Abstract
The oncogene p3k, coding for a constitutively active form of phosphatidylinositol 3-kinase (PI 3-kinase), strongly activates myogenic differentiation. Inhibition of endogenous PI 3-kinase activity with the specific inhibitor LY294002, or with dominant-negative mutants of PI 3-kinase, interferes with myotube formation and with the expression of muscle-specific proteins. Here we demonstrate that a downstream target of PI 3-kinase, serine-threonine kinase Akt, plays an important role in myogenic differentiation. Expression of constitutively active forms of Akt dramatically enhances myotube formation and expression of the muscle-specific proteins MyoD, creatine kinase, myosin heavy chain, and desmin. Transdominant negative forms of Akt inhibit myotube formation and the expression of muscle-specific proteins. The inhibition of myotube formation and the reduced expression of muscle-specific proteins caused by the PI 3-kinase inhibitor LY294002 are completely reversed by constitutively active forms of Akt. Wild-type cellular Akt effects a partial reversal of LY294002-induced inhibition of myogenic differentiation. This result suggests that Akt can substitute for PI 3-kinase in the stimulation of myogenesis; Akt may be an essential downstream component of PI 3-kinase-induced muscle differentiation.
Keywords: muscle-specific proteins, protein phosphorylation, transdominant negative mutant
The retroviral oncogene v-p3k codes for a homolog of the catalytic subunit p110α of phosphatidylinositol 3-kinase (PI 3- kinase); it induces oncogenic transformation of chicken embryo fibroblasts (CEF) in culture and causes hemangiosarcomas in young chicken (1). Overexpression of p3k in chicken embryo myoblasts (CEM) activates myotube formation and increases expression of muscle-specific proteins (2). PI 3-kinase is known to stimulate the differentiation of precursor cells of skeletal muscle (3, 4). Interference with PI 3-kinase activity, either by the chemical inhibitor LY294002 or with a dominant negative mutant of p85, which is the regulatory subunit of PI 3-kinase, blocks myotube formation and the expression of muscle-specific proteins (2–4). These results strongly support the conclusion that PI 3-kinase activity is essential for skeletal myogenesis. An important role for PI 3-kinase has also been suggested for smooth muscle differentiation (5).
Among the downstream targets of PI 3-kinase are phospholipase C (6–9), protein kinase C (10, 11), Rac (12–14), and the serine-threonine kinase Akt/protein kinase B. Akt was identified as the product of the oncogene v-akt in the lymphomagenic murine retrovirus, AKT8 (15). At the same time, Akt was named protein kinase B and RAC-PK (related to the A and C kinases) based on its homology with both protein kinase A and protein kinase C (16, 17). Akt is an important component of survival signals from several growth factors (18–26). It can induce oncogenic transformation of CEF and, like p3k, it causes hemangiosarcomas in chickens (27). Here we show that constitutively active forms of Akt activate myogenic differentiation and that Akt is an essential participant in PI 3-kinase-mediated myogenesis.
MATERIALS AND METHODS
Myoblast Cell Culture and Virus Production.
CEM were prepared as described previously (2). Akt proteins were expressed from the avian retroviral vector RCAS (27, 28). They included cellular Akt (c-Akt), a myristylated constitutively active form of c-Akt (Akt-Myr), and several Akt mutants (29) described in the Results section. For immune detection, Akt proteins were tagged with an epitope derived from the influenza virus hemagglutinin protein. The RCAS constructs were transfected into CEF, which then produced high-titer retroviral progeny with the respective insert (28). CEM were initially cultured in myoblast growth (MG) medium, a mixture of nutrient solutions M199 and F10 at a 2:1 ratio, supplemented with 10% fetal bovine serum and 5% chicken embryo extract. Two days after infection, CEM were switched to myoblast differentiation (MD) medium, which is identical to MG medium except for the substitution of 10% horse serum for fetal bovine serum.
Akt Kinase Assay.
CEM were infected with RCAS vector or RCAS-Akt-Myr. On day 5 after infection, cells were washed and scraped from the plates with ice-cold PBS. The in vitro kinase activity was determined after a published technique (29). The cells were incubated for 15 min on ice in lysis buffer (150 mM NaCl/20 mM Tris⋅HCl, pH 7.5/10% glycerol/1% Nonidet P-40/1 mM EDTA/10 mM NaF/1 mM DTT/1 mM phenylmethylsulfonyl fluoride/1 mM sodium vanadate/2 mM leupeptin/2 mM aprotinin), and centrifuged at 15,000 × g for 10 min to clarify the supernatants. Aliquots containing 200 μg of soluble protein were precleared with 30 μl of protein A/G agarose beads (Santa Cruz Biotechnology). The lysates were then incubated with monoclonal anti-hemagglutinin antibody 12CA5 and precipitated by using 30 μl of protein A/G agarose beads. To determine the endogenous Akt activity in CEM expressing the kinase-defective Akt mutant, Akt-K179M, lysates from CEM infected by RCAS or RCAS-Akt-K179M were immunoprecipitated three times with anti-hemagglutinin antibody 12CA5 to remove the Akt-K179M protein. The endogenous Akt was precipitated with rabbit anti-Akt antibodies and protein A/G agarose beads (Santa Cruz Biotechnology). Immunoprecipitates were washed five times with Nonidet P-40 lysis buffer and incubated at room temperature for 15 min in kinase reaction buffer (20 mM Hepes, pH 7.4/10 mM MgCl2/10 mM MnCl2/1 mM DTT/0.1 μg/ml histone H2B/2 μM ATP/10 μCi of [γ-32P]ATP (DuPont/NEN). Components of the kinase reaction were separated by 12% SDS/PAGE and electroblotted onto nitrocellulose membranes, and phosphorylation of histone H2B was visualized by autoradiography.
Immunoblot Analysis.
Immunoblot analysis was performed as described (2). CEM were lysed in RIPA buffer (150 mM NaCl/100 mM Tris⋅HCl, pH 8.0/1% Triton X-100/1% deoxycholic acid/0.1% SDS/5 mM EDTA/10 mM NaF) supplemented with 5 mM DTT/1 mM phenylmethylsulfonyl fluoride/1 mM sodium vanadate/20 μM leupeptin/100 μM aprotinin. The lysates were clarified by centrifugation at 15,000 × g for 10 min. Aliquots were resolved by SDS/PAGE and transferred to nitrocellulose membranes in 20 mM Tris⋅HCl, pH 8.0/150 mM glycine/20% (vol/vol) methanol. Membranes were blocked with 5% nonfat dry milk in PBS/0.05% Tween 20 buffer and incubated with antibodies specific for MyoD (Santa Cruz Biotechnology), myosin heavy chain (MHC), desmin (ICN), and actin (Sigma). Protein bands were detected by the incubation with horseradish peroxidase-conjugated antibodies (Amersham) and chemiluminescence reagent (DuPont/NEN).
RESULTS
Akt Stimulates the Formation of Myotubes in Cultures of CEM.
To determine whether ectopic expression of the Akt protein could affect MD, CEM were infected with the viral form of RCAS expressing Akt-Myr, a constitutively active form of Akt generated by fusing a myristylation signal to the amino terminus of the mouse c-Akt. As control, CEM were infected with the RCAS viral vector alone or with the Prague strain of Rous sarcoma virus expressing the Src kinase, a known inhibitor of myogenic differentiation (20). Successful infection of CEM and expression of Akt-Myr were verified 5 days after infection. Total cell extracts separated by PAGE and tested in an immunoblot showed the presence of the Akt-Myr protein in CEM. Immune complex kinase assays with histone H2B as a substrate demonstrated the enzymatic activity of the Akt-Myr construct (Fig. 1A). Successful infection in the vector-only control cultures was confirmed by the expression of the retroviral structural protein Gag at similar levels as in RCAS Akt-Myr-infected CEM. Starting with day 5 after infection, the Akt-Myr-expressing CEM showed increased fusion into postmitotic myotubes that were larger than the ones observed in control cultures infected with RCAS alone (Fig. 1B). As expected, CEM expressing the viral Src kinase did not exit the cell cycle and failed to fuse into myotubes (data not shown). These observations show that a constitutively active form of the Akt kinase with an affinity for the plasma membrane stimulates myogenic differentiation.
Figure 1.

(A) Increase of Akt kinase activity in CEM expressing Akt-Myr. CEM were prepared as described in Materials and Methods and were infected with the retroviral vector RCAS (lane 1) or with RCAS-Akt-Myr (lane 2). The cells were cultured for 2 days in MG medium, followed by 3 days in MD medium. On day 5 after infection, Akt kinase activities were determined as described in the Materials and Methods section. (B) Enhancement of myogenic differentiation by Akt-Myr. CEM were infected with the RCAS vector or RCAS-Akt-Myr and cultured as above. Representative fields were photographed 5 days after infection (6.5× objective, phase contrast). (C) Induction of muscle-specific proteins by Akt-Myr. Total proteins were prepared from CEM on day 8 after infection with RCAS vector (lane 1), RCAS-Akt-Myr (lane 2), or v-Src (lane 3). Muscle-specific protein levels were analyzed by immunoblot assay as described in the Materials and Methods section. Actin served as ubiquitously expressed control and was detected with rabbit antibodies from Sigma. Similar results were obtained in three independent experiments.
Akt Enhances the Expression of Muscle-Specific Proteins.
We used the muscle-specific proteins creatine kinase (CK), MyoD, MHC, and desmin as markers of myogenesis. CK activity in Akt-Myr expressing CEM became elevated on day 4 after infection and by day 9 was about 2.5 times the control value of CEM cultures infected with vector alone (data not shown). The protein levels of MyoD, MHC, and desmin were significantly increased in CEM expressing Akt-Myr as compared with CEM infected with vector alone (Fig. 1C). In CEM expressing Src, production of muscle-specific proteins was strongly inhibited. The morphological changes induced by Akt-Myr in CEM are therefore accompanied by elevated expression of muscle-specific proteins.
To determine whether CEF transformed by Akt-Myr secrete inducer(s) of myogenesis, conditioned medium prepared from Akt-transformed CEF was separated by ultracentrifugation into virus-free medium and virus pellet. A small amount of infectious Akt-Myr virus recovered from the pellet was able to induce myotube formation and increase expression of CK, MyoD, MHC, and desmin in CEM cultures. However, the virus-free conditioned medium failed to affect myotube fusion and expression of muscle-specific proteins. This result argues against a paracrine mechanism for the stimulation of myogenesis by Akt and suggests that Akt induces an intracellular signal that leads to differentiation.
Dominant Negative and Kinase-Negative Mutants of Akt Inhibit Myogenic Differentiation, CK Activity, and the Expression of Muscle-Specific Proteins.
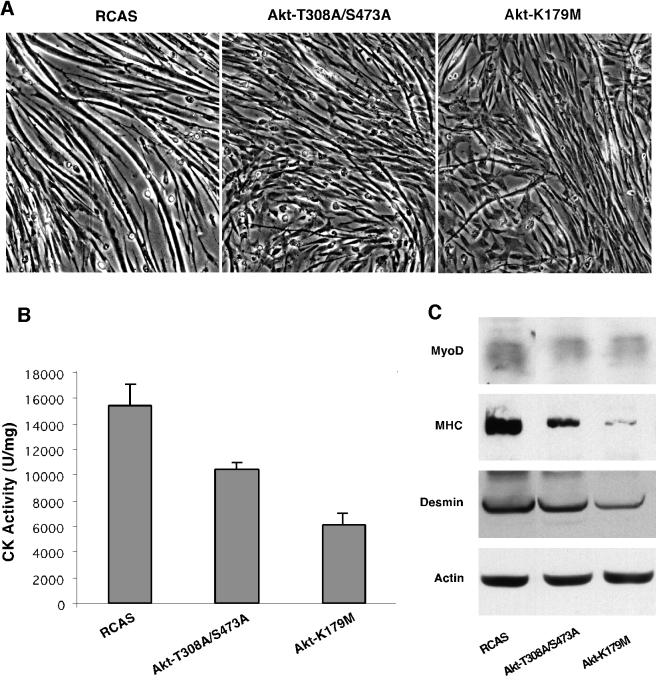
Akt kinase is activated by two phosphoinositide-dependent kinases, PDK-1 and PDK-2, which phosphorylate threonine 308 and serine 473 of Akt (30). The Akt mutant Akt-T308A/S473A, in which these two amino acids are replaced with alanines, cannot be activated by PDK-1 and PDK-2. This mutant functions as a dominant negative of Akt kinase, interferes with endogenous Akt activity, and inhibits PI 3-kinase-induced oncogenic transformation of CEF (27, 31). We have expressed Akt-T308A/S473A in CEM. The formation of myotubes was significantly reduced in these cultures as compared with the vector control (Fig. 2A). The inhibition was partial and not as drastic as that induced by the viral Src kinase. This observation suggests that Akt stimulates the process of myogenic differentiation only if it is phosphorylated by PDK1 and PDK2 and functions as an active kinase. As an additional test of the requirements for kinase activity of Akt in myogenic differentiation, we expressed the mutant Akt-K179M in CEM. Akt-K179M has an amino acid substitution in the kinase domain and lacks detectable Akt kinase activity (27, 29). On day 6 after infection, CK activity was markedly reduced in mutant-infected CEM as compared with vector control (Fig. 2B). Significant reductions were also seen in the expression levels of the muscle-specific proteins MyoD, MHC, and desmin, as determined by immunoblot analysis (Fig. 2C). To measure endogenous c-Akt activity, mutant-infected and control CEM were lysed in Nonidet P-40 buffer on day 5 after infection and immunoprecipitated three times with anti-hemagglutinin monoclonal antibody to deplete levels of Akt-K179M. In vitro kinase assays then revealed lowered endogenous Akt kinase activity as compared with vector control.
Figure 2.
Expression of Akt mutants inhibits myogenic differentiation. (A) The mutants Akt-T308A/S473A and Akt-K179M inhibit myotube formation. CEM were infected with RCAS or RCAS viruses expressing Akt-T308A/S473A or Akt-K179M. The cells were cultured in MG medium for 2 days after infection, followed by 3 days in MD medium to induce MD. Representative fields were photographed on day 5 after infection (6.5× objective, phase contrast). (B) Expression of Akt mutants inhibits CK activity. CK activity was determined on day 6 in CEM infected and cultured as described above. Mean ± SE values were from two experiments with four replicate assays. (C) Expression of Akt mutants reduces the levels of muscle-specific proteins. Muscle-specific proteins were analyzed in CEM on day 6 after infection with RCAS, RCAS-Akt-T308A/S473A, or RCAS-Akt-K179M viruses and assayed by immunoblot as in Fig. 1C. Similar results were obtained in replicate experiments.
Akt Can Substitute for PI 3-Kinase in the Induction of Myogenic Differentiation.
Recent studies have documented an essential role of PI 3-kinase in myogenic differentiation (2, 3). Inhibition of PI 3-kinase with LY294002 interferes with muscle differentiation. To test whether this inhibition could be reversed by Akt, we expressed Akt-Myr and wild-type c-Akt in CEM cultures and then treated these cells with the specific inhibitor LY294002. In the absence of inhibitor, myotube fusion was highly stimulated by Akt-Myr, but was not affected by c-Akt. In the presence of 25 μM LY294002, myotube formation in CEM infected by the RCAS vector alone was completely inhibited (Fig. 3). Expression of Akt-Myr reversed this inhibition and strongly stimulated myotube formation. Expression of c-Akt partially counteracted the PI 3-kinase inhibitor (Fig. 3). The levels of CK (Fig. 4A), MyoD, MHC, and desmin (Fig. 4B) were also significantly increased in CEM expressing Akt-Myr in the presence of inhibitor. c-Akt restored only the desmin level in inhibitor-treated CEM, a result consistent with the low kinase activity of c-Akt (27, 29). These observations demonstrate that disruption of myogenic differentiation by an inhibitor of PI 3-kinase can be reversed by the expression of Akt and suggest that Akt is an essential downstream component of the myogenic signal originating with PI 3-kinase.
Figure 3.
The inhibition of myotube formation by the PI 3-kinase-specific inhibitor LY294002 is counteracted by the expression of Akt. CEM cultured in MG medium were infected with RCAS, RCAS-Akt-Myr, or RCAS-c-Akt. On day 2 after infection, the cells were switched to MD medium and treated with 25 μM LY294002 (LY, +) or dimethyl sulfoxide solvent (LY, −) for 3 days. Representative fields were photographed on day 5 after infection (6.5× objective, phase contrast).
Figure 4.
Expression of Akt-Myr increases levels of muscle-specific proteins in the presence of LY294002. CK activity (A) and muscle-specific protein levels (B) were analyzed on day 5 after infection by using CEM treated as in Fig. 3.
DISCUSSION
The serine-threonine kinase Akt and its upstream activator, PI 3-kinase, share some cellular functions: they can stimulate cell growth and inhibit apoptosis. Both kinases have oncogenic potential; overexpression of constitutively active forms of PI 3-kinase or of Akt induces oncogenic transformation of cells in culture and tumors in animals (1, 27). In the context of muscle progenitors, PI 3-kinase and Akt signal exit from the cell cycle and initiate terminal differentiation. Constitutively active Akt is a strong stimulator of myogenesis, similar to PI 3-kinase. Akt greatly increases myotube formation in cultures of CEM and up-regulates the muscle-specific proteins CK, MyoD, MHC, and desmin. Overexpression of a dominant negative mutant of Akt, Akt-T308A/S473A, or of a kinase-defective mutant, Akt-K179M, interferes with endogenous Akt function, inhibits myotube formation, and prevents the up-regulation of muscle-specific proteins. A correlation between elevated protein levels of Akt-2 and muscle differentiation has also been described in a mammalian cell culture system (32). These results suggest that Akt plays an important and perhaps essential role in myogenic differentiation.
Akt has also been shown to stimulate the differentiation of adipocytes. It responds to signals from the insulin receptor that are transmitted by PI 3-kinase and result in translocation of Akt to the plasma membrane (33, 34). Constitutive membrane localization of Akt by fusion to a myristylation signal induces spontaneous differentiation of mouse preadipocytes (35, 36). Adipogenic differentiation was also observed in cultures of CEF expressing Akt-Myr (27).
Downstream targets of Akt include glycogen synthase kinase 3, FRAP (FK506-binding protein, rapamycin-associated protein, synonyms: mTOR, RAFT), and the apoptosis-inducing protein BAD. Glycogen synthase kinase 3 is an important component of Wnt signaling; it is inhibited by Akt-dependent phosphorylation (37, 38). This inhibition leads to increased levels of β-catenin, which then enters the nucleus and together with the lymphoid-enhancing factor family of DNA-binding proteins acts as a strong growth stimulator. FRAP is a member of the ataxia telangiectasia family of PI 3-kinases (39). It phosphorylates and activates both the ribosomal protein S6 kinase (p70 S6 kinase) and the protein that binds to the eukaryotic initiation factor 4E, 4E-BP1 (40). The result is enhanced translation of certain mRNAs. The apoptosis-inducing protein BAD is inactivated by Akt-dependent phosphorylation, improving cell survival (41, 42). It is currently unknown whether any of these Akt targets play a role in myogenesis. Activated forms of β-catenin and of LEF-1 have been described and could be tested for their effect on myogenic differentiation (43–45). For p70 S6 kinase, contradictory data have been published, some suggesting a role of p70 S6 kinase in myogenesis and others arguing against such a role (46, 47). Protection against apoptosis may also be important in muscle differentiation and could be tested by overexpressing in myoblasts known inhibitors of apoptosis, e.g., Bcl-2.
The PI 3-kinase-specific inhibitor LY294002, at a concentration of 25 μM, completely inhibited myogenic differentiation of CEM. This inhibition can be reversed by constitutively active Akt, which rescues the myogenic program in LY294002-treated CEM. Akt can therefore substitute for PI 3-kinase in signaling myogenic differentiation. The simplest interpretation of this result is that the myogenic signal issued by PI 3-kinase proceeds through and is mediated by Akt. This result is in apparent contradiction to studies that find an inhibitory effect of LY294002 and of wortmannin on myristylated Akt (48, 49). The effect probably results from the down-regulation of the PDK-1 and PDK-2 kinases, which are activated by PI 3-kinase and which in turn control Akt by phosphorylating it at threonine 308 and serine 473. However, the effect of the PI 3-kinase inhibitor on Akt is dose dependent. Whereas at 25 μM LY294002 Akt-Myr can overcome the inhibition of myogenesis, at 50 μM of inhibitor the rescue of myogenesis by Akt-Myr is only partial, and there is no rescue by c-Akt.
The nature of the myogenic signal travelling through PI 3-kinase and Akt is not known. The upstream stimulus for PI 3-kinase probably originates with the binding of a growth factor to its receptor; examples are insulin-like growth factor, platelet-derived growth factor, or epidermal growth factor. The signal generated by insulin-like growth factor appears to split into mitogenic activity, mediated by MAP kinases and myogenic activity, dependent on PI 3-kinase (46). PI 3-kinase and Akt signals also have two possible outcomes, oncogenic transformation or terminal differentiation into myotubes or adipocytes. Yet signals originating with these kinases are not generally tissue specific; additional factors must come into play that modulate the signal. The identification and characterization of these determinant factors now becomes an important task.
Acknowledgments
We thank Drs. Alfonso Bellacosa and Philip Tsichlis for generously providing Akt constructs and Dr. Richard A. Lerner for a gift of the 12CA5 monoclonal antibody. This work was supported by National Institutes of Health Grants CA 42564 and CA 78230. B.-H.J. is the recipient of a postdoctoral fellowship CA 77892 from the National Cancer Institute.
ABBREVIATIONS
- CEM
chicken embryo myoblasts
- PI 3-kinase
phosphatidylinositol 3-kinase
- CEF
chicken embryo fibroblasts
- CK
creatine kinase
- MHC
myosin heavy chain
- c-AKT
cellular AKT
- Akt-Myr
a myristylated constitutively active form of c-Akt
- MG
myoblast growth
- MD
myoblast differentiation
References
- 1.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 2.Jiang B-H, Zheng J Z, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14179–14183. doi: 10.1073/pnas.95.24.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaliman P, Canicio J, Shepherd P R, Beeton C A, Testar X, Palacin M, Zorzano A. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- 4.Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. J Biol Chem. 1998;273:28860–28867. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 6.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae Y S, Cantley L G, Chen C S, Kim S R, Kwon K S, Rhee S G. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 8.Barker S A, Caldwell K K, Pfeiffer J R, Wilson B S. Mol Biol Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratacap M P, Payrastre B, Viala C, Mauco G, Plantavid M, Chap H. J Biol Chem. 1998;273:24314–24321. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- 10.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 11.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C S, Newton A C, Schaffhausen B S, Toker A. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 13.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Nature (London) 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 14.Gringhuis S I, de Leij L F, Coffer P J, Vellenga E. Mol Cell Biol. 1998;18:1725–1735. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 16.Coffer P J, Woodgett J R. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 20.Franke T F, Cantley L C. Nature (London) 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- 21.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 22.Songyang Z, Baltimore D, Cantley L C, Kaplan D R, Franke T F. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulik G, Klippel A, Weber M J. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Nature (London) 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 25.Marte B M, Downward J. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 26.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 27.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 30.Anderson K E, Coadwell J, Stephens L R, Hawkins P T. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, et al. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calera M R, Pilch P F. Biochem Biophys Res Commun. 1998;251:835–841. doi: 10.1006/bbrc.1998.9566. [DOI] [PubMed] [Google Scholar]
- 33.Wijkander J, Holst L S, Rahn T, Resjo S, Castan I, Manganiello V, Belfrage P, Degerman E. J Biol Chem. 1997;272:21520–21526. doi: 10.1074/jbc.272.34.21520. [DOI] [PubMed] [Google Scholar]
- 34.Goransson O, Wijkander J, Manganiello V, Degerman E. Biochem Biophys Res Commun. 1998;246:249–254. doi: 10.1006/bbrc.1998.8602. [DOI] [PubMed] [Google Scholar]
- 35.Magun R, Burgering B M, Coffer P J, Pardasani D, Lin Y, Chabot J, Sorisky A. Endocrinology. 1996;137:3590–3593. doi: 10.1210/endo.137.8.8754791. [DOI] [PubMed] [Google Scholar]
- 36.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 37.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 38.Shaw M, Cohen P, Alessi D R. FEBS Lett. 1997;416:307–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- 39.Scott P H, Brunn G J, Kohn A D, Roth R A, Lawrence J C., Jr Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence J C, Jr, Abraham R T. Trends Biochem Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 41.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 42.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 43.Aoki, M., Hecht, A., Kruse, U., Kemler, R. & Vogt, P. K. (1999) Proc. Natl. Acad. Sci. USA96, in press. [DOI] [PMC free article] [PubMed]
- 44.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 45.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 46.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 47.Canicio J, Gallardo E, Illa I, Testar X, Palacin M, Zorzano A, Kaliman P. Endocrinology. 1998;139:5042–5049. doi: 10.1210/endo.139.12.6360. [DOI] [PubMed] [Google Scholar]
- 48.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Jr, Roth R A. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 49.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]