Abstract
Background
Circulating heat shock protein 70 (Hsp70) is present in the circulation of healthy individuals and in patients with various disorders, including chronic heart failure (CHF). However, the source and routes of release of Hsp70 is only partially characterised in clinical samples.
Aims
The purpose of this study was to study the clinical and biological correlates of Hsp70 in a CHF population and, for the first time, to investigate the association of HspA1B (also known as Hsp70-2) +1267 alleles with serum Hsp70 levels.
Methods
A total of 167 patients (123 men, 44 women) with <45% left ventricular ejection fraction (LVEF) were enrolled; serum Hsp70 level was determined by enzyme-linked immunosorbent assay and HspA1B +1267 polymorphism by polymerase chain reaction–restriction fragment length polymorphism.
Results
Increased Hsp70 levels were present in patients with severe CHF (NYHA III–IV) as compared to the group of NYHA I–II (p = 0.003). Hsp70 levels correlated with LVEF, NT-proBNP, serum bilirubin, aspartate aminotransferase, alanine aminotransferase, γGT (p < 0.05) concentrations in patients with severe CHF, although no correlation was observed between Hsp70 and CRP, TNF-α, or IL-6. HspA1B allele G was associated with higher Hsp70 levels (p = 0.001) in patients in NYHA IV class as compared to carriers of allele A.
Conclusions
Serum Hsp70 levels were associated with disease severity in heart failure patients. An interaction with the presence of HspA1B +1267 allele G was observed for Hsp70 concentrations. Hsp70 correlates with markers of heart function and hepatic injury, but not with signs of inflammation.
Keywords: Heart failure, Inflammation, Liver, Stress proteins
Introduction
Chronic heart failure (CHF) is a major health problem in the developed world, and its prevalence is still increasing (Kannel 2000; Stewart et al. 2003). Severe heart failure requiring hospitalisation has worse prognosis than many of the common types of cancer (Stewart et al. 2001). The opinion that the disease mainly consists of haemodynamic alterations or fluid and salt retention has largely changed. Nowadays, CHF is considered a complex syndrome; the constant hypoperfusion and hypoxia does affect most of the organs, causing inflammation, changes in the nutritional status, haemopoesis, liver and renal function, respiration and activation of the neurohormones.
Heat shock proteins (Hsp) are phylogenetically highly conserved molecules in their molecular structure, biochemical properties and partly even in their immunological structure. Hsps are traditionally classified by their molecular weight, the 70-kDa family (Hsp70) including constitutively expressed Hsp-8 and inducible Hsp70-1, also called HspA1B (Hsp70 in the following refers to the inducible form) members. Hsp70 is traditionally considered as intracellular cytoprotective chaperone, and its level can increase several-fold in response to stress (Prohaszka and Fust 2004), which can be infection, hypoxia and ischaemia, as well as reduced myocyte shortening characteristically seen in the failing heart (Knowlton et al. 1991). It has been recognised that Hsp70 are present in the peripheral circulation of healthy individuals (Pockley et al. 1998; Dhingra et al. 2006), and their levels increase after intensive exercise (Febbraio et al. 2002; Fehrenbach et al. 2005). Elevation of serum Hsp70 was reported in patients with soft tissue trauma (Pittet et al. 2002; Flohe et al. 2007), with peripheral and renal vascular disease (Wright et al. 2000), after acute myocardial infarction (AMI; Dybdahl et al. 2005; Satoh et al. 2006), or coronary artery bypass grafting (Dybdahl et al. 2002), preeclampsia (Molvarec et al. 2006) and haemolytic anemia elevated liver enzymes low platelet count (HELLP) syndrome (Molvarec et al. 2007). Genth-Zotz et al. (2004) found elevated serum Hsp70 levels in CHF patients and a correlation between the Hsp70 and disease severity and cachexia, but not with survival. One day after AMI, the elevated Hsp70 levels showed a moderate positive correlation with IL-6 and IL-8, with TNF-α and the levels of the Toll like receptor 4, although the association was absent in healthy control subjects. Circulating Hsp70 levels were positively related to markers of myocardial damage including cardiac troponin T and creatine kinase MB (Dybdahl et al. 2005; Satoh et al. 2006). Hsp70 can be released into the extracellular environment not only in response to tissue damage but also under physiologic conditions (Pockley et al. 1998).
HspA1B is one of the human Hsp70 genes (Milner and Campbell 1990); it encodes a heat-inducible protein Hsp70. The gene is polymorphic, potentially accounting for variation in its functions and susceptibility to stress tolerance (Zhou et al. 2005). An adenine (A) to guanine (G) polymorphism at HspA1B +1267 has been described; however, the polymorphism does not lead to any amino acid change in the Hsp70 protein. Carriage of the G allele is associated with increased risks and poor outcomes in different diseases (Balog et al. 2005; Giacconi et al. 2005; Klausz et al. 2005; Giacconi et al. 2006). Results about the effect of the polymorphism on the HspA1B messenger RNA (mRNA) levels are controversial (Pociot et al. 1993; Schroeder et al. 2000; Giacconi et al. 2005), whilst according to our best knowledge, the direct association with the serum Hsp70 level has never been reported.
The source of the serum Hsp70 has not been fully elucidated; however, increasing body of evidences suggest that Hsp70 may be released from viable cells within exosomes, and this mechanism can be induced by certain cytokines (Bausero et al. 2005). According to a recent report, Hsp72 (the inducible form of the Hsp70 family) can be released from the hepatosplanchnic tissue during exercise (Febbraio et al. 2002) or from the myocardium itself (St Rammos et al. 2002), lending credence to the hypothesis that free Hsp70 may also be discharged from necrotised cells (Basu et al. 2000).
The aim of our cross-sectional study was to identify the clinical associations and biological correlates of Hsp70 serum levels in patients with CHF. The influence of HspA1B genotypes on Hsp70 levels and on its associations to clinical data was also investigated.
Methods
Study population
The study was carried out in accordance of the Helsinki Declaration at the IIIrd Department of Internal Medicine, Semmelweis University based on a study protocol approved by the highest Ethical Committee of Hungary. Consecutive patients with clinical suspicion of CHF referred to trans-thoracic echocardiography were considered for inclusion. All patients with <45% left ventricular ejection fraction who provided written informed consent were included independently of the aetiology of the disease from the out- or inpatient cardiology departments. Patients with coexisting malignant or acute infectious conditions were not included. A total of 167 patients (123 men, 44 women) were enrolled between February 2005 and July 2006. The full clinical record of the patients was registered at inclusion with the detailed physical status and routine laboratory tests. Alcohol consumption was regarded regular if it was reported to be daily.
Blood samples were taken after 6 h of fasting between 8 and 10 a.m. by antecubital veinpuncture into native, ethylenediaminetetraacetic acid- or sodium citrate anticoagulated tubes. The samples were processed to obtain genomic DNA, and aliquots of serum and plasma were later stored at −70°C until further analysis.
Echocardiographic measurements
Left ventricular function was determined by two-dimensional echocardiography and Doppler examinations by the same investigator blinded to the results of other tests. The equipment used was a Hewlett-Packard Sonos 2500 echocardiography system with 2.5-MHz transducer. Pulsed, continuous and color-flow Doppler examinations were performed with the same transducer. Parasternal and apical views were obtained with the patient in a left lateral recumbent position. M-mode tracings were recorded on strip chart paper at a speed of 50 mm/s. Left ventricular end diastolic (Dd) and end systolic diameter (Ds) were measured, and the left ventricular ejection fraction (EF) was calculated as described by Quinones (EF = Dd2 − Ds2/Dd2).
Determination of serum soluble Hsp70
Soluble Hsp70 level was measured by using R&D System (USA, Cat No. DYC1663E) enzyme-linked immunosorbent assay (ELISA) kit. For Hsp70 family nomenclature, the suggestions of Tavaria et al. (1996) were used. Ninety-six-well microtitre plates were coated with mouse anti-human Hsp70 capture antibodies (100 μl, 2 μg/ml) in carbonate buffer (pH 9.5) overnight at 4°C. The capture antibody was tested for reactivity against the constitutive (Hsp-8) and inducible forms (Hsp70-1) of Hsp70 family, and two orders of magnitude higher reaction against the inducible form was observed (unpublished). Thus, this sandwich ELISA is specific for the inducible form of Hsp70 family. Plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 three times and non-specific binding sites blocked by incubation with 200 μl of PBS containing 0.5% gelatine and Tween 20 for 1 h at room temperature. After washing, 100 μl of the reference preparation (recombinant human Hsp70, 0–10 ng/ml) or serum samples (undiluted) were added, and the plates were incubated for 2 h at room temperature. Plates were subsequently washed, and Hsp70 binding was determined using biotinylated rabbit anti-human antibodies (100 μl, 0.5 μg/ml) in PBS gelatine. After 1.5 h at room temperature, plates were washed and incubated with streptavidin–horseradish peroxidase (1:200) in PBS gelatine for 20 min at room temperature. Plates were washed, and 100 μl of o-phenylene-diamine (Sigma) in citrate buffer was added. The optical density was measured at λ = 490 nm (reference at λ = 620 nm). The detection range of the assay was 0.05–10 ng/ml, the intra/inter-assay variability <10/<16%, respectively.
Determination of other laboratory parameters
Levels of NT-proBNP (Biomedica ELISA kit; Cat no. BI-20852), serum TNF-alpha (R&D System high sensitivity ELISA kit; Cat no. HSTA00C) and serum IL-6 (R&D System high sensitivity ELISA kit; Cat no. HS600B) were measured according to the manufacturer’s instructions. Standard laboratory parameters were measured by Roche Integra 800 (clinical chemistry, CRP) or by Cell-Dyn 3500 haematology analyser (complete blood count).
The HspA1B gene, also referred as Hsp70-1b or Hsp70-2 (Tavaria et al. 1996), which has a single nucleotide polymorphism A(1267)G, or rs1061581, was genotyped using polymerase chain reaction–restriction fragment length polymorphism technique exactly as described before by Vargas-Alarcon et al. (2002).
Statistical analysis
As most of the variables were not normally distributed, data are presented in the text and in the tables as median (25th–75th percentile) or as number (percent). Non-parametric tests were used for group comparisons; continuous variables between two groups were compared with the Mann–Whitney U test and between multiple groups with the Kruskal–Wallis test, whereas categorical variables were compared with the Pearson’s χ2 test. Spearman rank correlation coefficients were calculated for estimation of interrelations between sHsp70 and other variables. Simple linear regression was calculated between log-transformed sHsp70 and NT-proBNP, LV-EF. Multiple logistic regression analysis was applied to estimate interrelationship between variables as categorical predictors and severity of heart failure. Factorial analysis of variance (ANOVA) was used to analyse interaction effects between diseases severity (NYHA class categories) HspA1B genotypes and serum Hsp70 levels, and Dunnett’s post hoc test was applied. Results of the factorial ANOVA are presented as least squares of means with 95% confidence intervals. Statistical analyses were carried out using the software STATISTICA 7.0 (StatSoft Inc., Tulsa, OK, USA). Two-tailed p values were calculated and the significance level was put at a value of p < 0.05.
Results
Characteristics of the study population
The mean age of the participants was 68.6 (SD 11) years; 50.3% of the patients had a severe condition as assessed by New York Heart Association (NYHA) class III or IV, while 83 (49.7%) of the patients were ranged to the lower two NYHA classes. Nearly 24% of the patients were female. The characteristics of the study population, as stratified according to the severity of the disease, are presented in Table 1. As expected, heart rate, diastolic blood pressure, left ventricular ejection fraction, presence of peripheral oedema and pulmonary congestion, levels of NT-proBNP, serum sodium, carbamide, creatinine, total protein, albumin, C-reactive protein, TNF-alpha and haemoglobin were significantly associated with disease severity.
Table 1.
Characteristics of the study population as stratified according to severity of chronic heart failure
| NYHA I–II (n = 83) | NYHA III–IV (n = 84) | p value | |
|---|---|---|---|
| Male gender (%) | 61 (73.5%) | 68 (81%) | 0.590 |
| Age (years) | 67.14 (58.71–76.01) | 70.25 (63.34–78.0) | 0.147 |
| Heart rate (1/min) | 72 (66–84) | 80 (70–96) | 0.038 |
| Diastolic blood pressure (mmHg) | 80 (70–80) | 70 (63.5–80) | 0.004 |
| Left ventricular EF (%) | 38 (29–42) | 30 (25–38) | <0.001 |
| Periferial oedema | 25 (30.12%) | 45 (53.57%) | 0.003 |
| Pulmonary congestion | 19 (22.89%) | 55 (65.47%) | <0.001 |
| Etiology (ischaemic heart disease) | 43 (51.81%) | 55 (65.47%) | 0.071 |
| BMI (kg/m2) | 26.6 (24.44–30.9) | 26.12 (24.00–32.21) | 0.602 |
| Duration of CHF (years) | 4.23 (2.11–8.26) | 2.35 (0.55–5.82) | 0.029 |
| Consumed packs of tobacco | 11,506 (7,853–15,706) | 10,410 (2922–18308) | 0.489 |
| Regular alcohol consumption | 44 (53%) | 38 (45.2%) | 0.481 |
| Serum sodium (mmol/l) | 141 (139–143) | 139 (136–142) | 0.004 |
| Serum potassium (mmol/l) | 4.42 (4.02–4.80) | 4.38 (4.01–4.88) | 0.888 |
| Serum carbamide (mmol/l) | 7.6 (5.7–10.2) | 9.79 (7.11–16.90) | <0.001 |
| Serum creatinine (μmol/l) | 94 (77–112) | 110 (86–166) | 0.001 |
| Serum bilirubin (μmol/l) | 12.32 (8.90–18.60) | 13.20 (9.20–21.58) | 0.364 |
| AST (U/l) | 23 (19–32) | 22 (18–33) | 0.991 |
| ALT (U/l) | 23 (17–36) | 23 (16–38) | 0.949 |
| Gamma-GT (U/l) | 62 (29–123) | 80 (35–128) | 0.358 |
| Creatine kinase (U/l) | 68 (45–112) | 61.5 (38–105) | 0.532 |
| Serum total protein (g/l) | 73 (69–77) | 70 (62–74.5) | 0.002 |
| Serum albumin (g/l) | 42 (40–44) | 40 (36–42) | <0.001 |
| CRP (mg/l | 5.19 (2.67–10.69) | 7.43 (3.50–18.48) | 0.032 |
| TNF-alpha (pg/ml) | 2.03 (1.36-3.23) | 3.05 (2.08-4.95) | 0.003 |
| IL-6 (pg/ml) n = 138 (83 + 75) | 8.94 (4.79–14.20) | 11.24 (6.97–20.27) | 0.015 |
| White blood cells (109/l) | 6.92 (5.82–8.48) | 7.66 (6.65–8.49) | 0.043 |
| Red blood cells (1012/l) | 4.64 (4.20–4.98) | 4.41 (3.90–4.90) | 0.046 |
| Hemoglobin (g/l) | 144 (134–155) | 137 (121–150) | 0.005 |
| Red cell distribution width (%) | 13.95 (13.2–14.8) | 15 (14–16.2) | <0.001 |
| NT-proBNP (pmol/ml) | 0.604 (0.41–1.26) | 1.593 (0.71–3.05) | <0.001 |
| Soluble Hsp70 (ng/ml) | 0.30 (0.24–0.42) | 0.41 (0.28–0.73) | 0.003 |
| Carriers of HspA1B (1267) G allele | 49 (59%) | 41 (48.8%) | 0.121 |
| HspA1B (1267) allele frequencies (G/A) | 0.365/0.635 | 0.704/0.296 | 0.190 |
P values of Mann–Whitney or chi-square tests are presented. For continuous data median (interquartile range) are shown.
NYHA New York Heart Association, EF ejection fraction, BMI body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, γGT gamma-glutamyl transpeptidase, CRP C-reactive protein, CHF chronic heart failure, TNF tumour necrosis factor, IL-6 interleukin 6, NT-proBNP N-terminal pro-brain natriuretic peptide, Hsp heat shock protein
Association of sHsp70 levels with disease severity and clinical variables
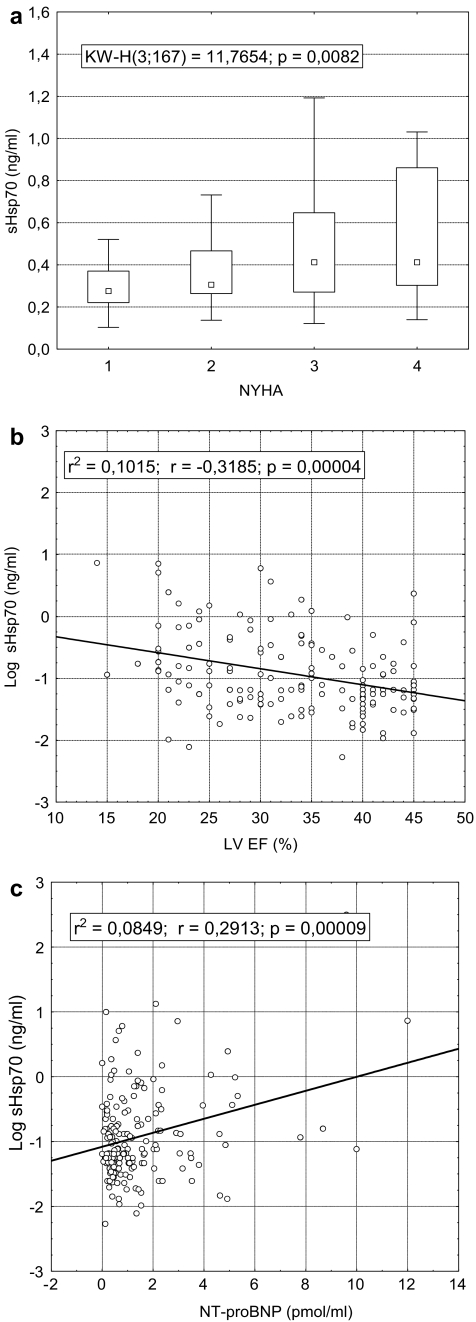
The concentration of soluble Hsp70 was significantly higher in patients with severe CHF (NYHA III–IV) as compared to patients with mild CHF (NYHA I–II, Table 1). At comparison of circulating Hsp70 levels in the four NYHA classes, significant difference was found by Kruskal–Wallis ANOVA by ranks test (p = 0.008; Fig. 1a). According to the results of the post hoc analysis, the increase of sHsp70 levels in NYHA classes III and IV were significant as compared to class I (p < 0.05, Dunnett’s test). In a univariate analysis, patients with sHsp70 level above the 75th percentile (0.557 ng/ml) had an almost 2.5-fold risk to belong to the NYHA III–IV group (OR 2.46, 95% CI 1.18–5.15). After adjustment for age, sex, sodium, creatinine, CRP, total protein, NT-proBNP levels and red blood cell count, the risk still remained significant (OR 2.92, 95% CI 1.21–7.01). As far as NYHA classification is based upon symptoms which might be dependent upon patient and physician bias in some cases, we validated the association of sHsp70 level and disease severity by left ventricular ejection fraction and NT-proBNP. Circulating Hsp70 levels showed significant association; p value was less than 0.0001 for both parameters (Fig. 1b and c).
Fig. 1.
Association of serum Hsp70 levels with NYHA functional classes (a), left ventricular ejection fraction (LV EF) (b) and NT-proBNP (c) in patients with chronic heart failure. Result of Kruskal–Wallis test (data are presented as medians with 25–75% percentiles and non-outlier ranges) and coefficients of linear regression are displayed. Dunnett’s post hoc analysis showed significant difference between Hsp70 levels in NYHA III and IV as compared to NYHA I (p = 0.019 and 0.036, respectively)
Analysing the correlations between sHsp70 levels on one hand and clinical or laboratory variables on the other (Table 2), there were significant correlations between sHsp70 concentrations and markers of liver injury [bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γGT] predominately in patients with severe disease. By contrast, the correlation between sHsp70 levels and markers of anaemia [red cell distribution width, haemoglobin, Table 2, or with haematocrit or red blood cell count (RBC), data not shown] was absent both in the patient populations with severe and mild condition. Most importantly, no correlation with CRP, TNF-alpha or IL-6 levels was observed in any groups analysed, and the same was found in case of serum creatinine (Table 2). There was no relationship between sHsp70 levels and blood pressure, heart rate or the duration of the disease (data not shown).
Table 2.
Correlation between serum Hsp70 levels and clinical and laboratory variables (total n = 167, NYHA I–II n = 83, NYHA III–IV n = 84)
| Variables | r (p value) | |
|---|---|---|
| Total | −0.29 (<0.001) | |
| Left ventricular ejection fraction | NYHA I–II | −0.17 (0.126) |
| NYHA III–IV | −0.27 (0.016) | |
| Total | 0.29 (<0.0001) | |
| Serum bilirubin | NYHA I–II | 0.28 (0.006) |
| NYHA III–IV | 0.30 (0.006) | |
| Total | 0.27 (<0.001) | |
| Aspartate aminotransferase | NYHA I–II | 0.16 (0.122) |
| NYHA III–IV | 0.42 (0.0001) | |
| Total | 0.20 (0.009) | |
| Alanine aminotransferase | NYHA I–II | 0.17 (0.101) |
| NYHA III–IV | 0.29 (0.009) | |
| Total | 0.38 (<0.0001) | |
| Gamma-glutamyl-transpeptidase | NYHA I–II | 0.22 (0.034) |
| NYHA III–IV | 0.42 (0.0001) | |
| Total | 0.01 (0.944) | |
| Serum creatinine | NYHA I–II | −0.17 (0.106) |
| NYHA III–IV | 0.05 (0.647) | |
| Total | 0.06 (0.423) | |
| TNF-alpha | NYHA I–II | −0.07 (0.637) |
| NYHA III–IV | 0.24 (0.073) | |
| Total | 0.13 (0.119) | |
| IL-6 (n = 138) | NYHA I–II | 0.03 (0.792) |
| NYHA III–IV | 0.16 (0.193) | |
| Total | 0.08 (0.303) | |
| Hemoglobin | NYHA I–II | 0.19 (0.071) |
| NYHA III–IV | 0.07 (0.529) | |
| Total | 0.13 (0.090) | |
| Red cell distribution width (%) | NYHA I–II | 0.11 (0.323) |
| NYHA III–IV | 0.04 (0.703) |
Spearman rank correlation coefficients with p values are displayed.
NYHA New York Heart Association, TNF tumour necrosis factor, IL-6 interleukin 6
Genotype specific interaction between sHsp70 levels and disease severity in CHF
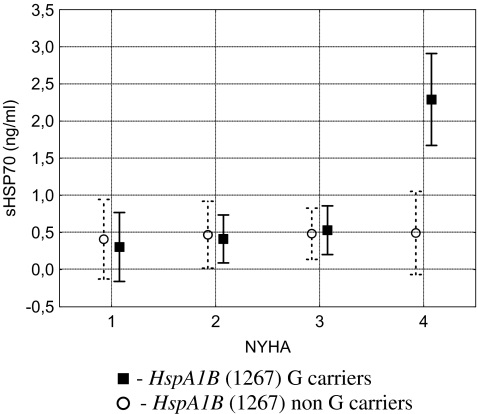
The (1267) A/G polymorphism in the HspA1B gene was found to be in Hardy–Weinberg equilibrium (data not shown). There was no association between HspA1B (1267) alleles and serum Hsp70 levels in the patients (Table 3). As Hsp70 levels were associated with heart function and disease severity, genotype effects were analysed in a factorial ANOVA model accordingly. Results of the allele-specific interaction between serum Hsp70 levels and NYHA classes are presented on Fig. 2. The association of variant allele G of HspA1B (1267) with increased serum Hsp70 levels was present mainly in patients with most severe disease (NYHA class IV, F = 5.42, p = 0.0014, factorial ANOVA). Results of the post hoc analysis (Dunnett’s test) indicated significant increase in sHsp70 levels in NYHA class IV patients as compared to class I (p < 0.0001) only in carriers of allele G of HspA1B.
Table 3.
Serum Hsp70 levels according to HspA1B (1267) alleles
| HspA1B (1267) | p value | ||
|---|---|---|---|
| AA | AG, GG | ||
| n | 69 | 90 | |
| Serum Hsp70 | 0.39 (0.29–0.52) | 0.31 (0.24–0.58) | 0.156 |
| Male gender(%) | 54 (78.3%) | 64 (71.1%) | 0.576 |
| Age | 73.3 (65.4–77.7) | 67 (57.2–77.7) | 0.049 |
| NYHA (I/II/III/IV) | 12/17/29/11 | 16/33/32/9 | 0.344 |
P values of Mann–Whitney and Pearson chi-square tests are displayed.
NYHA New York Heart Association, Hsp heat shock protein
Fig. 2.
HspA1B allele specific interaction between serum Hsp70 levels and disease severity in patients with chronic heart failure. Data are presented as LS means with 95% confidence intervals (vertical bars). Continuous line: HspA1B 1267 AA genotype, dotted line: HspA1B 1267 AG and GG genotypes. Dunnett’s post hoc test for Hsp70 levels between patients with NYHA I and non-HspA1B (1257) G carriers as compared to NYHA IV G carrier patients, highly significant (p < 0.0001) difference was found
Discussion
In the present study, two novel observations are reported. First, increased serum Hsp70 levels were observed in patients with severe heart failure (as reflected by NYHA III and IV classes or increased NT-proBNP levels/decreased LVEF values, respectively) as compared to patients with mild disease. The association of high sHsp70 levels with disease severity was influenced by the presence of HspA1B (1267) allele G. Second, the detailed characterisation of the patient population allowed us to identify significant correlations between sHsp70 levels and markers of heart function and hepatic injury. There was, however, no relationship between sHsp70 and the prototype acute phase reactant, C-reactive protein and the inflammatory cytokines TNF-alpha and IL-6.
There are only two studies known in the literature on serum Hsp70 levels in CHF. Our observations completely corroborate the results of Genth-Zotz et al. (2004), inasmuch as the concentration of Hsp70 was related to disease severity independently from the aetiology of CHF and did not show any association with TNF-α. In our study group, Hsp70 levels significantly correlated to left ventricular ejection fraction and NT-proBNP, indicating a direct relationship between worsening heart function and increased serum stress protein levels. The same associations were found by Giannessi et al. (2007). The increasing serum concentration of Hsp70 with the severity of the disease seemed to be independent of the condition of the kidneys and the liver, the anaemia and the inflammation, proven by a logistic regression model. Patient groups divided according to the NYHA classes (I–II vs III–IV) were compared using a logistic regression adjusted for the following nine variables: age, sex, creatinine, total protein, CRP, AST, RBC, NT-proBNP, and sodium levels. The risk to belong to the more severe heart failure group was more than two and a half times higher for those having high (>75% percentile, 0.557 ng/ml) serum Hsp70 levels as compared to those with low levels. This association was independent from the above variables. Furthermore, lack of significant correlation was observed between sHsp70 and serum creatinine, indicating that the elevation of sHsp70 parallel with the disease severity is not due to the impaired renal clearance of Hsp70.
There is a growing body of evidence that the 1267 polymorphism of the HspA1B gene associate with severity of different diseases, like, for example, Crohn’s disease (Klausz et al. 2005) and acute pancreatitis (Balog et al. 2005). The background of the associations is unclear. Our study is the first, however, investigating association between HspA1B genotypes and serum sHsp70 levels. In our CHF patient population, the frequency of the G allele did not differ between the severe and mild patient groups, but an increased serum Hsp70 level was found in carriers of allele G in patients with most severe disease. Heat shock proteins are primarily known to be intracellular proteins, but it is unknown whether serum Hsp70 concentration reflects intracellular concentration. Our study indicates an important interaction effect between disease severity, HspA1B genotypes and serum Hsp70 levels in patients with chronic heart failure. Carriage of allele G of HspA1B predisposes the affected population to have high serum Hsp70 levels if a severe stage hypoperfusion and ischaemia is present.
There are two mutually not exclusive explanations for our observations. HspA1B allele G might be associated with TNF2 allele (Chouchane et al. 1997; Mestiri et al. 2001; Galley and Lowe 2003), resulting in higher TNF response after inflammatory stimuli. This pronounced inflammatory response might result in higher level of tissue injury, resulting in the release of sHsp70. According to our unpublished results, the polymorphism of the TNF-308 and HspA1B (1267) are indeed connected (Pearson chi test p = 0.014), although TNF-308 is not directly associated with soluble Hsp70 level (Kruskal–Wallis test p = 0.436). However, the lack of relationship between HspA1B variant allele and TNF levels or the lack of correlation between sHsp70 levels and CRP and TNF-alpha do not support this possibility. Secondly, a direct influence through association with specific mRNA levels cannot be excluded; however, current reports are controversial whether HspA1B alleles are associated with increased [non-insulin-dependent diabetes mellitus (NIDDM) patients with atherosclerosis, Giacconi et al. 2005], decreased (NIDDM patients, Pociot et al. 1993) or unaltered (healthy volunteers, Schroeder et al. 2000) HspA1B mRNA levels after stimuli. Nevertheless, sHsp70 levels might also reflect intracellular Hsp70 concentrations especially if tissue damage is present, and this hypothesis is fueled by our observations on the correlation between markers of hepatopathy and tissue damage and sHsp70 levels. It is early to draw conclusions based on currently available data. Thus, further functional genetic studies are needed in the clinical setting to clear this question in more depth.
The source of circulating Hsps in healthy subjects, as well as in patients with pathological conditions, has not been determined yet. In CHF, the constant hypoxia and hypoperfusion is known to result in a systemic disease with failure of multiple organs. We aimed to describe biological correlates of sHsp70 and to characterise the source of sHsp70. Serum Hsp70 level correlated significantly with AST, ALT, γGT and serum bilirubin, suggesting that the source of Hsp70 may be the liver. On the contrary, there was no relationship between serum Hsp70 levels and markers of inflammation in any of the patients groups. These observations on the relationship between hepatopathy and increased sHsp70 levels are in concordance with our previous results (Molvarec et al. 2007; Madách et al., submitted) and with the group of Febbraio (Febbraio et al. 2002). In these studies, serum sHsp70 levels were found to strongly correlate with laboratory markers of tissue injury in HELLP syndrome and after strong exercise, but not with inflammatory markers.
Independently from the mechanism on how heat shock proteins are released into the circulation, Hsp70 might exert pro-inflammatory stimulus by the activation of the monocytes, leading to production of proinflammatory cytokines (IL-1β, IL-6, TNF-α; Asea et al. 2000), and by complement activation (Prohaszka et al. 2002). It is conceivable that Hsp70 can represent a protective mechanism as well. In animal models, overexpression of Hsp70 protects the heart against damaging effects of ischaemia (Trost et al. 1998) or against stress-induced injury by inhibiting Fas-mediated apoptosis of cardiomyocytes (Zhao et al. 2007).
In summary, according to our results, Hsp70 level in the circulation of CHF patients is related to disease severity, and it is associated with HspA1B polymorphism. Hsp70 levels show significant correlations with markers of hepatic injury markedly in patients with severe disease. However, it is still unclear whether Hsp70 is just a marker of the severity of CHF or plays a pathophysiological role in the progression of the disease.
Acknowledgements
This work was supported by the following grants: Hungarian Scientific Research Fund (OTKA T046837) and Ministry of Health (ETT 229/2006). The skilful technical assistance of Szigeti Antalné, Margit Kovács, Holeczky Rudolfné and Piroska Sturmann and the helpful suggestions of Katalin Udvarnoki regarding the sHsp70 assay are acknowledged with many thanks.
Abbreviations
- CHF
chronic heart failure
- Hsp
heat shock protein
- AMI
acute myocardial infarction
- HELLP syndrome
haemolytic anemia elevated liver enzymes low platelet count
- TNF
tumour necrosis factor
- Il-6
interleukin-6
- EF
ejection fraction
- BMI
body mass index
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- NYHA
New York Heart Association
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- γGT
gamma-glutamyl transpeptidase
- CRP
C-reactive protein
- RBC
red blood cell count
- NIDDM
non-insulin-dependent diabetes mellitus
References
- Asea A, Kraeft SK, Kurt-Jones EA et al (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442 [DOI] [PubMed]
- Balog A, Gyulai Z, Boros LG, Farkas G, Takacs T, Lonovics J, Mandi Y (2005) Polymorphism of the TNF-alpha, HSP70-2, and CD14 genes increases susceptibility to severe acute pancreatitis. Pancreas 30:e46–e50 [DOI] [PubMed]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546 [DOI] [PubMed]
- Bausero MA, Gastpar R, Multhoff G, Asea A (2005) Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol 175:2900–2912 [DOI] [PMC free article] [PubMed]
- Chouchane L, Ahmed SB, Baccouche S, Remadi S (1997) Polymorphism in the tumor necrosis factor-alpha promotor region and in the heat shock protein 70 genes associated with malignant tumors. Cancer 80:1489–1496 [DOI] [PubMed]
- Dhingra R, Larson MG, Benjamin EJ et al (2006) Cross-sectional correlates of serum heat shock protein 70 in the community. Am J Hypertens 19:227–231, discussion 232–223 [DOI] [PubMed]
- Dybdahl B, Wahba A, Lien E et al (2002) Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 105:685–690 [DOI] [PubMed]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A (2005) Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart 91:299–304 [DOI] [PMC free article] [PubMed]
- Febbraio MA, Ott P, Nielsen HB et al (2002) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544:957–962 [DOI] [PMC free article] [PubMed]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC (2005) Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med 26:552–557 [DOI] [PubMed]
- Flohe SB, Bangen JM, Flohe S, Agrawal H, Bergmann K, Schade FU (2007) Origin of immunomodulation after soft tissue trauma: potential involvement of extracellular heat-shock proteins. Shock 27:494–502 [DOI] [PubMed]
- Galley HF, Lowe PR (2003) Fishing in the gene pool. Crit Care Med 31:317–318 [DOI] [PubMed]
- Genth-Zotz S, Bolger AP, Kalra PR et al (2004) Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol 96:397–401 [DOI] [PubMed]
- Giacconi R, Caruso C, Lio D et al (2005) 1267 HSP70-2 polymorphism as a risk factor for carotid plaque rupture and cerebral ischaemia in old type 2 diabetes-atherosclerotic patients. Mech Ageing Dev 126:866–873 [DOI] [PubMed]
- Giacconi R, Cipriano C, Muti E et al (2006) Involvement of -308 TNF-alpha and 1267 Hsp70-2 polymorphisms and zinc status in the susceptibility of coronary artery disease (CAD) in old patients. Biogerontology 7:347–356 [DOI] [PubMed]
- Giannessi D, Colotti C, Maltinti M et al (2007) Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 12:265–274 [DOI] [PMC free article] [PubMed]
- Kannel WB (2000) Incidence and epidemiology of heart failure. Heart Fail Rev 5:167–173 [DOI] [PubMed]
- Klausz G, Molnar T, Nagy F, Gyulai Z, Boda K, Lonovics J, Mandi Y (2005) Polymorphism of the heat-shock protein gene Hsp70-2, but not polymorphisms of the IL-10 and CD14 genes, is associated with the outcome of Crohn’s disease. Scand J Gastroenterol 40:1197–1204 [DOI] [PubMed]
- Knowlton AA, Eberli FR, Brecher P, Romo GM, Owen A, Apstein CS (1991) A single myocardial stretch or decreased systolic fiber shortening stimulates the expression of heat shock protein 70 in the isolated, erythrocyte-perfused rabbit heart. J Clin Invest 88:2018–2025 [DOI] [PMC free article] [PubMed]
- Mestiri S, Bouaouina N, Ahmed SB, Khedhaier A, Jrad BB, Remadi S, Chouchane L (2001) Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein hsp70-2: susceptibility and prognostic implications in breast carcinoma. Cancer 91:672–678 [DOI] [PubMed]
- Milner CM, Campbell RD (1990) Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics 32:242–251 [DOI] [PubMed]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J Jr (2006) Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens 20:780–786 [DOI] [PubMed]
- Molvarec A, Prohaszka Z, Nagy B et al (2007) Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol 73:172–179 [DOI] [PubMed]
- Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC (2002) Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 52:611–617, discussion 617 [DOI] [PubMed]
- Pociot F, Ronningen KS, Nerup J (1993) Polymorphic analysis of the human MHC-linked heat shock protein 70 (HSP70-2) and HSP70-Hom genes in insulin-dependent diabetes mellitus (IDDM). Scand J Immunol 38:491–495 [DOI] [PubMed]
- Pockley AG, Shepherd J, Corton JM (1998) Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest 27:367–377 [DOI] [PubMed]
- Prohaszka Z, Fust G (2004) Immunological aspects of heat-shock proteins—the optimum stress of life. Mol Immunol 41:29–44 [DOI] [PubMed]
- Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G (2002) Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones 7:17–22 [DOI] [PMC free article] [PubMed]
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M (2006) Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail 8:810–815 [DOI] [PubMed]
- Schroeder S, Reck M, Lehmann LE, Book M, Hoeft A, Stuber F (2000) The Pstl polymorphism of the endotoxin-inducible heat-shock protein 70-2 gene does not affect messenger RNA level in human whole-blood cultures. Intensive Care Med 26:1139–1143 [DOI] [PubMed]
- Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ (2001) More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 3:315–322 [DOI] [PubMed]
- Stewart S, MacIntyre K, Capewell S, McMurray JJ (2003) Heart failure and the aging population: an increasing burden in the 21st century? Heart 89:49–53 [DOI] [PMC free article] [PubMed]
- St Rammos K, Koullias GJ, Hassan MO, Argyrakis NP, Voucharas CG, Scarupa SJ, Cowte TG (2002) Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc Surg 10:228–232 [DOI] [PubMed]
- Tavaria M, Gabriele T, Kola I, Anderson RL (1996) A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones 1:23–28 [DOI] [PMC free article] [PubMed]
- Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH (1998) Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest 101:855–862 [DOI] [PMC free article] [PubMed]
- Vargas-Alarcon G, Londono JD, Hernandez-Pacheco G et al (2002) Heat shock protein 70 gene polymorphisms in Mexican patients with spondyloarthropathies. Ann Rheum Dis 61:48–51 [DOI] [PMC free article] [PubMed]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG (2000) Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels 15:18–22 [DOI] [PubMed]
- Zhao Y, Wang W, Qian L (2007) Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones 12:83–95 [DOI] [PMC free article] [PubMed]
- Zhou F, Wang F, Li F et al (2005) Association of hsp70-2 and hsp-hom gene polymorphisms with risk of acute high-altitude illness in a Chinese population. Cell Stress Chaperones 10:349–356 [DOI] [PMC free article] [PubMed]