Abstract
The chaperonin containing TCP-1 (CCT) is required for the production of native actin and tubulin and numerous other proteins, several of which are involved in cell cycle progression. The mechanistic details of how CCT acts upon its folding substrates are intriguing: whilst actin and tubulin bind in a sequence-specific manner, it is possible that some proteins could use CCT as a more general binding interface. Therefore, how CCT accommodates the folding requirements of its substrates, some of which are produced in a cell cycle-specific manner, is of great interest. The reliance of folding substrates upon CCT for the adoption of their native structures results in CCT activity having far-reaching implications for a vast array of cellular processes. For example, the dependency of the major cytoskeletal proteins actin and tubulin upon CCT results in CCT activity being linked to any cellular process that depends on the integrity of the microfilament and microtubule-based cytoskeletal systems.
Keywords: CCT chaperonin, Cytoskeleton, Cell cycle, Actin, Tubulin
Introduction
The challenges faced by a newly synthesized protein are extensive: the effects of molecular crowding within the cell (where the protein concentration is in the region of 300 mg/ml) make the exposure of hydrophobic regions potentially very dangerous. This is especially so for large multi-domain proteins requiring long stretches of amino acids to be translated before folding can be completed. The group of proteins termed ‘molecular chaperones’ have evolved to combat the dangers of protein aggregation, many of them acting by recognising misfolded or partially folded proteins via exposed hydrophobic regions. This provides the cell with a general mechanism for rescuing non-native proteins before irreversible aggregation occurs. For example, some Hsp 70 chaperones work co-translationally, binding directly to the ribosome thereby ensuring the efficient capture of nascent polypeptides, whilst others bind to their folding substrates post-translationally (reviewed by Hartl and Hayer-Hartl 2002). This system provides the cell with a level of quality control where the exposure of hydrophobic residues both during translation and during post-translational folding can be recognised by a single mechanism. However, some proteins face particular challenges during their folding pathways and require specific interactions with molecular chaperones. For example, the highly abundant proteins actin and tubulin both require interactions with the chaperonin containing TCP-1 (CCT), also called TCP-1 ring complex (TRiC), in order to reach their native states (Sternlicht et al 1993). In addition to folding actin and tubulin, CCT has also been shown to interact with other proteins including members of the WD repeat containing protein family (reviewed by Gomez-Puertas et al 2004). Many structural aspects of CCT are well understood: the use of cryoelectron single-particle microscopy has allowed not only for the subunit arrangement of the CCT oligomer to be solved (Martin-Benito et al 2007), but for a great deal of information about the binding sites of actin and tubulin to be elucidated (Llorca et al 1999; Llorca et al 2000; Llorca et al 2001). However, much is still unknown about the exact mechanism of folding by CCT and the range of CCT folding substrates in vivo remains a matter for debate. This review will give an overview of CCT structure and its range of substrates and focus on how the activity of CCT may influence cell cycle progression and cytoskeletal organisation.
Chaperonins
The family of molecular chaperones termed chaperonins can be categorised based on their sequences as either group I or group II chaperonins. The first includes the eubacterial chaperone GroEL and chaperonins from mitochondria and chloroplasts; the second includes the archaeal chaperonins and CCT, which is found in the cytoplasm of all eukaryotic cells and is essential in yeast (Stoldt et al 1996). All chaperonins are large, barrel-shaped oligomers consisting of two rings of subunits stacked back-to-back surrounding a central cavity. In the case of the eubacterial chaperonin GroEL, the term ‘Anfinsen Cage’ has been adopted (Ellis 1996) to describe the protective environment provided by this central cavity in which proteins can proceed to their native state.
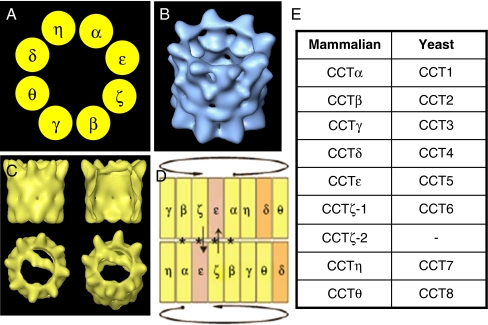
GroEL and the archaebacterial chaperonins are formed from one and three subunit species, respectively, whilst CCT consists of eight distinct subunits, which are the products of individual genes. The eight CCT subunits, named α, β, γ, δ, ɛ, ζ, η and θ (Cct1 to 8 in yeast), each occupy a fixed position in the chaperonin ring (Liou and Willison 1997) as shown in Fig. 1. Each consists of an equatorial domain that contains the ATP-binding site and both inter- and intra-ring contact sites, an apical, substrate-binding domain and an intermediate linker domain that relays nucleotide-induced conformational changes from the equatorial to the apical domains. These subunits display the most divergence in sequence in their apical, substrate-binding domains. Unlike GroEL, which is assisted by the lid-forming co-chaperone GroES, CCT has no such co-chaperone. Instead, a built-in lid is formed from helical protrusions that erupt from the apical domains of all eight CCT subunits and facilitate the encapsulation of folding substrates within the chaperonin cavity. The phasing of the two chaperonin rings has recently been solved using antibody labelling followed by cryoelectron single-particle microscopy and three-dimensional reconstructions (Martin-Benito et al 2007) as shown in Fig. 1. The proposed hierarchy amongst CCT subunits in their binding of ATP, as judged by mutational analysis in yeast (Lin and Sherman 1997) and by electron microscopy (Rivenzon-Segal et al 2005), is in good agreement with the overall structure of CCT. Therefore, the subunits thought to initiate ATP binding in one ring are in close proximity to those subunits at the end of this cascade in the other ring (Fig. 1).
Fig. 1.
Subunit arrangement and three-dimensional analysis of CCT. (A) Cartoon diagram to illustrate the positioning of the eight CCT subunits within the chaperonin ring according to (Liou and Willison 1997). (B) Cryoelectron single-particle reconstruction of the CCT oligomer (Llorca et al 2001) and (C) CCT–actin complexes with actin binding to CCT in an open conformation spanning the chaperonin cavity (Llorca et al 1999). (D) Inter- and intra-ring arrangement of CCT subunits as determined by immunolabelling and cryoelectron single-particle microscopy (Martin-Benito et al 2007). Circular arrows show the direction of intra-ring sequential changes (Lin and Sherman 1997). Asterisks indicate subunits that may be involved in possible inter-ring signalling, with a basic signalling mechanism indicated by arrows (Martin-Benito et al 2007). (E) Comparison of the nomenclature of CCT subunits in mammals and yeast
Substrate binding to CCT
Chaperonin containing TCP-1 is unique amongst the chaperonins in its composition of eight distinct subunits. Because these subunits each occupy fixed positions within the chaperonin ring, if one imagines CCT as a ‘binding interface’ for its substrates, these substrates are offered a complex array of sequence-specific and possibly geometry-specific binding sites.
It is probable that proteins interacting with CCT fall into several categories. First, the obligate folding substrates such as actin and tubulin that are dependent upon interactions with CCT in order to reach their native structure. Second, the opportunistic binding proteins-those that may on occasion make use of the chaperone activity of CCT but do not usually require interactions with CCT in order to reach their native state. Third, proteins that utilise CCT as a platform for complex assembly such as VHL tumor supressor protein (Mellville et al 2003), and fourth, proteins that regulate CCT activity.
Actin and tubulin
Unlike the eubacterial chaperonin GroEL, where the substrate recognition sequences are thought to be hydrophobic, there is a great deal of evidence to suggest that the interactions between CCT and its major folding substrates actin and tubulin are sequence-specific and electrostatic in nature. Surface loops in the actin sequence have been implicated in binding to CCT (Hynes and Willison 2000), and the substrate-binding region of the CCTγ subunit has been shown to be predominantly composed of polar and charged residues (Pappenberger et al 2002). Analysis of the other seven apical domains by sequence alignment indicates that this putative substrate-binding region is predominantly charged in all eight apical domains, whilst in GroEL, the corresponding region is hydrophobic (Pappenberger et al 2002). Further evidence for fundamental differences in how the Group I and Group II chaperonins process their substrates is demonstrated by in vitro experiments that have shown that substrate bound to GroEL remains protected from proteolysis (Weissman et al 1995). However, when actin is folded by CCT in rabbit reticulocyte lysate it is accessible to digestion by protease for at least part of its folding cycle (Grantham et al 2000).
The use of cryoelectron single-particle microscopy and three-dimensional reconstructions have provided detailed information not only about the overall arrangement of CCT architecture, but also regarding the way in which the major substrates, actin and tubulin, bind to and are folded by CCT (Llorca et al 1999; Llorca et al 2000; Llorca et al 2001). Actin and tubulin both bind to CCT in open conformations, binding directly to CCT subunits rather than simply being encompassed in the central cavity. In the case of actin, two CCT subunits are involved in binding at any one time and for tubulin, five CCT subunits, with both substrates displaying two modes of binding. In the absence of ATP, actin and tubulin are seen to bind to CCT in open conformations, with actin binding in a sequence-specific and geometry-dependent manner, interacting either with CCTδ and β or CCTδ and ɛ. Upon the binding of AMP-PNP to CCT, substrates become encapsulated by the built-in chaperonin lid and appear to move to one side of the chaperonin cavity, where the alignment of atomic structures with the substrate densities indicates that a near-native structure has been reached (Llorca et al 2001). The work of Neirynck et al (2006) is in agreement with the initial binding of actin to CCT occurring via CCTβ or CCTɛ followed by interactions with CCTδ, and identifies the actin-binding protein CAP as a possible co-factor in the final stages of actin folding.
Taken together, the binding of substrates to CCT in an electrostatic manner and the lack of complete encapsulation of substrate during the folding cycle are consistent with (at least in the case of actin and tubulin) CCT interacting with late-folding intermediates that have already formed a high degree of secondary structure. This theory is further supported by the work of Stemp et al (2005) and Pappenberger et al (2006) who both show that a stable-folding intermediate of actin formed in an Escherichia coli lysate in vitro translation system can reach its native form when in the presence of CCT. Furthermore, it is shown that CCT is necessary for the correct folding of newly synthesized actin and that the GroEL system cannot replace this activity (Stemp et al 2005).
The actin and tubulin monomers have very different native structures; however, they both need to interact with CCT in order to reach their native states. Why there is this dependency on CCT is not known; however, actin and tubulin both bind nucleotide, are very abundant proteins and form polymers. The way in which actin and tubulin bind to CCT via two binding sites gives obvious mechanical help to CCT in facilitating the movement of these substrates via conformational changes in the apical domains of CCT subunits. This would not be possible in the case of a substrate interacting with CCT via a single binding site. Whilst it is not known exactly what changes in conformation are achieved by actin and tubulin when interacting with CCT, it is probable that mechanical assistance from the CCT apical domains overcomes particular folding problems or holds these substrates in an open conformation such that nucleotide can bind. Indeed, in the case of tubulin, it has been demonstrated that the GTP-binding site becomes mature whilst tubulin is bound to CCT (reviewed by Lewis et al 1997).
Other CCT substrates
There are increasing reports of other proteins interacting with CCT. These include proteins involved in cell cycle regulation (Cdc20p, Cdh1p, Polo-like kinase 1 and cyclin E), the Von Hippel Lindau tumor suppressor protein and members of the WD repeat containing family (in particular those with seven bladed propellers), (reviewed by Valpuesta et al 2002; Horwich et al 2007). In the case of Cdc20p it was shown that binding to CCT occurs via the WD repeat region of the protein (Camasses et al 2003). Since actin and tubulin are the major folding substrates of CCT, their abundance may mask the binding of other much less abundant proteins in studies to identify other CCT substrates, making such substrates very difficult to detect, especially if their binding to CCT and release is rapid. However, studies that aim to trap folding substrates whilst bound to CCT may promote the binding of non-substrate proteins to CCT.
Caution should be taken when assigning a protein as a folding substrate for CCT based only upon observations of its binding to CCT. It is possible that over-expressed or tagged proteins may have a tendency to bind non-specifically to CCT. It has been demonstrated that several proteins in addition to actin and tubulin, when diluted out of denaturant, formed binary complexes with CCT, such as cofilin and actin depolymerising factor-1, but it is not known if these proteins interact with CCT in vivo (Melki et al 1997). Ideally, assays should be performed that can demonstrate the potential substrate protein can be released from CCT and then behave in a native fashion. For example, the elegant experiments of Pappenberger et al (2006) demonstrate that a folding intermediate of actin formed in an E. coli lysate system only reaches its native state upon binding and release by CCT. They were able to assess the native state of the actin released from CCT by both an antibody shift assay and a DNase I binding assay. Such assays are of great importance, confirming that the interaction of a protein with CCT has resulted in the adoption of a native conformation.
A specific chaperone and a general binding interface: a dual role for CCT
It is possible that some proteins utilise CCT as a general binding interface in a way similar to that of non-specific chaperones, binding only when an off-pathway folding event has occurred. Such an interaction may serve to stabilise vulnerable conformations that have exposed hydrophobic regions which, upon release from CCT (following ATP hydrolysis), are given the chance to refold to their native conformation. The analysis by Pappenberger et al (2002) of the amino acid composition of the lining of the CCT cavity shows that whilst there is an abundance of polar and charged residues (consistent with sequence-specific binding in the case of actin and tubulin), some CCT subunits do possess hydrophobic residues in this region (consistent with non-specific substrate binding). CCTα and CCTɛ (neighbours in the chaperonin ring) possess four and five hydrophobic residues, respectively, in the potential substrate-binding regions (Pappenberger et al 2002). It is therefore tempting to speculate that these two subunits could form a general binding site for hydrophobic interactions with substrates. Binding sites with substrates could also be formed with the hydrophobic amino acids found in the CCT helical protrusions, and the work of Spiess et al (2006) indicates that the Box1 region of VHL binds to CCT via a hydrophobic helix at the base of the helical protrusion, an interaction that is unique to Cct1p and Cct7p.
It would be of interest to determine the way in which proposed CCT substrates bind to CCT. One could predict that a substrate requiring specific input from CCT in order to overcome a particular folding difficulty or to be held in an appropriate conformation to allow ligand binding to occur may well bind in a specific orientation with more than one point of interaction with CCT, as demonstrated by actin and tubulin. On the other hand, the more opportunistic binding protein that is seeking assistance only when an off pathway event has occurred may instead bind without sequence specificity but via hydrophobic interactions. If the purpose of the interaction with CCT was simply to ‘buy time’ to give the substrate another chance to fold, it is probable that such an interaction would not require multiple binding sites with CCT.
Recent studies from several groups have demonstrated interactions between CCT and polyglutamine-expanded variants of the huntingtin protein (Behrends et al 2006; Kitamura et al 2006; Tam et al 2006). All of these studies demonstrated the ability of CCT to suppress the formation of polyglutamine aggregates. Tam et al (2006) discovered that over-expression of CCT1 (CCTα) and to a lesser degree, over expression of CCT4 (CCTδ) resulted in a change in aggregate morphology. Furthermore, the Cct1p apical domain was able to suppress polyglutamine aggregation in vitro, whereas other CCT apical domains had little or no effect. Apart from providing a stabilising binding interface, it is difficult to suggest how a lone apical domain can provide chaperone activity as it contains no ATP-binding site so it would not be able to undergo changes in conformation that would influence substrate folding. These observations indicate that subunits have differing capabilities in binding to aggregates. This suggests that CCT may well be providing two types of binding interface: that which utilises the charged residues of specific apical domains and a more general mode of binding that is able to recognise non-native hydrophobic regions.
CCT in vivo
Chaperonin containing TCP-1 is required for the production of native actin and tubulin and is also known to interact with a number of other proteins as described above. The integrity of the actin and tubulin-based cytoskeletal systems is essential in all cellular processes requiring cytoskeletal rearrangements, including cell division, normal and metastatic cell migration, growth cone guidance in neurons and developmental morphogenesis.
In tumor cells, the levels of CCT proteins are closely linked to the rates of cell growth (Yokota et al 1999) and CCT levels do not increase following neuronal differentiation in cultured cells (Roobol et al 1995). The levels of CCT subunits are not increased in the cellular response to heat shock, indicating that the primary function of CCT is in de novo protein folding during normal cell growth. Many proteins involved in the stress response utilise general mechanisms of recognition allowing them to interact with a wide range of unfolded proteins via exposed hydrophobic regions. Therefore, the lack of a requirement for increased CCT levels during the stress response is in keeping with CCT being required by a discreet subset of client proteins that require assistance to overcome particular folding issues.
CCT and cell cycle progression
Several proteins required for cell cycle progression have been shown to bind CCT, and extensive knock down of CCT by siRNA results in a block in cell cycle progression. Furthermore, the microinjection of an anti-CCTɛ antibody (that was shown to change the rate of actin and tubulin interactions with CCT upon translation in rabbit reticulocyte lysate) into cultured cells resulted in a delay in G1/S phase transition (Grantham et al 2006). Interestingly, these microinjection experiments demonstrated that subtle differences in the rates of actin and tubulin processing by CCT were sufficient to induce a significant delay of G1/S phase progression, suggesting that high levels of CCT activity are required by the cell. Considering that tubulin (the building block of the mitotic spindle) is a major CCT substrate, it is not surprising that CCT activity should be required for cell cycle progression. However, because CCT has also been shown to interact with Cdh1 and cyclin E (Camasses et al 2003; Passmore et al 2003; Won et al 1998), it is possible that altering interactions between CCT and these proteins is sufficient to delay cell cycle progression. It is important to note that cyclin E, which was found to bind to CCT in rabbit reticulocyte lysate and in cultured cells following removal of nucleotide to stabilise CCT–substrate interactions (Won et al 1998), was shown not to be dependent on interactions with CCT in order to be able to bind to Cdk-2 when translated in rabbit reticulocyte lysate in the presence of an anti-CCT antibody (Grantham et al 2006). Furthermore, Cyclin E was shown to gradually accumulate on CCT following translation in rabbit reticulocyte lysate rather than being processed in the same way that actin and tubulin are (Grantham et al 2006). This suggests that cyclin E may not rely on interactions with CCT to reach its native folded state.
Cdc20, which is required for cell cycle progression through mitosis via its activation of the anaphase-promoting complex, binds to CCT and is released in an ATP-dependent manner and requires these interactions with CCT in order to form complexes with anaphase-promoting complex (Camasses et al 2003). CCT was also shown to be required for the production of functional Polo-like kinase 1 (Liu et al 2005), which is required during late G2, indicating that CCT activity is important at different stages of the cell cycle. Yokota et al (1999) demonstrated that CCT is associated with tubulin in early S phase but not in G0/G1 arrested cells, and this was in line with increases in tubulin synthesis during cell cycle progression, whereas actin synthesis levels remained constant. It would appear therefore that at different stages of the cell cycle there is a need for CCT not only to deal with a constant flow of newly synthesized actin, but also to accommodate changes in levels of tubulin synthesis and other proteins that are expressed in a cell cycle-specific manner.
Dependency of actin and tubulin on CCT and implications for cytoskeletal organisation
Our recent work (Grantham et al 2006) described the effects of extensively reducing CCT levels by siRNA in cultured cells, where, in addition to a dramatic halt in cell cycle progression, a change in motility was observed. We assessed the number of proteins affected upon reducing levels of CCT by metabolic labelling followed by IEF PAGE. In contrast to the work of Horwich et al (1993) where the lack of the E. coli chaperonin GroEL resulted in 16 out of 35 proteins aggregating, we saw no such general loss of soluble proteins in cells where the CCT levels were reduced by approximately 90%. The levels of α- and β-tubulin were affected both at the level of protein synthesis and in terms of total protein. This is not surprising when one considers that the levels of tubulin are carefully regulated at the messenger RNA (mRNA) level (Cleveland and Sullivan 1985) and that the biogenesis of microtubules requires interactions from several chaperones, including CCT, and additional co-factors (reviewed by Lewis et al 1997). Therefore, this reduction in newly synthesized tubulin observed upon the reduction in CCT levels is probably due either to a direct reduction in tubulin synthesis or rapid degradation of non-native tubulin molecules. In contrast, we found that both the levels of total actin remained unchanged and that newly synthesised actin only declined slightly following a reduction in CCT levels for 3 days. However, the levels of native actin were much reduced and the actin cytoskeleton appeared disordered. It would therefore appear that unlike in the case of tubulin, the cell has no mechanism by which levels of non-native actin can be detected quickly, resulting in the shutdown of actin synthesis. Many cytoskeletal genes are regulated by a transcription factor named serum response factor (SRF), and G-actin is responsible for binding to and thereby inactivating MAL, a signal-regulated SRF cofactor (Posern and Treisman 2006). It is therefore likely that a disruption of levels of native actin could influence this system, especially as the actin gene itself is a target for SRF.
Considering that actin is a very abundant protein and is involved in an array of essential cellular functions, there appears to be a heavy reliance on CCT to ensure that actin is correctly folded. Perhaps even if a very small percentage of actin molecules were incorrectly folded, it would be easy to imagine that if such molecules could incorporate into microfilaments they may have far-reaching implications on the actin-based cytoskeletal system. A mutation in CCT4 (CCTδ) named anc2-1 in yeast results in a disorganised actin cytoskeleton and although microtubule structures appeared normal, an increase in sensitivity to benomyl was observed (Vinh and Drubin 1994). anc2-1 is a point mutation (G345D) located in the external surface of the apical (substrate-binding) domain of Cct4p (Llorca et al 1999). Shimon et al (2008) have demonstrated that this point mutation has a major effect on the allostery of the CCT ATPase, disrupting both inter- and intra-ring cooperativity. In vitro assays demonstrate that the production of native actin by this mutant CCT is reduced. Reduction in CCT levels by siRNA also leads to disorganisation of the actin-based cytoskeleton in mammalian cells (Grantham et al 2006). This could be due either to a lack of newly synthesized native actin monomers or due to an involvement of CCT directly in the organisation of the cytoskeleton. Indeed, in vitro, the CCT oligomer influences the kinetics of actin polymerisation with varying amounts of CCT subunits remaining associated with assembled actin filaments, whilst filament elongation assays using the short actin filaments of the red blood cell membrane-associated cytoskeleton as seeds for polymerisation indicated that CCT may act as a barbed-end capping protein (Grantham et al 2002). Additionally, muscle myosin has been shown to interact with CCT in rabbit reticulocyte lysate (Srikakulam and Winkelmann 1999) and misfolded myosin may also contribute to the disordered actin cytoskeleton observed when CCT levels are greatly reduced (Grantham et al 2006).
A potential role for CCT as monomers
Many studies regarding CCT utilise purified CCT oligomers, which appear to be quite stable. However, under more physiological conditions, in particular with respect to the concentration of ATP and potassium ions, the CCT oligomer is dynamic and able to undergo disassembly and reassembly (Roobol et al 1999a) and upon translation in rabbit reticulocyte lysate, CCT subunits are assembled into oligomers using CCT single-ring templates (Liou et al 1998).
Although it is clear that it is the CCT oligomer that is essential for the folding activity of actin and tubulin, there is increasing evidence that the CCT monomers themselves may have activity. Some CCT subunits (α, γ, ζ and θ) have been shown to behave as microtubule-associated proteins in vitro (Roobol et al 1999b) and the possibility of CCT involvement in cytoskeletal rearrangements is demonstrated by the work of Rademacher et al (1998), where growth of the yeast form of Candida albicans was found to be unaffected by over-expression of CCT8 (CCTθ) but hyphal morphogenesis (a process that requires assembly of actin and tubulin) was blocked. It is therefore possible that the role of CCT in the production of native actin and tubulin molecules extends beyond that of folding the newly synthesized actin and tubulin monomers and includes microtubule and microfilament organisation. CCTα localises at the centrosome (Brown et al 1996) and a microtubule structure unique to male germ cells, the manchette during spermiogenesis (Soues et al 2003). This suggests that either the CCT oligomer or the CCTα subunit in its monomeric form interacts with forms of assembled microtubule structures.
Intriguingly, over-expression of CCT6 (CCTζ) in Saccharomyces cerevisiae, which does not lead to an increase in the CCT oligomers, is able to suppress abnormal phenotypes caused by over-expression of Sit4p and Sap155p and by the conditional mutants tor2-21, lst8-2 and rsp5-9 (Kabir et al 2005). This suggests that the Cct6p monomer may be able to function by sequestering proteins that may normally bind to the CCT oligomer. These suppression activities were lost when the Cct6p ATP-binding motif was mutated, suggesting that a particular ‘active binding’ conformation of the CCT subunit determined by nucleotide binding may exist. The work of Tam et al (2006) demonstrated that the over-expression of either CCT1 or CCT4 subunits in yeast, whilst not resulting in an increase in the CCT oligomer, resulted in the formation of smaller polyglutamine aggregates and that in vitro, the apical domain of Cct1p suppressed polyglutamine aggregation. These observations are in support of CCT subunits in their monomeric form contributing to chaperone activity.
Regulation of CCT activity
A role for phosducin-like proteins as regulators of CCT activity has recently emerged. These proteins were first thought to be primarily involved in G-protein signalling as is phosducin, but it is now clear that unlike phosducin, which does not bind to CCT, this family of three related proteins, PhLP1, 2 and 3, have been shown to bind to CCT (reviewed by Willardson and Howlett 2007). Unlike classical CCT substrates, PhLP1, 2 and 3 bind to CCT in their native forms (McLaughlin et al 2002; Stirling et al 2006; Stirling et al 2007). PhLP1 has been shown to inhibit the folding of newly synthesised actin in vivo (McLaughlin et al 2002) and has been shown to interact with CCT via two different modes of binding, both bridging the central chaperonin cavity in a high arch rather than sitting within the central cavity (Martin-Benito et al 2004). Temperature sensitive mutants of the yeast homologue of PhLP2, PLP2, have been studied at their non-permissive temperatures where cytoskeletal and cell cycle defects are observed (Stirling et al 2007). This study demonstrated that these mutants are sensitive to both benomyl and latrunculin, suggesting that both microtubule and microfilament networks are affected, although budding defects and abnormal patch formation (indicating disrupted actin function or organisation) suggest that actin may be more severely affected than tubulin. plp2-1 mutants were shown to undergo a delay in DNA replication during cell cycle progression, suggesting that there is a link between CCT, Plp2p activity and the cell cycle (Stirling et al 2007). Deletions of PLP1, the yeast homologue of PhLP3, are able to rescue cells from the lethal over-expression of β-tubulin, presumably due to the excess tubulin not being correctly folded and therefore forming aggregates (Lacefield and Soloman 2003). Human PhLP3 was shown to form complexes with CCT–actin and CCT–tubulin and when present in excess, an inhibition of actin and tubulin folding was observed in vitro (Stirling et al 2006). The discovery of this group of proteins as potential regulators of CCT activity opens the way to understanding how the mechanisms of CCT action are coordinated.
CCT function is linked to an extensive range of cellular events
It is important to consider how much CCT is in the cell when assessing the range of potential CCT substrates and its function in vivo. The CCT oligomer is required to process newly synthesized molecules of the highly abundant proteins actin and tubulin as well as less abundant folding substrates such as Cdh1p. It is not possible to over-express actin and tubulin significantly (Weinstein and Soloman 1990), and this may be due to a ‘bottleneck’ effect created by the requirements of these two proteins to interact with CCT, suggesting that the chaperonin system is functioning at maximum capacity. Pappenberger et al (2006) estimate that in a haploid yeast cell the number of CCT oligomers is between 1,500 and 2,000. An important question is how many of these CCT oligomers are occupied with substrate interactions at any one time? Futcher et al (1999) calculated that 54 actin mRNA molecules were present in S. cerevisiae. In S. cerevisiae it is estimated that there are seven ribosomes bound to an actin mRNA molecule, indicating almost 400 newly synthesised actin molecules being present (Arava et al 2003) whilst Schizosaccharomyces pombe is estimated to have 5.4 ribosomes bound per actin mRNA (Lackner et al 2007). However, if actin translation was very active, with a maximum of 14 ribosomes bound per mRNA (i.e. one ribosome every 80 nucleotides), then the number of newly synthesised actin molecules present at any one time could be in the region of 760. If this were the case, then almost 50% of CCT oligomers may be occupied by actin. It is interesting to note that the study by Arava et al (2003) estimates that the synthesis rate of actin is more than 300 times higher than that of cdc20 (a low-abundance CCT substrate).
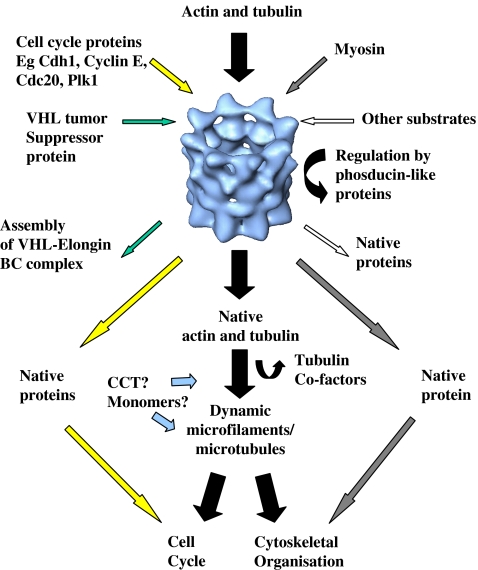
As shown in Fig. 2, CCT is central to the production of native actin and tubulin whilst also dealing with other folding substrates (some of which are produced in a cell cycle specific manner). This, taken together with the possibility that CCT (either in its oligomeric form or as individual subunits) may have a role in cytoskeletal organisation, demonstrates the importance of CCT activity for a vast array of essential cellular processes.
Fig. 2.
CCT coordinates an array of cellular functions via its folding substrates. CCT (shown here as a 3-dimensional reconstruction from single-particle electron microscopy, Llorca et al 2001) must be able to accommodate the folding of the major substrates actin and tubulin with additional substrates, some of which are required during cell cycle progression. Coloured arrows track the interactions of proteins with CCT
Acknowledgements
We acknowledge grants from Vetenskapsrådet, Assar Gabrielssons Fond and Carl Tryggers Stiftelse. We thank Thomas Nyström for comments on the manuscript and Per Sunnerhagen for helpful discussions.
References
- Arava Y, Wang Y, Storey JD, Liu CL, Brown PO, Herschlag D. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2003;100:3889–3894. doi: 10.1073/pnas.0635171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C, Langer CA, Boteva R, et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into non toxic oligomers. Mol Cell. 2006;23:887–897. doi: 10.1016/j.molcel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Brown CR, Doxsey SJ, Hong-Brown LQ, Martin RL, Welch WJ. Molecular chaperones and the centrosome: a role for TCP-1 in microtubule nucleation. J Biol Chem. 1996;271:824–832. doi: 10.1074/jbc.271.2.824. [DOI] [PubMed] [Google Scholar]
- Camasses A, Bogdanova A, Shevchenko A, Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell. 2003;12:87–100. doi: 10.1016/S1097-2765(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Sullivan KF. Molecular biology and genetics of tubulin. Ann Rev Biochem. 1985;54:331–366. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Revisiting the Anfinsen cage. Fold Des. 1996;1:R9–R15. doi: 10.1016/S1359-0278(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Futcher B, Latter GI, Monardo P, Mclaughlin CS, Garrels JI. A sampling of the yeast proteome. Mol Cell Biol. 1999;19:7357–7368. doi: 10.1128/mcb.19.11.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Puertas P, Martin-Benito J, Carrascosa JL, Willison KR, Valpuesta JM. The substrate recognition mechanisms in chaperonins. J Mol Recognit. 2004;17:85–94. doi: 10.1002/jmr.654. [DOI] [PubMed] [Google Scholar]
- Grantham J, Llorca O, Valpuesta JM, Willison KR. Partial occlusion of both cavities of the eukaryotic chaperonin with antibody has no effect upon the rates of beta-actin or alpha-tubulin folding. J Biol Chem. 2000;275:4587–4591. doi: 10.1074/jbc.275.7.4587. [DOI] [PubMed] [Google Scholar]
- Grantham J, Ruddock LW, Roobol A, Carden MJ. Eukaryotic chaperonin containing T-complex polypeptide 1 interacts with filamentous actin and reduces the initial rate of actin polymerization in vitro. Cell Stress Chaperones. 2002;7:235–242. doi: 10.1379/1466-1268(2002)007<0235:ECCTCP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J, Brackley KI, Willison KR. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res. 2006;312:2309–2324. doi: 10.1016/j.yexcr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Fenton WA, Chapman E, Farr GW. Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Low KB, Fenton WA, Hirshfield IN, Furtak K. Folding in vivo of bacterial cytoplasmic proteins: role of GroEL. Cell. 1993;74:909–917. doi: 10.1016/0092-8674(93)90470-B. [DOI] [PubMed] [Google Scholar]
- Hynes GM, Willison KR. Individual subunits of the eukaryotic cytosolic chaperonin mediate interactions with binding sites located on subdomains of beta-actin. J Biol Chem. 2000;275:18985–18994. doi: 10.1074/jbc.M910297199. [DOI] [PubMed] [Google Scholar]
- Kabir MA, Kaminska J, Segel GB, et al. Physiological effects of unassembled chaperonin Cct subunits in the yeast Saccharomyces cerevisiae. Yeast. 2005;22:219–239. doi: 10.1002/yea.1210. [DOI] [PubMed] [Google Scholar]
- Kitamura A, Kubota H, Pack C-G, et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol. 2006;8:1163–1170. doi: 10.1038/ncb1478. [DOI] [PubMed] [Google Scholar]
- Lacefield S, Soloman F. A novel step in beta-tubulin folding is important for heterodimer formation in Saccharomyces cerevisiae. Genetics. 2003;165:531–541. doi: 10.1093/genetics/165.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner DH, Beilharz TH, Marguerat S, Mata S, Watt S, Schubert F, Preiss T, Bähler J. A network of multiple regulatory layers shapes gene expression in fisson yeast. Mol Cell. 2007;26:145–155. doi: 10.1016/j.molcel.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Tian G, Cowan NJ. The alpha- and beta-tubulin folding pathways. Trends Cell Biol. 1997;7:479–484. doi: 10.1016/S0962-8924(97)01168-9. [DOI] [PubMed] [Google Scholar]
- Lin P, Sherman F. The unique hetero-oligomeric nature of the subunits in the catalytic cooperativity of the yeast Cct chaperonin complex. Proc Natl Acad Sci U S A. 1997;94:10780–10785. doi: 10.1073/pnas.94.20.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AKF, Willison KR. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of CCT micro-complexes. EMBO J. 1997;16:4311–4316. doi: 10.1093/emboj/16.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AKF, McCormack EA, Willison KR. The chaperonin containing TCP-1 (CCT) displays a single-ring mediated disassembly and reassembly cycle. Biol Chem. 1998;379:311–319. doi: 10.1515/bchm.1998.379.3.311. [DOI] [PubMed] [Google Scholar]
- Liu X, Lin C-Y, Lei M, Lan S, Zhou T, Erikson RL. CCT chaperonin complex is required for the biogenesis of functional Plk1. Mol Cell Biol. 2005;25:4993–5010. doi: 10.1128/MCB.25.12.4993-5010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, McCormack EA, Hynes G, et al. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 1999;402:693–696. doi: 10.1038/45294. [DOI] [PubMed] [Google Scholar]
- Llorca O, Martin-Benito J, Ritco-Vonsovici M, Grantham J, Hynes GM, Willison KR, Carrascosa JL, Valpuesta JM. Eukaryotic chaperonin CCT stabilizes actin and tubulin folding intermediates in open quasi-native conformations. EMBO J. 2000;19:5971–5979. doi: 10.1093/emboj/19.22.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, Martin-Benito J, Grantham J, Ritco-Vonsovici M, Willison KR, Carrascosa JL Valpuesta JM. The ‘sequential allosteric ring’ mechanism in the eukaryotic chaperonin- assisted folding of actin and tubulin. EMBO J. 2001;20:4065–4075. doi: 10.1093/emboj/20.15.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Benito J, Bertrand S, Hu T, Ludtke PJ, McLaughlin JN, Willardson BM, Carrascosa JL, Valpuesta JM. Structure of the complex between the cytosolic chaperonin CCT and phosducin-like protein. Proc Natl Acad Sci U S A. 2004;101:17410–17415. doi: 10.1073/pnas.0405070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Benito J, Grantham J, Boskovic J, Brackley KI, Carrascosa JL, Willison KR, Valpuesta JM. The inter-ring arrangement of the cytosolic chaperonin CCT. EMBO Rep. 2007;8:252–257. doi: 10.1038/sj.embor.7400894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JN, Thulin CD, Hart SJ, Resing KA, Ahn NG, Willardson BM. Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc Natl Acad Sci U S A. 2002;99:7962–7967. doi: 10.1073/pnas.112075699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R, Batelier G, Soulie S, Williams RC. Cytoplasmic chaperonin containing TCP-1: structural and functional characterisation. Biochemistry. 1997;36:5817–5826. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
- Mellville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Linau tumor suppressor complex. Mol Cell Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirynck K, Waterschoot D, Vandekerckhove J, Ampe C, Rommelaere H. Actin interacts with CCT via discrete binding sites: a binding transition-release model for CCT mediated actin folding. J Mol Biol. 2006;355:124–138. doi: 10.1016/j.jmb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Pappenberger G, Wilsher JA, Roe SM, Counsell DJ, Willison KR, Pearl LH. Crystal structure of the CCTgamma apical domain: implications for substrate binding to the eukaryotic cytosolic chaperonin. J Mol Biol. 2002;318:1367–1379. doi: 10.1016/S0022-2836(02)00190-0. [DOI] [PubMed] [Google Scholar]
- Pappenberger G, McCormack EA, Willison KR. Quantitative actin folding reactions using yeast CCT purified via an internal tag in the CCT3/γ subunit. J Mol Biol. 2006;360:484–496. doi: 10.1016/j.jmb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Passmore LA, McCormack EA, Au SWN, Paul A, Willison KR, Harper JW, Barford D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003;22:786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Triesman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Rademacher F, Kehren V, Stoldt VR, Ernst JF. A Candida albicans chaperonin subunit (CaCct8p) as a suppressor of morphogenesis and Ras phenotypes in C. albicans and Saccharomyces cerevisiae. Microbiology. 1998;144:2951–2960. doi: 10.1099/00221287-144-11-2951. [DOI] [PubMed] [Google Scholar]
- Roobol A, Holmes FE, Hayes NVL, Baines AJ, Carden MJ. Cytoplasmic chaperonin complexes enter neurites developing in vitro and differ in subunit composition within single cells. J Cell Sci. 1995;108:1477–1488. doi: 10.1242/jcs.108.4.1477. [DOI] [PubMed] [Google Scholar]
- Roobol A, Grantham J, Whitaker HC, Carden MJ. Disassembly of the cytosolic chaperonin in mammalian cell extracts at intracellular levels of K + and ATP. J Biol Chem. 1999;274:19220–19227. doi: 10.1074/jbc.274.27.19220. [DOI] [PubMed] [Google Scholar]
- Roobol A, Sahyoun ZP, Carden MJ. Selected subunits of the cytosolic chaperonin associate with microtubules assembled in vitro. J Biol Chem. 1999;274:2408–2415. doi: 10.1074/jbc.274.4.2408. [DOI] [PubMed] [Google Scholar]
- Rivenzon-Segal D, Wolf SG, Shimon L, Willison KR, Horovitz A. Sequential ATP-induced allosteric transitions of the cytoplasmic chaperonin containing TCP-1 revealed by EM analysis. Nat Struct Biol. 2005;12:233–237. doi: 10.1038/nsmb901. [DOI] [PubMed] [Google Scholar]
- Shimon L, Hynes GM, McCormack EA, Willison KR Horovitz A. ATP-induced allostery in the eukaryotic chaperonin CCT is abolished by the mutation G345D in CCT4 that renders yeast temperature sensitive for growth. J Mol Biol. 2008;377:469–477. doi: 10.1016/j.jmb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Soues S, Kann M-L, Fouquet J-P, Melki R. The cytosolic chaperonin CCT associates to cytoplasmic microtubular structures during mammalian spermiogenesis and to heterochromatin in germline and somatic cells. Exp Cell Res. 2003;288:363–373. doi: 10.1016/S0014-4827(03)00248-9. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–27273. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- Stemp MJ, Guha S, Hartl FU, Barral JM. Efficient production of native actin upon translation in a bacterial lysate supplemented with the eukaryotic chaperonin TRiC. Biol Chem. 2005;386:753–757. doi: 10.1515/BC.2005.088. [DOI] [PubMed] [Google Scholar]
- Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB. The t-complex polypeptide 1 is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling PC, Cuellar J, Alfaro GA, Khadali FE, Beh CT, Valpuesta JM, Melki R, Leroux MR. PhLP3 Modulates CCT-mediated actin and tubulin folding via ternary complexes with substrate. J Biol Chem. 2006;281:7012–7021. doi: 10.1074/jbc.M513235200. [DOI] [PubMed] [Google Scholar]
- Stirling PC, Srayko M, Takhar KS, Pozniakovsky A, Hyman AA, Leroux MR. Functional interaction between Phosducin-like Protein 2 and cytosolic chaperonin is essential for cytoskeletal protein function and cell cycle progression. Mol Biol Cell. 2007;18:2336–2345. doi: 10.1091/mbc.E07-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoldt V, Rademacher F, Kehren V, Ernst JF, Pearce DA, Sherman F. Review: the Cct eukaryotic chaperonin subunits of Saccharomyces cerevisiae and other yeasts. Yeast. 1996;12:523–529. doi: 10.1002/(SICI)1097-0061(199605)12:6<523::AID-YEA962>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tam S, Geller R, Speiss C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol. 2006;8:1155–1162. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valpuesta JM, Martin-Benito J, Gomez-Puertas P, Carrascosa JL, Willison KR. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 2002;529:11–16. doi: 10.1016/S0014-5793(02)03180-0. [DOI] [PubMed] [Google Scholar]
- Vinh DB, Drubin DG. A yeast TCP-1-like protein is required for actin function in vivo. Proc Natl Acad Sci U S A. 1994;91:9116–9120. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B, Soloman F. Phenotypic consequences of tubulin overproduction in Saccharomyces cerevisiae: differences between alpha-tubulin and beta-tubulin. Mol Cell Biol. 1990;10:5295–5304. doi: 10.1128/mcb.10.10.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JS, Hohl CM, Kovalenko O, et al. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signalling and chaperone assisted folding. Cell Signal. 2007;19:2417–2427. doi: 10.1016/j.cellsig.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KA, Schumacher RJ, Farr GW, Horwich AL, Reed SI. Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT. Mol Cell Biol. 1998;18:7584–7589. doi: 10.1128/mcb.18.12.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth. J Biol Chem. 1999;274:37070–37078. doi: 10.1074/jbc.274.52.37070. [DOI] [PubMed] [Google Scholar]