (Populus tricocarpa) reveals additional numbers of sHsp genes (Waters et al. 2008). The complexity of the plant sHsp families is further distinguished from that of other nonplant organisms by the fact that there are clear subfamilies of sHsps, some of which have been documented to be more than 400 million years old (Waters and Vierling 1999). These subfamilies are defined not only based on sequence variations within their ACD domains but also on specific conserved motifs in the ACD flanking sequences and on the presence of targeting sequences that determine localization of sHsps to distinct subcellular compartments (Scharf et al. 2001). Members of three subfamilies (designated CI, CII, and CIII) are localized in the cytoplasmic/nuclear compartment. Due to the presence of a functional nuclear localization signal, which is conserved in CIII sHsps, the localization of this sHsp is primarily nuclear (Siddique et al. 2003). The CI gene family is generally the largest sHsp subfamily in plants, and in A. thaliana, it has six gene members. In addition to the cytoplasmic/nuclear sHsps, there are four subfamilies encoding proteins targeted to the endoplasmic reticulum (ER), plastids (P), mitochondria (M), and peroxisomes (Po), respectively. The latter subfamily was identified initially based on the presence of a putative peroxisomal Type 1 targeting signal (PTS1; Scharf et al. 2001) and was subsequently shown to be localized to peroxisomes dependent on the functional C-terminal PTS1 (Ma et al. 2006).
These seven gene families of plant sHSPs (CI, CII, CIII, ER, P, M, and Po) are to date the best characterized of the plant sHsps. The in vivo localization of some of the plant sHsps has been monitored under hs and recovery conditions. Members of sHsp subfamilies CI and CII are found to incorporate into electro-dense particles of 40 nm in size, also called hs granules (HSG). HSG in turn assemble during hs into larger cytosolic complexes (HSCs), a process that appears to depend on the formation of heterooligomeric complexes between members from all three cytoplasmic/nuclear subfamilies (Kirschner et al. 2000; Siddique et al. 2003). HSCs are found to be associated with the Hsp70/40 chaperone machinery and to include a number of other proteins, consistent with the molecular chaperone model for sHsp function (Nover et al. 1983, 1989; Neumann et al. 1984; Scharf et al. 1998; Smýkal et al. 2000; Mogk et al. 2003). The ER, P, M, and Po sHsps have all been localized in vivo to the respective organelles and cannot be detected in the cytoplasm. Mitochondrial sHsps are reported to be involved with the protection of mitochondrial proteins during hs and with increased thermotolerance (Chou et al. 1989; Liu and Shono 1999; Sanmiya et al. 2004). Similarly, overexpression of the chloroplast-localized sHsp protects photosystem II under some stress conditions (Neta-Sharir et al. 2005; Guo et al. 2007).
In addition to 14 genes encoding members of these seven well-characterized plant sHsp gene families, there are five additional sHsp genes in the A. thaliana genome. In this paper, we investigated these five novel sHsps to determine their subcellular localization including their propensity to incorporate into HSGs, analyzed their pattern of expression during stress and development, and went on to purify three of the five to test their potential chaperone activity. We find that each of these proteins defines a new subfamily of sHsps in plants, including four additional cytoplasmic/nuclear sHsps and a second unique family of sHsps targeted to the mitochondria. Thus, higher plants have at least 12 conserved sHsp subfamilies, indicating these proteins have multiple protective roles throughout plant cells.
Materials and methods
General materials and methods
Growth and hs treatment of A. thaliana (ecotype Col-0) plants were performed under long-day conditions as described by Schramm et al. (2006).
For transient gene expression studies, tobacco (Nicotiana plumbaginifolia) leaf mesophyll protoplasts were used as described previously (Lyck et al. 1997; Scharf et al. 1998; Kirschner et al. 2000).
Monoclonal antibodies, clone 16B12 and 9E10 (Hiss Diagnostics), were used for immunodetection of 3HA and Myc-tagged proteins, respectively. Class-specific antibodies for immunodetection of endogenous class CI Hsps in tobacco protoplasts were used as described by Port et al. (2004), and polyclonal antibodies against rye catalase (Hertwig et al. 1992) were kindly provided by Matthias Schmidt (Goethe University, Frankfurt am Main). Secondary antibodies conjugated with horseradish peroxidase or with fluorescent dyes CY2 and CY3 were obtained from Sigma-Aldrich and Dianova, respectively.
Standard procedures were used for cloning and nucleic acid analyses. Polymerase chain reaction (PCR) fragments for subcloning were generated using the High Fidelity PCR Enzyme Mix (Fermentas).
Protein extraction, sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE), and immunoblot analysis were performed as described previously (Mishra et al. 2002; Port et al. 2004).
Sequence analyses and structure predictions
Protein sequence analyses were done with ClustalX 1.8.3, and phylogenetic relationships were visualized using TreeView 1.6.6. For secondary structure predictions, 3D-PSSM (http://www.sbg.bio.ic.ac.uk/~3dpssm/index2.html) was used.
Organellar targeting sequences and corresponding cleavage sites were predicted by iPSORT (http://psort.nibb.ac.jp/), TargetP (http://www.cbs.dtu.dk/services/TargetP/), and MITOP (http://www.mips.biochem.mpg.de/cgi-bin/proj/medgen/mitofilter).
For calculation of isoelectric points (pI), Clone Manger 8 was used.
Constructs for transient protein expression in plant protoplasts
Plant expression vectors were derived from the pRT series of vectors (Töpfer et al. 1988). Modified versions providing a Myc epitope for N-terminal immunotag fusions were described before (Kirschner et al. 2000; Siddique et al. 2003). For the generation of fusion constructs with a C-terminal Myc tag, pRTC-Myc was constructed by ligation of the double-stranded annealing product of oligonucleotides 589F (5′-aattcccggctagcgagcaaaagctgatttctgaggaggatctgtaact-3′) and 590R (5′-ctagagttacagatcctcctcagaaatcagcttttgctcgctagccggg-3′) into pRT104 digested with EcoRI and XbaI. Specific forward and reverse primers were used for amplification of complementary deoxyribonucleic acid (cDNA) fragments encoding the full-length open reading frames (ORFs) of sHsps analyzed in this study. Details of primer sequences and appropriate restriction sites inserted for ligation into pRTN-Myc or pRTC-Myc are summarized in Table S1.
RNA analyses and microarray datasets
For analysis of transcript levels by reverse transcriptase (RT)-PCR in different tissues and temperature treatments, harvesting of plant material and ribonucleic acid (RNA) isolation were performed as described by Schramm et al. (2006). Specific forward and reverse primer combinations for amplification of the corresponding cDNA fragments are summarized in Table S1.
For microarray-based expression analysis, primary datasets released by the AtGenExpress consortium (http://www.arabidopsis.org/info/expression/ATGenExpress.jsp) were normalized and further processed as described by Schramm et al. (2006). Microarray elements (Affymetrix ATH1 Gene Chip) selected for expression analysis are summarized in Table S2.
Subcellular localization of proteins in tobacco protoplasts
For indirect immunofluorescence microscopy in tobacco mesophyll protoplasts, procedures described by Scharf et al. (1998) were followed. The endogenous hs response of tobacco protoplasts was induced by preinduction (40°C for 15 min followed by 25°C for 3 h) and a second hs treatment at 40°C for 2 h.
As a mitochondrial marker, an expression construct was cotransformed encoding amino acid residues 1 to 78 of the β1-subunit of A. thaliana F1-ATPase (At5g08670) fused N-terminally to the ORF of green fluorescent protein (GFP). Nuclei were visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI).
Confocal laser scanning micrographs were captured using a Leica CLSM (Leica, Bensheim, Germany) and Imaris Software (Bitplane, Zurich).
Expression and purification of recombinant proteins
Bacterial expression constructs for Hsp18.5-CIV, Hsp21.7-CVI, Hsp26.5-MII, and Hsp15.7-Po are based on plasmid pJC20 (Clos and Brandau 1994) and were generated by PCR amplification using the corresponding plant expression constructs as template DNA. Details of primer sequences and appropriate restriction sites inserted for ligation into NdeI–BamHI linearized pJC20 are summarized in Table S1.
For Hsp26.5-MII, a N-terminally truncated version (Hsp26.5-MIIΔTP, amino acid residues 42–232) was generated to remove the putative mitochondrial target peptide (TP, amino acid residues 1–41) to express the predicted mature protein. For expression, Escherichia coli strain BL21(DE3) (Stratagene) was used, and the purification of recombinant sHsps was performed according to the protocol described by Lee and Vierling (1998) with following modifications.
Hsp18.5-CIV, Hsp21.7-CVI, and Hsp26.5-MIIΔTP were precipitated from cell lysates by adding ammonium sulfate to the concentration of 60% to 80% (w/w). Protein precipitates were dissolved in T25E1 buffer, dialyzed, and loaded on a diethylaminoethyl (DEAE) column (Sigma Fast Flow, DFF-100, DEAE Sepharose) as previously described (Lee and Vierling 1998). After elution of AtHsp21.7-CVI with TE25E1 buffer, the protein was used without any further purification. For elution of Hsp18.5-CIV and Hsp26.5-MIIΔTP gradients of NaCl in T25E1 buffer were required with 0 to 200 mM and 0 to 120 mM NaCl, respectively. Hsp26.5-MIIΔTP was further purified by hydroxyapatite chromatography (Bio-Gel HT, Biorad) according to Lee and Vierling (1998). For elution, a gradient from 25 to 400 mM NaPO4 was used.
Expression of Hsp15.7-Po resulted in the formation of inclusion bodies. After resolubilization with 5 M guanidinium–HCl in T25E1, the recombinant protein was further purified according to Lin and Cheng (1991). Solubilized protein samples were loaded on a DEAE chromatography column and eluted with buffer D (Lin and Cheng 1991). Eluted protein fractions were pooled and dialyzed against 5 M guanidinium–HCl in 25 mM NaPO4 buffer (pH 7.5) before application to a final purification step on hydroxyapatite.
Purified recombinant Hsp17.6C-CI was kindly provided by E. Basha (University of Arizona, USA). Protein concentrations were determined using calculated extinction coefficients according to Pace et al. (1995).
Luciferase protection assay
The firefly luciferase-refolding assay was done as previously described (Lee and Vierling 1998). Rabbit reticulocyte lysate (RRL) was acquired from Green Hectares (Oregon, USA) and QuantiLum Recombinant Luciferase from Promega. Heating reactions were carried out in 2.5 mM HEPES–KOH pH 7.5, 5 mM MgCl2, 150 mM KCl, and 2 mM dithiothreitol (DTT) in a total volume of 25 μl. For denaturation, samples containing 1 μM luciferase and various amounts of recombinant sHsp were incubated at 42°C for 8.5 min and cooled on ice for 5 min. After dilution of all samples into the RRL reactivation mixture (Lee and Vierling 1998) to a final luciferase concentration of 25 nM in a total volume of 40 μl, luciferase activity was determined by adding 2.5-μl aliquots of each reaction to 50 μl of luciferase-measuring buffer (20 mM Tricine–KOH pH 7.5, 2.7 mM MgSO4, 0.1 mM ethylenediamine tetraacetic acid, 33 mM DTT, 0.5 mM ATP, 255 μM coenzyme A, 100 μM luciferin). Luminescence was measured in a luminometer (Mikrolumat 2400; EG&G Berthold) with an integration time of 10 s. The activity, expressed as relative light units was compared to the corresponding activity before heating. Denaturation of luciferase was monitored immediately after heat treatment by centrifugation of sample aliquots at 15,000 rpm for 15 min. The resulting supernatant and pellet fractions were separated by 12% SDS-PAGE and stained with Coomassie brilliant blue.
Results
Identification and classification of sHsp members in the A. thaliana genome
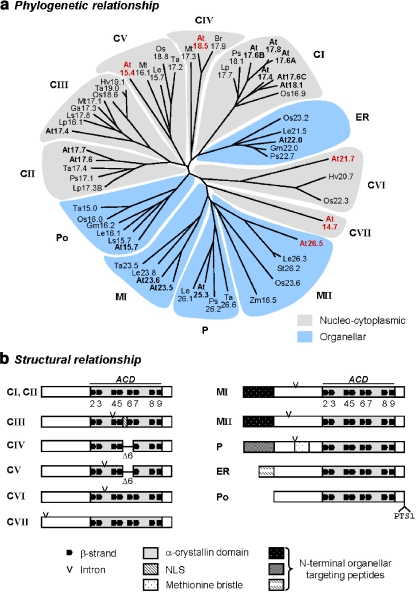
Searching the genome database of A. thaliana by BLASTP and BLASTN with known plant sHsp sequences as queries revealed 19 ORFs coding for putative sHsp genes (Scharf et al. 2001). This genomic search was complemented by EST database searches including those for other plant species (Table S3). Sequence comparison and parsimony analyses of amino acid residues in the conserved ACD regions revealed that the corresponding proteins are grouped into distinct clusters formed on separate branches in a phylogenetic tree (Fig. 1a). Based on this analysis and localization studies (see below), we defined five new subfamilies of sHsps (Fig. 1a, CIV, CV, CVI, CVII, MII) in addition to the previously known seven subfamilies CI, CII, CIII, ER, M, P, and Po (Scharf et al. 2001; Ma et al. 2006). In contrast to the CI, CII, and MI (former class M) subfamilies, which each consist of more than one member in A. thaliana, each of the newly defined subfamilies is represented by only one gene in A. thaliana, i.e., Hsp18.5-CIV, Hsp15.4-CV, Hsp21.7-CVI, Hsp14.7-CVII, and Hsp26.5-MII.
Fig. 1.
Classification and basic structures of plant sHsps. a Phylogenetic tree of plant sHsps based on sequence alignment of amino acid residues forming the α-crystallin domain (ACD). A. thaliana sHsps are in bold, and the newly identified members are highlighted in red. In total, 12 sHSP subfamilies are defined, i.e., seven cytoplasmic/nuclear subfamilies CI to CVII, one subfamily each of proteins targeted to the endoplasmic reticulum (ER), plastids (P), and peroxisomes (Po), respectively, and the two mitochondrial subfamilies MI and MII. b Block diagrams illustrate common and subfamily-specific elements of sHsp structures based on protein sequence alignments and secondary structure predictions. Size variations in the N- or C-terminal regions extending from the ACD are ignored. Predicted β-strands (β2–β9) are numbered according to the crystal structure of wheat Hsp16.9-CI. Amino acid residues involved in β6 formation are lacking in CIV and CV members (Δ6). Intron positions at the nucleotide level, targeting sequences, and the methionine bristle of the plastidial sHsps are indicated. For sequence alignments of the newly defined sHsp subfamilies, see Fig. S1. Data base accession numbers are given in Table S3. At Arabidopsis thaliana, Br Brassica rappa, Ga Gossypium arboretum, Gm Glycine max, Hv Hordeum vulgare, Le Lycopersicon esculentum, Lp Lycopersicon peruvianum, Ls Lactuca sativa or L. serriola, Mt Medicago truncatula, Os Oryza sativa, Ps Pisum sativum, St Solanum tuberosum, Ta Triticum aestivum, Zm Zea mays
For all but Hsp14.7-CVII, homologous genes were identified in other plant species (Fig 1a; Fig. S1 and Table S3). It is interesting to note that genes homologous to Hsp18.5-CIV were exclusively found in dicotyledonous plants, while homologues of Hsp21.7-CVI were only found for monocotyledonous plants. Except for genes in the CIV family, represented by Hsp18.5-CIV in A. thaliana, the other four genes all contain one intron, either in the ACD domain (Hsp15.4-CV and Hsp21.7-CVI) or in the N-terminal arm (Hsp14.7.CVII and Hsp26.5-MII; Fig. 1b). Altogether, these sequence data define 12 families of sHsps in plants.
Prediction of secondary structures and functional motifs
sHsps have a typical β-sheet structure within the ACD composed if eight β-strands (β2−β9) as seen in the X-ray structures of MjHsp16.5 and TaHsp16.9C-CI (Kim et al. 1998b; van Montfort et al. 2001b) (Fig. 1b). Predicted secondary structures within the evolutionary-conserved ACD of the plant orthologous sHsps revealed that irrespective of the species, most of the sHsps contain all these typical β-strands (Fig. 1b). Significant exceptions were found for members of the CIV (Hsp18.5-CIV in A. thaliana) and CV (Hsp15.4-CV in A. thaliana) subfamilies, which lack amino acid residues involved in strand β6 (Figs. 1b, S1). Due to the function of β6 in strand exchange for dimer formation (Kim et al. 1998b; van Montfort et al. 2001b), these sHsps would be predicted to have a different overall quaternary structure. No reliable secondary structure predictions were possible for sequences outside of the ACD for any of these proteins. However, with exception of subfamily CVI, the C-terminal ends of all other sHsps contain conserved sequence motifs, which are specific for each subfamily (Fig. S1 and Scharf et al. 2001, Fig. 1).
The sHsp transcriptome of A. thaliana
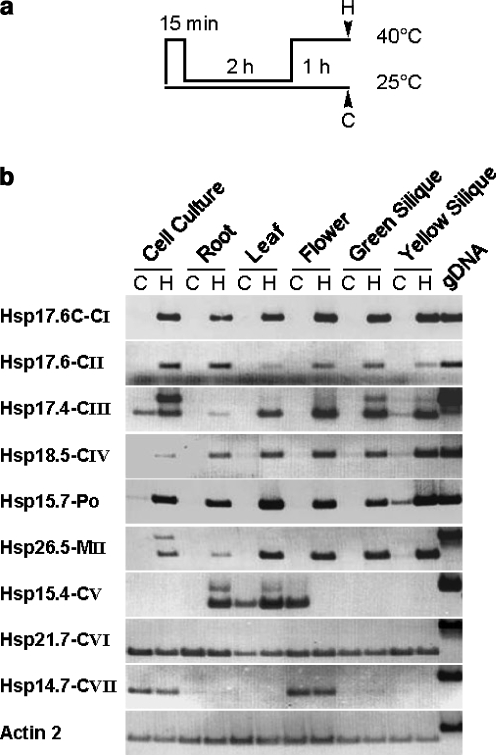
To understand the potential functional complexity of these diverse new families of sHsps, gene expression analysis by RT-PCR and microarray profiling were used to determine what abiotic stresses and developmental conditions specifically affect their expression. A. thaliana cell suspension cultures or whole plants were heat stressed as shown in Fig. 2a, and messenger RNA (mRNA) levels of the indicated genes were analyzed in different tissues by semiquantitative RT-PCR (Fig. 2b). Comparable to the expression of Hsp17-CI and Hsp17-CII sHsps, accumulation of transcripts for most of the newly identified sHsp members was induced or strongly enhanced by hs treatment irrespective of the analyzed tissue. It is surprising to note that the expression pattern of three members showed remarkable differences. For Hsp 15.4-CV, high transcript levels were already detectable in leaves and flowers of nonstressed plants. However, under hs, the expression of Hsp15.4-CV was induced in roots and significantly enhanced in leaves but strongly repressed in flowers. Expression of Hsp21.7-CVI was constitutive and ubiquitously present in all tissues, and transcript levels were not altered during hs. The expression of Hsp14.7-CVII was also found to be constitutive, but abundant transcript levels were only detectable in cultured cells and flowers.
Fig. 2.
RT-PCR analysis of sHsp expression. aA. thaliana plants or cell cultures were preinduced by a short heat stress treatment at 40°C for 15 min and incubation at 25°C for 2 h. Heat stress samples (H) were harvested after a second heat stress at 40°C for 1 h. Nonstressed control samples (C) were kept at room temperature. b Total RNA was extracted from the indicated tissues, and gene-specific mRNA levels were analyzed by RT-PCR. By comparison with PCR products generated on genomic template DNA, the accumulation of unspliced precursor forms becomes visible for some but not all intron-containing sHsp transcripts. Actin was used as a loading control and to confirm that RNA preparations were not contaminated by genomic DNA
Another interesting observation was the accumulation of nonspliced precursor transcripts under hs for some but not all intron-containing sHsp genes. Hsps 17.4-CIII, 15.4-CV, and 26.5-MII accumulated unspliced precursors, while Hsp21.7-CVI and Hsp14.7-CVII did not.
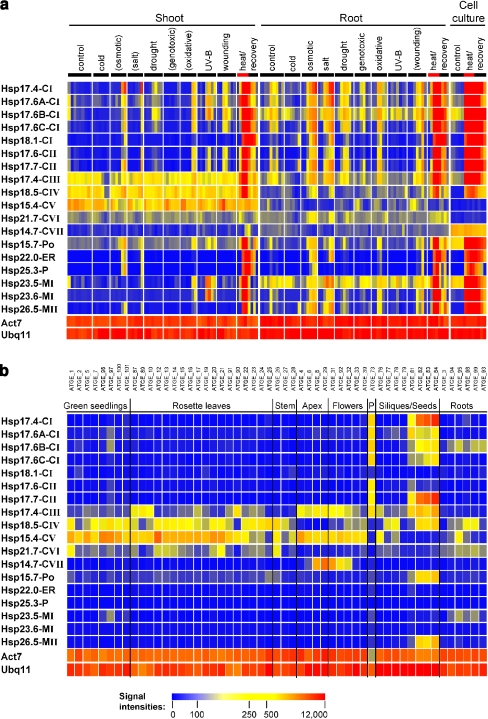
To obtain a broader overview of the expression behavior of all of the sHsp genes, microarray data provided by the AtGenExpress consortium were analyzed for A. thaliana plants and cell suspension cultures subjected to various abiotic stress conditions. With exception of the newly identified sHsps Hsp15.4-CV, 21.7-CVI, and 14.7-CVII, all other sHsps respond to different stressors to various extents, but the strongest accumulation of transcripts was observed under hs conditions (Fig. 3a). However, regarding the abundance and dynamic changes of transcript levels during ongoing stress treatments, remarkable differences in the expression behavior of individual sHsp genes were obvious. This was even evident for individual members belonging to the same sHsp family when compared over the time coarse of the applied stress treatments.
Fig. 3.
Expression profiling of sHsp expression under various abiotic stress conditions and during development. Normalized and averaged AtGenExpress microarray data (available at http://www.arabidopsis.org/info/expression/ATGenExpress.jsp) were selected for the indicated genes and visualized as heat maps (see “Materials and methods”). Due to the absence of a specific probe for Hsp17.8-CI on the 21K Affimetrix chip, only 18 sHsp genes are presented. The color code of signal intensities corresponds to the abundance of transcripts as indicated below and ranges from blue (no expression detectable) to red (high expression levels). For comparison, expression data for two constitutively active genes, Actin7 (Act7) and Ubiquitin11 (Ubq11), are included. a Transcript levels were analyzed in shoots and roots of A. thaliana seedlings grown under hydroponic conditions or in suspension culture cells after treatments with different abiotic stressors as indicated. Stressors given in brackets indicate that the corresponding tissue was not directly exposed to the stress treatment, and the observed response might be delayed or triggered by systemic signals. For heat stress, red bars mark the period of heat treatment preceding a subsequent recovery period at room temperature. b RNA sample codes for the developmental series are given on top in a spatial and temporal order corresponding to the indicated organs and developmental stages
The AtGenExpress experimental series also includes information on the A. thaliana transcriptome of different organs and developmental stages. Previous studies revealed that sHsp family members are also expressed under control conditions at specific plant developmental stages and in defined tissues (Prändl et al. 1995; Zarsky et al. 1995; zur Nieden et al. 1995; Wehmeyer et al. 1996; Wehmeyer and Vierling 2000; Lubaretz and zur Nieden 2002). In contrast to the majority of CI and CII sHsps, which show enhanced expression levels in pollen and during late stages of seed maturation, expression of Hsp18.5-CIV, Hsp26.5-MII, and Hsp15.7-Po were only detectable in seeds but not in pollen (Fig. 3b). it is interesting to note that none of the other newly identified or any other of the organellar sHsps shows a significant increase in transcript accumulation during these developmental stages. In agreement with our RT-PCR data, the expression pattern of most members of the newly defined cytoplasmic/nuclear families is much broader, and enhanced transcript levels of Hsps 18.5-CIV, 15.4-CV, and 21.7-CVI are visible in diverse nonstressed vegetative tissues, especially in rosette leaves. Also remarkable is the expression pattern of Hsp14.7-CVII, which is virtually restricted to late stages of shoot apex and early stages of flower development.
Subcellular localization of newly identified sHsps
After the classification of the newly identified A. thaliana sHsps 18.5-CIV, 15.4-CV, 21.7-CVI, 14.7-CVII, and 26.5-MII on the basis of their phylogenetic relationship, subcellular localization of these proteins was analyzed to verify this classification and to confirm the targeting predictions. Expression constructs encoding myc epitope-tagged fusion proteins were constructed, and the subcellular localization was studied in transiently transformed tobacco mesophyll protoplasts expressing the fusion proteins under the control of the constitutive CaMV 35S promoter.
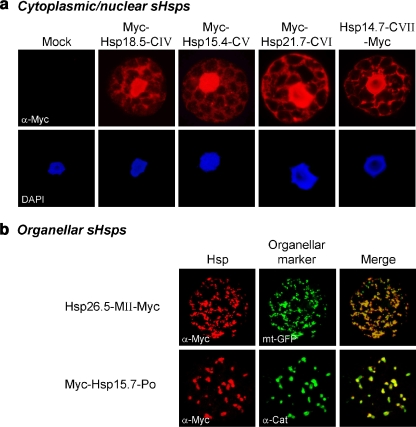
The results shown in Fig. 4a illustrate that Hsp18.5-CIV, Hsp15.4-CV, Hsp21.7-CVI, and Hsp14.7-CVII were distributed in the cytoplasmic/nuclear compartment. Both N- and C-terminal tagged versions were tested to minimize the possibility of mislocalization due to the position of the myc tag. However, in all cases, the intracellular distribution pattern was not altered by expression of the alternatively tagged variants (data not shown). As predicted, the C-terminally tagged Hsp26.5-MII was found exclusively in mitochondria, which is shown by its colocalization with a cotransformed GFP variant targeted to the mitochondria (Fig. 4b). For comparison, the intracellular distribution pattern of Hsp15.7-Po was similar, but its colocalization with endogenous catalase confirmed the expected targeting of this sHsp to peroxisomes (see also Ma et al. 2006). In contrast to Hsp26.5-MII-Myc, the N-terminal-tagged version accumulated in the cytoplasm by forming large protein aggregates, which is probably due to the masking of the N-terminal mitochondrial TP (data not shown).
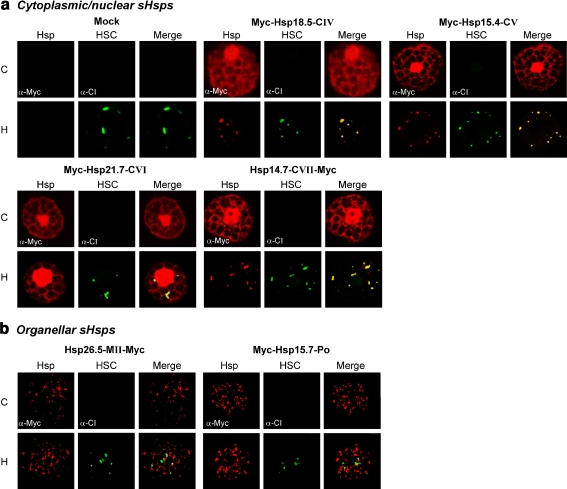
Fig. 4.
Subcellular localization of sHsps. Tobacco protoplasts were transiently transformed for expression of the indicated myc-tagged sHsps. For mock transformation pRTNeo was used. sHsps were fluorescently labeled using myc-specific antibodies (red channel). a Intracellular distribution pattern of cytoplasmic/nuclear sHsp members. DAPI staining shows the localization of nuclei in the same cells (blue channel). b Organellar sHsp members. As a marker for mitochondrial localization, an expression construct encoding GFP fused to a mitochondrial targeting signal (mt-GFP) was cotransformed, and immunodetection of endogenous catalase (α-Cat) was used to visualize peroxisomes (green channel). Merged images illustrate sites of colocalization in yellow
Localization of sHsps to cytoplasmic multichaperone complexes
sHsps of the cytoplasmic/nuclear subfamilies CI, CII, and CIII have been shown to be recruited to HSCs under hs where they may fulfill protective functions toward denatured proteins (Nover et al. 1989; Kirschner et al. 2000; Siddique et al. 2003). To analyze the localization of the newly identified sHsps under hs conditions and to determine if they are incorporated into endogenous tobacco HSCs, hs was applied to transformed protoplasts to induce the expression of endogenous sHsps. The formation of HSCs was followed by immunodetection of endogenous CI sHsps, which became clearly visible as a speckled pattern in the cytoplasm of heat-stressed protoplasts (see mock transformation in Fig. 5a). Changing of the nuclear–cytoplasmic distribution to the characteristic speckled pattern after hs and coimmunostaining with sHsp CI-specific antibodies indicated the recruitment of sHsps 18.5-CIV, 15.4-CV, and 14.7-CVII into HSCs (Fig. 5a). In contrast, the intracellular localization of Hsp21.7-CVI and of both organellar sHsps, Hsp26.5-MII and Hsp15.7-Po (Fig. 5b), was not altered by hs treatment.
Fig. 5.
Localization of sHsps under heat stress conditions. a, b Tobacco protoplasts were transformed for expression of the indicated myc-tagged proteins. After incubation for 18 h, samples were either subjected to a heat stress (sample H) to induce the expression of endogenous Hsps or were kept at room temperature (sample C). Myc-tagged sHsps were immunodetected in the red channel (α-Myc), and immunodetection of endogenous CI sHsps (α-CI) indicate the formation of cytoplasmic multichaperone complexes (HSC) in the green channel. Colocalization is illustrated in yellow in the merged images
In vitro chaperone assays
It is well known that sHsps can protect denatured proteins from irreversible aggregation by forming stable sHsp–substrate complexes (Lee et al. 1995; Ehrnsperger et al. 1997; Friedrich et al. 2004). To test the possible chaperone activity of the newly identified sHsps, we prepared purified recombinant Hsp18.5-CIV, Hsp21.7-CVI, and Hsp26.5-MII (Fig. S3; Craig 1988). Unfortunately, Hsp15.4-CV and Hsp14.7-CVII proved to be insoluble and could not be purified in sufficient quantity for analysis. We also purified recombinant Hsp15.7-Po, as this peroxisomal-localized protein had not previously been tested for chaperone activity.
All four of these purified proteins were tested for their ability to protect firefly luciferase from heat insolubilization and to support luciferase refolding in reticulocyte lysate in comparison to purified Hsp17.6-CI. The plant CI sHsps are well characterized for their chaperone activity (Lee et al. 1997; Lee and Vierling 1998; Basha et al. 2004).
As shown in Fig. 6a (sample C), the activity of nonheated luciferase remained constant during the assay. After denaturation by heating at 42°C for 8.5 min in the absence of any sHsp (sample H), the relative luciferase activity was reduced to less than 10%, and there was no measurable activity increase by subsequent incubation at room temperature. In contrast, by addition of increasing amounts of recombinant Hsp17.6C-CI, a concentration-dependent increase in recovery of luciferase activity was observed. These observations are consistent with the model that sHsps can stabilize luciferase during heat treatment by keeping the enzyme in a soluble state and thereby competent for refolding by ATP-dependent chaperones provided by the rabbit reticulocyte lysate (see also Fig. S2).
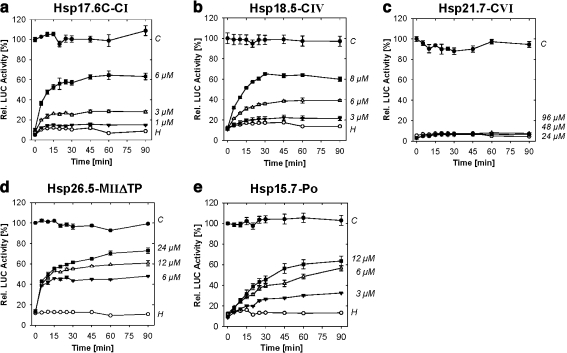
Fig. 6.
a–e In vitro chaperone assay – reactivation of sHsp-protected luciferase. Samples of 1 μM luciferase (LUC) were heated at 42°C for 8.5 min in the presence of increasing amounts of sHsps as indicated on the right margin of each panel. After dilution into rabbit reticulocyte lysate reactivation mixture to a final concentration of 25 nM, reactivation of LUC was monitored for 90 min. Values represent LUC activities relative to the activity before heating. Data are averages of three independent experiments, and standard deviations are shown as error bars. C Control samples with nonheated luciferase, H heat treatment of luciferase in absence of recombinant sHsps
Although different amounts of the individual sHsps were required to facilitate the same amount of luciferase refolding, comparable results were obtained with purified recombinant Hsp18.5-CIV, Hsp26.5-MIIΔTP, and Hsp15.7-Po (Fig. 6b,d,e). In contrast, Hsp21.7-CV did not show any protection capacity, although even higher amounts of the sHsp were tested (Fig. 6c).
Discussion
Complexity of the sHsp family in A. thaliana
In plants, sHsps are encoded by multiple genes forming a family of an unusually high complexity. This is explained, at least in part, by finding that specific plant sHsps are localized to distinct subcellular compartments including the cytoplasmic/nuclear compartment, the endomembrane system, plastids, mitochondria, and peroxisomes. Searching for sHsp-homologous genes in the complete genome sequence of A. thaliana revealed a total of 19 genes encoding putative members of the sHsp family. Most of them (14 genes) could be classified on the basis of sequence similarities with homologous plant sHsps described before (Scharf et al. 2001 and references therein) or subsequently studied in more detail (Siddique et al. 2003; Ma et al. 2006). However, five sHsp-encoding genes could not be assigned to any of the known sHsp subfamilies and were defined preliminary based on the phylogentic relationships of the conserved ACDs as related either to the cytoplasmic/nuclear subfamily CI (CI(r)) or to the plastidial subfamily P (P(r); Scharf et al. 2001). In this paper, we have shown that each of these newly defined sHsp members in A. thaliana represents a new subfamily with homologues also identified in other plant species (Figs. 1a, S1, Table S3). Supported by localization studies in transiently transformed tobacco protoplasts (Fig. 3), we propose their classification into four additional cytoplasmic/nuclear subfamilies (CIV to CVII) and a second subfamily of mitochondrial localized sHsps (MII). In addition to the seven subfamilies known before, a total of 12 sHsp subfamilies are now defined in plants.
Regulation of sHsp expression
The diversity of the plant sHsps and of their characteristic expression patterns indicates that sHsps have diverse functions in both the stress response and in the development of plants. Their putative roles range from protecting the cell from damaged protein aggregates accumulated during stress (Lee et al. 1997; Mogk et al. 2003) to potential functions in pollen and seed development (Waters et al. 1996; Wehmeyer et al. 1996; Lubaretz and zur Nieden 2002). Expression of plant sHsp genes, well known to be basically regulated on the transcriptional level, was previously found during hs and some other stressful conditions in most vegetative tissues as well as during maturation of pollen and seeds (Almoguera and Jordano 1992; Atkinson et al. 1993; Kobayashi et al. 1994; Prändl et al. 1995; Zarsky et al. 1995; Coca et al. 1996; Wehmeyer et al. 1996; Carranco et al. 1997). This typical expression behavior was only observed for two of the newly defined sHsps (Hsp18.5-CIV and Hsp26.5-MII). Like for all other hs-induced sHsps, the regulation of these two genes seems to be linked to the network of hs transcription factors (Hsfs) due to the complex patterns of Hsf-binding sites in the promoter regions (Scharf et al. 2001). This is also evident by the coordinated coexpression of these two genes with the same subset of sHsps during different stresses (Figs. 2 and 3a). However, differences in the accumulation of transcript levels during ongoing hs periods indicate that both genes might be specifically controlled by different Hsfs. Consistent with this idea, in HsfA2 knockout plants, the expression of Hsp26.5-MII was downregulated, but Hsp18.5-CIV expression was not affected (Schramm et al. 2006).
In addition to stress, both Hsp18.5-CIV and Hsp26.5-MII belong to the group of sHsp genes that are also expressed during seed maturation in A. thaliana (Fig. 3b). Hsp26.5-MII and the peroxisomal sHsp are the only organellar sHsps detectable in maturing seeds, and none of the organellar sHsps was expressed during pollen development. Since accumulation during seed maturation is usually specific for some but not all sHsp members and dependent on the developmental program of the particular plant species (Zur Nieden et al. 1995; Coca et al. 1996; Wehmeyer et al. 1996; Carranco et al. 1997; Kotak et al. 2007), it will be interesting to see whether the developmental regulation of mitochondrial MII members is conserved in other plant species.
Unexpectedly, the expression behavior of the other three newly defined sHsps was significantly different. They are constitutively expressed, and upon different stresses, transcript levels were not changed (Hsp21.7-CVI and Hsp14.7-CVII) or even downregulated (Hsp15.4-CV). While expression of Hsp21.7-CVI was found in all vegetative tissues, accumulation of Hsp14.7-CVII transcripts was only detectable during late stages of shoot apex and early stages of flower development. Since expression of the latter transcript was also observed in suspension culture cells, it is tempting to speculate that Hsp14.7-CVII and related proteins play a role in meristematic tissues. Very likely, both Hsp21.7-CVI and Hsp14.7-CVII might be involved in specific housekeeping functions of plant cells under normal growth conditions.
This conclusion is further consistent with another interesting observation, the accumulation of precursor transcripts for all intron-containing, hs-induced sHsps including Hsp17.4-CIII (Fig. 2) but not for the two, strictly constitutively expressed members. Whether this indicates a temperature sensitivity of splicing or the transcriptional arrest and storage of mRNAs during hs as described for genes with housekeeping functions remain open questions (Nover et al. 1989; Siddique et al. 2003). Contribution of differential splicing to the regulation of Hsps has been discussed for a long time (Yost and Lindquist 1986) but has not been investigated in plants.
Assembly of sHsps into cytoplasmic multichaperone complexes
Formation of cytoplasmic, highly ordered multichaperone complexes consisting of sHsps as the major protein components is a characteristic feature of plant cells exposed to higher temperatures, and they are assumed to function as storage sites for heat-sensitive proteins to prevent their irreversible aggregation under hs and to keep them in a renaturation competent state for refolding by ATP-dependent chaperone machines (Forreiter et al. 1997; Löw et al. 2000). By immunoflorescence microscopy studies, we have shown that in addition to CI, CII, and CIII sHsps (Kirschner et al. 2000; Siddique et al. 2003), also members of the newly defined cytoplasmic/nuclear sHsps CIV, CV, and CVII are recruited to HSCs upon hs (Fig. 4). However, the recruitment to HSCs seems not to be an intrinsic property of all cytoplasmic/nuclear sHsps. The intracellular localization of Hsp21.7-CVI was not altered under hs, and the typical formation of cytoplasmic foci and colocalization with Hsp17-CI was not observed. Similar results were reported earlier for the plastidial Hsp26.2-P from pea after truncation of the transit peptide, which leads to a cytoplasmic accumulation of the protein but not to the incorporation into HSCs (Kirschner et al. 2000). Masking or mutation of the organellar targeting sequences leads to a cytoplasmic retention of Hsp26.5-MII and Hsp15.7-Po, respectively, which leads to the formation of larger, irregular aggregates clearly distinguishable from the formation of HSCs (data not shown).
Previous studies have implicated distinct molecular functions for different sHsps in the assembly and resolubilization of HSCs. For Hsp17-CII, a scaffold function was proposed, which is required to recruit Hsp17-CI and Hsp17-CIII (Kirschner et al. 2000; Siddique et al. 2003). In contrast, Hsp17-CI was shown to prevent the aggregation of Hsp17-CII, observed by the overexpression in the absence of CI sHsps (Port et al. 2004). Since Hsp17-CIII was found to interact physically with both Hsp17-CI and Hsp17-CII, a mediator role in the organization of HSCs was discussed (Siddique et al. 2003). Such mediator or recruiting functions may also hold true for some members of the newly identified sHsps. However, to determine the specific functional and physical interactions of these proteins in the network of plant sHsps remains an intriguing matter for future investigations.
Chaperone activity of sHsps
Many studies on plant and cyanobacterial sHsps have influenced our knowledge of the function of sHsps (Nakamoto and Vigh 2007). Most sHsps investigated so far form large oligomeric complexes consisting of 8 to 12 or more monomers in the native state. Subunit exchange between different sHsp oligomers demonstrates that these large oligomers are dynamic rather than rigid structures (Bova et al. 1997, 2002; Sobott et al. 2002). Oligomer dissociation and subunit exchange upon hs are assumed to be essential factors triggering the exposure of hydrophobic amino acid residues for binding denatured proteins in both the amino-terminal domain and the ACD in an ATP-independent manner (Haslbeck et al. 1999; van Montfort et al. 2001b; Giese and Vierling 2002; Gu et al. 2002; Friedrich et al. 2004; Giese et al. 2005; Basha et al. 2006). Impairment of this balance caused by mutations that either prevent the dissociation of the oligomeric complexes or the formation of dimers as the basic building blocks for multimerization results in the loss of substrate binding and chaperone activity (Studer et al. 2002; Lentze et al. 2003; Giese and Vierling 2004).
The chaperone activity of CI and CII sHsps and their contribution to thermotolerance has been established in several in vivo (Forreiter et al. 1997; Löw et al. 2000) and in vitro studies (Lee et al. 1997; Lee and Vierling 2000; Basha et al. 2004) by using different heat-sensitive proteins as model substrates. In vitro, substrates denatured in the presence of CI sHsps can be refolded and reactivated by high-molecular-weight chaperones including Hsp70/DnaK, Hsp100/ClpB, and GroEL (Mogk et al. 2003; Basha et al. 2004; Friedrich et al. 2004).
By using purified proteins, we demonstrated that Hsp18.5-CIV and Hsp26.5-MII function as molecular chaperones in vitro (1) by preventing the heat induced aggregation of firefly luciferase (Fig. S2) and (2) by supporting renaturation and reactivation of this model substrate in RRL (Fig. 5). By comparison with Hsp17.6C-CI, their chaperone activities require similar stoichiometric substrate to sHsp ratios and appear to be as efficient as previously reported for other CI sHsps (Lee et al. 1995; Basha et al. 2004). By including the peroxisomal sHsp as an additional organellar sHsp, we provide direct evidence for its chaperone activity, which has not previously been reported. Hsp18.5-CIV was also an effective chaperone in protecting heat denatured luciferase in vitro, although it lacks the β-strand required for the formation of oligomeric complexes as seen in the wheat and Methanococcus sHsp oligomers, suggesting it may have unique mechanistic properties. In fact, preliminary data suggest Hsp18.5-CIV is dimeric rather than oligomeric as are all other plant sHsps characterized to date (Fig. S3). Further investigation of this apparently uniquely structured plant sHsp is in progress.
Among all sHsps investigated so far, Hsp21.7-CVI is the only example that has no protection capacity toward heat-denatured luciferase in vitro. Although all typical secondary structures were predicted, its oligomerization behavior was clearly distinct from all sHsps characterized so far but was similar to the active Hsp18.5-CIV (Fig. S3). However, in addition to its ubiquitous and constitutive expression and the failure to associate with cytoplasmic HSCs under hs, this finding supports the assumption of distinct, nonchaperone functions outside of the cellular stress response.
Summary
Analyses of five newly identified members of the sHsp family in A. thaliana as performed during this work provide an unexpected insight into an increasing multiplicity and functional diversity of sHsps in plants. These sHsps are representatives of new subfamilies with unique structural properties and probably specific functional features, as concluded from the observed expression patterns, intracellular localization, and chaperone activity. It is interesting to note that although even higher numbers of sHsp genes have been found by genome-wide searches in rice and poplar (Waters et al. 2008), all represent homologous genes belonging to the subfamilies identified in A. thaliana. Whether this evolutionary conservation is also extended generally to subfamily-specific functions within the plant kingdom remains a challenging question.
Electronic supplementary material
Below is the link to the electronic supplementary material.
A Cytoplasmic/nuclear subfamily CIV. B Cytoplasmic/nuclear subfamily CV. C Cytoplasmic/nuclear subfamily CVI. D Mitochondrial subfamily MII. E Peroxisomal subfamily (Po) (PDF 237 KB)
A Hsp17.6C-CI. B Hsp18.5-CIV. C Hsp21.7-CVI. D Hsp26.5-MIIΔTP. E Hsp15.7-Po (PDF 476 KB)
A Nucleo-cytoplasmic sHsps. B Organellar sHsps (PDF 508 KB)
Overview of sHsp expression constructs and used primers (DOC 49 KB)
Overview of microarray elements for expression analysis of sHsps (DOC 42 KB)
sHsps homologous to the newly identified A.thaliana members (DOC 147 KB)
Acknowledgments
We would like to thank Eman Basha (Tucson) for gifts of purified recombinant Hsp17.6C-CI and Lutz Nover (Frankfurt) for many helpful discussions. This work was supported by grants from the Deutsche Forschungsgemeinschaft to K.D.S. (Scha577/6) and P.v.K.D. (AFGN grant KO2888/1-2), from the US National Institute of Health (GM42762), US Department of Agriculture (NRICGP 3510014857), and US National Science Foundation (IBN-0213128) to E.V.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-008-0032-6) contains supplementary material, which is available to authorized users.
Contributor Information
Masood Siddique, Email: msiddiqu@biochem2.uni-frankfurt.de.
Sascha Gernhard, Email: Gernhard@biochem2.uni-frankfurt.de.
Pascal von Koskull-Döring, Email: doeringp@bio.uni-frankfurt.de.
Elizabeth Vierling, vierling@email.arizona.edu.
Klaus-Dieter Scharf, Phone: +49-69-79829285, FAX: +49-69-79829286, Email: scharf@bio.uni-frankfurt.de.
References
- Almoguera C, Jordano J (1992) Developmental and environmental concurrent expression of sunflower dry-seed-stored low-molecular-weight heat-shock protein and Lea mRNAs. Plant Mol Biol 19:781–792 [DOI] [PubMed]
- Atkinson BG, Raizada M, Bouchard RA, Frappier RH, Walden DB (1993) The independent stage-specific expression of the 18-kDa heat shock protein genes during microsporogenesis in Zea mays L. Dev Genet 14:15–26 [DOI] [PubMed]
- Basha E, Lee GJ, Demeler B, Vierling E (2004) Chaperone activity of cytosolic small heat shock proteins from wheat. Eur J Biochem 271:1426–1436 [DOI] [PubMed]
- Basha E, Friedrich KL, Vierling E (2006) The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J Biol Chem 281:39943–39952 [DOI] [PubMed]
- Bova MP, Ding LL, Horwitz J, Fung BK (1997) Subunit exchange of alphaA-crystallin. J Biol Chem 272:29511–29517 [DOI] [PubMed]
- Bova MP, Huang Q, Ding L, Horwitz J (2002) Subunit exchange, conformational stability, and chaperone-like function of the small heat shock protein 16.5 from Methanococcus jannaschii. J Biol Chem 277:38468–38475 [DOI] [PubMed]
- Carranco R, Almoguera C, Jordano J (1997) A plant small heat shock protein gene expressed during zygotic embryogenesis but noninducible by heat stress. J Biol Chem 272:27470–27475 [DOI] [PubMed]
- Chou M, Chen YM, Lin CY (1989) Thermotolerance of isolated mitochondria associated with heat shock proteins. Plant Physiol 98:617–621 [DOI] [PMC free article] [PubMed]
- Clos J, Brandau S (1994) pJC20 and pJC40-two high-copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in Escherichia coli. Protein Expr Purif 5:133–137 [DOI] [PubMed]
- Coca MA, Almoguera C, Thomas TL, Jordano J (1996) Differential regulation of small heat-shock genes in plants: analysis of a water-stress-inducible and developmentally activated sunflower promoter. Plant Mol Biol 31:863–876 [DOI] [PubMed]
- Craig WS (1988) Determination of quaternary structure of an active enzyme using chemical cross-linking with glutaraldehyde. Methods Enzymol 156:333–345 [DOI] [PubMed]
- de Jong WW, Caspers GJ, Leunissen JA (1998) Genealogy of the α-crystallin–small heat-shock protein superfamily. Int J Biol Macromol 22:151–162 [DOI] [PubMed]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 16:221–229 [DOI] [PMC free article] [PubMed]
- Fontaine JM, Rest JS, Welsh MJ, Benndorf R (2003) The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones 8:62–69 [DOI] [PMC free article] [PubMed]
- Forreiter C, Kirschner M, Nover L (1997) Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo. Plant Cell 9:2171–2181 [DOI] [PMC free article] [PubMed]
- Friedrich KL, Giese KC, Buan NR, Vierling E (2004) Interactions between small heat shock protein subunits and substrate in small heat shock protein–substrate complexes. J Biol Chem 279:1080–1089 [DOI] [PubMed]
- Giese KC, Vierling E (2002) Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J Biol Chem 277:46310–46318 [DOI] [PubMed]
- Giese KC, Vierling E (2004) Mutants in a small heat shock protein that affect the oligomeric state. J Biol Chem 279:32674–32683 [DOI] [PubMed]
- Giese KC, Basha E, Catague BY, Vierling E (2005) Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity. Proc Natl Acad Sci USA 102:18896–18901 [DOI] [PMC free article] [PubMed]
- Gu L, Abulimiti A, Li W, Chang Z (2002) Monodisperse Hsp16.3 nonamer exhibits dynamic dissociation and reassociation, with the nonamer dissociation prerequisite for chaperone-like activity. J Mol Biol 319:517–526 [DOI] [PubMed]
- Guo SJ, Zhou HY, Zhang XS, Li XG, Meng QW (2007) Overexpression of CaHSP26 in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J Plant Physiol 164:126–136 [DOI] [PubMed]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J (1999) Hsp26: a temperature-regulated chaperone. EMBO J 18:6744–6751 [DOI] [PMC free article] [PubMed]
- Haslbeck M, Miess A, Stromer T, Walter S, Buchner J (2005) Disassembling protein aggregates in the yeast cytosol. J Biol Chem 280:23861–23868 [DOI] [PubMed]
- Hertwig B, Streb P, Feierabend J (1992) Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol 100:1547–1553 [DOI] [PMC free article] [PubMed]
- Kampinga HH, Brunsting JF, Stege GJ, Konings AW, Landry J (1994) Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem Biophys Res Commun 204:1170–1177 [DOI] [PubMed]
- Kampinga HH, Brunsting JF, Stege GJ, Burgman PW, Konings AW (1995) Thermal protein denaturation and protein aggregation in cells made thermotolerant by various chemicals: role of heat shock proteins. Exp Cell Res 219:536–546 [DOI] [PubMed]
- Kappè G, Franck E, Verschuure P, Boelens WC, Leunissen JA, deJong WW (2003) The human genome encodes 10 α-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones 8:53–61 [DOI] [PMC free article] [PubMed]
- Kim R, Kim KK, Yokota H, Kim SH (1998a) Small heat shock protein of Methanococcus jannaschii, a hyperthermophile. Proc Natl Acad Sci USA 95:9129–9133 [DOI] [PMC free article] [PubMed]
- Kim KK, Kim R, Kim SH (1998b) Crystal structure of a small heat-shock protein. Nature 394:595–599 [DOI] [PubMed]
- Kirschner M, Winkelhaus S, Thierfelder JM, Nover L (2000) Transient expression and heat-stress-induced co-aggregation of endogenous and heterologous small heat-stress proteins in tobacco protoplasts. Plant J 24:397–411 [DOI] [PubMed]
- Kobayashi T, Kobayashi E, Sato S, Hotta Y, Miyajima N, Tanaka A, Tabata S (1994) Characterization of cDNAs induced in meiotic prophase in lily microsporocytes. DNA Res 1:15–26 [DOI] [PubMed]
- Kotak S, Vierling E, Bäumlein H, von Koskull-Döring P (2007) A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell 19:182–195 [DOI] [PMC free article] [PubMed]
- Lee GJ, Vierling E (1998) Expression, purification, and molecular chaperone activity of plant recombinant small heat shock proteins. Methods Enzymol 290:350–365 [DOI] [PubMed]
- Lee GJ, Vierling E (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol 122:189–198 [DOI] [PMC free article] [PubMed]
- Lee GJ, Pokala N, Vierling E (1995) Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem 270:10432–10438 [DOI] [PubMed]
- Lee GJ, Roseman AM, Saibil HR, Vierling E (1997) A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J 16:659–671 [DOI] [PMC free article] [PubMed]
- Lentze N, Studer S, Narberhaus F (2003) Structural and functional defects caused by point mutations in the alpha-crystallin domain of a bacterial alpha-heat shock protein. J Mol Biol 328:927–937 [DOI] [PubMed]
- Lin KH, Cheng SY (1991) An efficient method to purify active eukaryotic proteins from the inclusion bodies in Escherichia coli. Biotechniques 11:748–752 [PubMed]
- Liu J, Shono M (1999) Characterization of mitochondria-located small heat shock protein from tomato (Lycopersicon esculentum). Plant Cell Physiol 40:1297–1304 [DOI] [PubMed]
- Löw D, Brandle K, Nover L, Forreiter C (2000) Cytosolic heat-stress proteins Hsp17.7 class I and Hsp17.3 class II of tomato act as molecular chaperones in vivo. Planta 211:575–582 [DOI] [PubMed]
- Lubaretz O, zur Nieden U (2002) Accumulation of plant small heat-stress proteins in storage organs. Planta 215:220–228 [DOI] [PubMed]
- Lyck R, Harmening U, Höhfeld I, Treuter E, Scharf KD, Nover L (1997) Intracellular distribution and identification of the nuclear localization signals of two plant heat-stress transcription factors. Planta 202:117–125 [DOI] [PubMed]
- Ma C, Haslbeck M, Babujee L, Jahn O, Reumann S (2006) Identification and characterization of a stress-inducible and a constitutive small heat shock protein targeted to the matrix of plant peroxisomes. Plant Physiol 141:47–60 [DOI] [PMC free article] [PubMed]
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16:1555–1567 [DOI] [PMC free article] [PubMed]
- Mogk A, Schlieker C, Friedrich KL, Schonfeld HJ, Vierling E, Bukau B (2003) Refolding of substrates bound to small Hsps relies on a disaggregation reaction mediated most efficiently by ClpB/DnaK. J Biol Chem 278:31033–31042 [DOI] [PubMed]
- Nakamoto H, Vigh L (2007) The small heat shock proteins and their clients. Cell Mol Life Sci 64:294–306 [DOI] [PMC free article] [PubMed]
- Narberhaus F (2002) α-Crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev 66:64–93 [DOI] [PMC free article] [PubMed]
- Neta-Sharir I, Isaacson T, Lurie S, Weiss D (2005) Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell 17:1829–1838 [DOI] [PMC free article] [PubMed]
- Neumann D, Scharf KD, Nover L (1984) Heat shock induced changes of plant cell ultrastructure and autoradiographic localization of heat shock proteins. Eur J Cell Biol 34:254–264 [PubMed]
- Nover L (ed) (1991) Heat shock response. CRC, Boca Raton, FL
- Nover L, Scharf KD, Neumann D (1983) Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol 3:1648–1655 [DOI] [PMC free article] [PubMed]
- Nover L, Scharf KD, Neumann D (1989) Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9:1298–1308 [DOI] [PMC free article] [PubMed]
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4:2411–2423 [DOI] [PMC free article] [PubMed]
- Port M, Tripp J, Zielinski D, Weber C, Heerklotz D, Winkelhaus S, Bublak D, Scharf KD (2004) Role of Hsp17.4-CII as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2. Plant Physiol 135:1457–1470 [DOI] [PMC free article] [PubMed]
- Prändl R, Kloske E, Schöffl F (1995) Developmental regulation and tissue-specific differences of heat shock gene expression in transgenic tobacco and Arabidopsis plants. Plant Mol Biol 28:73–82 [DOI] [PubMed]
- Sanmiya K, Suzuki K, Egawa Y, Shono M (2004) Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett 557:265–268 [DOI] [PubMed]
- Scharf KD, Heider H, Höhfeld I, Lyck R, Schmidt E, Nover L (1998) The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol Cell Biol 18:2240–2251 [DOI] [PMC free article] [PubMed]
- Scharf KD, Siddique M, Vierling E (2001) The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing alpha-crystallin domains (Acd proteins). Cell Stress Chaperones 6:225–237 [DOI] [PMC free article] [PubMed]
- Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Döring P (2006) The heat stress transcription factor HsfA2 serves as a regulatory amplifier of as subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol 60:759–772 [DOI] [PubMed]
- Siddique M, Port M, Tripp J, Weber C, Zielinski D, Calligaris R, Winkelhaus S, Scharf KD (2003) Tomato heat stress protein Hsp16.1-CIII represents a member of a new class of nucleocytoplasmic small heat stress proteins in plants. Cell Stress Chaperones 8:381–394 [DOI] [PMC free article] [PubMed]
- Smýkal P, Hrdy I, Pechan PM (2000) High-molecular-mass complexes formed in vivo contain smHSPs and HSP70 and display chaperone-like activity. Eur J Biochem 267:2195–2207 [DOI] [PubMed]
- Sobott F, Benesch JL, Vierling E, Robinson CV (2002) Subunit exchange of multimeric protein complexes. J Biol Chem 277:38921–38929 [DOI] [PubMed]
- Stamler R, Kappe G, Boelens W, Slingsby C (2005) Wrapping the [alpha]-crystallin domain fold in a chaperone assembly. J Mol Biol 353:68–79 [DOI] [PubMed]
- Stege GJ, Brunsting JF, Kampinga HH, Konings AW (1995) Thermotolerance and nuclear protein aggregation: protection against initial damage or better recovery? J Cell Physiol 164:579–586 [DOI] [PubMed]
- Studer S, Obrist M, Lentze N, Narberhaus F (2002) A critical motif for oligomerization and chaperone activity of bacterial alpha-heat shock proteins. Eur J Biochem 269:3578–3586 [DOI] [PubMed]
- Töpfer R, Schell J, Steinbiss HH (1988) Versatile cloning vectors for transient gene expression and direct gene transfer in plant cells. Nucleic Acids Res 16:8725 [DOI] [PMC free article] [PubMed]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E (2001a) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol 8:1025–1030 [DOI] [PubMed]
- van Montfort RLM, Slingsby C, Vierling E (2001b) Structure and function of the small heat shock protein/a-crystallin family of molecular chaperones. Adv Protein Chem 59:105–156 [DOI] [PubMed]
- Vierling E (1991) The role of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 44:385–410
- Waters ER, Vierling E (1999) The diversification of plant cytosolic small heat shock proteins preceded the divergence of mosses. Mol Biol Evol 16:127–139 [DOI] [PubMed]
- Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 296:325–338 [DOI]
- Waters ER, Aevermann BD, Sanders-Reed Z (2008) Comparative analysis of the small heat shock proteins in three angiosperm genomes identifies new subfamilies and reveals diverse evolutionary patterns. Cell Stress Chaperones DOI 10.1007/s12192-008-0023-7 [DOI] [PMC free article] [PubMed]
- Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol 122:1099–1108 [DOI] [PMC free article] [PubMed]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E (1996) Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol 112:747–757 [DOI] [PMC free article] [PubMed]
- Yost HJ, Lindquist S (1986) RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell 45:185–193 [DOI] [PubMed]
- Zarsky V, Garrido D, Eller N, Tupy J, Vincente O, Schöffl F, Herberle-Bors E (1995) The expression of a small heat shock gene is activated during induction of tobacco pollen embryogenesis by starvation. Plant Cell Environ 18:139–147 [DOI]
- Zur Nieden U, Neumann D, Bucka A, Nover L (1995) Tissue-specific localization of heat-stress proteins during embryo development. Planta 196:530–538 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
A Cytoplasmic/nuclear subfamily CIV. B Cytoplasmic/nuclear subfamily CV. C Cytoplasmic/nuclear subfamily CVI. D Mitochondrial subfamily MII. E Peroxisomal subfamily (Po) (PDF 237 KB)
A Hsp17.6C-CI. B Hsp18.5-CIV. C Hsp21.7-CVI. D Hsp26.5-MIIΔTP. E Hsp15.7-Po (PDF 476 KB)
A Nucleo-cytoplasmic sHsps. B Organellar sHsps (PDF 508 KB)
Overview of sHsp expression constructs and used primers (DOC 49 KB)
Overview of microarray elements for expression analysis of sHsps (DOC 42 KB)
sHsps homologous to the newly identified A.thaliana members (DOC 147 KB)