Abstract
The pleiotropic cytokine interleukin-6 (IL-6) has been demonstrated to increase during exercise. Little is known regarding the response of the soluble IL-6 receptors (sIL-6R and sgp130) during such exercise. The aim of the current study was to investigate the response of plasma IL-6, sIL-6R and sgp130 during fatiguing submaximal exercise in humans. Twelve participants underwent an incremental exercise test to exhaustion and one week later performed a submaximal exercise bout (96 ± 6% lactate threshold) to volitional exhaustion. Blood samples taken at rest and immediately post exercise were analyzed for IL-6, sIL-6R and sgp130. IL-6 increased (P < 0.01) by 8.4 ± 8.9 pg ml−1 (75.7%) during the exercise period. sIL-6R and sgp130 also increased (P < 0.05) by 2.7 ± 3.9 ng ml−1 (9.6%) and 37.7 ± 55.6 ng ml−1 (9.6%), respectively. The current study is the first investigation to demonstrate that alongside IL-6, acute exercise stress results in an increase in both sIL-6R and sgp130.
Keywords: Exercise stress, Interleukin-6, Receptors, Fatigue
Introduction
Since 1991, when Northoff and Berg (1991) first discovered that the myokine IL-6 increased during exercise, numerous studies have demonstrated that IL-6 can increase up to 100 fold during exercise, in a duration and intensity dependent manner (Ostrowski et al. 1998; Pedersen and Fischer 2007). In addition to its well established role in the regulation of immune function (for review see Jones 2005) recent work has identified a number of additional roles for this pleiotropic cytokine. One of those emerging for IL-6 is that it crosses the blood brain barrier leading to hyperalgesia (DeLeo et al. 1996), increased feelings of fatigue (Nishimoto et al. 2000), increased feelings of depression and a reduced ability to concentrate (Spath-Schwalbe et al. 1998). These central effects of IL-6 can be highlighted during exercise where infusion of IL-6 reduces the performance of a 10K time trial, possibly due to an increased serotonergic activity in the central nervous system (Robson-Ansley et al. 2004).
Further work has demonstrated that infusion of IL-6 increases lipolysis and fat oxidation in adipocytes at rest (Petersen et al. 2005) but not during low-intensity exercise (Hiscock et al. 2005). In contrast, IL-6 infusion has been found to have equivocal effects on glucose metabolism at rest (Carey et al. 2006; Geiger et al. 2007) but increases endogenous glucose production during exercise (Febbraio et al. 2004). Critically, none of these aforementioned investigations, looking into the biological effects of IL-6, have taken into account the possible role of the receptors through which IL-6 signalling is mediated (e.g. Chow et al. 2001).
Under normal conditions IL-6 signals through the membrane bound IL-6R and gp130 receptors (Heinrich et al. 2003). Whilst gp130 is ubiquitously expressed (Hibi et al. 1990) IL-6R expression is limited mainly to hepatocytes and leucocytes (Jones 2005), the midbrain, hypothalamus (Miyahara et al. 2000) and approximately 60% of adipocytes (Bastard et al. 2002). With respect to skeletal muscle, at rest and during exercise there is relatively little IL-6R expression with this increasing in the recovery period and peaking several hours post-exercise (Keller et al. 2005a, b). In tissues where IL-6R is devoid or lacking, IL-6 can signal through a system of soluble receptors, sIL-6R and sgp130, with the formation of an IL-6/sIL-6R complex acting agonistically to increase IL-6 activity, a process named trans-signalling (Jones et al. 2001, 2005). On the other hand, signalling via this complex can be blocked by the antagonistic sgp130, by forming a tertiary IL-6/sIL-6R/sgp130 complex which is biologically inactive (Jostock et al. 2001). These soluble receptors have previously been found to regulate the activity of IL-6 in many situations including the inflammatory processes involved in many chronic diseases (Scheller et al. 2006).
It is surprising, therefore, that there have been very few investigations which have investigated the effect of exercise on these soluble IL-6 receptors. In the only study, to our knowledge, investigating sIL-6R levels during exercise there appeared to be a trend for an increase with exercise, with relatively low subject numbers (n = 6) (Keller et al. 2005b). No investigations to date, have measured sgp130 levels during exercise. The aim of the present study, therefore, was to investigate the response of plasma IL-6 and its soluble receptors during an acute exercise stress to fatigue.
Results
The criterion for 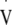 O2max was met by all participants in the preliminary test. This physiological maximum was determined based on meeting at least three of the following four criteria: 1) change in
O2max was met by all participants in the preliminary test. This physiological maximum was determined based on meeting at least three of the following four criteria: 1) change in 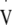 O2 < 2 ml kg−1 min−1 between the last minute of the test and the last minute of the previous workload; 2) respiratory exchange ratio ≥1.15; 3) achieving age predicted maximal heart rate (220-age); 4) maximum blood lactate >8 mmol l−1. The data from this preliminary test is detailed in Table 1.
O2 < 2 ml kg−1 min−1 between the last minute of the test and the last minute of the previous workload; 2) respiratory exchange ratio ≥1.15; 3) achieving age predicted maximal heart rate (220-age); 4) maximum blood lactate >8 mmol l−1. The data from this preliminary test is detailed in Table 1.
Table 1.
Descriptive data obtained from an incremental exercise stress. Twelve sedentary men, not participating in any regular exercise, (age 50.6 ± 7.8 yr, height 174.9 ± 9.0 cm, mass 75.9 ± 9.0 kg, means ± SD) initially performed an incremental exercise test to determine lactate threshold (LT) and 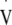 O2max (Jones 1998) on an electromagnetically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands)
O2max (Jones 1998) on an electromagnetically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands)
| Variable | Mean ± S.D. |
|---|---|
| Maximum oxygen uptake (ml kg−1 min−1) | 30.0 ± 6.5 |
| Maximum heart rate (beats min−1) | 174.7 ± 7.4 |
| Maximum blood lactate (mmol l−1) | 9.1 ± 2.2 |
| Maximum power output (Watts) | 167.9 ± 41.5 |
| RER at VO2max | 1.22 ± 0.12 |
| Oxygen uptake at LT ml kg−1 min−1) | 10.6 ± 2.7 |
| Power output at LT (Watts) | 119.8 ± 35.5 |
| LT as percentage of VO2max (%) | 68.4 ± 19.3 |
During this test the workload was increased from an initial 65 W by 15 W in 2 min stages, with a fingertip blood lactate (Lactate Pro, Akray KDK, Koyota, Japan) and heart rate (Polar s810i, Polar Electro Oy, Kempele, Finland) measurement taken at the end of every stage. Expired air was measured continuously using an on-line breath-by-breath gas analysis system (Oxycon Pro, Jaeger, Hoechberg, Germany). 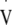 O2max was identified as the oxygen uptake (
O2max was identified as the oxygen uptake (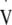 O2) averaged over the highest 30 s period during the test. LT was calculated by simultaneously solving two linear regression equations relating blood lactate to workload following identification of the visually observed breakpoint in blood lactate by two experienced reviewers (Beaver et al. 1985), and was used to calculate the workload required to elicit approximately 95%
O2) averaged over the highest 30 s period during the test. LT was calculated by simultaneously solving two linear regression equations relating blood lactate to workload following identification of the visually observed breakpoint in blood lactate by two experienced reviewers (Beaver et al. 1985), and was used to calculate the workload required to elicit approximately 95% 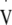 O2max which was used in the subsequent experimental trial.
O2max which was used in the subsequent experimental trial.
During the submaximal exercise participants on average fatigued after 56.3 ± 26.0 min of cycling. This acute exercise stress resulted in an increase (P = 0.007) in IL=6 from 11.1 ± 10.3 pg ml−1 at rest to 19.5 ± 14.1 pg ml−1, a rise of approximately 81%. Circulating levels of sIL-6R also increased (P = 0.036) from 28.1 ± 6.6 ng ml−1 at rest to 30.8 ± 9.1 ng ml−1, a rise of approximately 10%, in response to the experimental protocol. Similarly sgp130 levels increased (P = 0.024) from resting levels of 391.6 ± 124.3 ng ml−1 to 429.3 ± 166.1 ng ml−1 at the end of exercise, an increase of approximately 10%. Blood glucose levels decreased (P = 0.006) from 5.7 ± 0.5 mmol l−1 to 5.2 ± 0.6 mmol l−1 in response to the acute exercise stress.
Discussion
The current investigation has demonstrated that during a single submaximal exercise bout to fatigue there is a significant increase in not only plasma IL-6 but also in its soluble receptors sIL-6R and sgp130 (Fig. 1). Several investigations have demonstrated that in response to exercise circulating levels of IL-6 increase and the current investigation supports these findings (e.g. Pedersen et al. 2004). Little work, however, had previously investigated the response of the soluble IL-6 receptors to exercise. This is surprising since these receptors extend the half-life of IL-6, mediate its biological activity and are involved in many biological processes (for review see Jones et al. 2005). Previous work has investigated the response of sIL-6R during exercise and found that whilst exercise stimulated membrane bound IL-6R production there was no statistically significant effect of exercise on circulating levels of sIL-6R (Keller et al. 2005b). However, there were only six subjects participating in this investigation and, in addition, there were no measures of sgp130. The current data (n = 12) demonstrate that both sIL-6R and sgp130 also increase after an acute exercise stress concurrent with a decrease in circulating glucose levels.
Fig. 1.
The response of IL-6, sIL-6R and sgp130 to a submaximal exercise bout to fatigue. * denotes a significant difference from rest, P < 0.05 (n = 12). At least one week after the initial test, subjects visited the laboratory after a 12 h fast and having avoided any alcohol, caffeine or strenuous exercise for 24 h prior to testing. To ensure euhydration, subjects consumed 1 l of water the night before and 0.5 l 2 h before the experiment. Previous work from our laboratory has shown that this preparation ensures euhydration, as measured by urine specific gravity (Marshall et al. 2006). Subjects then performed cycling exercise at 96 ± 6% LT until volitional exhaustion with water permitted ad libitum throughout. Blood samples were obtained by venepuncture (20 G Insyte-W, BD, USA) from an antecubital vein at rest and immediately post-exercise. Blood samples were collected in K+EDTA tubes. For glucose analysis blood was aliquoted into perchloric acid, centrifuged and the supernatant frozen at −80°C until analysis. For cytokine determination blood samples were centrifuged, the plasma removed and frozen at −80°C until analyses. Samples were assayed in duplicate for IL-6 (CV = 6.4%), sIL-6R (CV = 3.4%) and sgp130 (CV = 3.4%) via enzyme-linked immunosorbent assay using commercially available antibody pairs (IL-6:OptEIA IL-6 set; sIL-6R: M5 capture Ab and M182 detection Ab, and sgp130:A1 capture Ab and D2 detection antibody, all from BD Biosciences, Oxford, UK). Blood glucose (CV = 1.7%) was also measured spectrophotometrically using commercially available kits (Randox, UK). Pre and post-exercise blood samples were compared via paired t-tests. Significance was accepted at P < 0.05. Data are presented as Mean ± SD
The current study has investigated a single bout of exercise. A different response of IL-6 and the receptors is noted after repeated exercise (training) where sIL-6R levels have been found to be decreased in patients with chronic heart failure (Adamopoulos et al. 2002) and also decreased in obese women when exercise is combined with a hypocalorific diet (You et al. 2004). A similar response in IL-6 has also been found after an exercise intervention (Starkweather 2007) on several, but not all, occasions (Olson et al. 2007), highlighting the difference in the response of IL-6 and its receptors to acute and chronic exercise stress.
Soluble IL-6R is produced by either proteolytic cleavage (PC) or domain splicing (DS) from the membrane bound receptor with resting levels being mainly DS sIL-6R with PC sIL-6R increasing with Ca2+ mobilization (Jones et al. 1998, 2001). Once in the circulation sIL-6R acts agonistically to augment the activity of IL-6 (Jones and Rose-John 2002). This would suggest, therefore, that in the current investigation the previously demonstrated effects of IL-6 on endogenous glucose production (Febbraio et al. 2004) and fatigue (Robson-Ansley et al. 2004) may in part be mediated by the concurrent increase in sIL-6R, leading to a greater IL-6/sIL-6R complex. Indeed, in their study, Febbraio and colleagues (2004) concluded that the glucose production modulating properties of IL-6 are regulated by an as yet unidentified contraction-induced factor. This increase in sIL-6R during exercise stress, when membrane bound IL-6R expression is low (Keller et al. 2005b), make it a possible candidate to fulfil this criteria of the contraction induced factor, increasing IL-6 signalling via an enhanced trans-signalling. However, when the response of sgp130 receptors is considered this may not be the case.
Alongside the increase in IL-6 and sIL-6R observed in the present study we have shown for the first time that there was also a 10% increase in sgp130 during exercise. This receptor is generated by a shedding mechanism with the majority being the result of alternative mRNA splicing (Diamant et al. 1997). The precise mechanism which initiates the generation of sgp130 is unknown and so we are unable to suggest the pathway by which exercise has resulted in this increased production of sgp130. Soluble gp130 acts as a buffer for any increase in the IL-6/sIL-6R complex, serving to inhibit transsignalling and decrease the biological activity of IL-6 (Jostock et al. 2001). Our previous suggestion of an increase in transignalling during exercise would appear, therefore, to be offset by the concurrent rise in the agonistic sgp130. It has been estimated, however, that within its normal concentration range the buffering capacity of sgp130 is limited (Dimitrov et al. 2006) and may therefore be unable to significantly affect the increase in transignalling via the IL-6/sIL-6R complex.
Although the current study is the first study to investigate the response of the major aspects of the circulating IL-6 signalling system to exercise, further investigation into the precise role of these responses is required. Indeed, while enzyme-linked immunosorbent assays measure levels of IL-6, sIL-6R and sgp130, they can only provide a measure of the total content of each of these cytokines and do not provide any information on the levels of these which are free or in either the binary or tertiary complex. For this reason, new methods have recently been developed which allow the accurate measurement of the amount of IL-6/sIL-6R complex (Scheller et al. 2004). The application of methods such as these is crucial to further our understanding of the response of the circulating IL-6 system during exercise stress.
One possible limitation to the current data is that changes in plasma volume during the exercise period were not accounted for. The plasma volume changes should, however, be minimal as subjects were allowed to consume water ad libitum during exercise, resulting in no change in body mass over the hour (data not shown). From similar exercise protocols in our laboratory plasma volume changes have been between 3 and 5% (Layden et al. 2004) accounting for around half of the 10% increase in sIL-6R and sgp130 observed. Furthermore, it has been argued that the level of the parameter at the time, irrespective of plasma volume shifts, is the important factor as it is this that determines the body’s response (Kargotich et al. 1998), i.e. an increase in transignalling.
In conclusion, the present investigation has demonstrated that submaximal cycling exercise to fatigue, in sedentary middle-aged men, results in an increase in not only IL-6 but also in its soluble receptors, sIL-6R and sgp130. Further work is required to determine the effect of these changes on the biological activity of IL-6.
References
- Adamopoulos S, Parissis J, Karatzas D, Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou K, Coats AJ, Kremastinos DT (2002) Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol 39:653–663 [DOI] [PubMed]
- Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert J-J, Capeau J, Hainque B (2002) Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 87:2084–2089 [DOI] [PubMed]
- Beaver WL, Wasserman K, Whipp BJ (1985) Improved detection of lactate threshold during exercise using a log–log transformation. J Appl Physiol 59:1936–1940 [DOI] [PubMed]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA (2006) Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55:2688–2697 [DOI] [PubMed]
- Chow D, He X, Snow AL, Rose-John S, Garcia KC (2001) Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291:2150–2155 [DOI] [PubMed]
- DeLeo JA, Colburn RW, Nichols M, Malhotra A (1996) Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J Interferon Cytokine Res 16:695–700 [DOI] [PubMed]
- Diamant M, Rieneck K, Mechti N, Zhang XG, Svenson M, Bendtzen K, Klein B (1997) Cloning and expression of an alternatively spliced mRNA encoding a soluble form of the human interleukin-6 signal transducer gp130. FEBS Lett 412:379–384 [DOI] [PubMed]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J (2006) Sleep enhances IL-6 trans-signaling in humans. FASEB J 20:2174–2176 [DOI] [PubMed]
- Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK (2004) Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53:1643–1648 [DOI] [PubMed]
- Geiger PC, Hancock CR, Wright DC, Han DH, Holloszy JO (2007) IL-6 increases muscle insulin sensitivity only at super-physiological levels. Am J Physiol Endocrinol Metab 292:1842–1846 [DOI] [PubMed]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20 [DOI] [PMC free article] [PubMed]
- Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T (1990) Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63:1149–1157 [DOI] [PubMed]
- Hiscock N, Fischer CP, Sacchetti M, van Hall G, Febbraio MA, Pedersen BK (2005) Recombinant human interleukin-6 infusion during low-intensity exercise does not enhance whole body lipolysis or fat oxidation in humans. Am J Physiol Endocrinol Metab 289:E2–E7 [DOI] [PubMed]
- Jones AM (1998) A five year physiological case study of an olympic runner. Br J Sports Med 32:39–43 [DOI] [PMC free article] [PubMed]
- Jones SA (2005) Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 175:3463–3468 [DOI] [PubMed]
- Jones SA, Rose-John S (2002) The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta 1592:251–263 [DOI] [PubMed]
- Jones SA, Horiuchi S, Novick D, Yamamoto N, Fuller GM (1998) Shedding of the soluble IL-6 receptor is triggered by Ca2+ mobilization, while basal release is predominantly the product of differential mRNA splicing in THP-1 cells. Eur J Immunol 28:3514–3522 [DOI] [PubMed]
- Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM (2001) The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 15:43–58 [DOI] [PubMed]
- Jones SA, Richards PJ, Scheller J, Rose-John S (2005) IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res 25:241–253 [DOI] [PubMed]
- Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S (2001) Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 268:160–167 [DOI] [PubMed]
- Kargotich S, Goodman C, Keast D, Morton AR (1998) The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med 26:101–117 [DOI] [PubMed]
- Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK (2005a) Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol 99:2075–2079 [DOI] [PubMed]
- Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, Hidalgo J, Pedersen BK (2005b) Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J 19:1181–1183 [DOI] [PubMed]
- Layden JD, Malkova D, Nimmo MA (2004) Fat oxidation after acipimox-induced reduction in plasma nonesterified fatty acids during exercise at 0 degrees C and 20 degrees C. Metabolism 53:1131–1135 [DOI] [PubMed]
- Marshall HC, Ferguson RA, Nimmo MA (2006) Human resting extracellular heat shock protein 72 concentration decreases during the initial adaptation to exercise in a hot, humid environment. Cell Stress Chaperones 11:129–134 [DOI] [PMC free article] [PubMed]
- Miyahara S, Komori T, Fujiwara R, Shizuya K, Yamamoto M, Ohmori M, Okazaki Y (2000) Effects of repeated stress on expression of interleukin-6 (IL-6) and IL-6 receptor mRNAs in rat hypothalamus and midbrain. Life Sci 66:L93–L98 [DOI] [PubMed]
- Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K (2000) Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood 95:56–61 [PubMed]
- Northoff H, Berg A (1991) Immunologic mediators as parameters of the reaction to strenuous exercise. Int J Sports Med 12(Suppl 1):S9–S15 [DOI] [PubMed]
- Olson TP, Dengel DR, Leon AS, Schmitz KH (2007) Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes.(Lond) 31:996–1003 [DOI] [PubMed]
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK (1998) A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513:889–894 [DOI] [PMC free article] [PubMed]
- Pedersen BK, Fischer CP (2007) Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care 10:265–271 [DOI] [PubMed]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M (2004) The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor. Proc Nutr Soc 63:263–267 [DOI] [PubMed]
- Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, Pedersen BK (2005) Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab 288:E155–E162 [DOI] [PubMed]
- Robson-Ansley PJ, Milander L, Collins M, Noakes TD (2004) Acute interleukin-6 infusion impairs athletic performance in healthy, trained male runners. Can J Appl Physiol 29:411–418 [DOI] [PubMed]
- Scheller J, Kovaleva M, Rabe B, Eichler J, Kallen K, Rose-John S (2004) Development of a monoclonal anti-body based enzyme-linked immunoabsorbent assay for the binding of gp130 to the IL-6/IL-6R complex and its competitive inhibition. J Immunol Methods 291:93–100 [DOI] [PubMed]
- Scheller J, Ohnesorge N, Rose-John S (2006) Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 63:321–329 [DOI] [PubMed]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J (1998) Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab 83:1573–1579 [DOI] [PubMed]
- Starkweather AR (2007) The Effects of exercise on perceived stress and IL-6 levels among older adults. Biol Res Nursing 8:186–194 [DOI] [PubMed]
- You T, Berman DM, Ryan AS, Nicklas BJ (2004) Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab 89:1739–1746 [DOI] [PubMed]



