Abstract
In superior cervical ganglion (SCG) neurons calcium-induced calcium release (CICR), mediated by ryanodine receptors (RyRs), contributes to stimulation-evoked intracellular calcium ([Ca2+]i) transients. Hypothesis: The contribution of CICR to electrical field stimulation (EFS)–evoked [Ca2+]i transients in SCG cells declines with senescence and may be partially recovered in the presence of caffeine. We measured EFS-evoked [Ca2+]i transients in isolated fura-2–loaded SCG cells from Fischer-344 rats aged 6, 12, and 24 months with either the RyR antagonist ryanodine to block the contribution of CICR to [Ca2+]i transients or caffeine to sensitize CICR to EFS. EFS-evoked [Ca2+]i transients increased from 6 to 12 months and declined at 24 months and ryanodine decreased [Ca2+]i transients in SCG cells from 6- and 12-month-old animals only. Caffeine significantly increased EFS-evoked [Ca2+]i transients in all age groups. These data suggest that CICR declines with senescence and residual CICR function may be reclaimed in senescent cells with caffeine.
Keywords: Ryanodine receptors, Aging and calcium release, Aging and function of superior cervical ganglia
AN emerging body of data suggests that normal aging of physiological systems is a subtle alteration in function that cannot be attributed to pathology (1). However, the remodeling of a physiological system with age correlates with an initiation of certain pathological processes. Thus, a more complete understanding of the process of “normal” aging could lead to the clarification of the processes that precede degenerative disease. During aging the maintenance of constant cerebral blood flow (CBF) in the face of rising systolic blood pressure (SBP) may likely result from a combination of compensatory mechanisms. However, as the descent into senescence continues, these mechanisms may no longer keep CBF within a normal range, which in time will result in pathological consequences such as stroke.
CBF is primarily governed by myogenic autoregulation of cerebral blood vessels at a SBP between 60 and 140 mm Hg (2,3). Above 140 mmHg, activation of sympathetic nerves arising from the superior cervical ganglion (SCG) provides an additional protective vasoconstrictor response mediated in part by norepinephrine in cerebral blood vessels (2,4–6). Overall, the evidence suggests that cerebral blood vessels and neuronal innervation function as a “unit” which helps to modulate CBF within the bounds of normal and pathophysiology (6).
The risk of hemorrhagic stroke increases with age, and the single most important factor is a rising SBP that occurs in both the rat model and in humans (2,3,7–12). Thus, in the face of an age-related rising SBP, proper function of the cerebral neurovascular unit is important to moderate stroke risk (4–6,13).
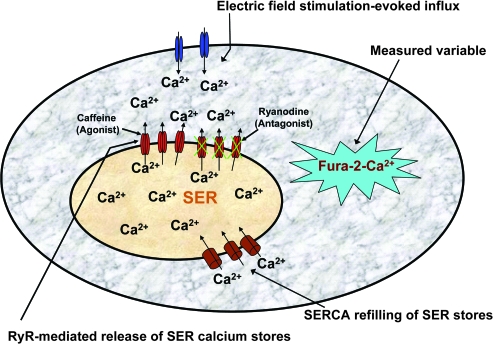
Calcium signaling in neurons conveys numerous signals including neurotransmitter release, gene expression, plasticity, and cell death (14–22). The release of neurotransmitters from neurons and in particular those of the SCG is initiated with opening of voltage-gated calcium channels. Much of this signal is damped by calcium-buffering proteins (23–25). However, calcium signaling is sustained and amplified by calcium-induced calcium release (CICR), through the activation of channels called ryanodine receptors (RyRs) located on the smooth endoplasmic reticulum (SER) (Figure 1) (26–34). In addition, CICR can be blocked with RyR antagonists such as ryanodine (27) and “sensitized” with agonists such as caffeine, which lowers the threshold of [Ca2+]i necessary to evoke CICR and increases its contribution to stimulation-evoked [Ca2+]i transients (33,34).
Figure 1.
Model of the superior cervical ganglion (SCG) cell and illustrated protocol used in this study. In SCG cells, calcium transients are modulated by numerous mechanisms including influx via voltage-gated calcium channels, calcium-induced calcium release (CICR), and transport of calcium by smooth endoplasmic reticulum Ca2+-ATPases, which both buffers [Ca2+]i transients and refills smooth endoplasmic reticulum (SER) calcium stores to maintain regenerative CICR. CICR is mediated via ryanodine receptors, which can be inhibited or activated using ryanodine or caffeine, respectively (see Methods). In this study, electrical field stimulation was used to evoke calcium influx, which activates CICR from SER calcium stores. Influx and CICR contribute to the magnitude of the measured variable, [Ca2+]i as detected by fura-2 fluorescence.
As RyRs appear to be the primary mediators of CICR in the rat SCG, pharmacological blockade or sensitization of these channels in combination with the measurement of stimulation-evoked [Ca2+]i transients may reveal the functional impact of the RyRs on calcium dynamics with advancing age (Figure 1). In this study we measured electrical field stimulation (EFS)–evoked [Ca2+]i dynamics in isolated SCG cells from Fischer-344 (F-344) rats aged 6, 12, and 24 months in the absence and presence of the RyR antagonist ryanodine. In addition, we utilized the RyR agonist caffeine to sensitize the CICR process to EFS at low frequency. We tested the hypothesis that the contribution of CICR to EFS-evoked [Ca2+]i transients in SCG cells increases at middle age and declines with senescence and that CICR may be partially recovered in senescent cells in the presence of the RyR agonist caffeine.
METHODS
Experimental Animals
All procedures used in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University, and the approved guidelines were adhered to throughout the study. Animals were allowed to eat and drink at will and were maintained on a 12:12-hour light–dark cycle under controlled temperature (72–77°F). Male F-344 rats aged 6 months (young adult), 12 months (middle age), and 24 months (senescent) were obtained from the National Institutes of Health–National Institute on Aging breeding colony (Harlan–Sprague–Dawley, Indianapolis, IN). This age range was chosen as the median life span of the F-344 rat is 24 months and represents the aging process from maturity to senescence providing a clearer overview of how advancing age affects the parameters of interest (35).
Superior Cervical Ganglion Preparation
Rats were anaesthetized with CO2 (45 seconds) followed by decapitation. The dissection of the superior cervical ganglia and preparation of isolated cells have been previously described (25,36,37). SCGs were dissected from the carotid artery bifurcation and promptly placed in sterile cold Tyrode's solution, which contained (in mM) 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES), and 10 glucose. The ganglia were then acutely dissociated in 5 mL of Earle's balanced salt solution containing trypsin (6000 U/mL), collagenase D (1 mg/mL), DNAse-1 type IV (0.1 mg/mL), HEPES (20 mM), glucose (10 mM), and NaHCO3 (10 mM) and adjusted to pH 7.4 with NaOH (1 M). After incubation in a shaking water bath for 45 minutes at 34°C, the digestion reaction was stopped by the addition of 5 mL of modified Hank's balanced salt solution (HBSS) with 10% fetal calf serum, 1.3 mM CaCl2, and 5 mM HEPES and adjusted to pH 7.4 with NaOH (1 M). Dissociated cells were centrifuged at 60 g for 5 minutes and resuspended in 5 mL of fresh HBSS. Cells were centrifuged again at 60 g for 5 minutes and redispersed in 5 mL of HBSS (containing 10% fetal calf serum and 5 mM HEPES adjusted to pH 7.4 with NaOH [1 M]). Cells were centrifuged again, and HBSS was decanted to 1 mL. The cells were dispersed in sterile Pasteur pipettes, and 0.5 mL of the dispersed cells was placed onto Cell-Tak (3.5 mg/cm2; BD Biosciences, Bedford, MA)-coated glass coverslips with a Sylgard adhesive (Dow Corning, Inc, Midland, MI) attached a oval 2-cm ring. The coverslips were placed in 3.5-cm Petri dishes, and placed between the three metal prongs on a Beckman S4180 rotor and centrifuged for an additional 5 minutes at 60 g, which increases the number of cells that adhere to the plates. Cells were then incubated for 12–14 hours at room temperature overnight in the fresh HBSS medium containing 10% fetal calf serum.
Cell Health and Measurement of Intracellular Calcium
We have measured [Ca2+]i in isolated SCG cells in young and aged animals for more than 15 years (38). The protocol for cell isolation produces healthy cells in all age groups, and this has been determined by lack of uptake of trypan blue, stable baseline [Ca2+]i, and uniform loading of fura-2 in SCG cells from all age groups (25,36,37). In the present study healthy cells are likely to have robust esterase activity, and if there are no age-related differences in esterase activity, we would anticipate uniform loading of fura-2 in cells from all age groups. To assess this possibility, the intensity of the 380-nm fluorescence signal is proportional to the amount of loaded dye (39). In the present study the 380-nm fluorescent signals in resting cells are 153.48 ± 10.19, 159.04 ± 10.80, 159.99 ± 10.58 and are not significantly different in 6-, 12-, and 24-month-old animals, respectively. These data are consistent with our past studies (25,36,37), suggesting that the cells chosen for observation represent healthy cells in all age groups and suggest that the isolation method does not compromise the data.
Our methods of measuring [Ca2+]i have been previously described (25,36,37). SCG cells were loaded with 10 μM fura-2 acetoxymethylester (fura-2/AM) for 20 min at room temperature, then washed with Tyrode's buffer containing (in mM) 138 NaCl, 2 CaCl2, 1 MgCl2, 5 KCl, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH (1 M). Incubation was continued for an additional 20 minutes to allow intracellular esterases to convert the fura-2/AM dye into the free acid form (40,41).
Coverslips were mounted into a superfusion chamber attached to the stage of a Nikon inverted microscope (Nikon Instruments, Tokyo, Japan). The microscope was attached to a Universal Imaging System running MetaFluor version 6.2 (Universal Imaging Corporation, a subsidiary of Molecular Devices, West Chester, PA). The perfusion system allowed the chamber volume (∼250 μL) to be exchanged at the rate of two times per second (500 μL/s). The fura-2 probe was illuminated by a xenon lamp and the fluorescence was excited alternately at wavelengths 340 and 380 nm by a Lambda DG-4 hyperswitch (Sutter Instruments, Novato, CA), and a Photometric Cool Snap 12-bit digital camera (Roper Scientific, Tucson, AZ) measured the emission fluorescence at 510 nm. Adjusting the microscope stage where no cells were in the field of view corrected for background light levels. Before loading fura-2/AM, cellular autofluorescence was examined in SCG cells as previously described (25) and was below the limits of detection by our imaging system. During the experiment, background fluorescence was subtracted, the 340- and 380-nm fluorometric signals were collected and the [Ca2+]i was calculated and logged to a Dynamic Data Exchange Excel file at a rate of ∼3 Hz. Ambient light levels were minimized, and SCG cells were only illuminated during data acquisition to minimize photobleaching of the dye.
Intracellular calcium was estimated by both in vitro and in vivo calibration methods as we have previously reported (25,36,37). We used the experimental fluorescent intensity ratios (R) to [Ca2+]i over the physiological range by iterative fit to the equation: [Ca2+]i = Kd [R − Rmin]/[Rmax − R] Sf. Rmin is the 340/380 ratio at zero [Ca2+] and Rmax is the 340/380 ratio at 40 μM [Ca2+]. Kd is the dissociation constant of fura-2, whereas Sf is a correction factor representing the ratio Fmin/Fmax, where Fmin is the emission intensity at 380 nm when fura-2 is in the free form (0 [Ca2+]), Fmax is the emission intensity at 380 nm when fura-2 is in the bound form (40 μM [Ca2+]) (42). We routinely carry out calibrations and have kept a running average of the Rmin, Rmax, Sf, and Kd values for fura-2 for more than 15 years. For this report we used multiple calibrations, and the [Ca2+]i was estimated using averaged in vitro values for Sf (15.6), Rmin (0.286), Rmax (5.33), and Kd (266 nM). We applied the same Kd for both young and old SCG neurons, because previous reports have shown no significant change in fura-2 Kd values for young and old neurons (43). In addition, the in vitro calibration for Kd obtained in these studies is comparable with the in vivo values in other neuronal cell models (40,43).
Electric Field Stimulation Apparatus
Electric field stimulation (EFS) is a method utilizing the delivery of graded stimuli to individual cells. This method renders an advantage over the use of various nonphysiological chemicals or buffers containing nonphysiological concentrations of ions such as K+ (eg, 50 mM K+). Although these methods induce relatively intense [Ca2+]i transients, EFS more approximates the behavior of cells in normal physiological buffers (41,44). EFS was delivered to isolated cells through parallel platinum electrodes as previously described via a Grass S48 Stimulator (Grass Medical Instruments, Quincy, MA) (41,44). A 10-ohm resistor was placed in series with the platinum electrodes, and the delivered current was monitored as the voltage drop across the resistor with a Tektronix 5103N oscilloscope (Tektronix, Wilsonville, OR). The current was ramped from a minimum of 50 mA to a maximum of 325 mA (Protocol 1) in the absence and presence of 100 μM of the RyR antagonist, 9,21-dehydro-ryanodine (Calbiochem, a brand of EMD Biosciences, La Jolla, CA), to analyze blockade of Ca2+ release from SER stores. In a separate protocol (Protocol 2) we fixed the current at 300 mA and delivered 24 pulses of EFS at 3 Hz in the absence and presence of the RyR agonist, caffeine.
Protocol 1: Progressive current ramp stimulation in the presence and absence of 100 μM ryanodine blockade.—
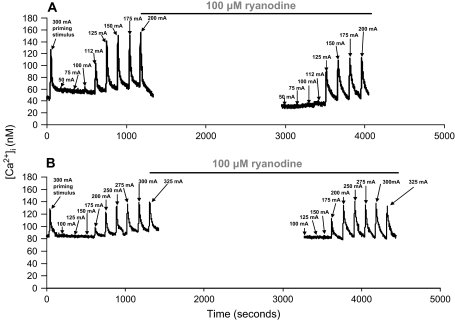
Figure 2 shows representative data in a single SCG cell from 6- and 24-month-old animals demonstrating the response to progressive ramping of EFS current before and after the addition of 100 μM 9,21-dehydro-ryanodine. Graded EFS-produced transients were on average threefold greater than baseline. These data are consistent with the magnitude of EFS-evoked [Ca2+]i transients seen in other studies in isolated hippocampal and sensory neurones (41, 44). Ryanodine is a well-studied and effective molecular tool to measure the contribution of CICR on stimulation-evoked [Ca2+]i transients in numerous neuronal models due to its selectivity for the RyRs (26,27,29,45–47). Studies have demonstrated this selectivity showing that micromolar concentrations of ryanodine do not appear to alter the function of plasma membrane ion channels (33). In addition, ryanodine has complex actions on the RyR, where nanomolar concentrations activate the channel, locking it into an open low-conductance state, whereas micromolar concentrations block the channel (27,48,49). We therefore initially performed dose–response experiments and found that 100 μM ryanodine maximally blocked the RyR. The incubation time necessary to see the full effects of ryanodine is inversely proportional to the concentration. Thus, incubation times vary widely from 5 to 60 minutes depending on the drug concentration (60 minutes at 1 μM to 10 minutes at 500 μM) and cell type (48,49). In our hands, a 30-minute incubation resulted in maximal inhibitory effects of 100 μM ryanodine on EFS-evoked [Ca2+]i transients. Initially, 50 pulses (5 Hz, 10 seconds, 300 mA) of a supermaximal priming stimulus were applied followed by a 2-minute equilibration period to ensure uniform loading of SER Ca2+ stores (47). For the control stimulations, 50-pulse trains at 5 Hz were delivered at currents ranging from 50 to 325 mA with a 2-minute equilibration period separating each stimulation. During the last control supermaximal stimulation, Tyrode's buffer containing 100 μM ryanodine was rapidly superfused onto the cells and left to incubate for 30 minutes. The addition of ryanodine during the last simulation was to ensure maximal blockade of the receptors, as ryanodine binding to RyR is dependent on receptor activity, which would be greatest during cell stimulation (21,50). Following the addition of ryanodine, the cells were stimulated using the same pulse-train parameters. Time-dependent controls were also performed to ensure that the observed effects of ryanodine were not simply due to rundown of EFS-evoked [Ca2+]i transients. Rundown was estimated by comparing each [Ca2+]i transient in control stimulations (S1), followed by a 30-minute time interval, and stimulations were repeated (S2). The ratio of S2/S1 was compared and this did not vary by more than 10%, demonstrating that the effect of ryanodine on stimulation-evoked [Ca2+]i transients was not due to a significant rundown in any of the age groups (data not shown).
Figure 2.
Electrical field stimulation (EFS) evokes ryanodine-sensitive calcium responses in rat superior cervical ganglion (SCG) cells. Representative data in a single SCG cell from a (A) 6-month-old and (B) 24-month-old animal. Fifty pulses of EFS (5 Hz) were delivered at varied currents with a 2-minute equilibration separating each stimulation. Following the last stimulation, cells were exposed to Tyrode's buffer containing 100 μM of the ryanodine receptor antagonist ryanodine for 30 minutes, and the stimulation protocol was repeated (see Methods).
Protocol 2: Aging and sensitization of CICR to EFS in the presence of caffeine.—
This protocol was developed on the basis that the contribution of CICR to EFS-evoked [Ca2+]i transients at low frequencies can be enhanced in the presence of caffeine (33,34). With this protocol we tested the hypothesis that CICR is enhanced with caffeine and can be partially recovered in senescent cells. As in Protocol 1, a priming supermaximal stimulus (50 pulses, 10 seconds, 5 Hz, 300 mA) was delivered to the cells to load SER calcium stores. Next the frequency was reduced to 3 Hz, and 24 pulses (300 mA) were delivered to the cells as a control stimulus. Twenty-four pulses produced the maximal [Ca2+]i transient at 3 Hz in all age groups, and this was empirically determined by ramping the delivered number of pulses from 3 to 30 (data not shown). Following the control stimulation, cells were allowed to equilibrate for 10 minutes and then were exposed to Tyrode's solution containing 5 mM caffeine. The cells were then allowed to incubate in the presence of caffeine for 10 minutes to sensitize RyRs. While still in the presence of 5 mM caffeine, a train of 24 pulses at 3 Hz was again delivered to the cells.
Data Analysis
In Protocol 1, calcium transients were analyzed by using Origin 6.1 software (Origin Lab Inc., Northamptom, MA) in all age groups. Peak [Ca2+]i was determined by subtracting basal [Ca2+]i from maximum stimulation–evoked [Ca2+]i for all transients. The rate of rise of [Ca2+]i was determined by linear fit (r = .99) from basal [Ca2+]i to the maximum stimulation–evoked [Ca2+]i for all transients. The IC50 current data (one-half maximal delta [Ca2+]i) was calculated using Origin 6.1 software by plotting peak [Ca2+]i versus stimulus current with a best sigmoid fit (r = .99). In Protocol 2, peak [Ca2+]i transients were analyzed as in Protocol 1.
Statistics
The impact of age on all measured parameters was determined by analysis of variance and Fischer-PLSD. Because each group of cells acted as their own control, the effects of drug treatments on measured [Ca2+]i parameters within each group were compared using a paired t test. Statistical analysis was done using StatView 5.0 software (Abacus Concepts, Berkeley, CA).
RESULTS
Aging and Effect of Blockade of RyRs on EFS-Evoked [Ca2+]i Transients
EFS-evoked [Ca2+]i transients with changes in applied current followed a graded fashion over the current range utilized (Figure 2). The small changes in [Ca2+]i at lower currents generally reflect calcium influx, and the larger and more abrupt increases in [Ca2+]i shown between 112 and 125 mA reflect the contribution of CICR to EFS-evoked [Ca2+]i transients (33,34,44). In Figure 2, we show representative data to demonstrate the effect of the RyR antagonist ryanodine on EFS-evoked [Ca2+]i transients in an SCG cell from a 6- and 24-month-old animal. Ryanodine addition decreases the magnitude of EFS-evoked [Ca2+]i transients at all applied currents in 6-month-old cells with a complete lack of effect at 24 months. Furthermore, the maximal [Ca2+]i transient is reached at lower currents as compared with 24 months. In cells from 6-month-old animals, although inhibition of CICR decreased the magnitude of EFS-evoked [Ca2+]i transients, the transients broadened, suggesting that these cells recovered more slowly. This finding is consistent with other studies in isolated sympathetic neurons demonstrating that inhibition of CICR with ryanodine slows the rate of repolarization and the rate of recovery of subsequent [Ca2+]i transients (51). In that study CICR was shown to activate calcium-dependent K+ channels, which in turn modulated the rate of recovery from depolarization and the rate of recovery of the subsequent [Ca2+]i transient. Taken together, the current and past studies suggest that CICR modulates the shape of [Ca2+]i transients by contributing to both the magnitude and recovery dynamics of EFS-evoked [Ca2+]i transients.
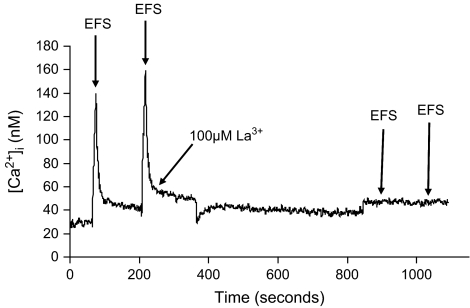
Numerous studies suggest that CICR is initiated by stimulation-evoked calcium influx due to the activation of voltage-gated calcium channels (26–31,33,34). This was assessed, as a control, by stimulating cells with EFS (50 pulses, 5 Hz, 300 mA) in the absence and presence of lanthanum (La3+). Figure 3 shows representative data in an SCG cell from a 6-month-old animal with consecutive EFS-evoked [Ca2+]i transients in the absence of La3+, and then following a 10-minute incubation in Tyrode's buffer containing 100 μM La3+ to block all calcium influx. We utilized three different concentrations of La3+ (50, 100, and 150 μM) and chose 100 μM La3+ as the maximal concentration as there was no significant difference (p > .05) in the lack of EFS-evoked [Ca2+]i response between 100 and 150 μM. In the presence of La3+, EFS did not evoke a [Ca2+]i transient. These data suggest that calcium influx is necessary to activate CICR and that release of calcium from the SER is not occurring by some other mechanism.
Figure 3.
Dependence of electrical field stimulation (EFS)–induced calcium responses on extracellular calcium influx. These data serve as a control to demonstrate that in superior cervical ganglion (SCG) cells, all responses to EFS are lost when calcium influx is blocked with lanthanum (La3+). SCG cells were activated with EFS (50 pulses, 5 Hz, 300 mA) in the absence and presence of 100 μM La3+. Following two consecutive EFS-evoked [Ca2+]i transients, the cells were incubated with 100 μM La3+ for 10 minutes to block all calcium influx. When EFS was reapplied, simulation-evoked [Ca2+]i transients were not observed. Representative data from a group of seven cells from a 6-month-old animal.
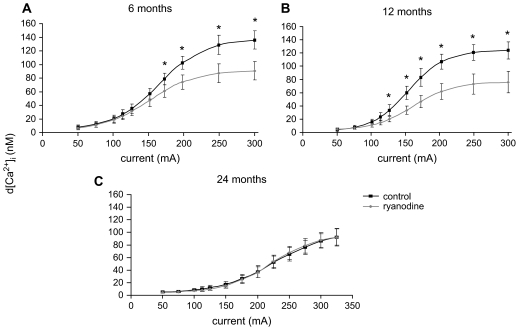
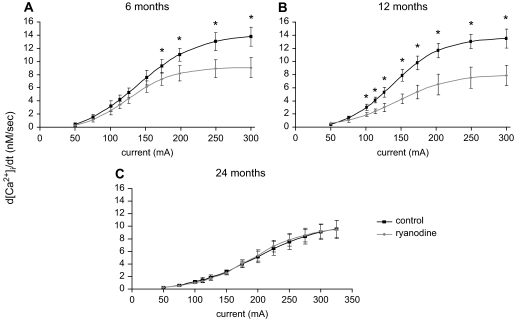
Figures 4 and 5 show the progressive effect of increasing current on the dynamics of EFS-evoked [Ca2+]i transients in SCG cells. Progressive increases in current enhanced both the magnitude (Figure 4) and rate of rise (Figure 5) of [Ca2+]i transients in all age groups. Consistent with the role of CICR to the EFS-evoked [Ca2+]i transients, ryanodine significantly depressed both parameters in 6- and 12-month-old animals, but had no effect in SCG cells from 24-month-old animals. Furthermore, the efficacy of ryanodine was greater in SCG cells from 12-month-old animals as compared with 6-month-old animals at currents lower than 150 mA. In addition, the maximal change in magnitude and rate of rise of EFS-evoked [Ca2+]i occurs at lower current levels in SCG cells from 6- and 12-month-old as compared with 24-month-old animals (Figures 4 and 5).
Figure 4.
Impact of advancing age on electrical field stimulation–evoked [Ca2+]i transients in the absence and presence of ryanodine in superior cervical ganglion cells from 6- (A), 12- (B), and 24- (C) month-old animals. Cells were activated at various applied currents as per Protocol 1 in the absence and presence of 100 μM ryanodine (see Methods). N = 19–31 cells from eight animals in each age group. *Significantly different from ryanodine treatment, p < .01 by paired t test.
Figure 5.
Impact of advancing age on rate of rise (d[Ca2+]i/dt) of electrical field stimulation–evoked [Ca2+]i transients in the absence and presence of ryanodine in superior cervical ganglion cells from 6- (A), 12- (B), and 24- (C) month-old animals. Cells were activated at various applied currents as per Protocol 1 in the absence (black) and presence (gray) of 100 μM ryanodine (see Methods). N = 19–31 cells from eight animals in each age group. *Significantly different from ryanodine treatment, p < .01 by paired t test.
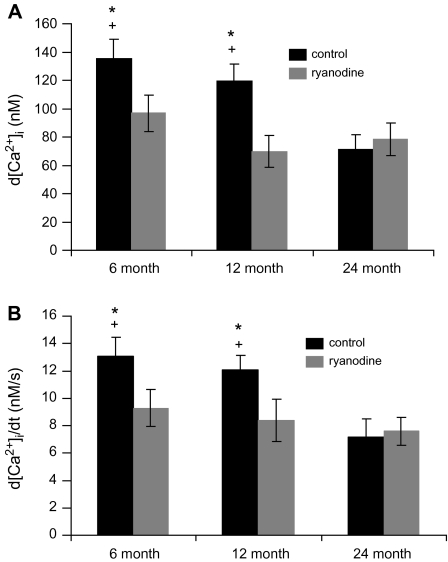
Because the magnitudes of stimulation-evoked [Ca2+]i transients are likely to reflect a combination of calcium influx and CICR (27,51), we compared the d[Ca2+]i and rate of rise of EFS-evoked [Ca2+]i transients at maximal current in the absence and presence of ryanodine (Figure 6). Clearly, the maximal d[Ca2+]i and rate of rise of EFS-evoked [Ca2+]i transients significantly declined at 24 months as compared with 6 and 12 months. Ryanodine depressed the magnitude of both parameters in SCG cells from 6- and 12-month-old animals with no effect at 24 months.
Figure 6.
Summarized responses of the impact of late maturation and senescence on the magnitude (A) and rate of rise (B) of maximal electrical field stimulation–evoked [Ca2+]i transients. Bars represent the peak values derived from curves in Figures 4 and 5 for control (black) and in the presence of ryanodine (gray). N = 19–31 cells from eight animals in each age group. *Significantly different from ryanodine treatment, p < .01, by paired t test. +Significantly different from 24 months, p < .02, by analysis of variance and Fisher-protected least significant differences test.
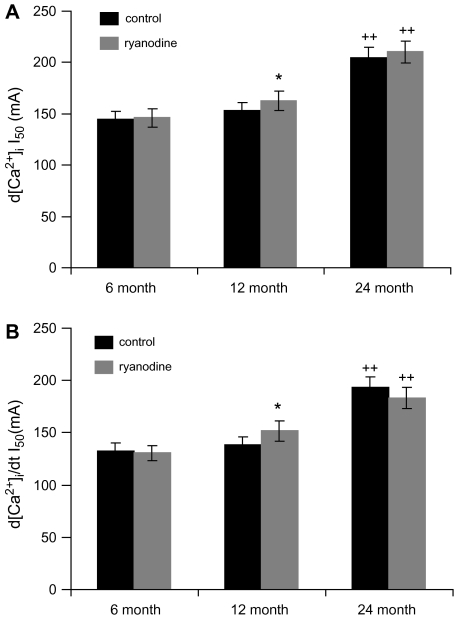
As a measurement of the sensitivity of SCG cells to EFS, we determined the currents necessary to evoke ½ maximal d[Ca2+]i and rate of rise of [Ca2+]i (Figure 7). For both parameters, the currents necessary were significantly greater in SCG cells from 24-month-old animals as compared with cells from 6- and 12-month-old animals. In addition, ryanodine significantly decreased the sensitivity of the SCG cells to EFS only in 12-month-old animals.
Figure 7.
Aging alters electrical field stimulation current (I50) necessary to evoke ½ maximal d[Ca2+]i (A) and rate of rise of [Ca2+]i (B). N = 19–31 cells from eight animals in each age group. *Significantly different from control, p < .05, by paired t test. ++Significantly different from 6 and 12 months, p < .02, by analysis of variance and Fisher-PLSD test.
Aging and Effect of Caffeine Sensitization of CICR on EFS-Evoked [Ca2+]i Transients
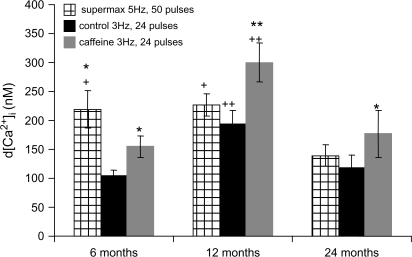
Caffeine, a RyR agonist, has been shown to reduce the threshold of EFS-evoked increases in calcium influx necessary to trigger the CICR mechanism (33,34). We compared the maximal EFS–evoked [Ca2+]i transients in each age group using two different frequencies and different pulse trains (Figure 8). This was done to test the hypothesis that CICR contributes to EFS-evoked [Ca2+]i transients to a greater extent at higher as compared with lower stimulation frequencies and decreases with advancing age. The maximal EFS–evoked [Ca2+]i transient at 5 Hz and 50 pulses, 300 mA, was significantly reduced in SCG cells from 24-month-old animals. In comparison, the maximal EFS–evoked [Ca2+]i transient at 3 Hz was obtained at 24 pulses in all age groups and was significantly less only in SCG cells from 6-month-old animals compared with the maximal [Ca2+]i transient obtained at 5 Hz. At 3 Hz and in the presence of caffeine, the maximal EFS–evoked [Ca2+]i transients were significantly greater in SCG cells in all age groups, with the greatest effect at 12 months. In addition, in the presence of caffeine, the EFS-evoked [Ca2+]i transients in SCG cells from 24-month-old animals were similar to those observed at 6 months.
Figure 8.
Impact of late maturation and senescence, on the magnitude (d[Ca2+]i), of electrical field stimulation–evoked [Ca2+]i transients. Comparisons were made between supermaximal stimulation (5 Hz, 50 pulses) to the stimulation at a lower frequency (3 Hz, 24 pulses) in the absence and presence of caffeine. N = 16–22 cells from five to six animals from each age group. *Significantly different from control, p < .03, by paired t test. **Significantly different from control and supermax, p < .01, by paired t test. +Significantly different from 24 months, p < .05, by analysis of variance (ANOVA) and Fisher-PLSD test. ++Significantly different from 6 and 24 months, p < .03, by ANOVA and Fisher-PLSD test.
DISCUSSION
One of the main observations gathered in this study was that the contribution of CICR to EFS-evoked [Ca2+]i transients was greater at middle age than in either young adults or senescent animals in the SCG cell model. Furthermore, the contribution of CICR to EFS-evoked elevations in [Ca2+]i may be partly restored in senescent animals by the RyR agonist, caffeine. Overall, these data suggest that coupling of membrane depolarization and calcium entry to RyR activation increases during middle age, reflecting increased CICR, and the contribution of CICR to EFS-evoked [Ca2+]i transients is significantly compromised with advancing age. However, caffeine can still augment this coupling during the onset of senescence suggesting that some residual CICR capacity still remains.
These conclusions are reinforced by the lack of effect of ryanodine on EFS-evoked [Ca2+]i dynamics at all current levels in SCG cells from 24-month-old animals and the increased effect of ryanodine at lower currents in SCG cells from 12-month-old animals as compared with 6-month-old animals (Figures 4 and 5). Furthermore, at a lower EFS frequency, caffeine enhances [Ca2+]i transients in SCG cells from senescent animals, making them more similar to SCG cells from 6-month-old animals. Even still, the greatest augmentation was observed in SCG cells from 12-month-old animals (Figure 8). Overall, the data suggest that in young adults and during middle age, calcium influx and CICR are a coupled process and that this coupling declines with senescence, leaving calcium influx as the primary signaling mechanism in senescent cells.
CICR With Late Maturation and Advancing Age in the SCG Cell Model
Maximal EFS–evoked [Ca2+]i transients declined when the frequency was reduced from 5 to 3 Hz only in SCG cells from 6-month-old animals and caffeine significantly increased the maximal EFS–evoked [Ca2+]i transient at the lower frequency (Figure 8). These data suggest that in the SCG cells from the youngest animals, CICR may not contribute as greatly to the magnitude of EFS-evoked [Ca2+]i transient at low input frequencies. These data are consistent with other studies suggesting that CICR is triggered by a critical threshold [Ca2+]i level (33,34). Furthermore, caffeine sensitizes CICR to EFS-evoked [Ca2+]i elevations at low frequencies, thus increasing the magnitude of the [Ca2+]i transient (Figure 8) (33,34). These data combined with data obtained in the presence of ryanodine suggest that CICR is important to EFS-evoked [Ca2+]i transients in SCG cells from 6-month-old animals. Furthermore, in cells from these animals, activation of CICR may require greater [Ca2+]i elevations than in cells from 12-month-old animals, thus necessitating the higher input frequency.
In SCG cells from 12-month-old animals, the similar responses to EFS at 5 and 3 Hz and the greater effect of caffeine at 3 Hz and ryanodine at lower input currents as compared with 6 months suggest that the sensitivity of CICR to EFS-evoked elevations in [Ca2+]i is enhanced at middle age (Figures 4,5, and 8). Overall, regardless of the input current or frequency, CICR in SCG cells from 12-month-old animals may be more sensitive to elevations in [Ca2+]i evoked by EFS as compared with SCG cells from 6-month-old animals.
The data obtained from SCG cells from senescent animals are much different than the data in the younger age groups. At maximal current or 5-Hz input frequency, stimulation-evoked [Ca2+]i was significantly less than that observed at 6 and 12 months (Figures 6 and 8). When the frequency was reduced to 3 Hz, EFS-evoked [Ca2+]i elevations remained similar to those obtained at 5 Hz. These data, when combined with a complete lack of effect of ryanodine on EFS-evoked increases in [Ca2+]i (Figures 4 and 5), suggest that CICR does not appreciably contribute to the magnitude and shape of EFS-evoked [Ca2+]i transients in SCG cells from 24-month-old animals. Interestingly, caffeine partially restores CICR function in SCG cells from 24-month-old animals, suggesting that there is some residual CICR function in SCG cells from senescent animals. However, this function can only be observed when RyR activity is augmented with caffeine.
It is interesting that augmenting RyR activity with caffeine enhances EFS-evoked [Ca2+]i transients, whereas inhibiting RyR activity with ryanodine is without effect in senescent SCG cells. This may be due to independent ryanodine- and caffeine-binding sites on the RyRs. Ryanodine binds to activated RyR and does not competitively antagonize caffeine. Caffeine on the other hand binds to a different site on the RyR, which increases the open probability of the RyR channel (27,31). Thus, the data in the present study suggest that caffeine still sensitized the RyRs in senescent SCG cells which then allows CICR to contribute to EFS-evoked [Ca2+]i signaling.
A decline in the coupling of CICR to membrane depolarization may explain our previous finding that high K+-evoked [Ca2+]i transients are increased during middle age but severely compromised in SCG cells from senescent animals (25). In addition, these data are consistent with our finding that the capacity of SCG cells to release calcium from the SER stores increases at middle age and then declines with senescence (25). As the magnitude and duration of stimulation-evoked [Ca2+]i transients are dependent on calcium influx and release (27,32), the suppression of EFS-evoked [Ca2+]i transients in the senescent SCG cells appears to be mostly due to loss of CICR as opposed to a decline in calcium influx through voltage-gated calcium channels. This conclusion is further supported by the comparison of EFS-evoked d[Ca2+]i transients in the presence of ryanodine (Figure 4). When ryanodine is present, CICR is blocked and the remaining [Ca2+]i transient is most likely to reflect EFS-evoked calcium influx. In the presence of ryanodine, the d[Ca2+]i in 6-, 12-, and 24-month-old SCG cells are 90.9 ± 13.3, 80.0 ± 16.1, and 90.1 ± 11.9, respectively. These data are not significantly different, and although the data do not represent a direct measure of calcium influx, overall, our previous and present studies do not support the idea that calcium influx is significantly altered with age in the SCG model. Other published studies support the idea that the reduction in stimulation-evoked [Ca2+]i transients that occurs with advancing age is not necessarily due to changes in calcium influx. For example, in central neurons, Ca2+ influx as measured from current densities has been reported to increase with age (52–54) remain unchanged (55) or decline in sensory neurons (56). Taken together, there are no conclusive data that support a general age-related decline in calcium influx in various neuronal models including the SCG.
The data in this study compare well with other studies, suggesting that there is a general decline in the contribution of RyR-mediated calcium release with senescent aging in central neurons (57). However, the findings of the current study contrast some studies in central nervous system neurons. In the present study, CICR increases from 6 to 12 months of age, suggesting a change in CICR that occurs during the transition from young to middle age. These data are consistent with a recent study performed on hippocampal CA1 neurons (48). In that study, slow after-hyperpolarizations, which are dependent on CICR, increased from 4 to 12 months and remained elevated in senescent animals (23 months). In contrast, CICR in the SCG declines from 12 to 24 months. Thus, in the SCG there appears to be two phases of CICR function, first an increase during the transition from young adult to middle age and then a dramatic decline from middle age to senescence. The functional significance of this contrast is difficult to interpret in terms of calcium signaling and may be due to differing function between SCG and CA1 neurons. In the study by Gant and coworkers (48), the increase in CICR is thought to contribute to an age-related decline in calcium homeostasis which can lead to neuronal dysfunction. Likewise, the biphasic changes in CICR in the SCG may also contribute to age-related decrements in the function of cerebrovascular neurons. Indeed, SCG neurons help protect the cerebral blood vessels from rupture in the face of age-related hypertension, and thus, the decline in CICR may be partly related to increased stroke risk in elderly populations (7).
Mechanisms Underlying Age-Related Alterations in CICR
The data from this study suggest that RyR activation increases at middle age and declines with senescence. Thus, the contribution of CICR to EFS-evoked [Ca2+]i transients in this study and K+-evoked [Ca2+]i transients in our previous study (25) similarly increases at middle age and then declines with senescence. Although the data in the present study do not necessarily address the specific mechanisms underlying how CICR function changes, our previous work discussed in light of the current studies illuminates some possibilities.
There are numerous intracellular modulators of RyR function. For example FKBP proteins alter RyR channel activity depending on the binding status, where the channels are activated by dissociation and inhibited by binding of FKBP proteins. In addition, RyR phosphorylation and interactions with intracellular molecules such as cyclic adenosine diphosphate ribose (cADPr) modulate CICR by altering the sensitivity of RyRs to changes in cellular calcium levels, much like exogenous caffeine (27,58,59). In the case of cADPr, its cellular levels are modulated by the release of nitric oxide via neuronal nitric oxide synthase (nNOS) (60). Thus, the cellular levels of nNOS and its activity in turn modulate cADPr levels through the activity of ribosyl cyclase, which synthesizes cADPr (60).
It is possible that the cellular levels of the modulators of CICR and their interactions with the RyRs are altered during middle age and senescence. To our knowledge, the levels of FKBP-binding proteins and/or cADPr and their affinities for the RyRs have not been studied with aging. However, our research group has examined the impact of age on the genetic expression and protein levels of specific RyR isoforms, total RyR phosphorylation, and nNOS levels, which in turn regulate cADPr levels, in the SCG. The genetic expression and protein levels of the RyR3 isoform and nNOS increased from 6 to 12 months and then declined back to the 6-month level at 24 months (61). Although these data suggest that there is an interplay between RyR expression and function, additionally levels and/or affinity of the RyR for modulators such as FKBP-binding proteins and cADPr may be substantially different in senescent SCG neurons, accounting for reductions in RyR activity and their contribution to calcium signaling.
Alterations in the filling state of the SER also contribute to the development of CICR, where decreases in calcium in the SER stores lead to reductions in CICR activity. For example repetitive caffeine-evoked calcium release from the SER will reduce CICR. In addition, smooth endoplasmic reticulum calcium-ATPase (SERCA) function is a key component to this process, and blockade of SERCA function with antagonists such as thapsigargin or cyclopiazonic acid leads to reduced SER calcium content and concomitantly, CICR (29,33,45,62). These studies suggest that activation of the CICR mechanism is not only dependent on changes in [Ca2+]i and other molecular modulators, but upon calcium levels within the SER.
The impact of aging on the magnitude of caffeine and high K+–evoked [Ca2+]i transients in our previous study (25) and EFS-evoked [Ca2+]i transients in the current study are parallel. These calcium transients increase at middle age and then decline with senescence. In the former study, we measured the capacity of the SER to release calcium with exposure to supramaximal caffeine and the time to maximally refill SER calcium stores mediated by SERCA (25). Clearly, these two processes declined with senescence. As the magnitude of high K+ and EFS-evoked [Ca2+]i transients reflect calcium influx coupled to RyR-mediated CICR, the data suggest that the contribution of CICR is compromised during senescence due in part to a decline in SERCA-mediated filling of SER calcium stores. This conclusion is derived in light of our previous studies focusing directly and indirectly on SERCA function with senescence (25,36,37,63,64). Overall, the increase in CICR at middle age and the decline with senescence may well involve multiple mechanisms, including changes in SERCA function altering the SER calcium loading levels.
SUMMARY
Neurotransmitter release in the SCG is critically dependent on calcium signaling, which entails both stimulation-evoked calcium influx and subsequent release of calcium from SER stores, by CICR. In this study, we show that during late maturation, stimulation-evoked CICR increases at middle age and then declines with senescence. In addition, residual CICR function may be enhanced in senescent cells by augmenting RyR activity with caffeine. Overall, these data have important implications for the function of adrenergic nerves during the aging process.
FUNDING
This work was supported in part by National Institutes of Health, HED# P01 31226.
Acknowledgments
The authors wish to acknowledge the technical expertise of Mr Charles Hewitt in the development and execution of measurement of [Ca2+]i with our imaging system.
References
- 1.Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 2.Faraci FM, Heistad DD. Regulation of cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Fugleholm K, Day LB, Ye S, Weller RO, Day IN. Molecular pathogenesis of subarachnoid hemorrhage. Int J Biochem Cell Biol. 2003;35:1341–1360. doi: 10.1016/s1357-2725(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 4.Busija DW, Heistad DD, Marcus ML. Effects of sympathetic nerves on cerebral vessels during acute moderate increases in arterial pressures in dogs and cats. Circ Res. 1980;46:696–702. doi: 10.1161/01.res.46.5.696. [DOI] [PubMed] [Google Scholar]
- 5.Furuichi S, Endo S, Haji A, Takeda R, Nisijima M, Takaku A. Related changes in sympathetic activity, cerebral blood flow and intracranial pressure, and effect of an α-blocker in experimental subarachnoid haemorrhage. Acta Neurochir (Wien) 1999;141:415–424. doi: 10.1007/s007010050318. [DOI] [PubMed] [Google Scholar]
- 6.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RD, Curb JD, Rodriguez BL, et al. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;56:479–486. doi: 10.1016/s0895-4356(02)00611-x. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda S, Tsuchikura S, Iida H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 2004;53:67–72. doi: 10.1538/expanim.53.67. [DOI] [PubMed] [Google Scholar]
- 9.Kocemba J, Kawecka-Jaszcz K, Gryglewska B, Grodzicki T. Isolated systolic hypertension: pathophysiology, consequences and therapeutic benefits. J Hum Hypertens. 1998;12:621–626. doi: 10.1038/sj.jhh.1000676. [DOI] [PubMed] [Google Scholar]
- 10.Nagai R, Nagata S, Fukuya F, Higaki J, Rakugi H, Ogihara T. Changes in autonomic activity and baroreflex sensitivity with the hypertension process and age in rats. Clin Exp Pharmacol Physiol. 2003;30:419–425. doi: 10.1046/j.1440-1681.2003.03852.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776–785. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 12.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 13.Bill A, Linder J. Sympathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiol Scand. 1976;96:114–121. doi: 10.1111/j.1748-1716.1976.tb10176.x. [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ. Calcium signaling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 16.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 17.Cunnane TC, Smith AB. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faure AV, Grunwald D, Moutin MJ, et al. Developmental expression of the calcium release channels during early neurogenesis of the mouse cerebral cortex. Eur J Neurosci. 2001;14:1613–1622. doi: 10.1046/j.0953-816x.2001.01786.x. [DOI] [PubMed] [Google Scholar]
- 19.Ginty DD. Calcium regulation of gene expression: isn't that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 20.Malenka RC, Kauer JA, Perkel DJ, Nicoll RA. The impact of postsynaptic calcium on synaptic transmission—its role in long-term potentiation. Trends Neurosci. 1989;12:444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- 21.Narita K, Akita T, Hachisuka J, Huang SM, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals: amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitzer NC, Ribera AB. Development of electrical excitability in embryonic neurons: mechanism and roles. J Neurobiol. 1998;37:190–197. [PubMed] [Google Scholar]
- 23.Kostyuk PG. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28:253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- 24.Trouslard J, Marsh SJ, Brown DA. Calcium entry through nicotinic receptor channels and calcium channels in cultured rat superior cervical ganglion cells. J Physiol (Lond) 1993;468:53–71. doi: 10.1113/jphysiol.1993.sp019759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanterpool CK, Pearce WJ, Buchholz JN. Advancing age alters rapid and spontaneous refilling of caffeine-sensitive calcium stores in sympathetic superior cervical ganglion cells. J Appl Physiol. 2005;99:963–971. doi: 10.1152/japplphysiol.00343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Fill M, Copello JA. Ryanodine receptor and calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 28.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrion NV, Adams PR. Release of intracellular calcium and modulation of membrane currents by caffeine in bull-frog sympathetic neurones. J Physiol. 1992;445:515–535. doi: 10.1113/jphysiol.1992.sp018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa Y, Kurebayashi N, Murayama T. Putative roles of type 3 ryanodine receptor isoforms (RyR3) Trends Cardiovasc Med. 2000;10:65–70. doi: 10.1016/s1050-1738(00)00050-5. [DOI] [PubMed] [Google Scholar]
- 32.Tully K, Treistman SN. Distinct intracellular calcium profiles following influx through N-versus L-type calcium channels: role of Ca2+-induced Ca2+ release. J Neurophysiol. 2004;92:135–143. doi: 10.1152/jn.01004.2003. [DOI] [PubMed] [Google Scholar]
- 33.Usachev YM, Thayer SA. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J Neurosci. 1997;17:7404–7414. doi: 10.1523/JNEUROSCI.17-19-07404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usachev YM, Thayer SA. Controlling the urge for a Ca2+ surge: all-or-none Ca2+ release in neurons. Bioessays. 1999;21:743–750. doi: 10.1002/(SICI)1521-1878(199909)21:9<743::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Coleman P, Finch C, Joseph J. The need for multiple time points in aging. Neurobiol Aging. 1990;2:1–2. doi: 10.1016/0197-4580(90)90055-5. [DOI] [PubMed] [Google Scholar]
- 36.Pottorf WJ, Duckles SP, Buchholz JN. Adrenergic nerves compensate for a decline in calcium buffering during ageing. J Auton Pharmacol. 2000;20:1–13. doi: 10.1046/j.1365-2680.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 37.Pottorf WJ, Duckles SP, Buchholz JN. SERCA function declines with age in adrenergic nerves from the superior cervical ganglia. J Auton Pharmacol. 2000;20:281–290. doi: 10.1046/j.1365-2680.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 38.Buchholz JN, Behringer EJ, Pottorf WJ, Pearce WJ, Vanterpool CK. Age-dependent changes in Ca2+ homeostasis in peripheral neurones: implications for changes in function. Aging Cell. 2007;6:285–296. doi: 10.1111/j.1474-9726.2007.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negulescu PA, Machen TE. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 1990;192:38–81. doi: 10.1016/0076-6879(90)92062-i. [DOI] [PubMed] [Google Scholar]
- 40.Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 41.Pottorf WJ, Thayer SA. Transient rise in intracellular calcium produces a long-lasting increase in plasma membrane calcium pump activity in rat sensory neurons. J Neurochem. 2002;83:1002–1008. doi: 10.1046/j.1471-4159.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- 42.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 43.Murchison D, Griffith WH. Increased calcium buffering in basal forebrain neurons during aging. J Neurophysiol. 1998;80:350–364. doi: 10.1152/jn.1998.80.1.350. [DOI] [PubMed] [Google Scholar]
- 44.Pottorf WJ, Johanns TM, Derrington SM, Strehler EE, Enyedi A, Thayer SA. Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem. 2006;98:1646–1656. doi: 10.1111/j.1471-4159.2006.04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friel DD, Tsien RW. A caffeine and ryanodine-sensitive Ca2+ store in bull frog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llano I, Dipolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 47.Messutat S, Heine M, Wicher D. Calcium-induced calcium release in neurosecretory insect neurons: fast and slow responses. Cell Calcium. 2001;30:199–211. doi: 10.1054/ceca.2001.0227. [DOI] [PubMed] [Google Scholar]
- 48.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 50.Holmberg SR, Williams AJ. The cardiac sarcoplasmic reticulum calcium release channel: modulation of ryanodine binding and single-channel activity. Biochim Biophys Acta. 1990;1022:187–193. doi: 10.1016/0005-2736(90)90113-3. [DOI] [PubMed] [Google Scholar]
- 51.Akita T, Kuba K. Functional triads consisting of ryanodine receptors, Ca2+ channels, and Ca2+-activated K+ channels in bullfrog sympathetic neurons. J Gen Physiol. 2000;116:697–720. doi: 10.1085/jgp.116.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landfield PW, Pitler TA. Prolonged Ca2+-dependent after-hyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 53.Pitler TA, Landfield PW. Aging-related prolongation of Ca2+ spike duration in rat hippocampal slice neurons. Brain Res. 1990;508:1–6. doi: 10.1016/0006-8993(90)91109-t. [DOI] [PubMed] [Google Scholar]
- 54.Porter NM, Thibault O, Thibault V, Chen KY, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murchison D, Griffith WH. High-voltage-activated calcium currents in basal forebrain neurons during ageing. J Neurophysiol. 1996;76:158–174. doi: 10.1152/jn.1996.76.1.158. [DOI] [PubMed] [Google Scholar]
- 56.Kostyuk PG, Pronchuk N, Savchenko A, Verkhratsky A. Calcium currents in aged rat dorsal root ganglion neurones. J Physiol (Lond) 1993;461:467–483. doi: 10.1113/jphysiol.1993.sp019523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimers disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HC. Physiologic functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 59.Marx SO, Marks AR. Regulation of the ryanodine receptor in heart failure. Basic Res Cardiol. 2002;97(suppl 1):I49–I51. doi: 10.1007/s003950200029. [DOI] [PubMed] [Google Scholar]
- 60.Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxide/cGMP pathway. J Biol Chem. 1996;271:17739–17745. doi: 10.1074/jbc.271.30.17739. [DOI] [PubMed] [Google Scholar]
- 61.Vanterpool CK, Vanterpool EA, Pearce WJ, Buchholz JN. Advancing age alters the expression of the ryanodine receptor 3 isoform in adult rat superior cervical ganglia. J Appl Physiol. 2006;101:392–400. doi: 10.1152/japplphysiol.00167.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kostyuk P, Verkhratsky A. Calcium stores in neurons and glia. Neuroscience. 1994;63:381–404. doi: 10.1016/0306-4522(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 63.Buchholz J, Tsai H, Foucart S, Duckles SP. Advancing age alters intracellular calcium buffering in rat adrenergic nerves. Neurobiol Aging. 1996;17:885–892. doi: 10.1016/s0197-4580(96)00179-0. [DOI] [PubMed] [Google Scholar]
- 64.Tsai H, Pottorf WJ, Buchholz JN, Duckles SP. Adrenergic nerve smooth endoplasmic reticulum calcium buffering declines with age. Neurobiol Aging. 1998;19:89–96. doi: 10.1016/s0197-4580(98)00008-6. [DOI] [PubMed] [Google Scholar]