Abstract
A number of structurally divergent proteins with J domains, called J proteins, interact with and activate the ATPase of Hsp70s, thereby harnessing the ATPase activity for conformational work on target proteins. The precise role of most mammalian J proteins remains undefined. In this paper, we demonstrate that transient expression of the J protein, Rdj2, in HEK 293 cells increased cellular cyclic adenosine monophosphate (cAMP) levels in the presence of the β-adrenergic agonist isoproterenol. In CNS-derived catecholaminergic neuronal cell line (CAD) neuroblastoma cells, expression of Rdj2 increased isoproterenol-stimulated phosphorylation of cAMP response element binding protein (CREB). Moreover, we have characterized the binding properties of Rdj2 and observed a direct interaction between Rdj2 and receptor-coupled trimeric GTP-binding proteins (G proteins). We further show that the composition of the Rdj2-chaperone complex and the cysteine string protein (CSPα)-chaperone complex, another J protein, is distinct. Our data demonstrate that Rdj2 modulates G protein signaling and further suggest that chaperoning G proteins is an emerging theme of the J protein network.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-008-0056-y) contains supplementary material, which is available to authorized users.
Keywords: Rdj2, J protein, CSPα, Cysteine string protein, G protein
Introduction
Over 40 human proteins contain a J domain, a domain found in the bacterial J protein, DnaJ, which is required for bacteriophage λ DNA replication in Escherichia coli. A J domain is a ~70 amino acid region of homology comprised of four helices with a highly conserved tripeptide of histidine, proline, and aspartic acid (HPD motif) located between helices II and III. A number of mammalian J proteins (e.g., auxilin (Ungewickell et al. 1995), CSPα (Braun et al. 1996), Hsj1 (Cheetham et al. 1994)) have been shown to stimulate the ATPase activity of the Hsp70 protein family. Hsp70’s ATPase activity is coupled to a broad range of folding processes, including: the folding of newly synthesized proteins, the transport of proteins across membranes, the refolding of misfolded proteins, the disassembly of protein complexes, in addition to conformational re-arrangements of components in signal transduction, cell cycle, and apoptotic pathways. There is no functional one-to-one correspondance between members of the Hsp70 family and J proteins (Zhao et al. 2008).
The functions of most mammalian proteins with J domains remain undefined. Within the cell, Hsp70:J protein-folding machines are almost certainly highly regulated; however, the mechanistic basis of this regulation remains to be solved. Outside of the J domain, J proteins vary significantly in their amino acid sequence, relative abundance, and localization (Craig et al. 2006). The mammalian J protein studied in most detail is auxilin, a protein necessary for dynamic conformational rearrangements of clathrin during clathrin-mediated endocytosis (Ungewickell et al. 1995; Eisenberg and Greene 2007). Current evidence indicates that other proteins with J domains harness Hsp70s for conformational work related to a number of other processes including: keratin cytoskeleton homeostasis (Mrj), sorting to the proteosome (Hsj1), endocytosis (Rme-8), and cell survival (Hsp40) (Zhao et al. 2008). How J protein driven conformational changes are optimized for different cellular roles remains an enigma. A major challenge is to define the cellular role(s) of mammalian J proteins.
We have previously shown that cysteine string protein (CSPα), a synaptic vesicle-tethered J protein, regulates heterotrimeric GTP-binding protein (G protein) function (Magga et al. 2000; Natochin et al. 2005). Activation of G proteins involves an exchange of GDP for GTP on 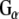 subunits and the release of GTP-bound
subunits and the release of GTP-bound 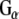 and
and 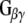 to interact with effector molecules. CSPα, in association with Hsc70 and SGT (small glutamine rich tetratricopeptide repeat domain protein), preferentially targets the inactive GDP-bound form of
to interact with effector molecules. CSPα, in association with Hsc70 and SGT (small glutamine rich tetratricopeptide repeat domain protein), preferentially targets the inactive GDP-bound form of 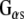 and promotes GDP/GTP exchange, which leads to increases in cAMP production. CSPα also enhances G protein inhibition of N-type calcium channels (Magga et al. 2000; Miller et al. 2003b). CSPα is selective for
and promotes GDP/GTP exchange, which leads to increases in cAMP production. CSPα also enhances G protein inhibition of N-type calcium channels (Magga et al. 2000; Miller et al. 2003b). CSPα is selective for 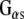 , and, as such, is the first identified guanine nucleotide exchange factor (GEF) for
, and, as such, is the first identified guanine nucleotide exchange factor (GEF) for 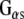 (Natochin et al. 2005). Dupre and colleagues (Dupre et al. 2007) have recently demonstrated that DRiP78 (dopamine receptor interacting protein, also called HDJ3, LIP6 and DnaJC14), an endoplasmic reticulum resident protein that contains a J domain interacts with and chaperones
(Natochin et al. 2005). Dupre and colleagues (Dupre et al. 2007) have recently demonstrated that DRiP78 (dopamine receptor interacting protein, also called HDJ3, LIP6 and DnaJC14), an endoplasmic reticulum resident protein that contains a J domain interacts with and chaperones 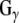 . These studies raise the possibility that G proteins are targeted by proteins that contain a J domain, such as CSPα and DRiP78.
. These studies raise the possibility that G proteins are targeted by proteins that contain a J domain, such as CSPα and DRiP78.
In this study, we have investigated Rdj2, a 48 kDa J domain-containing protein of unknown function (Andres et al. 1997). We demonstrate, for the first time, that Rdj2 (also called DjA2, Dj3, Dnj3, Cpr3, Hirip4 and DnaJA2) associates directly with G proteins and modulates G-protein mediated signal transduction. We identify five highly conserved protein components of the Rdj2:G protein complex. Our work establishes Rdj2 as a G protein chaperone and suggests a central role for J protein:Hsc70 chaperone machines in controlling the conformational changes undertaken by cellular G proteins.
Materials and methods
cAMP Assays Human embryonic kidney tsa-201 (HEK) cells were seeded into 12-well plates and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. At ~70% confluency, cells were transiently transfected with β2 adrenergic receptor, Rdj2, or CSPα as indicated. Forty-eight hours post transfection, cells were incubated for 3 min at 37°C with serum-free medium or 50 μM isoproterenol and then washed with phosphate-buffered saline. Cells were lysed with 280 μl of 0.1 M HCl. Lysates were centrifuged at 600 × g and the supernatant was collected. The cAMP level of each supernatant (100 μl) was quantified using a Direct cAMP Enzyme Immunoassay kit (Sigma).
Preparation of rat brain membrane and soluble fractions Whole rat brains were homogenized in 20-mM Tris–HCl buffer (pH 7.4), 2 mM MgSO4, 1 mM phenylmethylsulfonyl fluoride (PMSF) and ethylenediaminetetraacetic acid (EDTA)-free inhibitor cocktail as previously described (Beck et al. 2006). The homogenate was centrifuged at 100,000 × g for 1 h at 4°C. The resultant soluble fraction was removed and designated the soluble cytosolic fraction (S). The remaining pellet was solubilized in homogenizing buffer containing 1% (w/v) n-dodecyl-β-d-maltoside (Calbiochem) for 60 min at 4°C. Following centrifugation at 100,000 × g for 1 h at 4°C, the resulting supernatant constituted the detergent-solubilized membrane particulate fraction (P). To evaluate the distribution of Rdj2, rat brain regions were dissected and solubilized in 50 mM Tris (pH 6.8), 1 mM EDTA, 2% SDS, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin. All procedures were carried out in strict accordance with a protocol approved by the University of Calgary Animal Care Committee.
Preparation of fusion proteins Glutathione-S-transferase (GST) fusion proteins GST-Rdj2, GST-CSPα, GST-CSPα1–82 and GST-Hsc70 were prepared by sub-cloning polymerase chain reaction (PCR) products into the bacterial expression plasmids pGEX-KG or pGEX-4T, as previously described (Braun and Scheller 1995; Beck et al. 2006). The GST-auxilin construct was a kind gift from Dr. E. Lafer. Following sequence verification, DNA was transformed into AB1899 or DH10B E. coli. Expression of GST fusion proteins was induced with 100 μM isopropyl-β-d-thiogalactosidase (IPTG) for 5 h at 37°C. Bacteria were suspended in phosphate-buffered saline (PBS), 0.05% (v/v) Tween 20, 2 mM EDTA, 0.5 mM PMSF and 0.1% (v/v) β-mercaptoethanol and lysed by two passages through a French press (Spectronics Instruments Inc.) GST fusion proteins were recovered by binding to glutathione-sepharose beads (GE Healthcare Biosciences). Beads were suspended as a 50% (v/v) slurry in 20 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 4.5 mM Mg acetate, 150 mM KCl, and 0.2% (v/v) Triton X-100. The concentrations of recombinant GST fusion proteins were estimated by Coomassie blue staining of SDS-polyacrylamide gels using bovine serum albumin (BSA) as a standard. Equimolar amounts of the soluble GST fusion proteins were evaluated for possible association with the indicated proteins.
In vitro ‘pull-down’ assays The detergent-solubilized particulate fraction (P) and the 100,000 × g soluble cytosolic fraction (S) isolated from rat brain were incubated with bead-immobilized GST-tagged proteins in 20 mM MOPS, 4.5 mM Mg acetate, 150 mM KCl, 0.5% Triton X-100, and 2 mM ATP or 2 mM GDP in a final volume of 400 μl, for 1 h at 37°C. Beads were washed twice with 200 μl ml of ice-cold 20 mM MOPS, 4.5 mM Mg acetate, 150 mM KCl, 0.2% Triton X-100. Bound proteins were eluted in Laemmli sample buffer, fractionated by SDS-PAGE and analyzed by Western blotting.
Immunoprecipitation The detergent-solubilized membrane fraction (P) and the cytosolic fraction (S) were pre-cleared by incubation with GammaBind G-Sepharose for 1 h at 4°C. Immunoprecipitation was achieved by incubating the pre-cleared fractions with anti-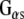 antibody overnight at 4°C followed by GammaBind G-Sepharose (Amersham Biosciences) for 30 min at 25°C. The mixture was then centrifuged at 5,000 rpm for 5 min, and the pellets were washed three times with 200 μl of 20 mM Tris–HCl (pH 7.4) buffer containing 130 mM NaCl, 2 mM MgSO4, and 50 μM GDP. Samples were resuspended in Laemmli sample buffer prior to resolution by SDS-PAGE and immunoblotting.For the association with GTP agarose (Sigma), the membrane fraction (P) or cytosolic fraction (S), or both, were incubated with GTP-agarose overnight at 4°C followed by centrifugation at 5000 rpm for 5 min, and the pellets were washed three times with 200 μl of 20 mM Tris–HCl (pH 7.4) buffer containing 130 mM NaCl, and 2 mM MgSO4. Samples were resuspended in Laemmli sample buffer prior to resolution by SDS-PAGE and immunoblotting
antibody overnight at 4°C followed by GammaBind G-Sepharose (Amersham Biosciences) for 30 min at 25°C. The mixture was then centrifuged at 5,000 rpm for 5 min, and the pellets were washed three times with 200 μl of 20 mM Tris–HCl (pH 7.4) buffer containing 130 mM NaCl, 2 mM MgSO4, and 50 μM GDP. Samples were resuspended in Laemmli sample buffer prior to resolution by SDS-PAGE and immunoblotting.For the association with GTP agarose (Sigma), the membrane fraction (P) or cytosolic fraction (S), or both, were incubated with GTP-agarose overnight at 4°C followed by centrifugation at 5000 rpm for 5 min, and the pellets were washed three times with 200 μl of 20 mM Tris–HCl (pH 7.4) buffer containing 130 mM NaCl, and 2 mM MgSO4. Samples were resuspended in Laemmli sample buffer prior to resolution by SDS-PAGE and immunoblotting
Sequence analysis DnaJ from E. coli (accession number: AAC73126.1), Ydj1 from Saccharomyces cerevisiae (accession number: CAA95937.1), Rdj2 from Rattus norvegicus (accession number: EDL87492.1) and CSPα from Rattus norvegicus (accession number: P60905) were selected for analysis. Alignment was performed using clustal-W with default settings in place and homology was performed by EMBOSS pairwise global alignment using an implementation of the Needleman–Wunsch algorithm (Needleman and Wunsch 1970). Domains were identified with InterproScan (IPR).
Immunoblotting Proteins were electro-transferred (70 V for 45 min at 4°C) from SDS-polyacrylamide gels to nitrocellulose (0.45 um) in 20 mM Tris, 150 mM glycine, and 12% methanol. Membranes were blocked with 4% milk solution (prepared in PBS with 0.1% Tween 20 or TBS with 0.1% Tween 20 for phosphoCREB analysis) and incubated with primary antibody in blocking buffer for 2 h at room temperature or overnight at 4°C. The membranes were washed in blocking solution and incubated with horseradish peroxidase-coupled secondary antibody. The signal was developed using West Pico Pierce reagent (Pierce Biotechnology, Inc.) and exposed to Amersham Hyperfilm™ ECL (GE Healthcare, Ltd).
PhosphoCREB Analysis Caspase-activated DNase mouse neuroblastoma cells were seeded into six well plates and grown in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% Penicillin/streptomycin. At ~70% confluency, DMEM/F12 was replaced with Opti-MEM-1 serum-free medium and cells were transfected with Rdj2, or CSPα using lipofectamine-™2000 (Invitrogen). Twenty four hours post transfection, cells were incubated for 15 min with either DMEM/F12 medium or 50 μM isoproterenol and then washed with phosphate-buffered saline. Cells were lysed in Laemmli sample buffer and whole-cell lysates were resolved by SDS-PAGE. PhosphoCREB and CREB protein levels were then detected by Western blot analysis.
Results
Rdj2 stimulates cAMP generation
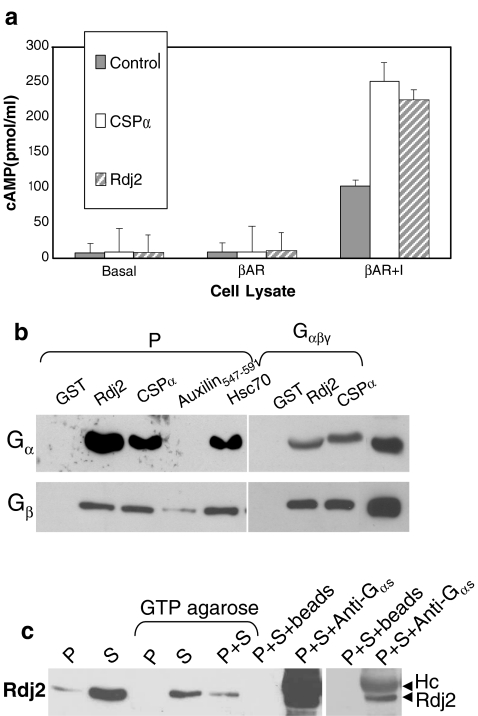
During our analysis of J protein family members as regulators of G-protein mediated signal transduction, we observed that the type I J-protein, Rdj2, enhanced isoproterenol-stimulated cAMP levels, similar to that previously described for the type III J-protein, CSPα (Natochin et al. 2005). Human epithelial kidney (HEK) cells were transfected with Rdj2 or CSPα in the presence and absence of co-transfected β2 adrenergic receptor. Figure 1a shows that in Rdj2+β2 adrenergic receptor transfected HEK cells, isoproterenol evoked a 22-fold increase in cellular cAMP levels compared to a 12-fold increase in cells transfected with β adrenergic receptor alone, demonstrating that Rdj2 modulates β-adrenergic receptor mediated signaling. Rdj2 is thus the second member of the J protein family found to promote G protein signaling, as we have previously reported that isoproterenol evoked a 27-fold increase in cAMP levels in cells expressing CSPα+β adrenergic receptor (Natochin et al. 2005).
Fig. 1.
Rdj2 interacts with G proteins and stimulates cAMP generation. (a). HEK cells were transfected with plasmids encoding the β2 adrenergic receptor, CSPα1–198 or Rdj21–412 as indicated. Approximately 48 h later, cells were incubated for 3 min with serum-free medium (basal), or 50 μM isoproterenol, lysed, and the amount of cellular cAMP determined under each condition. Results are expressed as means ± SE for a total of four separate experiments. (b) Western blot analysis showing the binding of 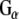 and
and 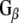 to GST, GST-CSPα, GST-Rdj2, GST-auxilin547–591 or GST–Hsc70 fusion proteins immobilized on glutathione-sepharose. Fusion proteins were incubated in the presence of 400 μg of solubilized rat brain membranes (P) or 2.5 μg of purified bovine
to GST, GST-CSPα, GST-Rdj2, GST-auxilin547–591 or GST–Hsc70 fusion proteins immobilized on glutathione-sepharose. Fusion proteins were incubated in the presence of 400 μg of solubilized rat brain membranes (P) or 2.5 μg of purified bovine 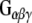 (Calbiochem) at 37°C for 30 min. The beads were washed, and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis. Purified bovine
(Calbiochem) at 37°C for 30 min. The beads were washed, and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis. Purified bovine 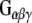 (1 μg) is shown in the right-most hand lane. The nitrocellulose membrane was probed with either an anti-
(1 μg) is shown in the right-most hand lane. The nitrocellulose membrane was probed with either an anti-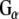 polyclonal (Calbiochem) or anti-
polyclonal (Calbiochem) or anti-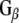 monoclonal antibody (transduction labs). (c) Western analysis showing the association of Rdj2 to GTP-agarose and co-immunoprecipitation of Rdj2 with anti-
monoclonal antibody (transduction labs). (c) Western analysis showing the association of Rdj2 to GTP-agarose and co-immunoprecipitation of Rdj2 with anti-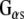 polyclonal (Santa Cruz). The nitrocellulose membrane was probed with a mouse anti-Rdj2 monoclonal antibody (Abnova). Twenty micrograms of the detergent-solubilized membrane fraction (P) and 20 μg of the 100,000 × g cytosolic fraction (S) prepared from rat brain are shown in the left-hand lanes; 230 µg of the solubilzed membrane fraction (P) or 250 µg of the cytosolic fraction (S), or both, were incubated with GTP-agarose, GammaBind G-Sepharose (beads), or anti-
polyclonal (Santa Cruz). The nitrocellulose membrane was probed with a mouse anti-Rdj2 monoclonal antibody (Abnova). Twenty micrograms of the detergent-solubilized membrane fraction (P) and 20 μg of the 100,000 × g cytosolic fraction (S) prepared from rat brain are shown in the left-hand lanes; 230 µg of the solubilzed membrane fraction (P) or 250 µg of the cytosolic fraction (S), or both, were incubated with GTP-agarose, GammaBind G-Sepharose (beads), or anti-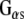 polyclonal antibody (Santa Cruz) and GammaBind G-sepharose as indicated. The right-hand panel shows a lighter exposure of the last two lanes
polyclonal antibody (Santa Cruz) and GammaBind G-sepharose as indicated. The right-hand panel shows a lighter exposure of the last two lanes
In order to investigate the possibility of an association between G proteins and Rdj2, glutathione-S-transferase (GST) fusion proteins consisting of either Rdj2, CSPα, auxilin547–591 or Hsc70 were coupled to glutathione-sepharose beads and used in an in vitro binding assay. Figure 1b shows that recombinant immobilized Rdj2 interacts with 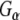 and
and 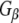 subunits present in a solubilized membrane fraction from rat brain. We further observed that these G-protein subunits also associated with CSPα and Hsc70 in vitro, as previously reported (Magga et al. 2000; Miller et al. 2003a, b; Natochin et al. 2005). No
subunits present in a solubilized membrane fraction from rat brain. We further observed that these G-protein subunits also associated with CSPα and Hsc70 in vitro, as previously reported (Magga et al. 2000; Miller et al. 2003a, b; Natochin et al. 2005). No 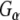 association was observed with GST alone or auxilin547–591, a region of auxilin that encodes the evolutionary conserved J domain, demonstrating the specificity of this
association was observed with GST alone or auxilin547–591, a region of auxilin that encodes the evolutionary conserved J domain, demonstrating the specificity of this 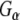 –Rdj2 protein interaction. Similarly,
–Rdj2 protein interaction. Similarly, 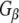 did not associate with GST and only very low levels were detected to associate with auxilin, demonstrating the specificity of the
did not associate with GST and only very low levels were detected to associate with auxilin, demonstrating the specificity of the 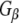 –Rdj2 association. While these data are consistent with a direct interaction between Rdj2 and G proteins, they do not rule out the possibility that G proteins and Rdj2 associate indirectly. To investigate this possibility, we examined the ability of fusion proteins consisting of GST, full-length Rdj2 and full-length CSPα to interact with purified G proteins. In each binding assay, equal amounts of fusion protein were immobilized to glutathione-sepharose beads, as confirmed by Ponceau S staining. The presence of G-protein subunits was analyzed by Western blotting. As shown in Fig. 1b, recombinant Rdj2 was able to bind purified bovine
–Rdj2 association. While these data are consistent with a direct interaction between Rdj2 and G proteins, they do not rule out the possibility that G proteins and Rdj2 associate indirectly. To investigate this possibility, we examined the ability of fusion proteins consisting of GST, full-length Rdj2 and full-length CSPα to interact with purified G proteins. In each binding assay, equal amounts of fusion protein were immobilized to glutathione-sepharose beads, as confirmed by Ponceau S staining. The presence of G-protein subunits was analyzed by Western blotting. As shown in Fig. 1b, recombinant Rdj2 was able to bind purified bovine 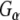 and
and 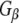 protein subunits, indicating that the Rdj2–G protein interaction is indeed a direct physical interaction between G proteins and Rdj2. No G protein–GST association was observed, while robust
protein subunits, indicating that the Rdj2–G protein interaction is indeed a direct physical interaction between G proteins and Rdj2. No G protein–GST association was observed, while robust 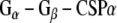 interactions were evident, consistent with our previous reports (Magga et al. 2000; Miller et al. 2003b). The amino acid identity between Rdj2 and CSPα is 51% within the J domain. Outside of the J domain, Rdj2 and CSPα display only limited amino acid conservation. Consistent with a Rdj2:G protein association, Fig. 1c shows that native rat brain Rdj2 co-immunoprecipitates with
interactions were evident, consistent with our previous reports (Magga et al. 2000; Miller et al. 2003b). The amino acid identity between Rdj2 and CSPα is 51% within the J domain. Outside of the J domain, Rdj2 and CSPα display only limited amino acid conservation. Consistent with a Rdj2:G protein association, Fig. 1c shows that native rat brain Rdj2 co-immunoprecipitates with 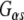 and associates with GTP-agarose. The observation that Rdj2 associates with GTP agarose and that Rdj2-binding is reduced in the presence of the membrane (P) fraction which contains G proteins, implies that Rdj2 association with GTP agarose does not occur indirectly via G proteins (but may indirectly occur through another protein). Furthermore, a component of the membrane (P) fraction appears to inhibit or compete, resulting in reduced Rdj2-GTP agarose binding. In Fig. 1c, the nitrocellulose membrane was probed with an anti-Rdj2 monoclonal antibody; detection of the anti-
and associates with GTP-agarose. The observation that Rdj2 associates with GTP agarose and that Rdj2-binding is reduced in the presence of the membrane (P) fraction which contains G proteins, implies that Rdj2 association with GTP agarose does not occur indirectly via G proteins (but may indirectly occur through another protein). Furthermore, a component of the membrane (P) fraction appears to inhibit or compete, resulting in reduced Rdj2-GTP agarose binding. In Fig. 1c, the nitrocellulose membrane was probed with an anti-Rdj2 monoclonal antibody; detection of the anti-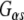 antibody heavy chain (Hc) by the HRP-linked secondary antibody is also evident. Taken together, these results indicate that Rdj2 specifically and directly interacts with
antibody heavy chain (Hc) by the HRP-linked secondary antibody is also evident. Taken together, these results indicate that Rdj2 specifically and directly interacts with 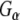 and
and 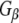 and promotes isoproterenol-stimulated elevation of cAMP levels.
and promotes isoproterenol-stimulated elevation of cAMP levels.
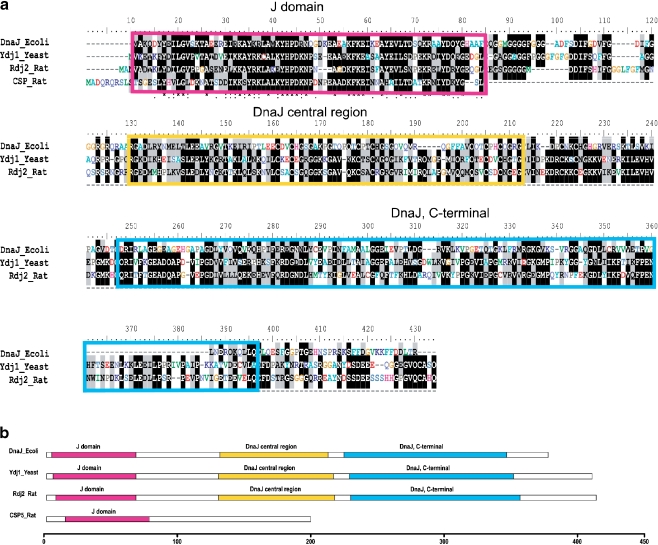
Members of the J protein family are found in a wide variety of species from E. coli to man. In addition to the J domain, a number of other domains have been identified (Ohtsuka and Hata 2000; Cheetham and Caplan 1998; Zhao et al. 2008). DnaJ, an E. coli J protein is essential for bacteriophage λ DNA replication (Georgopoulos et al. 1980). Figure 2a shows that in contrast to CSPα, which contains only one ancient domain (i.e., the J domain), Rdj2 has extensive regions of homology with E. coli DnaJ and Saccharomyces cerevisiae Ydj1, an abundant yeast J protein whose deletion causes severe growth defects (Caplan and Douglas 1991). The amino acid identities between the full-length sequences of DnaJ-Rdj2, and Ydj1-Rdj2 shown in Fig. 2a are 32% and 49%, respectively (supplementary Figure 1). The amino acid identities between the J domain of DnaJ-Rdj2, Ydj1-Rdj2 and CSPα-Rdj2 are 52%, 66%, and 51%, respectively. DnaJ, Ydj1, and Rdj2 have three main domains as identified by Interproscan (IPR): a J domain, DnaJ central region and DnaJ, C-terminal with the IPR number IPR001623, IPR001305 and IPR002939, respectively (Fig. 2b). Thus, while CSPα and Rdj2 both have the ancient J domain, the absence of the DnaJ central region and the DnaJ C-terminal region suggests that CSPα has been fine tuned to acquire specific chaperone features.
Fig. 2.
Comparison of rat Rdj2 with E. coli DnaJ, S. cerevisia Ydj1 and rat CSPα amino acid sequence. (a) Comparison of the amino acid sequences of DnaJ, Ydj1, Rdj2, and the N-terminal region of CSPα (amino acids 1 to 82). The locations of three domains are highlighted in corresponding colors in panel b. Alignments of sequences were obtained using Clustal-W with default settings in place. (b) Domain alignments of the DnaJ_Ecoli, Ydj1_Yeast, Rdj2_Rat, and CSPα_Rat. InterProScan has been used to identify the domains. Scale bar marks the length measured by amino acids (see Supplementary Figure 1)
Rdj2 is widely expressed in the brain
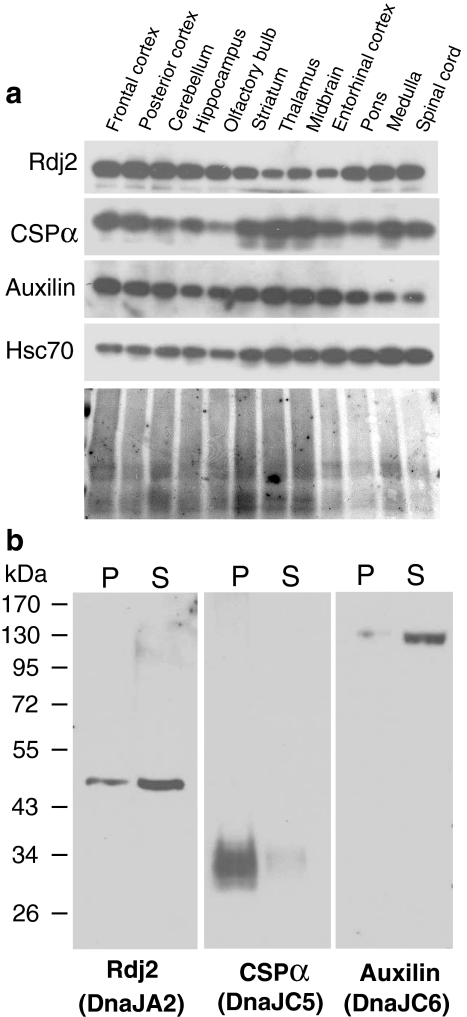
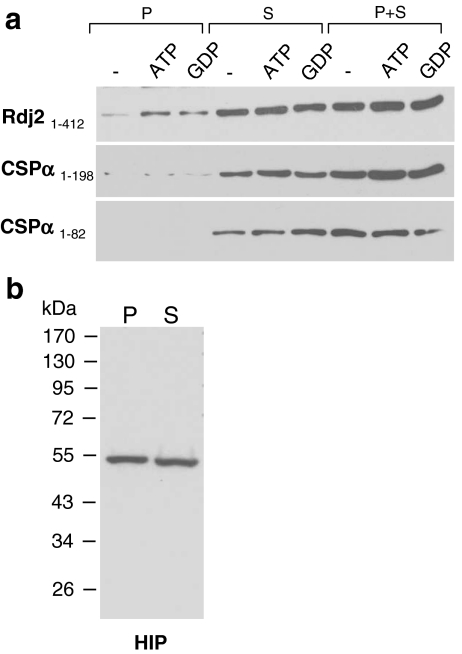
Next, we investigated the distribution of Rdj2 in rat brain. Rdj2, CSPα, auxilin, and Hsc70 were found in all regions of the rat brain examined (Fig. 3a). Lesser amounts of Rdj2 were detected in thalamus and midbrain regions; in contrast, CSPα and auxilin were notably abundant in these areas. As expected, Hsc70 was present in all regions of the rat brain. Although Rdj2, CSPα and auxilin are all constitutively expressed in the brain, they differ in their cellular localization. Figure 3b shows that Rdj2, a 48-kDa protein, is found in both the cytosolic (S) and membrane particulate (P) fractions of rat brain; in contrast CSPα is localized primarily within the membrane fraction (P) and auxilin is abundant in the cytosolic fraction (S). The presence of Rdj2 in the membrane fraction is likely due to its lipid modification (Andres et al. 1997). These results suggest that Rdj2, CSPα, and auxilin may coordinate Hsc70 conformational activity in distinct cellular regions.
Fig. 3.
Rdj2 is widely expressed in the brain. (a) Western blot analysis showing the distribution Rdj2, CSPα, auxilin, and Hsc70 in rat brain. Twenty-five micrograms of unfractionated tissue homogenate isolated from the indicated regions of rat brain were separated by SDS-PAGE, transferred to PVDF and probed with the indicated antibodies. Ponceau S staining of the membrane is shown in the lower panel. (b) Western blot analysis showing the expression of J proteins in a solubilized membrane fraction (45 µg) (lanes denoted by P) and a cytosolic fraction (45 µg) (lanes denoted by S) prepared from whole rat brain. Individual nitrocellulose membranes were probed with a mouse anti-Rdj2 monoclonal antibody (Abnova); rabbit anti-CSPα polyclonal antibody; rabbit anti-auxilin polyclonal antibody, and mouse anti-Hsc70 monoclonal antibody (Sigma). The electrophoretic positions of molecular weight standards (in kDa) are indicated on the left-hand side of the blot. Names in brackets are from Qui et al. 2006
Hsc70, Hsp90, and Hsp110 are components of the Rdj2 complex
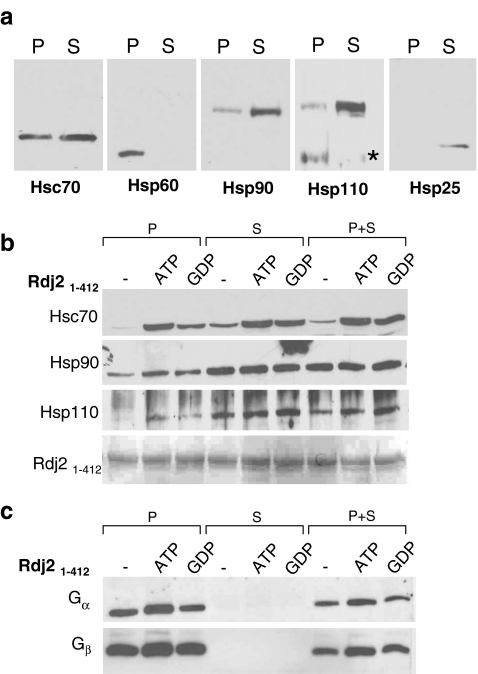
In order to begin to understand the mode of action of Rdj2, we examined the molecular nature of the Rdj2 complex, specifically, its possible assembly with other chaperones. Identification of the components of the Rdj2 complex sets the stage for determining Rdj2’s cellular function. First, we established the relative distribution of chaperones in the rat brain fractions utilized (Fig. 4a). Mammalian Hsp110 (heat shock protein 110) is a divergent relative of Hsp70. Although the precise role of Hsp110 in mammalian cells is not yet defined, a number of reports have designated the Hsp110s as NEFs (nucleotide exchange factors) for the Hsp70s (reviewed: Shaner and Morano 2007). Hsp110 and the ATPase Hsp90 (Heat Shock Protein 90) (reviewed: Prodromou and Pearl 2003) were abundant in the cytosolic (S) fraction from the unstressed rat brain. Hsc70 was abundant in both the membrane (P) and cytosolic (S) fractions; however, the stress-inducible Hsp70 was not detectable in our rat brain preparations (data not shown). The mitochondrial chaperone, Hsp60 (Heat Shock Protein 60), was found in the membrane (P) fraction. The stress-inducible Hsp25 was detected at low levels in the cytosolic fraction.
Fig. 4.
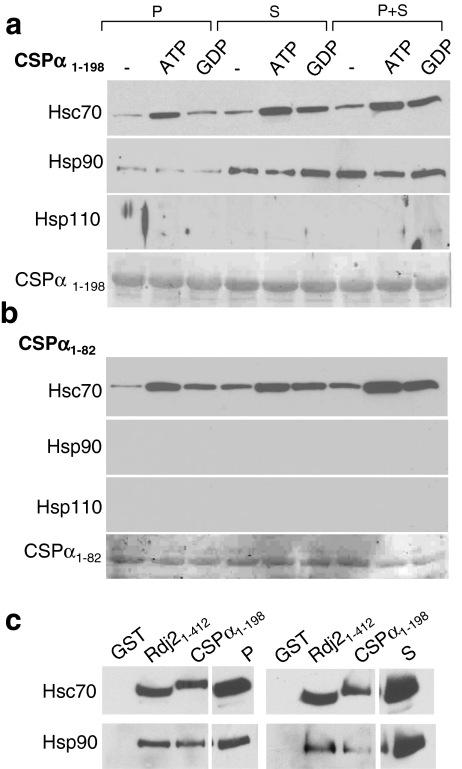
Rdj2 interacts with Hsc70, Hsp90, and Hsp110. (a) Western analysis showing the expression of chaperone systems in a rat brain membrane fraction (45 µg) (lanes denoted P), and rat brain cytosolic fraction (45 µg) (lanes denoted S). Nitrocellulose membranes were probed with a mouse anti-Hsc70 monoclonal antibody (Sigma), a mouse anti-Hsp60 monoclonal antibody (Sigma), a rat anti Hsp90 monoclonal antibody (Stressgen), a rabbit anti-Hsp110 polyclonal antibody (Stressgen), and a rabbit anti-Hsp25 polyclonal antibody (Stressgen). A non-specific immunoreactive band is indicated with an asterisk. (b) Western blot analysis showing the association of chaperones with Rdj21–412. Fusion proteins were immobilized on glutathione-sepharose beads and incubated in the presence of 230 μg of a rat brain detergent solubilized membrane fraction (P) or 250 μg of a rat brain cytosolic fraction (S) or both (P+S) in either the presence or absence of 2 mM ATP or GDP. The beads were washed and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis with the indicated antibodies. The bottom panel shows the Ponceau S staining profile of GST-Rdj2. (c) Western blot analysis showing the association of 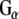 and
and 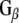 with GST-Rdj2 containing complexes. The nitrocellulose membrane was probed with either a rabbit anti-
with GST-Rdj2 containing complexes. The nitrocellulose membrane was probed with either a rabbit anti-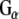 polyclonal antibody (Calbiochem) or mouse anti-
polyclonal antibody (Calbiochem) or mouse anti-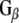 monoclonal antibody (Transduction labs). For
monoclonal antibody (Transduction labs). For 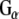 , the pixel values from left to right were 30,405; 37,983; 20,622; 0; 0; 0; 14,854; 28,310; 17,997. For
, the pixel values from left to right were 30,405; 37,983; 20,622; 0; 0; 0; 14,854; 28,310; 17,997. For 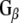 , the pixel values were 80,229; 83,480; 71,667; 220; 0; 5,053; 45,356; 61,599; 48,696. The results shown are representative of three independent experiments
, the pixel values were 80,229; 83,480; 71,667; 220; 0; 5,053; 45,356; 61,599; 48,696. The results shown are representative of three independent experiments
Next, we evaluated the possible association of Hsp110 and Hsp90 with Rdj2. GST-Rdj2 was coupled to glutathione-sepharose beads and incubated with either a solubilized membrane fraction (P) or cytosolic fraction (S) or both (P+S) in the presence and absence of either 2 mM ATP or 2 mM GDP. Figure 4b demonstrates that Hsc70, Hsp90, and Hsp110 were able to bind GST-Rdj2, suggesting that Rdj2 targets a multimeric chaperone complex to G proteins. In the presence of either ATP or GDP, the interaction of Rdj2 with both membrane-associated and cytosolic Hsc70 was observed to increase. These observations are consistent with previous reports showing an ATP-dependent increase in the Rdj2–Hsc70 association (Beck et al. 2006). In contrast, the interaction of Hsp90 and Hsp110 with Rdj2 was robust and not nucleotide dependent. As expected, more Hsp90 and Hsp110 were found to complex with Rdj2 when GST-Rdj2 was incubated with a cytosolic fraction (S) compared with a membrane fraction (P), reflecting the greater amounts of these proteins in the cytosolic fraction (Fig. 4a, b). Assembly of the Rdj2 multimeric chaperone complex did not alter the Rdj2–G protein interaction, which was maintained in the presence of ATP and GDP (Fig. 4c). These data indicate that the Rdj2–G protein association is part of a multi-protein evolutionary conserved chaperone complex.
Next, we compared the association of Hsp110 and Hsp90 in vitro with full-length GST-CSPα, and GST-CSPα1–82. CSPα1–82 encodes the conserved J domain, the region of highest similarity between CSPα and Rdj2 (Fig. 2). Previously, we demonstrated that ATP promotes a CSPα-Hsc70 association (Magga et al. 2000), and that CSPα preferentially interacts with the GDP-bound conformation of 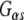 (Natochin et al. 2005). In pull-down assays using a rat brain cytosolic fraction, both ATP and GDP increased the association of CSPα with Hsc70, similar to that found for Rdj2 (Fig. 5a). In pull-down assays using a solubilized membrane particulate fraction, ATP, but not GDP, increased the CSPα–Hsc70 interaction, as previously reported (Miller et al. 2003b). In contrast to Rdj2, Hsp110 was not observed to associate with either CSPα or CSPα1–82. The Hsp90 association with CSPα1–198 was more evident when GST-CSPα was incubated with a rat brain cytosolic fraction (S) and weaker in a solubilized membrane fraction (P). Hsp90 was not found to associate with CSPα1–82, indicating that regions outside of the J domain are involved in its assembly and/or stability with full-length CSPα.
(Natochin et al. 2005). In pull-down assays using a rat brain cytosolic fraction, both ATP and GDP increased the association of CSPα with Hsc70, similar to that found for Rdj2 (Fig. 5a). In pull-down assays using a solubilized membrane particulate fraction, ATP, but not GDP, increased the CSPα–Hsc70 interaction, as previously reported (Miller et al. 2003b). In contrast to Rdj2, Hsp110 was not observed to associate with either CSPα or CSPα1–82. The Hsp90 association with CSPα1–198 was more evident when GST-CSPα was incubated with a rat brain cytosolic fraction (S) and weaker in a solubilized membrane fraction (P). Hsp90 was not found to associate with CSPα1–82, indicating that regions outside of the J domain are involved in its assembly and/or stability with full-length CSPα.
Fig. 5.
CSPα interacts with Hsc70 but not Hsp110. Western blot analysis showing the association of chaperones with (a) CSPα1–198, (b) CSPα1–82 or (c) CSPα1–198, Rdj21–412 or GST. Fusion proteins were immobilized on glutathione-sepharose beads and incubated in the presence of 230 µg of a rat brain detergent-solubilized membrane fraction (P) or 250 µg of a rat brain cytosolic fraction (S) or both (P+S) in either the presence or absence of 2 mM ATP or GDP. The beads were washed and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis. (a and b) The bottom panels show the Ponceau S staining profile of GST-CSPα1–198 and GST-CSPα1–82 respectively. (c) Lane 4 shows 20 µg of a rat brain detergent-solubilized membrane fraction (P) and lane 8 shows 20 µg of a rat brain cytosolic fraction (S). The results shown are representative of three to five independent experiments
There is currently no consensus as to whether Hsp90 can be coupled to the CSPα–Hsc70 chaperone system. Previous work utilizing biochemical cross-linking strategies identified a CSPα–Hsp90 complex (Sakisaka et al. 2002); however, the CSPα–Hsp90 interaction was not observed by yeast two-hybrid analysis (Stahl et al. 1999). We directly compared the Rdj2–Hsp90 and CSPα–Hsp90 complexes using our in vitro pull-down analysis. In each binding assay, an equal amount of fusion protein was immobilized to glutathione-sepharose beads, as confirmed by Ponceau S staining. Figure 5c shows that in vitro the association of Hsp90 with Rdj2 is greater than with CSPα. In contrast, Hsp90, and Hsc70 were not found to associate with GST alone, demonstrating the specificity of the protein–protein interactions. Exposure times of the P and S panels in Fig. 5c are not identical. Taken together, these results indicate that although the J proteins, Rdj2 and CSPα, both form complexes with G proteins; in vitro Hsp90 is more abundant in the Rdj2 complex than the CSPα complex. Furthermore, Hsp110 is present in the Rdj2 complex but absent from the CSPα complex.
The regulatory cofactors HOP and HIP are components of the Rdj2 complex
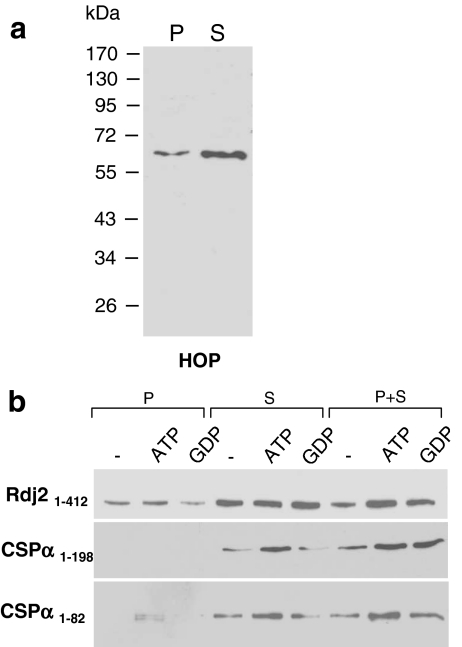
Next, we examined the Rdj2 chaperone complex for the presence of the cofactor Hsp70 organizing protein (HOP). HOP has a TPR domain, a degenerate 34 amino acid sequence found in more than 50 proteins that possess binding sites for an EEVD (glutamic acid, glutamic acid, valine, aspartic acid) motif, which is found in both Hsc70 and Hsp90. Therefore, HOP is a likely candidate cofactor for the coupling of Hsp90 to the Rdj2-Hsc70 chaperone system. It remains to be established if other TPR proteins (e.g., SGT) compete with HOP at these EEVD sites. Figure 6 clearly demonstrates that HOP is a major component of the GST–Rdj2 chaperone complex. HOP was detected in both the solubilized membrane (P) and cytosolic (S) rat brain fractions; however, the Rdj2–HOP association was greater in the cytosolic fraction. Taken together, these observations suggest that the ATPase Hsp90 is coupled to the Rdj2–Hsc70 ATPase chaperone machine through HOP.
Fig. 6.
Association of HOP with CSPα and Rdj2 chaperone complexes. Western blot analysis showing the association of HOP with Rdj21–412, CSPα1–198, and CSPα1–82 fusion proteins. (a) The expression of HOP in a solubilized membrane fraction (45 µg) (lane denoted by P), and rat brain cytosolic fraction (45 µg) (lane denoted by S). The electrophoretic positions of molecular weight standards (in kDa) are indicated on the left-hand side of the blot. The data shown are representative of three similar experiments. (b) Fusion protein was immobilized on glutathione-sepharose beads and incubated with 230 µg of a rat brain detergent-solubilized membrane fraction (P) or 250 µg of a rat brain cytosolic fraction (S) in either the presence or absence of 2 mM ATP or 2 mM GDP, as indicated. The beads were washed, and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis using a mouse anti-HOP monoclonal antibody (Stressgen)
CSPα and CSPα1–82 were also found to interact with cytosolic HOP (Fig. 6b). Like the CSPα–Hsc70 association, the CSPα-HOP and CSPα1–82–HOP interactions were moderately increased in the presence of ATP. Since HOP is predicted to interact with Hsc70, the ATP-dependent increase in association with CSPα was anticipated. Taken together, these observations suggest that while HOP may couple Hsp90 to Hsc70, in vitro the Rdj2–HOP–Hsp90 complex is more abundant than the CSPα–HOP–Hsp90 complex.
The Rdj2–G protein association is robust and stable, suggesting the presence of an Hsc70 regulatory cofactor that stabilizes the interaction of Rdj2 with its target protein. To investigate this possibility, we evaluated the Rdj2 complex for Hsp70 interacting protein (HIP). HIP binds to the ATPase domain of Hsc70 and has been proposed to stabilize the ADP-bound form (high affinity) of Hsc70, thereby preventing Hsc70’s discharge of client protein (Frydman and Hohfeld 1997). Figure 7b shows that HIP was detected in both the solubilized membrane (P) and cytosolic (S) rat brain fractions; however, the Rdj2-HIP association was greater in the cytosolic fraction. CSPα and CSPα1–82 were also found to associate with HIP (Fig. 7a). It is possible that in the membrane fraction HIP is in complex with other chaperones and therefore not freely available for association with CSPα or alternately that CSPα/HIP assembly is detergent sensitive. Taken together, these observations demonstrate that the protein cofactor HIP is a component of the J protein–Hsc70 folding machines: Rdj2–Hsc70, and CSPα-Hsc70. Further experimentation is required to establish the exact (direct versus indirect) interactions among the components of the Rdj2 complex identified and the potential involvement of HIP in the stability of the protein–protein interactions.
Fig. 7.
Association of HIP with CSPα and Rdj2 chaperone complexes. (a) Western blot analysis showing the association of HIP with Rdj21–412, CSPα 1–198, and CSPα1–82 fusion proteins. Fusion proteins were immobilized on glutathione-sepharose beads and incubated with 230 µg of a detergent-solubilized rat brain membrane fraction (P) or 250 µg of a rat brain cytosolic fraction (S) in the presence or absence of either 2 mM ATP or 2 mM GDP, as indicated. The beads were washed, and bound proteins were eluted in sample buffer, fractionated by SDS-PAGE and subjected to Western blot analysis with a mouse anti-HIP monoclonal antibody. (b) The bottom panel shows the expression of HIP in a solubilized rat brain membrane fraction (45 µg) (lane denoted by P), and rat brain cytosolic fraction (45 µg) (lane denoted by S). The electrophoretic positions of molecular weight standards (in kDa) are indicated on the left-hand side of the blot. The results shown are representative of at least three experiments
Estrogen does not alter Rdj2 expression in CAD mouse neuroblastoma cells
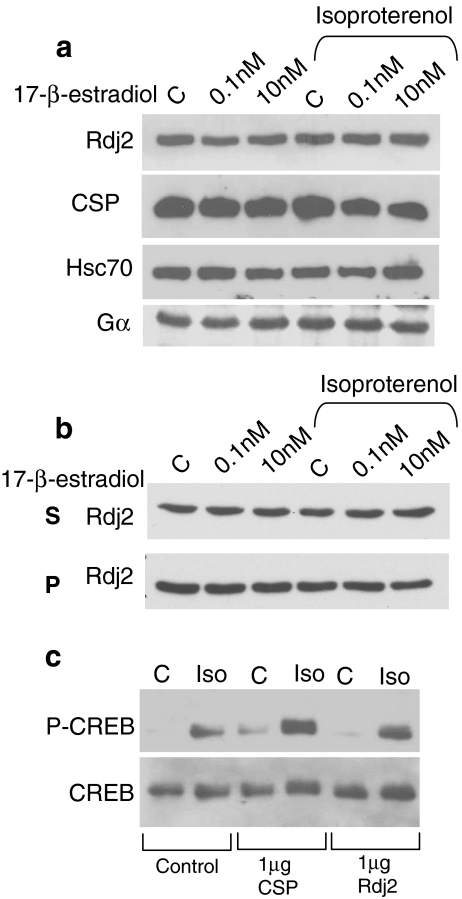
It has been suggested that Rdj2 expression is regulated by estrogen (Ohlsson et al. 2001). To explore these possible links, we examined the expression of Rdj2 in murine CAD neuroblastoma cells treated with 17-β-estradiol. No change in Rdj2 expression was observed in CAD cells exposed to 0.1 nM or 10 nM estradiol for 24 h (Fig. 8a). To better understand the role of J protein–Hsc70 chaperone machines in G-protein signaling, we examined isoproterenol-based signaling in CAD cells. Following treatment with estradiol for 24 h, a second group of cells were further exposed to 50 μM isoproterenol for 15 min. Neither 17-β-estradiol nor isoproterenol were observed to trigger changes in Rdj2, CSPα, Hsc70, or 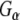 expression (Fig. 8a). We also evaluated CAD cell particulate and soluble fractions for preferential changes in Rdj2 expression after treatment with estradiol and isoproterenol. Figure 8b shows that no changes were observed in either the particulate or soluble fractions.
expression (Fig. 8a). We also evaluated CAD cell particulate and soluble fractions for preferential changes in Rdj2 expression after treatment with estradiol and isoproterenol. Figure 8b shows that no changes were observed in either the particulate or soluble fractions.
Fig. 8.
Rdj2 promotes CREB phosphorylation in CAD neuroblastoma cells. (a) Western blot analysis showing the expression of Rdj2, CSPα, and Hsc70 in CAD murine neuroblastoma cells treated with 0.1 nM or 10 nM 17-β-estradiol and/or 50 μM isoproterenol for 15 min. (b) Western blot analysis showing the expression of Rdj2 in pellet and soluble fractions of CAD cells treated with 0.1 nM or 10 nM 17-β-estradiol and/or 50 μM isoproterenol for 15 min. (c) Western blot analysis showing the expression of phosphoCREB and total CREB in CAD cells transiently transfected as indicated or lipofectamine alone (control), treated with 50 µM isoproterenol and 100 μM IBMX for 15 min. Twenty-five micrograms of protein was resolved by SDS-PAGE. For phosphoCREB, the pixel values from left to right were 13,820; 34,682; 12,216; 49,559; 100; and 35,079. Isoproterenol increased CREB phosphorylation by 1.5-fold in control cells, 3.0- fold in cells transfected with CSPα and 3.5-fold in cells transfected with Rdj2
Rdj2 promotes CREB phosphorylation in CAD neuroblastoma cells
To gain further insight into the molecular function of Rdj2, we evaluated the activation of cellular mechanisms downstream of G proteins. CAD cells were examined for the phosphorylation of cAMP response element-binding protein (CREB). Activation of the transcription factor CREB by phosphorylation serves as an independent readout of signaling through G proteins. We examined the role of Rdj2 in isoproterenol-stimulated CREB phosphorylation in CAD cells transfected with 1 μg of myc-tagged Rdj2 or myc-tagged CSPα. Isoproterenol-induced CREB phosphorylation was greater in CSPα (three-fold) and Rdj2 (3.5-fold) transfected CAD cells (Fig. 8c) than control cells (1.5-fold). By comparison, total CREB, myc-tagged Rdj2, and myc-tagged-CSPα levels do not change under the conditions evaluated. These data are consistent with the conclusion that Rdj2 enhances 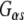 -mediated signaling.
-mediated signaling.
In summary, our experiments indicate that the multimeric Rdj2 chaperone complex enhances G-protein signaling.
Discussion
We have found that Rdj2, an ancient type-I J protein, directly interacts with heterotrimeric G proteins and that expression of Rdj2 in HEK cells increases isoproterenol-stimulated cAMP levels. Furthermore, in CAD cells, expression of Rdj2 increases isoproterenol-stimulated phosphorylation of CREB, which would be anticipated in response to elevated cAMP. Our data show that the Rdj2–G protein complex is a biochemically stable multimeric chaperone complex that includes the evolutionary conserved proteins Hsc70, Hsp110, and Hsp90, as well as the regulatory cofactors HIP and HOP. Rdj2 may target a number of proteins in the signaling pathway; however, the existence of a direct physical interaction between G proteins and Rdj2 leads us to favor a mechanism in which Rdj2 chaperones the activation of G proteins, perhaps as a GEF. While the association of Rdj2 with Hsp90 and Hsp110 is ATP-independent, the assembly of Hsc70 with this complex is ATP-sensitive, emphasizing the dynamic nature of the Rdj2 multimeric chaperone complex. Within the cell, it is anticipated that conformational work of the Rdj2–Hsc70–Hsp90 system is highly regulated through the assembly and disassembly of the ATPases with the protein cofactors. Several functional parallels between Rdj2 and the synaptic vesicle protein CSPα are evident. First, Rdj2 and CSPα are both J proteins that are abundantly expressed in the brain. Second, Rdj2 and CSPα directly interact with G proteins and increase isoproterenol-stimulated G-protein signaling. Finally, Rdj2 and CSPα both form multimeric chaperone complexes. Yet, notable differences between Rdj2 and CSPα are also apparent. Rdj2 is present in both membrane and cytosolic fractions of rat brain, while CSPα is found on synaptic vesicles (Mastrogiacomo et al. 1994; Blondeau et al. 2004) in neurons, as well as on secretory granules in exocrine (Braun and Scheller 1995; Zhao et al. 1997), endocrine (Brown et al. 1998), and neuroendocrine (Kohan et al. 1995; Chamberlain et al. 1996) tissues. Second, the nucleotide binding protein, Hsp110 is a component of the Rdj2, but not the CSPα complex. Finally, Rdj2 is type I, while CSPα is structurally distinct and classified as a type-III J protein (Qiu et al. 2006; Zhao et al. 2008). It has been proposed that the J protein family is comprised of “generalists and specialists” (Sahi and Craig 2007), raising the possibility that Rdj2 acts as a generalist with a role in surveillance. Notably, Rdj2 has been shown to associate with PrPC (Beck et al. 2006). A general chaperone activity for Rdj2 is further supported by its significant homology with Saccharomyces cerevisae Ydj1, a generalist J protein (Sahi and Craig 2007) and E. coli DnaJ. While CSPα and Rdj2 both have the ancient J domain, the absence of the DnaJ central region and the DnaJ C-terminal region suggests that the structure/function of CSPα has been fine-tuned to acquire specific features. The biological significance of the differences observed in these two biochemically stable and evolutionarily conserved J protein–G protein complexes and the precise roles they play in integrating G-protein signaling will undoubtedly be the basis of further investigation.
Our data demonstrate that Rdj2 regulates G-protein signaling is consistent with the emerging theme that J proteins are important GTPase regulators. We have previously shown that CSPα, the synaptic vesicle type-III J protein, is a 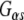 GEF (Magga et al. 2000; Miller et al. 2003a, b; Natochin et al. 2005). Dupre and colleagues have identified DRiP78, an endoplasmic reticulum type-III J protein as a
GEF (Magga et al. 2000; Miller et al. 2003a, b; Natochin et al. 2005). Dupre and colleagues have identified DRiP78, an endoplasmic reticulum type-III J protein as a 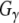 chaperone (Dupre et al. 2007). Also, genetic interactions between the type-III J proteins, auxilin and Rme-8, and the GTPase dynamin, have been identified (Chang et al. 2004; Sever et al. 2006). In addition to their association with GTPases, CSPα also interacts with voltage-gated calcium channels (Magga et al. 2000; Miller et al. 2003a, b), DRiP78 also interacts with G-protein coupled receptors (Dupre et al. 2007), and auxilin interacts with clathrin. Also, Hsj1b, a type-II J protein, has been shown to modulate the processing of the G-protein coupled receptor, rhodopsin (Chapple and Cheetham 2003). These results collectively suggest that several J protein–Hsc70 complexes chaperone cellular GTPases and associated proteins. It is tempting to speculate that J proteins have supported the evolutionary diversity of cellular GTPases. In general, chaperones are thought to function as evolutionary “capacitors” as a result of their ability to buffer misfolded proteins (Mayer and Bukau 2005; Arndt et al. 2007). Further experimentation is required to establish the full extent of the role the J protein network and their partner Hsp70s with regard to cellular GTPases.
chaperone (Dupre et al. 2007). Also, genetic interactions between the type-III J proteins, auxilin and Rme-8, and the GTPase dynamin, have been identified (Chang et al. 2004; Sever et al. 2006). In addition to their association with GTPases, CSPα also interacts with voltage-gated calcium channels (Magga et al. 2000; Miller et al. 2003a, b), DRiP78 also interacts with G-protein coupled receptors (Dupre et al. 2007), and auxilin interacts with clathrin. Also, Hsj1b, a type-II J protein, has been shown to modulate the processing of the G-protein coupled receptor, rhodopsin (Chapple and Cheetham 2003). These results collectively suggest that several J protein–Hsc70 complexes chaperone cellular GTPases and associated proteins. It is tempting to speculate that J proteins have supported the evolutionary diversity of cellular GTPases. In general, chaperones are thought to function as evolutionary “capacitors” as a result of their ability to buffer misfolded proteins (Mayer and Bukau 2005; Arndt et al. 2007). Further experimentation is required to establish the full extent of the role the J protein network and their partner Hsp70s with regard to cellular GTPases.
Although neurons are thought to be particularly vulnerable to the detrimental effects of misfolded proteins, our current knowledge of chaperones in the nervous system is fragmentary and many questions regarding neural chaperones remain. It is not yet known which of the 40 J proteins identified in humans are involved in neural function, the identity of their client proteins, or how these J proteins differ in function. J-protein folding machines are undoubtedly regulated by several cellular processes including: the heat shock response, the translocation of Hsc70 (Manzerra and Brown 1996; Manzerra et al. 1997; Bai et al. 2007), and the presence of protein cofactors. As such, a comprehensive molecular model of cellular J protein activity does not exist at present. Despite the current lack of molecular detail underlying J protein chaperone networks, in animal models, chaperones are the most powerful known inhibitors of neurodegeneration (Arndt et al. 2007; Muchowski 2002).
Members of the J protein family are found in a wide variety of species from E. coli to man. DnaJ, an E. coli J protein is essential for bacteriophage λ DNA replication (Georgopoulos et al. 1980). Figure 2 shows that in contrast to CSPα, which contains only one ancient domain (i.e., the J domain), Rdj2 has extensive regions of homology with E. coli DnaJ and S. cerevisiae Ydj1, an abundant yeast J protein whose deletion causes severe growth defects (Caplan and Douglas 1991).
In conclusion, the identification of neural chaperones and the proteins they regulate in vivo remains an important biological question. Our results reveal that Rdj2 binds to G proteins and enhances G protein-mediated signal transduction. We establish the identity of the components of the Rdj2 chaperone machine, an essential step that sets the stage toward understanding its function. The Rdj2 chaperone machinery is a multimeric chaperone complex that differs, in part, from the CSPα chaperone complex. The regulation of G-protein function by chaperones such as Rdj2 and CSPα may represent an important paradigm with regard to the control of neurotransmitter release and synaptic efficacy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(A) Length of the J domain, DnaJ central region, and DnaJ C-terminal region for DnaJ, Ydj1, Rdj2, and CSPα. InterProScan was used to identify the domains. (B) Amino acid homology between the J domains of DnaJ, Ydj1, Rdj2, and CSPα. (C) Amino acid homology between the full-length sequences of DnaJ, Ydj1, and Rdj2 (PDF 93.2 KB)
Acknowledgments
This work was supported by funding from the Alberta Prion Research Institute and the Canadian Institute of Health Research. We are grateful to Dr. L. Greene (National Institute of Health) for anti-auxilin polyclonal, to Dr. E. Lafer (University of Texas) for GST-auxilin construct, to Dr. M. Nyugen (University of Calgary) for CAD neuroblastoma cells, and Dr. M Bouvier (Université de Montréal) for the pcDNA3 β2 adrenergic receptor construct. APB is an Alberta Heritage Foundation for Medical Research Senior Scholar. JEAB is an Alberta Heritage Foundation for Medical Research Senior Scholar.
References
- Andres DA, Shao H, Crick DC, Finlin BS. Expression cloning of a novel farnesylated protein, RDJ2, encoding a DnaJ protein homologue. Arch Biochem Biophys. 1997;346:113–124. doi: 10.1006/abbi.1997.0296. [DOI] [PubMed] [Google Scholar]
- Arndt V, Rogon C, Hohfeld J. To be, or not to be – molecular chaperones in protein degradation. Cell Mol Life Sci. 2007;64(19–20):2525–2541. doi: 10.1007/s00018-007-7188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Swayne LA, Braun JE. The CSPalpha/G protein complex in PC12 cells. Biochem Biophys Res Commun. 2007;352:123–129. doi: 10.1016/j.bbrc.2006.10.178. [DOI] [PubMed] [Google Scholar]
- Beck KE, Kay JG, Braun JE. Rdj2, a J protein family member, interacts with cellular prion PrP(C) Biochem Biophys Res Commun. 2006;346:866–871. doi: 10.1016/j.bbrc.2006.05.185. [DOI] [PubMed] [Google Scholar]
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JE, Scheller RH. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology. 1995;34:1361–1369. doi: 10.1016/0028-3908(95)00114-L. [DOI] [PubMed] [Google Scholar]
- Braun JE, Wilbanks SM, Scheller RH. The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem. 1996;271:25989–25993. doi: 10.1074/jbc.271.42.25989. [DOI] [PubMed] [Google Scholar]
- Brown H, Larsson O, Branstrom R, Yang S, Leibiger B, Leibiger I, Fried G, Moede T, Deeney JT, Brown GR, Jacobsson G, Rhodes CJ, Braun JE, Scheller RH, Corkey BE, Berggren P, Meister B. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. EMBO J. 1998;17:5048–5058. doi: 10.1093/emboj/17.17.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Douglas MG. Characterization of YDJ1: a yeast homologue of the bacterial dnaJ protein. J Cell Biol. 1991;114:609–621. doi: 10.1083/jcb.114.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Henry J, Burgoyne RD. Cysteine string proteins are associated with chromaffin granules. J Biol Chem. 1996;271:19514–19517. doi: 10.1074/jbc.271.32.19514. [DOI] [PubMed] [Google Scholar]
- Chang HC, Hull M, Mellman I. The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J Cell Biol. 2004;164:1055–1064. doi: 10.1083/jcb.200311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J Biol Chem. 2003;278:19087–19094. doi: 10.1074/jbc.M212349200. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:SFAEOD>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham ME, Jackson AP, Anderton BH. Regulation of 70-kDa heat-shock-protein ATPase activity and substrate binding by human DnaJ-like proteins, HSJ1a and HSJ1b. Eur J Biochem. 1994;226:99–107. doi: 10.1111/j.1432-1033.1994.tb20030.x. [DOI] [PubMed] [Google Scholar]
- Craig EA, Huang P, Aron R, Andrew A. The diverse roles of J-proteins, the obligate Hsp70 co-chaperone. Rev Physiol Biochem Pharmacol. 2006;156:1–21. doi: 10.1007/s10254-005-0001-0. [DOI] [PubMed] [Google Scholar]
- Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282:13703–13715. doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/S0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos CP, Lundquist-Heil A, Yochem J, Feiss M. Identification of the E. coli dnaJ gene product. Mol Gen Genet. 1980;178:583–588. doi: 10.1007/BF00337864. [DOI] [PubMed] [Google Scholar]
- Kohan SA, Pescatori M, Brecha NC, Mastrogiacomo A, Umbach JA, Gundersen CB. Cysteine string protein immunoreactivity in the nervous system and adrenal gland of rat. J Neurosci. 1995;15:6230–6238. doi: 10.1523/JNEUROSCI.15-09-06230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magga JM, Jarvis SE, Arnot MI, Zamponi GW, Braun JE. Cysteine string protein regulates G-protein modulation of N-type calcium channels. Neuron. 2000;28:195–204. doi: 10.1016/S0896-6273(00)00096-9. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Brown IR. The neuronal stress response: nuclear translocation of heat shock proteins as an indicator of hyperthermic stress. Exp Cell Res. 1996;229:35–47. doi: 10.1006/excr.1996.0341. [DOI] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170:130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A, Parsons SM, Zampighi GA, Jenden DJ, Umbach JA, Gundersen CB. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994;263:981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Swayne LA, Chen L, Feng ZP, Wacker JL, Muchowski PJ, Zamponi GW, Braun JEA. Cysteine String Protein (CSP) inhibition of N-type calcium channels is blocked by mutant huntingtin. J Biol Chem. 2003;278:53072–53081. doi: 10.1074/jbc.M306230200. [DOI] [PubMed] [Google Scholar]
- Miller LC, Swayne LA, Kay JG, Feng ZP, Jarvis SE, Zamponi GW, Braun JEA. Molecular detrminants of cysteine string protien modulation of N-type calcium channels. J Cell Sci. 2003;116:2967–2974. doi: 10.1242/jcs.00595. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for moleclar chaperones. Neuron. 2002;35:9–12. doi: 10.1016/S0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- Natochin M, Campbell TN, Barren B, Miller LC, Hameed S, Artemyev NO, Braun JE. Characterization of the G alpha(s) regulator cysteine string protein. J Biol Chem. 2005;280:30236–30241. doi: 10.1074/jbc.M500722200. [DOI] [PubMed] [Google Scholar]
- Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Brunner N, Engelholm LH, Lundholt BK, Weidle U, Briand P, Lykkesfeldt AE. Identification of two estrogen regulated genes associated with growth regulation of human breast cancer. Mol Cell Endocrinol. 2001;182:1–11. doi: 10.1016/S0303-7207(01)00559-7. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Hata M. Mammalian HSP40/DNAJ homologs: cloning of novel cDNAs and a proposal for their classification and nomenclature. Cell Stress Chaperones. 2000;5:98–112. doi: 10.1379/1466-1268(2000)005<0098:MHDHCO>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahi C, Craig EA. Network of general and specialty J protein chaperones of the yeast cytosol. Proc Natl Acad Sci USA. 2007;104:7163–7168. doi: 10.1073/pnas.0702357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Meerlo T, Matteson J, Plutner H, Balch WE. rab-alphaGDI activity is regulated by a Hsp90 chaperone complex. EMBO. 2002;21:6125–6135. doi: 10.1093/emboj/cdf603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S, Skoch J, Newmyer S, Ramachandran R, Ko D, McKee M, Bouley R, Ausiello D, Hyman BT, Bacskai BJ. Physical and functional connection between auxilin and dynamin during endocytosis. EMBO J. 2006;25:4163–4174. doi: 10.1038/sj.emboj.7601298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl B, Tobaben S, Sudhof TC. Two distinct domains in hsc70 are essential for the interaction with the synaptic vesicle cysteine string protein. Eur J Cell Biol. 1999;78:375–381. doi: 10.1016/S0171-9335(99)80079-X. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SEH, Linder R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Zhao CM, Jacobsson G, Chen D, Hakanson R, Meister B. Exocytotic proteins in enterochromaffin-like (ECL) cells of the rat stomach. Cell Tissue Res. 1997;290:539–551. doi: 10.1007/s004410050960. [DOI] [PubMed] [Google Scholar]
- Zhao X, Braun AP, Braun JE (2008) Biological roles of neural J proteins. Cell Mol Life Sci, in press [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(A) Length of the J domain, DnaJ central region, and DnaJ C-terminal region for DnaJ, Ydj1, Rdj2, and CSPα. InterProScan was used to identify the domains. (B) Amino acid homology between the J domains of DnaJ, Ydj1, Rdj2, and CSPα. (C) Amino acid homology between the full-length sequences of DnaJ, Ydj1, and Rdj2 (PDF 93.2 KB)